Abstract
Neprilysin is a transmembrane metalloendopeptidase that degrades neuropeptides that are important for both growth and contraction. In addition to promoting carcinogenesis, decreased levels of neprilysin increases inflammation and neuroendocrine cell hyperplasia, which may predispose to vascular remodeling. Early pharmacological studies showed a decrease in chronic hypoxic pulmonary hypertension with neprilysin inhibition. We used a genetic approach to test the alternate hypothesis that neprilysin depletion increases chronic hypoxic pulmonary hypertension. Loss of neprilysin had no effect on baseline airway or alveolar wall architecture, vessel density, cardiac function, hematocrit, or other relevant peptidases. Only lung neuroendocrine cell hyperplasia and a subtle neuropeptide imbalance were found. After chronic hypoxia, neprilysin-null mice exhibited exaggerated pulmonary hypertension and striking increases in muscularization of distal vessels. Subtle thickening of proximal media/adventitia not typically seen in mice was also detected. In contrast, adaptive right ventricular hypertrophy was less than anticipated. Hypoxic wild-type pulmonary vessels displayed close temporal and spatial relationships between decreased neprilysin and increased cell growth. Smooth muscle cells from neprilysin-null pulmonary arteries had increased proliferation compared with controls, which was decreased by neprilysin replacement. These data suggest that neprilysin may be protective against chronic hypoxic pulmonary hypertension in the lung, at least in part by attenuating the growth of smooth muscle cells. Lung-targeted strategies to increase neprilysin levels could have therapeutic benefits in the treatment of this disorder.
Chronic hypoxic pulmonary hypertension (PHTN) is a major clinical problem, complicating most lung and heart disorders.1,2 In large animal models of chronic hypoxic PHTN that closely resemble human disease, the earliest pulmonary artery (PA) smooth muscle cell (SMC) proliferative changes occur at the medial/adventitial border.3 Growth and migration of SMC and myofibroblasts in distal vessels is also a prominent feature.4,5 These structural changes, together with derangements in vascular tone, are major contributors to the severity of chronic hypoxic PHTN.1,2,3,4,5,6 However, mechanisms that regulate susceptibility to, and severity of, chronic hypoxic PHTN and vascular remodeling remain poorly understood. Currently available treatments for chronic hypoxic PHTN are also inadequate.
Mouse models of chronic PHTN have provided many insights into pathogenesis.7,8 Murine susceptibility to chronic hypoxic PHTN depends on genetic background.5 Additionally, inflammation due to viral infection, hypoxia, or other forms of injury is important.9,10 Targeted manipulation of selected genes can increase the acute or chronic PHTN response to hypoxia.11 Some models are notable for a modest rise in baseline right ventricular (RV) pressure,8,12 while others are not.13,14,15 Typically, a proportional RV hypertrophic response is observed. However, there is a precedent for uncoupling of the pulmonary vascular and cardiac responses suggesting independent or tissue-specific regulatory mechanisms may be operative.16,17 Interestingly, a unique paradigm, that of exaggerated PHTN and vascular remodeling together with less than expected RV hypertrophy, has not to our knowledge been reported.
Neprilysin (NEP; neutral endopeptidase; CD10) is a transmembrane metallopeptidase present in the lung, brush-border membrane of renal tubules, intestine, adrenal gland, brain, heart, and peripheral blood vessels.18,19 Within the lung vasculature, NEP is expressed in SMCs, fibroblasts, and endothelial cells. NEP hydrolyzes bioactive neuropeptides, including bombesin-like peptides (BLPs), endothelin-1 (ET-1), and substance P (sub P).20 Four other enzymes found in the lung [angiotensin converting enzyme (ACE), endothelin converting enzyme (ECE), aminopeptidase N, and dipeptidyl peptidase IV (DPPIV)] share some substrates with NEP.21 Thus, NEP contributes to the maintenance of a delicate balance of neuropeptides in the lung and elsewhere; disruption of that balance could alter susceptibility to hypoxic injury.22,23
The role of NEP in chronic hypoxic PHTN remains uncertain. Early studies, conducted with inhibitors of NEP, suggested that this peptidase may contribute to chronic hypoxic PHTN.24,25 However, recent observations in other systems support the possibility that NEP could actually be protective against PHTN, through both peptidase-dependent (eg, degradation of selected vasoactive neuropeptides) and peptidase-independent (eg, complex formation of NEP’s intracellular cytosolic domain with signaling molecules) mechanisms.26 Even the peptidase-dependent effects may extend beyond neuropeptide targets.27 Because lung NEP expression and activity varies widely in humans,28 we speculate that individuals could differ in their susceptibility to chronic hypoxic PHTN depending on their level of NEP expression/activity.
Early NEP inhibitors may have had both on- and off-target effects, due in part to local bioavailability and specificity for NEP versus other peptidases. Newer NEP antagonists have been tested alone and in combination with ACE and ECE inhibitors for their cardioprotective effects. These agents have been shown to improve cardiac function, limit cardiac hypertrophy and decrease systemic blood pressure.29,30,31,32 Even these newer NEP inhibitors may have complex effects. The use of gene deletion of NEP could help reconcile these divergent observations.33 Studies with NEP null mice have already suggested an important role for NEP in the regulation of systemic blood pressure, permeability, inflammation, and amyloid β protein levels.33,34,35
A number of observations link neuroendocrine cell (NEC) hyperplasia, NEP inhibition, and PHTN. NECs are present within the airway epithelium of the lung, often adjacent to small pulmonary vessels.36 They synthesize and secrete a variety of neuropeptides and amines, including BLPs, ET-1, 5-HT, and likely sub P.37,38 Hyperplasia of lung NECs has been described in association with exposure to injurious stimuli, leading to structural remodeling of the PA wall and PHTN.37,39,40,41,42,43,44 In addition, hyperplasia of NECs has been detected in response to NEP inhibition.45 Thus, a strong link exists between NEC hyperplasia, NEP inhibition, and settings where increased hypoxic PHTN is observed; however, to our knowledge, these factors have not been reported together in a genetically defined mouse model.
The goal of this study was to use a genetic approach to determine whether a role for NEP exists in the regulation of susceptibility to and the development of chronic hypoxic PHTN. We tested the hypothesis that NEP protects the lung vasculature from the development of PHTN in response to chronic hypoxia at least in part by suppressing the growth of PA SMCs. Our approach was to first carefully examine important baseline normoxic features of wild-type (NEP+/+) and NEP null (NEP−/−) mouse lung and heart. Then, we tested the effect of NEP deletion on susceptibility to chronic hypoxic PHTN and vascular remodeling. We defined temporal and spatial changes in NEP expression in response to hypoxia in NEP+/+ mice, and compared the pattern of medial/adventitial remodeling and growth of PA SMCs from NEP+/+ and NEP−/− mice. Finally, we tested the effect of NEP replacement strategies (recombinant and lentiviral) on growth of PA SMCs from NEP−/− mice.
Materials and Methods
Materials
Recombinant human NEP (rNEP) was provided by Dr. Catherine Magill, AXYS Pharmaceuticals, Inc (South San Francisco, CA). Other chemicals were from Sigma (St. Louis, MO) unless specified.
Animals
Breeder mice for the C57BL/6 NEP null (NEP−/−) colony were originally provided by Drs. NP and C Gerard.33 C57BL/6 NEP+/+ littermate controls and NEP−/− mice were born at Denver altitude. Additional wild-type (NEP+/+) mice were obtained from Jackson Laboratories (Bar Harbor, ME) and Harlan (Indianapolis, IN). At 11 to 14 weeks of age, mice were randomly assigned to normoxic (Nx) and hypoxic (Hx) treatment groups. Each gender was equally represented. Approval of animal protocols was obtained from the University of Colorado and Denver VA Medical Center Institutional Animal Care and Use Committees.
Genotyping
Amplification of genomic DNA from mouse tail was performed using primer sets specific for NEP and Neomycin (NEP forward: 5′-CCAAACTTAAGCCTATTCTTAC-3′, reverse: 5′-CCATTATGAACCTCCAGGAC-3′ and Neomycin forward: 5′-GATGGATTGCACGCAGGTTCT-3′) according to the following PCR protocol: 94°C for 45 seconds, 55°C for 1 minute, 72°C for 2 minutes plus 10 seconds’ autoextension repeated for 30 cycles, and 1 cycle of 72°C for 3 minutes (Figure 1). NEP expression was confirmed in mouse lung by demonstrating the presence or absence of NEP by immunoblot (as shown in Figure 1) and immunostaining. Finally, NEP activity was detected in lung lysate from NEP+/+, but not NEP−/−, mice.
Figure 1.
Genotyping and Western analysis of NEP+/+ vs NEP−/− mice. A: Results of genotyping NEP+/+ and NEP−/− mouse tail DNA by PCR shows an NEP band of 200 bp and a Neomycin band of 1400 bp. B: Western analysis of mouse lung homogenates with an NEP antibody shows a protein band of 100 kDa in NEP+/+, but not NEP−/−, samples (30 μg/lane).
Hypoxia Exposure
NEP+/+ and NEP−/− mice were exposed to normoxia (5200 ft, Denver altitude) or hypoxia (18,000 ft, hypobaric chamber) for up to 5 weeks with interruptions of <1 hour every 3 to 4 days for animal maintenance. In preliminary studies, C57BL/6 mice were maintained in a simulated sea level chamber (hyperbaric normoxia; pressurized to 1 atmosphere) for 5 weeks and the impact of Denver altitude on RSVP was found to be minimal (1 to 3 mmHg). Simulated sea level was achieved by the use of inline compressed air directed through a chamber fabricated with fixed and variable resistance valves. In parallel with the fixed leak from the chamber was a solenoid valve controlled leak. A Stratham transducer regulated the solenoid valve to maintain pressure in the chamber at 5.8 inches of mercury relative to Denver altitude (equal to sea level). The air in the chamber exchanged at least 10 times per hour.
Invasive Hemodynamic Measurements
At the end of normoxic or hypoxic exposures, mice were anesthetized with Ketamine-Rompun (100 and 15 mg/kg; Fort Dodge, Madison, NJ and Miles Laboratories, Elkhart, IN), and while spontaneously breathing, underwent closed-chest measurements of right ventricular systolic pressure (RVSP) using a pressure transducer (Statham; n = 3 to 5 measurements/mouse) as previously described.17,46,47 In preliminary studies, this transthoracic approach yielded the same RVSP results as an intravascular Millar catheter technique with more consistency and less time expended. The transthoracic approach was therefore used routinely here.
Baseline Echocardiographic Assessment
Transthoracic echocardiography was performed using 10 and 13 MHz ultrasound probes with a Vivid Five System (General Electrics Vingmed Ultrasound, Horton, Norway). Data were analyzed with EchoPac 6.3.6 software.17 The mice were anesthetized with Ketamine-Rompun. Left heart dimensions were obtained in short-axis view. Intraluminal diameter of PA and flow in the PA was obtained in the parasternal longitudinal axis. Cardiac output and index were calculated using standardized methods.48 Echocardiographic measurements were only made as part of the baseline assessment.
Blood and Tissue Harvesting
After hemodynamic measurements were completed, blood was collected from the RV via percutaneous stick, for measurement of hematocrit and preparation of serum. Then the chest was opened and 100 units of heparin were injected into the RV. After gentle perfusion of the pulmonary circulation with PBS (pressure = 40 cmH2O), the left lung was removed and quick-frozen in liquid nitrogen. The right lung was inflated to an airway pressure of 30 cmH20 with a mixture of 1% prewarmed agarose (GIBCO, Grand Island, NY) and 4% paraformaldehyde in PBS before preparation of sections for staining.17
Measurement of RV Hypertrophy
Heart ventricles were dissected, dried for 7 days, and weighed.17 Hearts not dissected were fixed with paraformaldehyde and either processed for histological analysis with H&E to assess cardiac structure or dissected and examined for valvular and PA outflow tract anomalies.
Quantitation of Neuropeptide Levels by RIA/Enzyme Immunoassay
Lung lysates and serum from NEP+/+ and NEP−/− mice exposed to normoxia and 3 and 7 days hypoxia were prepared for quantitation of selected neuropeptides. BLP levels were measured by RIA; ET-1 and sub P levels were measured by enzyme immunoassay (all from Phoenix Pharmaceuticals, St. Joseph, MO).
Tissue and Immunostaining
Paraformaldehyde-fixed, paraffin-embedded sections (4 μm) of mouse lung and heart tissues were prepared and routinely stained with H&E or for increased definition of distal airway structure in selected cases immunostained for pan-cytokeratin (AE1/AE3, Ventana, Tucson, AZ).17 For immunostaining of lung sections, antigen retrieval with citrate buffer was performed and tissue was blocked with 1% H2O2, avidin, biotin, and/or mouse-on-mouse blocking reagent (Vector Lab, Burlingame, CA), as appropriate. Sections were incubated with diluted primary antibodies: anti-α-SMA (Thermo, Waltham, MA), anti-Von Willebrand Factor VIII (Dako, Glostrup, Denmark), anti-human NEP (RDI Systems, Flauder, NJ), anti-mouse Ki-67 Tec3 (Dako), or anti-rat calcitonin gene-related peptide to localize NECs (Peninsula Laboratories, San Carlos, CA). In selected experiments, sections from lungs were fixed with methyl Carnoy’s solution and incubated with anti-smooth muscle-specific myosin heavy chain (Dr. Maria Frid, University of Colorado, Denver, CO). The tissue was then incubated in appropriate secondary antibody. For α-SMA and calcitonin gene-related peptide detection, sections were stained with alkaline phosphatase (Vector Lab). For all other antigens, detection was with the ABC method (Vector) followed by diaminobenzidine (Biogenex, San Ramon, CA). Slides were counterstained with Gill’s Hematoxylin #1. MicroBrightfield digital image analysis system (Colchester, VT) was used to evaluate the pattern and intensity of staining. A lung pathologist examined stained sections blindly.
Morphometric Analysis of Lung Vasculature
The total number of factor VIII-positive 10 to 50 μm distal vessels was counted per unit area of lung tissue to determine vessel density using Stereo Investigator software (MicroBrightField) as previously described.17 The number of partially and fully muscularized distal pulmonary vessels was then counted in α-SMA-positive stained sections and expressed as a percentage of the total number of factor VIII-positive vessels, consecutively stained in the same section, as previously described.17 At least one hundred 10- to 50-μm vessels per mouse were examined. The medial and adventitial wall thicknesses of proximal vessels (50 to 125 μm) were measured and expressed as a percentage of the diameter of the vessel’s external elastic lamina using Stereo Investigator software as previously described.17 At least ten 50- to 125-μm vessels per mouse were examined.
Western Analyses
Quick frozen lung tissue or PA SMCs were homogenized (PowerGen 700, Pittsburgh, PA) in buffers containing protease inhibitors. After low speed centrifugation, supernatants [whole lung homogenate (30 to 50 μg) or PA SMC lysate (20 μg)] were electrophoresed on 8% SDS-polyacrylamide electrophoresis gels and transferred to nitrocellulose membranes. Blots were blocked with 1% bovine serum albumin and incubated in primary antibodies: anti-ACE (Santa Cruz, Santa Cruz, CA), anti-ECE (Dr. Ivy Dunbar, U. Colorado, Denver, CO), anti-aminopeptidase N (Santa Cruz), anti-DPPIV (R&D Systems, Minneapolis, MN), anti-human NEP (clone 56C6, Labvision, Fremont, CA), anti-mouse NEP (R&D Systems), anti-rat NEP (Dr. Louis Hersh, U Kentucky, preadsorbed against NEP−/− mouse lung lysate), anti-proliferating cell nuclear antigen (PCNA; Santa Cruz), anti-phosphorylated extracellular signal-regulated kinase (pERK; Cell Signaling), anti-C-fos (Santa Cruz), or anti-C-jun (Santa Cruz). After incubation with appropriate secondary antibody, blots were developed via chemiluminescence and assessed by densitometry. All anti-NEP antibodies, regardless of species specificity, identified the same 100-kDa NEP protein in our mouse pulmonary tissues or cells.
Measurement of NEP Catalytic Activity
NEP activity was measured using a modified fluorometric assay described by Li and Hersh.49 PA SMC lysate supernatants (1 to 5 μg of protein) were incubated with glutaryl-Ala-Ala-Phe-4-methoxy-2-naphthylamide for 60 minutes at 37°C. NEP releases Phe-4-methoxy-2-naphthylamide, which is converted to the fluorescent 4-methoxy-2-naphthylamide on the inclusion of recombinant aminopeptidase N. Control reactions contained the NEP inhibitor phosphoramidon. Phosphoramidon also inhibits ECE but requires over an order of magnitude more inhibitor for this effect.50 Under the conditions of the assay used here, phosphoramidon had no effect on background activity measured in lysates prepared from NEP −/− lung or isolated PA SMCs. Therefore, the activity measurements reported in this study are specific for NEP. Fluorescence was measured with an excitation wavelength of 340 nm and an emission wavelength of 425 nm. Activities were from the linear portion of the graphs for protein concentration and incubation time.
Isolation and Characterization of C57BL/6 Mouse PA SMCs
SMCs from the main PA media of NEP+/+ and NEP−/− mice were isolated as previously described.47 Cells were characterized by light microscopic appearance, growth characteristics, and stained for α-SMA and smooth muscle-specific myosin between passage 3 and 6. For staining, PA SMCs were fixed in cold methanol and, after incubation with the primary antibody, cells were incubated in the dark with secondary antibody (Alexa 594 or Alexa 488). Cells were used for studies between passage 4 and 16.
Measurement of PA SMC Proliferative Indices
For measurement of proliferation-associated markers (PCNA, pERK, c-fos, c-jun), lysates of subconfluent PA SMCs under conditions of early serum withdrawal (2% ×24 hours) were prepared for Western analysis. For quantitation of cell proliferation, cells were sparsely seeded (3 × 103/well of 24 well plate) in 10% fetal bovine serum (FBS)-containing media and incubated for 24 hours. Then fresh media containing 0.1 to 10% FBS was added. Measurements of cell number were taken in quadruplicate every 1 to 2 days thereafter by manual hemocytometry or Coulter counter (Beckman) as described previously.51 3H-thymidine incorporation was also used as an index of DNA synthesis and cell proliferation as previously described and validated.51 Briefly, SMCs were seeded at a density of 30 to 50 × 103/well in 24-well plates, and then serum-deprived using Dulbecco’s Modification of Eagle’s Medium/F12 media containing 0.1% serum for 2 days. Fresh media (containing 0% serum), 3H-thymidine, and test conditions were then added for 24 hours. In select experiments, PA SMCs were treated with neuropeptides (BLP: Bachem, Torrance, CA; ET-1: Peptides International, Louisville, KY; sub P: Bachem) and exposed to either 20% or 3% oxygen for the incubation period as previously described.52 For replacement studies, recombinant NEP was added 30 minutes before neuropeptides.
Induced Expression of NEP by Wild-Type NEP Lentivirus Infection
NEP−/− mouse PA SMCs were infected with lentivirus (cytomegalovirus promoter driving expression of the human NEP cDNA; 1 to 20 μl; titer = ∼1 × 108colony forming units/ml) in Dulbecco’s Modification of Eagle’s Medium/F12 medium containing 0.3% FBS and 8 μg/ml polybrene, and incubated at 37°C for 1 hour. Dulbecco’s Modification of Eagle’s Medium/F12 medium containing 10% FBS was then added, and changed 24 hours later to medium containing 0.1% FBS for an additional 48 hours. Cells were then either harvested for Western analysis and NEP activity determinations, or assayed for neuropeptide-stimulated DNA synthesis.
Data Analysis
All data are expressed as the mean ± SEM. For animal studies the ‘n’ is the number of mice per experimental group. For cell culture studies the ‘n’ represents the number of cell populations each from a different mouse tested or the number of replicate wells per treatment with the number of additional experiments on different populations noted in the legend. Statistical comparisons (SuperANOVA software program, Abacus Concepts, Berkeley, CA) were performed by t-test or one- or two-way analysis of variance, followed by the Scheffé’s multiple-comparison tests, as appropriate. Data were considered significantly different if P < 0.05.
Results
C57BL/6 NEP+/+ and NEP−/− Mice Have Similar Lung Airway and Vasculature Structure under Baseline Conditions
Pan-cytokeratin-stained lung sections from normoxic NEP+/+ and NEP−/− mice were prepared and reviewed by a blinded lung pathologist, who analyzed sections for abnormalities that could contribute to PHTN. No differences in distal airway or alveolar structure were observed (Figure 2A and B). Hematocrits, which could affect blood viscosity, pulmonary vascular resistance, and cardiac function, were the same under baseline normoxic conditions for both NEP+/+ and NEP−/− mice (Figure 2C). The density of distal pulmonary vessels (10 to 50 μm) was also examined in lungs from normoxic NEP+/+ and NEP−/− mice and found to be similar in both groups (Figure 2D). Likewise, there were no baseline differences in intraluminal main PA diameter in vivo or in cardiac index, which could alter susceptibility to PHTN, as assessed by echocardiography (Figure 2, E and F).
Figure 2.
Initial characterization of C57BL/6 NEP+/+ and NEP−/− mice under baseline conditions. A and B: Representative NEP+/+ and NEP−/− lung sections stained for pan-cytokeratin showing terminal bronchiolar airway branches (triangle) and alveolar walls. Positive signal is brown [DAB] (n = 3). Magnification = original ×100. For C–F open bar is NEP+/+ and closed bar is NEP−/−. C: Hematocrit (n = 11). D: Density of distal (10 to 50 μm) pulmonary vessels in lung sections stained for factor VIII (n = 4 to 5). E and F: Intraluminal diameter of the main PA in vivo and cardiac index estimated by transthoracic echocardiography (n = 8 to 9). At baseline C57BL/6 NEP+/+ and NEP−/− mice displayed no significant differences in these key structural and functional parameters (P = NS).
NEP and Other Peptidase Expression in C57BL/6 NEP+/+ and NEP−/− Mouse Lung
The baseline normoxic pattern of NEP expression in NEP+/+ mouse lung is shown in Figure 3A and B. A blinded investigator scored stain intensity (0 to 4+) at four sites in NEP+/+ mouse lungs (distal vessels, alveolar walls, proximal vessels, and proximal airways). Distal vessels that remodel early in response to hypoxia stained strongly for NEP (4+). Alveolar walls stained less intensely and more variably (3+). There was a prominent NEP signal around proximal airways next to the outer medial wall of adjacent proximal PAs (4+). The signal within the proximal PA media was more modest (3+). These studies show that NEP is normally expressed where most of the vascular structural change occurs in the lung in response to hypoxia. No NEP was detected in NEP−/− mouse lung by immunostaining as shown here (Figure 3C) or by Western blotting (Figure 1B) or activity assay. Potential adaptive or compensatory changes in baseline protein expression levels of other lung peptidases that share substrates with NEP were examined by Western analysis (Figure 3D). No differences in lung levels of ACE, ECE, aminopeptidase N, or DPPIV were found in NEP+/+ vs NEP−/− mice, suggesting that differential hypoxic responsiveness of the pulmonary vasculature would not likely be due to compensation in expression of the four other pulmonary cell-surface peptidases.
Figure 3.
Localization of NEP and detection of other relevant peptidases in C57BL/6 NEP+/+ vs NEP−/− mouse lung under baseline conditions. For NEP stain the positive signal is brown (DAB). The signal is slightly muted by counterstain. Small arrow, (A and C), distal vessel; large arrow, (B), proximal vessel, arrowhead, (B), adjacent proximal airway. A: Distal vessel and alveolar walls of NEP+/+ mouse lung. B: Proximal vessel and adjacent airway of NEP+/+ mouse lung. C: Distal vessel and alveolar walls of NEP−/− mouse lung. Staining NEP+/+ mouse lung without primary antibody yields the same negative result. Magnification for (A–C) = original ×400 (n = 5). D: Expression of other relevant lung peptidases in NEP+/+ and NEP−/− lung. Aminopeptidase N (APN). Representative Western analysis shown (n = 4; P = NS).
C57BL/6 NEP−/− Mouse Lungs Have Increased Density of NECs at Baseline
Quantitation of the basal density of NECs in the lungs of NEP+/+ vs NEP−/− mice is shown in Figure 4. NEC density is expressed as calcitonin gene-related peptide-positive cells/mm airway. Figure 4A shows a representative NEC along an airway near a vessel in NEP−/− mouse lung. A significant increase in number of lung NECs was found in the NEP−/− mice compared with NEP+/+ mice (NEP+/+: 0.19 ± 0.05 vs NEP−/−: 0.33 ± 0.04 NEC/mm airway) (Figure 4B).
Figure 4.
Increased numbers of NECs in lungs of C57BL/6 NEP−/− mice compared with NEP+/+ mice. Lung NECs were localized using rabbit anti-rat calcitonin gene-related peptide antibody. Positive signal (alkaline phosphatase, red). A: Representative positive NEC along an airway near a vessel in NEP−/− mouse lung. Large arrow points to stained NEC. B: NEP−/− mice have more NECs per length of airway than NEP+/+ mice (n = 4; *P < 0.05).
Levels of Selected Neuropeptides in Lung Lysates and Serum from C57BL/6 NEP+/+ and NEP−/− Mice under Baseline Conditions
The basal concentration of selected neuropeptides (BLP, ET-1, sub P) was measured in the lungs and serum of NEP+/+ and NEP−/− mice by RIA or enzyme immunoassay. No significant differences in BLP or ET-1 were found in NEP+/+ vs NEP−/− mouse lung (BLP: 1.7 ± 0.3 vs. 1.27 ± 0.06 pg/μg, n = 7; ET-1: 0.15 ± 0.04 vs. 0.17 ± 0.01 pg/mg, n = 4; P = NS). A modest increase in the baseline level of sub P was detected in the NEP−/− lungs (NEP+/+: 0.09 ± 0.02 vs NEP−/−: 0.18 ± 0.02 pg/mg; n = 7; P < 0.05). Serum concentrations for BLP, ET-1 and sub P were similar in NEP+/+ vs NEP−/− mice (BLP: 47 ± 6 vs. 60 ± 3 pg/mg, n = 3; ET-1: 0.18 ± 0.03 vs. 0.22 ± 0.03 pg/mg; sub P: 0.10 ± 0.03 vs. 0.12 ± 0.03 pg/mg, respectively, n = 7; P = NS).
C57BL/6 NEP−/− Mice Have Increased RVSP in Response to Chronic Hypoxia as Compared with C57BL/6 NEP+/+ Mice
As part of the hemodynamic assessment, hematocrit was measured in NEP+/+ and NEP−/− mice exposed to normoxia and 5 weeks of hypoxia. There was no difference at baseline (44 ± 1 and 42.8 ± .01%; P = NS; n = 10) and both showed the same degree of reactive polycythemia to hypoxia (57 ± 1 and 56 ± 2%; P = NS; n = 10). RVSP was measured via closed-chest measurements, using a pressure transducer (Figure 5A). Following 5 weeks of hypoxia, NEP−/− mice have increased RVSP compared with their NEP+/+ littermates (NEP+/+ vs NEP−/−: normoxia, 30 ± 1 vs. 30 ± 1; hypoxia, 43 ± 3 vs. 50 ± 2 mmHg). Heart rates were similar at the time of these measurements (420 ± 20, 420 ± 40, 430 ± 20, and 480 ± 40 BPM respectively; P = NS). In preliminary control experiments, baseline echocardiographic measurement of cardiac indices (Figure 2) and parallel assessments by Millar catheter of RVSP were made. Cardiac indices were the same. Intravascular Millar catheter measurements further validated the reliability of the transthoracic approach. In a side-by-side analysis, both approaches yielded the same RVSP results (transthoracic versus Millar intravascular NEP+/+: normoxia 32 ± 2 vs. 33 ± 3; 5 weeks hypoxia 42 ± 1 vs. 46 ± 7 mmHg, respectively; n = 2 to 4). Finally, we previously demonstrated a close relationship between increases in RVSP detected with the transthoracic technique and RV hypertrophy in mice with a normal cardiac phenotype.17 These findings, when combined with the inspection of cardiac structure (including RV outflow tract) that was unrevealing, suggest that the RVSP measurements made in this study accurately reflect changes in PA pressure.
Figure 5.
Increased RVSP and pulmonary vascular remodeling in response to chronic hypoxia in C57BL/6 NEP−/− mice. NEP+/+ (open bar) and NEP −/− (closed bar). Adult mice (10 to 12 weeks) were exposed to either Nx (Denver altitude; 5280 ft) or Hx (18,000 ft) for 5 weeks. A: Measurements of RVSP, an indirect index of PA pressure, were made by percutaneous cardiac puncture in anesthetized, spontaneously breathing mice (n = 6 to 8; *P ≤ 0.05 vs all others). B: Lung sections were stained for both factor VIII (endothelial cell marker) and α-SMA; α-SMA-positive vessels were expressed as a percentage of factor VIII positive (total) vessels. Data were pooled and represents both the partially and fully muscularized vessels (n = 4 to 5; *P < 0.05 vs all others). C: Hypoxia selectively induced a thickening of the adventitia in NEP−/− mice (n = 5; *P < 0.05 vs all others). A similar pattern was noted in the media.
C57BL/6 NEP−/− Mice Exhibit Exaggerated Pulmonary Vascular Remodeling in Response to Chronic Hypoxia
We found that the increased RVSP in chronically hypoxic NEP−/− mice is accompanied by exaggerated vascular remodeling of distal (10 to 50 μm) and to a lesser extent proximal (50 to 125 μm) pulmonary vessels. Lung sections were analyzed for evidence of vascular remodeling by immunolocalization of factor VIII and α-SMA. Distal (10 to 50 μm) vessel density was similar between NEP+/+ and NEP−/− mice at baseline and after exposure to hypoxia (NEP+/+ vs NEP−/−: normoxia, 10.4 ± 0.6 vs. 9.3 ± 0.5; 5 weeks hypoxia, 10 ± 1 vs. 11.1 ± 0.5 vessels/mm2; n = 8–9; P = NS). The quantitation of α-SMA positive vessels, expressed as a percentage of the total number of vessels in the same section stained simultaneously with factor VIII, is shown in Figure 5B. Staining for α-SMA shows that NEP+/+ and NEP−/− mice have similar numbers of partially and fully muscularized distal vessels at baseline. There was a marked increase in α-SMA positive vessels in the chronically hypoxic NEP−/− lungs relative to those from NEP+/+ mice (percent actin positive 10 to 50 μm vessels in NEP+/+ vs NEP−/− mice: normoxia, 23 ± 2 vs. 24 ± 3%; hypoxia, 29 ± 3 vs. 41.3 ± 0.8%). While the total response (partially and fully muscularized vessels combined) in NEP+/+ mice was subtle, if the analysis was separated by vessel type, then an easily detectable increase in the partially, but not fully, muscularized vessel number in response to hypoxia was appreciable (data not shown). In additional methyl Carnoy’s-fixed lungs, the baseline percentage of myosin positive distal vessels was measured and found to be lower in NEP−/− vs NEP+/+ mouse lungs (NEP+/+ vs NEP−/−: 4 ± 1% vs 1.8 ± 0.4%). On exposure to hypoxia the percentage increased to the same extent in NEP+/+ vs NEP−/− mouse lungs (6.4 ± 0.6 vs. 3.6 ± 0.5%; n = 3). As is typically observed with C57BL/6 mice, no change in proximal medial or adventitial thickness was found in the NEP+/+ mice following 5 weeks of hypoxia (NEP+/+ medial: 11.9 ± 0.7 vs. 12.0 ± 0.6%; adventitial: 9.6 ± 0.8 vs. 9.1 ± 0.5% total vessel diameter; n = 5). At baseline, NEP−/− mice show a trend toward slightly reduced medial and adventitial thicknesses. However, in response to hypoxia, the NEP−/−, but not NEP+/+, mice showed a significant increase in medial and, to a greater extent, adventitial thicknesses (NEP−/− medial: 9.3 ± 0.4 vs. 12 ± 0.6%; adventitial: 7.7 ± 0.5 vs. 12.6 ± 0.9% total vessel diameter). Figure 5C shows the adventitial data. Figures 6 and 7 show representative hypoxia-induced structural changes observed in the distal and proximal pulmonary vasculature of NEP+/+ vs NEP−/− mice.
Figure 6.
Increased α-SMA expression in distal vessels (10 to 50 μm) of NEP−/− mice compared with NEP+/+ mice in response to chronic hypoxia. Representative α-SMA (alkaline phosphatase, red) staining shown in mouse lung sections. A: NEP+/+ Nx. B: NEP+/+ 5 weeks Hx. C: NEP−/− Nx. D: NEP−/− 5 weeks Hx. Magnification = original ×200.
Figure 7.
Histological demonstration of chronic hypoxia-induced structural changes in 50- to 125-μm pulmonary vessels from C57BL/6 NEP+/+ and NEP−/− mice. Formalin-fixed, paraffin-embedded lung sections were stained for factor VIII (DAB, brown) and α-SMA (alkaline phosphatase, red). A: NEP+/+ Nx. B: NEP+/+ 5 weeks Hx. C: NEP−/− Nx. D: NEP−/− 5 weeks Hx. Magnification = original ×640.
NEP+/+ and NEP−/− Mice Have a Similar RV Hypertrophic Response to Chronic Hypoxia Despite Differences in RVSP and Pulmonary Vascular Remodeling
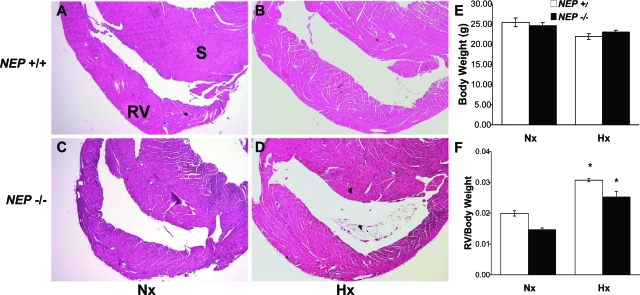
Examination of the hearts from NEP+/+ and NEP−/− mice for evidence of structural differences at baseline and following 5 weeks of hypoxia revealed that the extent of RV hypertrophy was lower than anticipated in NEP−/− mice, considering the magnitude of pulmonary vascular changes observed (representative cross section images: Figure 8, A–D). NEP+/+ and NEP−/− mice had similar total body weight under normoxic and chronic hypoxic conditions (Figure 8E). The total cardiac mass of the NEP+/+ and NEP−/− mice was 23.6 ± 0.9 and 22.4 ± 0.8 mg at baseline and 27 ± 1 vs. 24 ± 1 mg following 5 weeks of hypoxia. RV mass at baseline was slightly reduced in NEP−/− mice compared with NEP+/+ mice. The RV hypertrophic response to chronic hypoxia was similar in both NEP+/+ and NEP−/− mice (Figure 8F), consistent with recent observations on the role of NEP in the heart and systemic vasculature (where NEP depletion limits ventricular hypertrophy and systemic hypertension).33,53
Figure 8.
RV histology and mass and body weight under baseline conditions and following exposure to chronic hypoxia. Heart sections were prepared and stained with H&E. A: NEP+/+ Nx, RV: right ventricular, S: Septum. B: NEP+/+ 5 weeks Hx. C: NEP−/− Nx. D: NEP−/− 5 weeks Hx. NEP+/+ and NEP−/− hearts show no structural differences under normoxic conditions (A and C) or after hypertrophic response to chronic Hx (B and D). For (E and F) NEP+/+ (open bar), NEP−/− (closed bar). E: Body weight of NEP+/+ and NEP−/− mice under normoxic and hypoxic conditions. No differences were found. F: RV mass to body weight ratios for NEP+/+ and NEP−/− mice under normoxic and hypoxic conditions. RV mass was slightly reduced in NEP−/− mice compared with NEP+/+ mice under baseline Nx conditions. RV mass increased similarly in NEP+/+ and NEP−/− mice in response to chronic hypoxia (n = 6 to 11; *P ≤ 0.05 vs same Nx group).
Hypoxic C57BL/6 NEP+/+ Mice Exhibit a Transient Decrease in Lung NEP Expression
To determine the temporal and spatial relationship between wild-type lung NEP expression and hypoxia-induced pulmonary vascular remodeling and cell growth, C57BL/6 NEP+/+ mice were exposed to normoxia or increasing durations of hypoxia (1, 3, 10, and 35 days). Lung lysates were prepared and probed for NEP expression by Western blot, which showed a decrease in aggregate whole lung expression at 3 days of hypoxia (Figure 9A). Subsequently, lungs from mice exposed to normoxia or 3 days hypoxia were immunostained to localize NEP (Figure 9, B–C). This serial dilution study was done in the absence of counterstain to increase sensitivity. A marked decrease in the NEP signal in distal vessels (4+ to 1+) and adjacent alveolar walls (3+ to 1+) was observed (n = 4). A more modest decrease was found in proximal airways (4+ to 3+) and vessels (3+ to 2+) suggesting local heterogeneity in the response. Thus a more substantial decrease in NEP expression at the site where distal vessels are present was observed after 3 days hypoxia than was detected by Western analysis of whole lung.
Figure 9.
Transient early decrease in lung NEP expression in NEP+/+ mice in response to hypoxia. A: Nx (open box) and Hx (closed triangle). Relative NEP expression in whole lung lysates determined by Western blot and densitometry (n = 4 to 12; *P < 0.05 vs same Nx group). Insert: Representative Western blot of NEP expression in whole lung lysates at baseline and following 3 days Hx. B and C: Immunolocalization of NEP in distal vessels (10 to 50 μm) and alveolar walls in NEP+/+ mouse lung at baseline (B) and after 3 days Hx (C). Lungs were stained for NEP (DAB, brown), but in contrast to Figure 3, no counterstain was used. We performed serial dilution to show maximal difference in signal at distal vascular site (see arrows). Magnification = original ×400.
Proliferation of PA SMCs in NEP−/− Mice Compared with NEP+/+ Mice: In Vivo and in Vitro Assessment
Lung sections from NEP+/+ and NEP−/− mice exposed to 0, 3, and 35 days hypoxia were stained for the proliferation-associated marker, Ki-67. The goal was to detect in vivo differences in vascular cell growth and relate them temporally to decreases in NEP expression observed in 3 days hypoxia-exposed WT mice. Figure 10A shows a representative Ki-67 positive cell in PA from NEP+/+ mouse lung after 3 days hypoxia. In vivo, the baseline Ki-67 signal is similar in the pulmonary vasculature of NEP+/+ and NEP−/− mice (Figure 10B). However, following 3 days of hypoxia a transient increase in proliferation of both NEP+/+ and NEP−/− mouse pulmonary vascular cells in vivo was detected, with a larger (3.5 vs 0.8 fold) increase in Ki-67 staining detected in the NEP−/− mice (NEP+/+ vs −/−: normoxia 1.0 ± 0.1 vs. 1.1 ± 0.1; hypoxia 1.8 ± 0.3 vs. 3.9 ± 0.5 cells/mm2). By 35 days of hypoxia the proliferative signals trended back toward baseline and were of similar magnitude (NEP +/+ and −/−: 1.6 ± 0.4 and 1.5 ± 0.2 cells/mm2 respectively; n = 4 to 10; P = NS).
Figure 10.
Quantification of proliferative changes in pulmonary vessels in vivo and isolated PA SMCs in vitro for NEP+/+ and NEP−/− mice. Staining lung sections for the proliferation-associated marker, Ki-67, was done under baseline normoxic conditions and following 3 days and 35 days hypoxic exposure. Isolated NEP+/+ and NEP−/− PA SMCs, grown under normoxic conditions, were stained for α-SMA and analyzed by fluorescence microscopy. A: Representative positive Ki-67 cell (arrow) in PA of NEP+/+ mouse lung after 3 days Hx exposure (DAB, brown). Magnification = original ×400. B: Positive Ki-67 cells localized to pulmonary vessels were counted per unit area. Pulmonary vascular cell proliferation was transiently increased in NEP−/− mice compared with NEP+/+ mice in response to 3 days of hypoxia (n = 4 to 6; *P < 0.05 vs all others). C: α-SMA expression in NEP+/+ PA SMCs (0.1% serum × 1 day). D: α-SMA expression in NEP−/− PA SMCs. Magnification = original ×200. E: PA SMCs from NEP−/− mice have increased expression of proliferation markers (PCNA, pERK, c-fos, and c-jun) as compared with those from NEP+/+ mice by Western analysis (0.2% serum ×1 day; n = 3; *P < 0.05 vs NEP+/+). F: Isolated PA SMCs from NEP−/− mice grow faster than NEP+/+ derived cells (10% serum; n = 3; *P < 0.05 vs NEP+/+). G: Exogenous rNEP (0.01 to 0.10 μg/μl) inhibits the enhanced growth of PA SMCs isolated from NEP−/− mice. Cells were treated every other day with human rNEP (1% serum; n = 3; *P < 0.05 vs no rNEP).
To further investigate the increased pulmonary vascular remodeling and cell proliferation observed in vivo, PA SMCs from normoxic NEP+/+ and NEP−/− mice were isolated from the proximal medial PA. Representative staining of the PA SMC for α-SMA is shown in Figure 10 C and D. The PA SMCs from the NEP−/− mice are slight smaller in appearance and have a less intense but clearly detectable smooth muscle-specific myosin signal (data not shown). Under conditions of early serum withdrawal (0.2% × 24 hours), PA SMCs from NEP−/− mice have increased levels of proliferation markers (PCNA, pERK, c-fos, and c-jun) compared with their NEP+/+ counterparts as shown by Western analysis (Figure 10E). The NEP−/− PA SMCs also exhibit increased growth in the presence of 0.1 to 10% serum compared with PA SMCs from NEP+/+ mice (data for 10% serum shown: Figure 10F) (NEP+/+ vs NEP−/−: 1 day, 0.31 ± 0.03 vs. 0.29 ± 0.02; 5 days, 4.5 ± 0.9 vs. 14.9 ± 0.2 cells × 104 per well). The enhanced growth is at least partially reversed in a dose-dependent manner by the addition of 0.01 to 0.1 μg/μl recombinant NEP (data for 1% serum shown: Figure 10G).
Other Adaptive Responses to Hypoxia that May Predispose to Increased PA Remodeling and SMC Growth
NEC density in both NEP+/+ and NEP−/− mouse lungs tended to increase only slightly with 5 weeks of hypoxia (NEP+/+ vs NEP−/−: normoxia, 0.19 ± 0.05 vs. 0.33 ± 0.04; 5 weeks hypoxia, 0.22 ± 0.05 vs. 0.36 ± 0.08 cells/mm airway; n = 6–7). Lung levels of selected neuropeptides (BLP, ET-1, and sub P) were measured on exposure to 3 days of hypoxia. This duration was chosen to temporally match with the Ki-67 and NEP expression data and preliminary ET-1 measurements on 35 days samples revealed only modest increases over control of similar magnitude in both types of mice (data not shown). After 3 days of hypoxia BLP levels trended up slightly in NEP−/− lung (NEP+/+ vs NEP−/−: normoxia, 1.7 ± 0.3 vs. 1.27 ± 0.06 pg/μg; hypoxia, 1.4 ± 0.2 vs. 1.8 ± 0.2 pg/μg lung protein; n = 7). The increase in ET-1 levels was greater in NEP−/− mouse lungs compared with their NEP+/+ counterpart but was still modest in magnitude (NEP+/+ vs NEP−/−: normoxia, 0.15 ± 0.04 vs. 0.17 ± 0.01; hypoxia, 0.24 ± 0.04 vs. 0.32 ± 0.05 pg/mg lung protein; n = 4). There was a similar increase in sub P in both groups (NEP+/+ vs NEP−/−: normoxia, 0.09 ± 0.02 vs. 0.18 ± 0.02; hypoxia, 0.24 ± 0.06 vs. 0.24 ± 0.03 pg/mg lung protein; n = 7). The levels of these neuropeptides (BLP, ET-1, and sub P) were then measured after 7 days of hypoxia. BLP and ET-1 levels were similar to the 3 days findings. A modest further upward trend in sub P levels was noted in the null lungs (n = 3; data not shown). NEP−/− PA SMCs were more growth responsive to these same neuropeptides (BLP [1 to 100 nmol/L]; Bombesin used as BLP for all studies, ET-1 [1 to 10 nmol/L], and sub P [1 to 100 nmol/L]) than NEP+/+ cells (NEP+/+ vs NEP−/−: 8 ± 2 vs. 30 ± 10%, −10 ± 10 vs. 17 ± 7%, and −10 ± 20 vs. 30 ± 10%, respectively; reported as percent change compared with control; pooled from n = 4 experiments on different populations, n = 7 replicate wells). The growth signals were further potentiated by exposure to hypoxia in vitro (3% O2 for 24 hours) following priming with the same neuropeptides (same concentrations BLP, ET-1, and sub P; NEP+/+ vs NEP−/−: 13 ± 6 vs. 190 ± 80%, −22 ± 9 vs. 130 ± 60%, and −10 ± 8 vs. 130 ± 80%, respectively; reported as percent change compared with control; pooled from 2 experiments on different populations, n = 3 replicates).
NEP Replacement Attenuates Neuropeptide-Induced DNA Synthesis in NEP−/− Mouse PA SMCs
Finally, we tested whether complementary NEP replacement strategies could attenuate increased growth of PA SMCs from NEP−/− mice. As shown in Figure 11A–C, pretreatment of NEP−/− PA SMCs with a submaximal concentration of rNEP (0.01 μg/μl) attenuates the increased neuropeptide-stimulated proliferative responses observed (without versus with rNEP: BLP, 1.00 ± 0.08 vs. 0.58 ± 0.05; ET-1, 1.0 ± 0.2 vs. 0.41 ± 0.06; sub P, 1.0 ± 0.2 vs. 0.6 ± 0.1 with stimulated value normalized to 1.0). Also, NEP expression was induced in NEP−/− PA SMCs by lentiviral infection with a vector containing the cDNA for human NEP. This induction of NEP expression was characterized by Western analysis for NEP and assay for NEP catalytic activity (Figure 11D). The expression and activity of NEP increased proportionally to the lentiviral multiplicity of infection in NEP−/− PA SMCs. PA SMCs were then treated with BLP (100 nmol/L), ET-1 (10 nmol/L), or sub P (100 nmol/L) with or without prior NEP lentiviral (multiplicity of infection 30), and DNA synthesis was measured. Infection with NEP lentivirus decreased the proliferative response to these same neuropeptides (% decrease: BLP: 80 ± 3, ET-1: 94 ± 7, and sub P: 100 ± 7%, respectively). The normalized BLP data are shown in Figure 11E.
Figure 11.
Decreased proliferative response to selected neuropeptides in NEP−/− PA SMCs by replacement NEP strategies. Quiescent PA SMCs (0.1% serum ×2 days) were incubated with different neuropeptides (BLP [Bombesin used for all studies], ET-1, or sub P) ±0.01 μg/μl rNEP in serum-free media for 24 hours; DNA synthesis was measured as per Materials and Methods, in response to (A) BLP (100 nmol/L), (B) ET-1 (10 nmol/L), and (C) sub P (100 nmol/L). open bar = −rNEP, closed bar = + rNEP; n = 3 experiments each with 3 to 10 replicate wells on different cell populations; *P < 0.05 vs no rNEP. D: Expression of NEP in NEP−/− PA SMCs via transduction with a lentivirus containing the human cDNA for NEP. Top sub panel shows Western analysis of NEP in NEP−/− PA SMCs treated 3 days prior with varying multiplicity of infection of NEP lentivirus. Bottom sub panel shows the NEP catalytic activity of these same NEP−/− PA SMCs. E: NEP−/− PA SMCs were treated with BLP (100 nmol/L) ± NEP lentivirus (30× MOI) and DNA synthesis was measured. open bar = −NEP lentivirus, closed bar + NEP lentivirus; n = 3 to 11 replicate wells; similar results obtained in three other experiments; *P < 0.05 vs no NEP lentivirus. Similar inhibitory effect on ET-1 (10 nmol/L) and sub P (100 nmol/L) responses found.
Discussion
In the current study, we tested the hypothesis that genetic depletion of NEP would increase susceptibility to PHTN and vascular remodeling in response to chronic hypoxia, at least in part, by increasing the growth of PA SMCs. We found that, at baseline, the absence of NEP makes little difference on measures of pulmonary and cardiac structure and function. Lungs and hearts from normoxic NEP+/+ and NEP−/− mice do not differ in airway or alveolar wall architecture, hematocrit, vessel density or muscularization, estimated intraluminal proximal PA diameter by echocardiography, or RVSP. Direct morphometric analysis indicates that, at baseline, the proximal PA wall thickness of NEP−/− mice may be slightly reduced as compared with that of NEP+/+ mice. Hearts from normoxic NEP+/+ and NEP−/− mice do not differ in cardiac index by echocardiography or structure of the RV outflow tract or of the aorta. RV mass is also slightly reduced in the NEP−/− vs NEP+/+ mouse at baseline, which is consistent with the original finding of Lu et al demonstrating a slight overall decrease in total cardiac mass in NEP−/− vs NEP+/+ mice.33
We found that NEP−/− mice have a higher baseline pulmonary NEC density than do NEP+/+ mice. This is particularly relevant because hyperplasia of NECs has been described in association with PHTN, particularly in the early stages of this disorder.54,55 Lung levels of sub P were modestly increased at baseline while those of ET-1 and BLP were not. We also found no change in the baseline serum levels of these neuropeptides in NEP+/+ vs NEP−/− mice. The subtle neuropeptide imbalance resulting from the deletion of NEP suggests that changes in peptide levels and turnover in the local lung microenvironment may influence vascular cell responsiveness.23 Since the aggregate changes in neuropeptide levels are surprisingly subtle here though, we speculate that increased cell responsiveness to neuropeptides may be more important in the pathogenesis of increased PHTN. Alternately, an “unidentified” pro-remodeling factor could be responsible for the exaggerated PA remodeling observed in hypoxic NEP−/− mice. The lung expression of other peptidases that share some substrates with NEP (aminopeptidase N, DPPIV, ACE, and ECE), do not differ between NEP+/+ and NEP−/− mice at baseline, suggesting that the hypoxic phenotype observed in NEP−/− mice was not due to adaptive changes in other peptidases.
On hypoxic exposure of C57BL/6 NEP−/− mice, we observed noticeably augmented PHTN and vascular remodeling, in comparison with that which is normally seen with hypoxic wild-type mice of this strain. In particular, we observed a particularly striking increase in muscularization of the distal (10 to 50 μm) pulmonary vessels and appreciable medial/adventitial thickening of the proximal PA. Importantly, NEP+/+ mice exhibit an early (3 days), transient decrease in lung NEP expression in response to hypoxia that is most impressive in distal vessels and alveolar walls. In a few other systems a decrease in NEP activity and/or expression following exposure to hypoxia has been reported.56,57,58 Here we have provided temporal and spatial definition in the mouse lung and related it to vascular cell proliferation. We observed a parallel transient increase in the in vivo proliferation of pulmonary vascular cells (as measured by the Ki-67 proliferation marker), demonstrating the axiom that hypoxia might act as a switch for increased growth. Our data suggest that decreased NEP can function in much the same way as hypoxia (ie, as an injurious priming stimulus). In the absence of hypoxia in vivo mechanisms must be operative that prevent increased growth or expression of a pro-proliferative phenotype. In vitro, we however have found that, when NEP is genetically eliminated, PA SMCs exhibit greater expression of select proliferation-associated biomarkers (PCNA, pERK, c-fos, and c-jun).59,60 This suggests that NEP depletion promotes a proliferative milieu. In addition, it was found that PA SMCs from NEP−/− mice grow much faster than do PA SMCs from NEP+/+ mice, which corroborates our observation of enhanced expression of proliferation-associated biomarkers in these cells. Proliferative responses to selected neuropeptides are up-regulated in NEP−/− PA SMCs compared with their NEP+/+ counterparts. These signals are further potentiated by hypoxia. Finally, NEP replacement strategies decrease the exaggerated growth of isolated NEP−/− PA SMCs. The data demonstrate that deletion of NEP augments the murine pulmonary hypertensive response to chronic hypoxia and that in the lung, NEP may exert a protective effect against chronic hypoxic PHTN, at least in part, by attenuating the growth of PA SMCs.
It is well known that the pulmonary and systemic circulations normally respond to hypoxia in opposite manners. For example, pulmonary vessels respond to hypoxia by contracting to redirect blood flow to better oxygenated areas of the lung, whereas systemic vessels dilate to increase flow of oxygenated blood to areas of tissue hypoxia or ischemia. The RV falls in between. It responds to PHTN with an adaptive hypertrophic response, the magnitude of which typically parallels the pressure elevation in the pulmonary circulation. In a few instances, uncoupling of the extent of PHTN and cardiac hypertrophy has been observed.16,17 These reports suggest that mechanisms regulating adaptive responses of PA SMC and RV myocytes (including hypertrophy/hyperplasia) may be different. Our studies also demonstrate that the pulmonary and systemic circulations of NEP−/− mice do not respond to hypoxia in the same manner. The cardiac findings in our study are consistent with observations made with more specific NEP antagonists alone and in combination with ACE inhibitors29,30,53 and with the original description of the cardiac phenotype of the NEP−/− mouse.33 There was no evidence of differential structural change in the left ventricle of NEP+/+ or NEP−/− mice following chronic hypoxia, making increased cardiac dysfunction an unlikely explanation for the pulmonary vascular and right heart findings here. With the augmented PHTN and vascular remodeling that was observed in hypoxic NEP−/− mice, a greater extent of RV hypertrophy would have been expected. This can be explored further in future studies with echocardiographic measurements after 5 weeks of hypoxia. Consistent with what is known about NEP in the heart and systemic circulation, NEP depletion seemed to limit this RV hypertrophy. Thus, our data suggests also, that NEP may play a different role in the lung than in the heart. Finally, to our knowledge, the direction of uncoupling between PHTN and remodeling versus RV hypertrophy is unique compared with previous reports.
Along with the increased susceptibility of the C57BL/6 NEP−/− mouse to hypoxia-induced remodeling, we hypothesize that a shift to a more proliferative, less differentiated SMC phenotype has occurred. Indeed, morphometric analysis suggests there were increases in α-SMA positive/smooth muscle-specific myosin negative cells in distal (10 to 50 μm) pulmonary vessels in the NEP−/− mice following exposure to chronic hypoxia. Since smooth muscle-specific myosin is a marker for more fully differentiated SMCs, proliferation or migration of de-differentiated SMCs or myofibroblasts into the distal circulation may be a prominent feature of the exaggerated remodeling seen in the NEP−/− mouse. Decreased NEP expression may be important at these sites of phenotypic switching and de-differentiation.
The mechanism of the hypoxia-induced decrease in NEP activity and expression is unknown, but a likely contributor is oxidant stress, which may be selectively increased during hypoxia.61,62 The transcription factor, hypoxia-inducible factor 1, is increased during hypoxia and could also play a key role in the hypoxia-induced decrease in NEP expression we have observed.63 Alterations in NEP degradation may be important, although this is an understudied area.64 Many other factors may influence and contribute to the down-regulation of NEP expression, including inflammation.65
In the present study, we have focused on how NEP could alter susceptibility to hypoxic PHTN. At baseline, we found only a modest increase in lung, but not serum, sub P levels in NEP−/− vs NEP+/+ mice. We suspect that local derangements in availability of selected neuropeptides at the cell surface, or differential regulation of neuropeptide levels under hypoxic conditions, may contribute more to the exaggerated chronic hypoxia-induced pulmonary vascular remodeling observed in the NEP−/− mouse. Measurements of BLP, ET-1, and sub P after 3 and 7 days of hypoxia support this concept. Each may contribute to the PHTN/vascular remodeling observed under hypoxic conditions, in both the NEP−/− and NEP+/+ mice.66,67 However, it is not known if the small increases in BLP, ET-1, and sub P lung levels found here at baseline or in response to hypoxia is sufficient to account for the augmented PHTN and pulmonary vascular remodeling we observed in hypoxic NEP−/− mice. More studies with appropriate mice and antagonists are needed. However, no single neuropeptide-specific antagonist strategy is likely to attenuate the entire response observed.
Additional studies are needed to examine how NEP modulates levels of selected neuropeptide receptors for BLP, ET-1, and sub P and on critical signaling events in cell proliferation, migration, and contraction, such as phospholipase D, PLA2, and PKC cascades, and pathways that activate Rho Kinase and lead to tyrosine phosphorylation of p125FAK and paxillin.68,69 Experiments to determine the relative importance of PA SMC versus other cell types in the observed cytoprotective effects of NEP are being developed. In addition, we are expanding the current observations made in mice to the study of relevant human disease states.70 These conditions include chronic lung and heart conditions complicated by secondary pulmonary hypertension, like chronic obstructive lung disease.71
In summary, we have found that targeted deletion of NEP in the C57BL/6 mouse predisposes to exaggerated PHTN and vascular remodeling in response to chronic hypoxia. Thus, NEP may protect the lung against hypoxia-induced vascular remodeling, in large part by limiting the magnitude of neuropeptide-induced proliferative, migratory and/or contractile responses. The resulting hypoxia-induced vascular remodeling is substantial and demonstrates proliferation and migration of de-differentiated SMCs or myofibroblasts into the distal pulmonary circulation, as well as, proximal changes at the medial/adventitial border. Changes in the pulmonary vasculature are found in the hypoxic NEP−/− mouse, which are similar to those found in large animal models of hypoxic PHTN that closely parallel human disease and are usually not associated with mouse models of chronic hypoxic PHTN.1,2,3,4,5,6 We believe that further work with the NEP−/− mouse model of chronic hypoxic PHTN may lead to the identification of new therapeutic strategies or targets to limit or reverse this important clinical problem.
Acknowledgments
We thank Cheryl Oliver-Pickett, Cassana Littler, and Marian Maslak for expert general administrative and technical assistance, Julie Harral for assistance with intravascular RVSP measurements, Dr. Catherine Magill for providing human rNEP, and Drs. Ivan F. McMurtry and John V. Weil for encouragement during the early stages of this project.
Footnotes
Address reprint requests to Edward C. Dempsey, M.D., Cardiovascular Pulmonary Research Laboratory; B-133, University of Colorado Denver, 12700 E. 19th Ave, Aurora, CO 80046. E-mail: edward.dempsey@ucdenver.edu.
Supported by grants from the National Heart Lung and Blood Institute PPG #HL14985 and RO-1 #HL078927 to E.C.D.
Current address of S.L., Department of Anesthesiology and Intensive Care Medicine, University of Leipzig Medical Faculty, Leipzig, Germany.
References
- Chaouat A, Bugnet AS, Kadaoui N, Schott R, Enache I, Ducolone A, Ehrhart M, Kessler R, Weitzenblum E. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–194. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- Said SI. Mediators and modulators of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L547–L558. doi: 10.1152/ajplung.00546.2005. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Orton EC, Ensley B, Tucker A, Stenmark KR. Hypoxia increases bromodeoxyuridine labeling indices in bovine neonatal pulmonary arteries. Am J Respir Cell Mol Biol. 1997;16:366–371. doi: 10.1165/ajrcmb.16.4.9115746. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol. 1981;240:H62–H72. doi: 10.1152/ajpheart.1981.240.1.H62. [DOI] [PubMed] [Google Scholar]
- Hales CA, Kradin RL, Brandstetter RD, Zhu YJ. Impairment of hypoxic pulmonary artery remodeling by heparin in mice. Am Rev Respir Dis. 1983;128:747–751. doi: 10.1164/arrd.1983.128.4.747. [DOI] [PubMed] [Google Scholar]
- Dempsey EC, Stevens T, Durmowicz AG, Stenmark KR. Haddad GG, Lister G, editors. 1996:225–274. [Google Scholar]
- Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, Jiang YP, Chen JJ, Waschek JA, Kort S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation. 2007;115:1260–1268. doi: 10.1161/CIRCULATIONAHA.106.681718. [DOI] [PubMed] [Google Scholar]
- Merklinger SL, Wagner RA, Spiekerkoetter E, Hinek A, Knutsen RH, Kabir MG, Desai K, Hacker S, Wang L, Cann GM, Ambartsumian NS, Lukanidin E, Bernstein D, Husain M, Mecham RP, Starcher B, Yanagisawa H, Rabinovitch M. Increased fibulin-5 and elastin in S100A4/Mts1 mice with pulmonary hypertension. Circ Res. 2005;97:596–604. doi: 10.1161/01.RES.0000182425.49768.8a. [DOI] [PubMed] [Google Scholar]
- Gillespie MN, Hartsfield CL, O'Connor WN, Cohen DA. Pulmonary hypertension in a murine model of the acquired immunodeficiency syndrome. Am J Respir Crit Care Med. 1994;150:194–199. doi: 10.1164/ajrccm.150.1.8025749. [DOI] [PubMed] [Google Scholar]
- Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, Jones RC, Picard MH, Zapol WM. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest. 1998;101:2468–2477. doi: 10.1172/JCI2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Mason NA, Strange JW, Walker H, Wilkins MR. Beneficial effects of phosphodiesterase 5 inhibition in pulmonary hypertension are influenced by natriuretic peptide activity. Circulation. 2003;107:234–237. doi: 10.1161/01.cir.0000050653.10758.6b. [DOI] [PubMed] [Google Scholar]
- Pascaud MA, Griscelli F, Raoul W, Marcos E, Opolon P, Raffestin B, Perricaudet M, Adnot S, Eddahibi S. Lung overexpression of angiostatin aggravates pulmonary hypertension in chronically hypoxic mice. Am J Respir Cell Mol Biol. 2003;29:449–457. doi: 10.1165/rcmb.2002-0120OC. [DOI] [PubMed] [Google Scholar]
- Frank DB, Lowery J, Anderson L, Brink M, Reese J, de Caestecker M. Increased susceptibility to hypoxic pulmonary hypertension in Bmpr2 mutant mice is associated with endothelial dysfunction in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol. 2008;294:L98–L109. doi: 10.1152/ajplung.00034.2007. [DOI] [PubMed] [Google Scholar]
- Vermeersch P, Buys E, Pokreisz P, Marsboom G, Ichinose F, Sips P, Pellens M, Gillijns H, Swinnen M, Graveline A, Collen D, Dewerchin M, Brouckaert P, Bloch KD, Janssens S. Soluble guanylate cyclase-alpha1 deficiency selectively inhibits the pulmonary vasodilator response to nitric oxide and increases the pulmonary vascular remodeling response to chronic hypoxia. Circulation. 2007;116:936–943. doi: 10.1161/CIRCULATIONAHA.106.677245. [DOI] [PubMed] [Google Scholar]
- Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee ME. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler CM, Wehling CA, Wick MJ, Fagan KA, Cool CD, Messing RO, Dempsey EC. Divergent contractile and structural responses of the murine PKC-epsilon null pulmonary circulation to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1083–L1093. doi: 10.1152/ajplung.00472.2004. [DOI] [PubMed] [Google Scholar]
- Shipp MA, Richardson NE, Sayre PH, Brown NR, Masteller EL, Clayton LK, Ritz J, Reinherz EL. Molecular cloning of the common acute lymphoblastic leukemia antigen (CALLA) identifies a type II integral membrane protein, Proc Natl Acad Sci USA. 1988;85:4819–4823. doi: 10.1073/pnas.85.13.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ, Tanzawa K. Mammalian membrane metallopeptidases: nEP. ECE, KELL, and PEX. FASEB J. 1997;11:355–364. doi: 10.1096/fasebj.11.5.9141502. [DOI] [PubMed] [Google Scholar]
- Koehne P, Schaper C, Graf K, Kunkel G. Neutral endopeptidase 24.11: its physiologic and possibly pathophysiologic role in inflammation with special effect on respiratory inflammation. Allergy. 1998;53:1023–1042. doi: 10.1111/j.1398-9995.1998.tb03812.x. [DOI] [PubMed] [Google Scholar]
- van der Velden VH, Hulsmann AR. Peptidases: structure, function and modulation of peptide-mediated effects in the human lung. Clin Exp Allergy. 1999;29:445–456. doi: 10.1046/j.1365-2222.1999.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday ME, Hua J, Torday JS, Reyes B, Shipp MA. CD10/neutral endopeptidase 24.11 in developing human fetal lung. Patterns of expression and modulation of peptide-mediated proliferation, J Clin Invest. 1992;90:2517–2525. doi: 10.1172/JCI116145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ, Nalivaeva NN. Proteinase dysbalance in pathology: the neprilysin (NEP) and angiotensin-converting enzyme (ACE) families. Cell Mol Biol (Noisy-le-grand) 2006;52:40–48. [PubMed] [Google Scholar]
- Klinger JR, Petit RD, Warburton RR, Wrenn DS, Arnal F, Hill NS. Neutral endopeptidase inhibition attenuates development of hypoxic pulmonary hypertension in rats. J Appl Physiol. 1993;75:1615–1623. doi: 10.1152/jappl.1993.75.4.1615. [DOI] [PubMed] [Google Scholar]
- Thompson JS, Morice AH. Neutral endopeptidase inhibitors and the pulmonary circulation. Gen Pharmacol. 1996;27:581–585. doi: 10.1016/0306-3623(95)02051-9. [DOI] [PubMed] [Google Scholar]
- Sumitomo M, Iwase A, Zheng R, Navarro D, Kaminetzky D, Shen R, Georgescu MM, Nanus DM. Synergy in tumor suppression by direct interaction of neutral endopeptidase with PTEN. Cancer Cell. 2004;5:67–78. doi: 10.1016/s1535-6108(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Febbraio M, Simantov R, Zheng R, Shen R, Silverstein RL, Nanus DM. Neprilysin inhibits angiogenesis via proteolysis of fibroblast growth factor-2. J Biol Chem. 2006;281:33597–33605. doi: 10.1074/jbc.M602490200. [DOI] [PubMed] [Google Scholar]
- Cohen AJ, Bunn PA, Franklin W, Magill-Solc C, Hartmann C, Helfrich B, Gilman L, Folkvord J, Helm K, Miller YE. Neutral endopeptidase: variable expression in human lung, inactivation in lung cancer, and modulation of peptide-induced calcium flux. Cancer Res. 1996;56:831–839. [PubMed] [Google Scholar]
- Xu J, Carretero OA, Liu YH, Yang F, Shesely EG, Oja-Tebbe N, Yang XP. Dual inhibition of ACE and NEP provides greater cardioprotection in mice with heart failure. J Card Fail. 2004;10:83–89. [PubMed] [Google Scholar]
- Maki T, Nasa Y, Yamaguchi F, Yoshida H, Mori M, Takada T, Horikawa E, Okano K, Takeo S. Long-term treatment with neutral endopeptidase inhibitor improves cardiac function and reduces natriuretic peptides in rats with chronic heart failure. Cardiovasc Res. 2001;51:608–617. doi: 10.1016/s0008-6363(01)00258-9. [DOI] [PubMed] [Google Scholar]
- Corti R, Burnett JC, Jr, Rouleau JL, Ruschitzka F, Luscher TF. Vasopeptidase inhibitors: a new therapeutic concept in cardiovascular disease? Circulation. 2001;104:1856–1862. doi: 10.1161/hc4001.097191. [DOI] [PubMed] [Google Scholar]
- Daull P, Jeng AY, Battistini B. Towards triple vasopeptidase inhibitors for the treatment of cardiovascular diseases. J Cardiovasc Pharmacol. 2007;50:247–256. doi: 10.1097/FJC.0b013e31813c6ca5. [DOI] [PubMed] [Google Scholar]
- Lu B, Figini M, Emanueli C, Geppetti P, Grady EF, Gerard NP, Ansell J, Payan DG, Gerard C, Bunnett N. The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat Med. 1997;3:904–907. doi: 10.1038/nm0897-904. [DOI] [PubMed] [Google Scholar]
- Sturiale S, Barbara G, Qiu B, Figini M, Geppetti P, Gerard N, Gerard C, Grady EF, Bunnett NW, Collins SM. Neutral endopeptidase (EC 3.4.24.11) terminates colitis by degrading substance P. Proc Natl Acad Sci USA. 1999;96:11653–11658. doi: 10.1073/pnas.96.20.11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Cutz E. Introduction to pulmonary neuroendocrine cell system, structure-function correlations. Microsc Res Tech. 1997;37:1–3. doi: 10.1002/(SICI)1097-0029(19970401)37:1<1::AID-JEMT1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Gosney JR. Pulmonary neuroendocrine cell system in pediatric and adult lung disease. Microsc Res Tech. 1997;37:107–113. doi: 10.1002/(SICI)1097-0029(19970401)37:1<107::AID-JEMT11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Van Lommel A, Bolle T, Fannes W, Lauweryns JM. The pulmonary neuroendocrine system: the past decade. Arch Histol Cytol. 1999;62:1–16. doi: 10.1679/aohc.62.1. [DOI] [PubMed] [Google Scholar]
- Nylen ES, Becker KL. Chronic hyperoxia and hamster pulmonary neuroendocrine cell bombesin and calcitonin. Anat Rec. 1993;236:248–252. doi: 10.1002/ar.1092360130. [DOI] [PubMed] [Google Scholar]
- Springall DR, Collina G, Barer G, Suggett AJ, Bee D, Polak JM. Increased intracellular levels of calcitonin gene-related peptide-like immunoreactivity in pulmonary endocrine cells of hypoxic rats. J Pathol. 1988;155:259–267. doi: 10.1002/path.1711550312. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Lock JE, Elde RP, Thompson TR. Pulmonary neuroendocrine cells in hyaline membrane disease and bronchopulmonary dysplasia. Pediatr Res. 1982;16:446–454. doi: 10.1203/00006450-198206000-00009. [DOI] [PubMed] [Google Scholar]
- Heath D, Yacoub M, Gosney JR, Madden B, Caslin AW, Smith P. Pulmonary endocrine cells in hypertensive pulmonary vascular disease. Histopathology. 1990;16:21–28. doi: 10.1111/j.1365-2559.1990.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Aguayo SM, King TE, Jr, Waldron JA, Jr, Sherritt KM, Kane MA, Miller YE. Increased pulmonary neuroendocrine cells with bombesin-like immunoreactivity in adult patients with eosinophilic granuloma. J Clin Invest. 1990;86:838–844. doi: 10.1172/JCI114782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday ME, Yoder BA, Cuttitta F, Haley KJ, Emanuel RL. Bombesin-like peptide mediates lung injury in a baboon model of bronchopulmonary dysplasia. J Clin Invest. 1998;102:584–594. doi: 10.1172/JCI2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett CG, Shahsafei A, Graham SA, Sunday ME. CD10/neutral endopeptidase inhibition augments pulmonary neuroendocrine cell hyperplasia in hamsters treated with diethylnitrosamine and hyperoxia. Am J Respir Cell Mol Biol. 1999;21:13–20. doi: 10.1165/ajrcmb.21.1.3389. [DOI] [PubMed] [Google Scholar]
- Fagan KA, Fouty BW, Tyler RC, Morris KG, Jr, Hepler LK, Sato K, LeCras TD, Abman SH, Weinberger HD, Huang PL, McMurtry IF, Rodman DM. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest. 1999;103:291–299. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler CM, Morris KG, Jr, Fagan KA, McMurtry IF, Messing RO, Dempsey EC. Protein kinase C-epsilon-null mice have decreased hypoxic pulmonary vasoconstriction. Am J Physiol Heart Circ Physiol. 2003;284:H1321–H1331. doi: 10.1152/ajpheart.00795.2002. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Tanonaka K, Daicho T, Nawa M, Oikawa R, Nasa Y, Takeo S. Preferable anesthetic conditions for echocardiographic determination of murine cardiac function. J Pharmacol Sci. 2005;99:95–104. doi: 10.1254/jphs.fp0050343. [DOI] [PubMed] [Google Scholar]
- Li C, Hersh LB. Neprilysin: assay methods, purification, and characterization. Methods Enzymol. 1995;248:253–263. doi: 10.1016/0076-6879(95)48018-8. [DOI] [PubMed] [Google Scholar]
- Kukkola PJ, Savage P, Sakane Y, Berry JC, Bilci NA, Ghai RD, Jeng AY. Differential structure-activity relationships of phosphoramidon analogues for inhibition of three metalloproteases: endothelin-converting enzyme, neutral endopeptidase, and angiotensin-converting enzyme. J Cardiovasc Pharmacol. 1995;26 Suppl 3:S65–S68. [PubMed] [Google Scholar]
- Dempsey EC, Badesch DB, Dobyns EL, Stenmark KR. Enhanced growth capacity of neonatal pulmonary artery smooth muscle cells in vitro: dependence on cell size, time from birth, insulin-like growth factor I, and auto-activation of protein kinase C. J Cell Physiol. 1994;160:469–481. doi: 10.1002/jcp.1041600310. [DOI] [PubMed] [Google Scholar]
- Dempsey EC, McMurtry IF, O'Brien RF. Protein kinase C activation allows pulmonary artery smooth muscle cells to proliferate to hypoxia. Am J Physiol. 1991;260:L136–L145. doi: 10.1152/ajplung.1991.260.2.L136. [DOI] [PubMed] [Google Scholar]
- Farina NK, Johnston CI, Burrell LM. Reversal of cardiac hypertrophy and fibrosis by S21402, a dual inhibitor of neutral endopeptidase and angiotensin converting enzyme in SHRs. J Hypertens. 2000;18:749–755. doi: 10.1097/00004872-200018060-00013. [DOI] [PubMed] [Google Scholar]
- Heath D, Madden B, Yacoub M. Pulmonary endocrine cells in plexogenic pulmonary arteriopathy. Physiol Bohemoslov. 1990;39:309–313. [PubMed] [Google Scholar]
- Madden BP, Gosney J, Coghlan JG, Kamalvand K, Caslin AW, Smith P, Yacoub M, Heath D. Pretransplant clinicopathological correlation in end-stage primary pulmonary hypertension. Eur Respir J. 1994;7:672–678. doi: 10.1183/09031936.94.07040672. [DOI] [PubMed] [Google Scholar]
- Carpenter TC, Stenmark KR. Hypoxia decreases lung neprilysin expression and increases pulmonary vascular leak. Am J Physiol Lung Cell Mol Physiol. 2001;281:L941–L948. doi: 10.1152/ajplung.2001.281.4.L941. [DOI] [PubMed] [Google Scholar]
- Fisk L, Nalivaeva NN, Boyle JP, Peers CS, Turner AJ. Effects of hypoxia and oxidative stress on expression of neprilysin in human neuroblastoma cells and rat cortical neurones and astrocytes. Neurochem Res. 2007;32:1741–1748. doi: 10.1007/s11064-007-9349-2. [DOI] [PubMed] [Google Scholar]
- Oh-hashi K, Nagai T, Tanaka T, Yu H, Hirata Y, Kiuchi K. Determination of hypoxic effect on neprilysin activity in human neuroblastoma SH-SY5Y cells using a novel HPLC method. Biochem Biophys Res Commun. 2005;334:380–385. doi: 10.1016/j.bbrc.2005.06.095. [DOI] [PubMed] [Google Scholar]
- Iatropoulos MJ, Williams GM. Proliferation markers. Exp Toxicol Pathol. 1996;48:175–181. doi: 10.1016/S0940-2993(96)80039-X. [DOI] [PubMed] [Google Scholar]
- Kajanne R, Miettinen P, Mehlem A, Leivonen SK, Birrer M, Foschi M, Kahari VM, Leppa S. EGF-R regulates MMP function in fibroblasts through MAPK and AP-1 pathways. J Cell Physiol. 2007;212:489–497. doi: 10.1002/jcp.21041. [DOI] [PubMed] [Google Scholar]
- Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- Shinall H, Song ES, Hersh LB. Susceptibility of amyloid beta peptide degrading enzymes to oxidative damage: a potential Alzheimer’s disease spiral. Biochemistry. 2005;44:15345–15350. doi: 10.1021/bi050650l. [DOI] [PubMed] [Google Scholar]
- Greijer AE, van der Groep P, Kemming D, Shvarts A, Semenza GL, Meijer GA, van de Wiel MA, Belien JA, van Diest PJ, van der Wall E. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). J Pathol. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- Gaczynska M, Osmulski PA. Small-molecule inhibitors of proteasome activity. Methods Mol Biol. 2005;301:3–22. doi: 10.1385/1-59259-895-1:003. [DOI] [PubMed] [Google Scholar]
- Hwang L, Leichter R, Okamoto A, Payan D, Collins SM, Bunnett NW. Downregulation of neutral endopeptidase (EC 3.4.24.11) in the inflamed rat intestine. Am J Physiol. 1993;264:G735–G743. doi: 10.1152/ajpgi.1993.264.4.G735. [DOI] [PubMed] [Google Scholar]
- Zee ED, Schomberg S, Carpenter TC. Hypoxia upregulates lung microvascular neurokinin-1 receptor expression. Am J Physiol Lung Cell Mol Physiol. 2006;291:L102–L110. doi: 10.1152/ajplung.00286.2005. [DOI] [PubMed] [Google Scholar]
- Springer J, Fischer A. Substance P-induced pulmonary vascular remodelling in precision cut lung slices. Eur Respir J. 2003;22:596–601. doi: 10.1183/09031936.03.00027903. [DOI] [PubMed] [Google Scholar]
- Yeon DS, Kim JS, Ahn DS, Kwon SC, Kang BS, Morgan KG, Lee YH. Role of protein kinase C- or RhoA-induced Ca(2+) sensitization in stretch-induced myogenic tone. Cardiovasc Res. 2002;53:431–438. doi: 10.1016/s0008-6363(01)00496-5. [DOI] [PubMed] [Google Scholar]
- Sinnett-Smith J, Lunn JA, Leopoldt D, Rozengurt E. Y-27632, an inhibitor of Rho-associated kinases, prevents tyrosine phosphorylation of focal adhesion kinase and paxillin induced by bombesin: dissociation from tyrosine phosphorylation of p130(CAS). Exp Cell Res. 2001;266:292–302. doi: 10.1006/excr.2001.5219. [DOI] [PubMed] [Google Scholar]
- Dempsey EC, Cool CD, Littler CM. Lung disease and PKCs. Pharmacol Res. 2007;55:545–559. doi: 10.1016/j.phrs.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Barr EJ, Wehling CA, Miller YE, Voelkel NF, Dempse EC. Lung neprilysin activity and expression are decreased in a human model of chronic hypoxic pulmonary hypertension. 2007 American Thoracic Society, San Francisco, California. Am J Respir Crit Care Med. 2007;Vol. 175:A44. (abstract 322) [Google Scholar]