Abstract
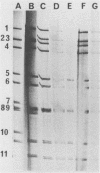
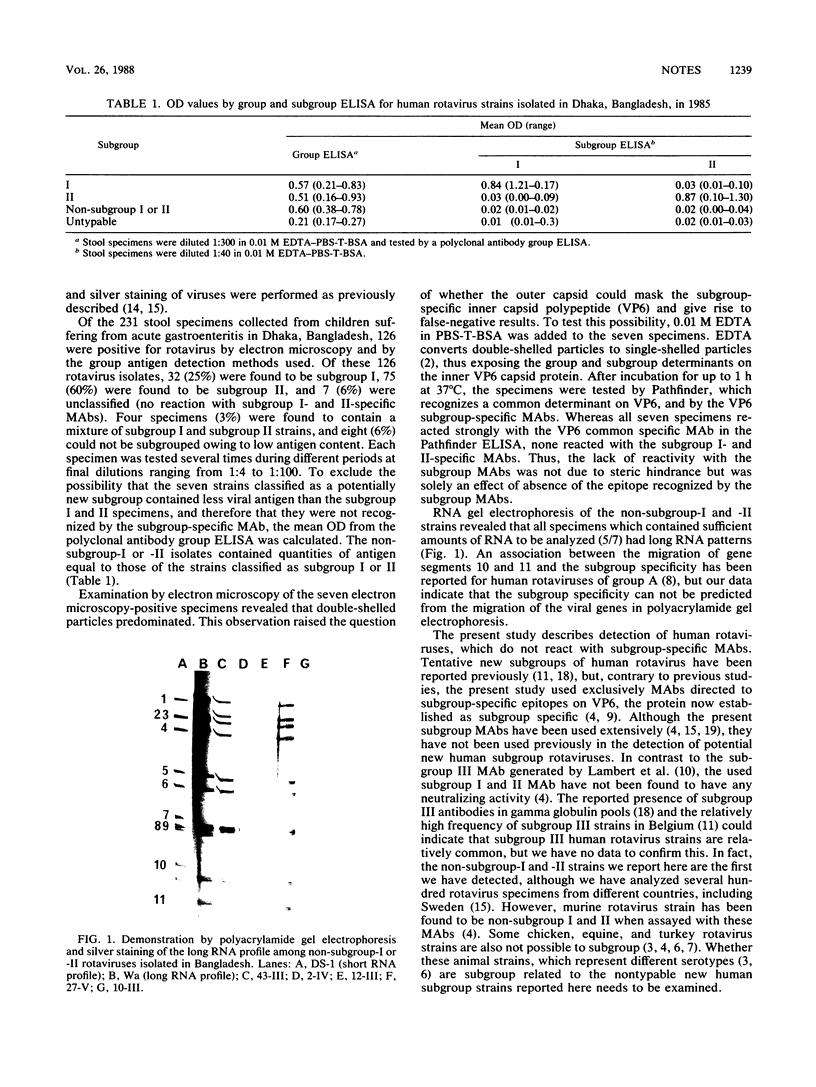
Of 126 rotavirus-positive specimens, 7 could not be subgrouped (I or II). These strains showed a distinct reaction with a monoclonal antibody recognizing a common region on VP6, but they did not react with VP6 subgroup-specific monoclonal antibodies although they contained as much viral antigen as the subgrouped strains.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark H. F., Hoshino Y., Bell L. M., Groff J., Hess G., Bachman P., Offit P. A. Rotavirus isolate WI61 representing a presumptive new human serotype. J Clin Microbiol. 1987 Sep;25(9):1757–1762. doi: 10.1128/jcm.25.9.1757-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Isolation, propagation, and characterization of a second equine rotavirus serotype. Infect Immun. 1983 Sep;41(3):1031–1037. doi: 10.1128/iai.41.3.1031-1037.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Espejo R. T., Flores J., Wyatt R. G., Kapikian A. Z., Chanock R. M. Distinctive ribonucleic acid patterns of human rotavirus subgroups 1 and 2. Infect Immun. 1981 Sep;33(3):958–961. doi: 10.1128/iai.33.3.958-961.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Lambert J. P., Marbehant P., Marissens D., Zissis G. Monoclonal antibodies directed against different antigenic determinants of rotavirus. J Virol. 1984 Jul;51(1):47–51. doi: 10.1128/jvi.51.1.47-51.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. P., Marissens D., Marbehant P., Zissis G. Prevalence of subgroup 1, 2, and 3 rotaviruses in Belgian children suffering from acute diarrhea (1978-1981). J Med Virol. 1983;11(1):31–38. doi: 10.1002/jmv.1890110105. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L., Grandien M., Pettersson C. A. Comparison of solid-phase immune electron microscopy by use of protein A with direct electron microscopy and enzyme-linked immunosorbent assay for detection of rotavirus in stool. J Clin Microbiol. 1983 Nov;18(5):1244–1249. doi: 10.1128/jcm.18.5.1244-1249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L., Uhnoo I., Grandien M., Wadell G. Molecular epidemiology of rotavirus infections in Uppsala, Sweden, 1981: disappearance of a predominant electropherotype. J Med Virol. 1986 Feb;18(2):101–111. doi: 10.1002/jmv.1890180202. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985 May;66(Pt 5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Urasawa S. Reactivity patterns to human rotavirus strains of a monoclonal antibody against VP2, a component of the inner capsid of rotavirus. Brief report. Arch Virol. 1986;87(1-2):135–141. doi: 10.1007/BF01310550. [DOI] [PubMed] [Google Scholar]
- Tufvesson B. Detection of a human rotavirus strain different from types 1 and 2--a new subgroup? Epidemiology of subgroups in a Swedish and an Ethiopian community. J Med Virol. 1983;12(2):111–117. doi: 10.1002/jmv.1890120205. [DOI] [PubMed] [Google Scholar]
- White L., Perez I., Perez M., Urbina G., Greenberg H., Kapikian A., Flores J. Relative frequency of rotavirus subgroups 1 and 2 in Venezuelan children with gastroenteritis as assayed with monoclonal antibodies. J Clin Microbiol. 1984 Apr;19(4):516–520. doi: 10.1128/jcm.19.4.516-520.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]