Abstract
The pathological hallmarks of idiopathic pulmonary fibrosis include proliferating fibroblasts and myofibroblasts, as well as excessive collagen matrix deposition. In addition, both myofibroblast contraction and remodeling of the collagen-rich matrix contribute to the abnormal structure and function of the fibrotic lung. Little is known, however, about collagen-associated proteins that promote fibroblast and myofibroblast retention, as well as the proliferation of these cells on the extracellular matrix. In this study, we demonstrate that aortic carboxypeptidase-like protein (ACLP), a collagen-associated protein with a discoidin-like domain, is expressed at high levels in human fibrotic lung tissue and human fibroblasts, and that its expression increases markedly in the lungs of bleomycin-injured mice. Importantly, ACLP-deficient mice accumulated significantly fewer myofibroblasts and less collagen in the lung after bleomycin injury, as compared with wild-type controls, despite equivalent levels of bleomycin-induced inflammation. ACLP that is secreted by lung fibroblasts was retained on fibrillar collagen, and ACLP-deficient lung fibroblasts that were cultured on collagen exhibited changes in cell spreading, proliferation, and contraction of the collagen matrix. Finally, the addition of recombinant discoidin-like domain of ACLP to cultured ACLP-deficient lung fibroblasts restored cell spreading and increased the contraction of collagen gels. Therefore, both ACLP and its discoidin-like domain may be novel targets for anti-myofibroblast-based therapies for the treatment of pulmonary fibrosis.
Idiopathic pulmonary fibrosis (IPF) is a fatal disease with no known effective therapy that is characterized by a progressive decline in lung function and a median survival of only 36 months.1 The key pathological feature of IPF is fibroblastic foci, areas rich in fibroblasts, myofibroblasts, and excess extracellular matrix (ECM). Myofibroblasts, through their secretion of ECM molecules including fibrillar collagens, fibronectin, elastin, and proteoglycans, contribute significantly to the formation of fibrotic lesions.2,3
The cellular origin(s) of lung myofibroblasts is still uncertain, but they likely arise through differentiation of fibroblasts that originate from numerous sources, including resident lung fibroblasts, and mesenchymal and bone marrow-derived progenitor cells, and from epithelial and endothelial cells via transdifferentiation.4 Fibroblast to myofibroblast differentiation is classically defined by expression of the pro-contractile, cytoskeletal protein α-smooth muscle actin (α-SMA); however, other important differentiation events include expression of specialized matrix proteins, such as the extra-domain A of fibronectin, and formation of mature focal adhesions and actin stress fibers.5 Although numerous cytokines and growth factors, including transforming growth factor-β1 (TGF-β1), are known to regulate the fibrotic process,6 the ECM also influences myofibroblast proliferation and differentiation via biochemical and mechanical signaling.5,7 For example, on tissue culture plastic, a collagen-poor and stiff planar surface, fibroblast to myofibroblast differentiation occurs predominantly via fibronectin-mediated contacts.5,8 On a deformable in vitro collagen lattice, fibroblast proliferation and differentiation to myofibroblasts depends on at least two major processes: 1) cell adhesion and spreading on the matrix, and 2) generation of tension in the matrix mediated by fibroblast contraction.5,7 Although collagen-binding integrin receptors mediate, in part, fibroblast adhesion to collagen, the potent morphological, proliferative, and differentiating effects of collagen on fibroblasts indicate a role for additional mediators of cell–collagen matrix interactions.5,7,9
We previously identified aortic carboxypeptidase-like protein (ACLP), an ECM-associated protein highly expressed in collagen-rich tissues and secreted by fibroblasts, myofibroblasts, and smooth muscle cells.10,11,12 ACLP, a modular protein, contains a lysine- and proline-rich extensin domain, a putative collagen binding discoidin-like domain (DLD), and a catalytically inactive metallocarboxypeptidase domain at its carboxyl terminus.10 In addition to its expression in collagen-rich tissues, ACLP expression markedly increases in the injured dermis and vasculature.11,13 Most ACLP-deficient (ACLP−/−) mice have a lethal defect in closure of the ventral abdominal wall called gastroschisis; ACLP−/− mice that do not have gastroschisis survive to adulthood but exhibit a significant delay in healing experimentally induced dermal wounds.13 Given the cellular and molecular similarities between wound healing and pulmonary fibrosis,4,5,6 we hypothesize that ACLP is an important mediator of pulmonary fibrosis. In this study, we show that ACLP is highly expressed in fibrotic lungs, both in humans with IPF and in bleomycin-treated mice, and that ACLP−/− mice are substantially protected from bleomycin-induced lung fibrosis, including lessened accumulation of lung myofibroblasts. Moreover, we demonstrate that ACLP mediates lung fibroblast cell spreading and proliferation on collagen and collagen matrix contraction, in part via its DLD.
Materials and Methods
Mice
Wild-type (ACLP+/+) and ACLP−/− mice were obtained through breeding ACLP heterozygote (ACLP+/−) mice as previously described.13 ACLP−/− mice on a pure C57Bl/6 background die perinatally from gastroschisis (unpublished observations). We therefore studied mice on a mixed 129Sv-C57Bl/6 background that had been inbred for >12 generations and are genetically homogenous. Wild-type littermate siblings were used for all controls. The Harvard Medical Area Standing Committee on Animals approved all animal experimental procedures.
Bleomycin Injury
Equal numbers of 10- to 12-week-old male and female ACLP+/+ and ACLP−/− mice were given 50 μl of sterile normal saline or 0.0011 U/g body weight of bleomycin sulfate (NOVAPLUS, Irvine, CA) via a 22-gauge oral endotracheal catheter under intraperitoneal avertin anesthesia. This dose of bleomycin produced consistent lung fibrosis with a mortality of <10% and is consistent with doses used in the murine bleomycin model.14
Human Lung Specimens
Lung tissue from six patients with idiopathic pulmonary fibrosis was obtained at the time of lung transplant surgery. All patients had a presurgical diagnosis of IPF and the diagnosis was confirmed by clinical pathological review of the explanted lung tissue. Control lung tissue was obtained through the Center for Organ Recovery and Education (Pittsburgh, PA) from normal regions of lungs of six organ donors whose lungs were not used for transplantation. The Institutional Internal Review Board at the Brigham and Women’s Hospital (Boston, MA) approved the experimental protocol.
Histology and Immunohistochemistry
Lungs were perfused with PBS, inflation-fixed at 25 cm H2O pressure with methyl Carnoy’s solution or 10% neutral buffered formalin and then processed and embedded in paraffin. Human and mouse lung sections (5 μm) were stained for collagen with Masson’s trichrome according to the manufacturer’s instructions (Sigma, St Louis, MO). Parallel sections were immunostained for ACLP and α-SMA as described.12,15 Where indicated, immunostaining was quantified by measuring the area of staining per microscopic field using ImageJ software.
Hydroxyproline Assay
Total right lung collagen content was determined by measuring the amount of hydroxyproline in 6 N HCl lung hydrosylates and expressed as μg hydroxyproline/mg lung.14,16
Bronchoalveolar Lavage Fluid Analysis
After anesthetizing mice, bronchoalveolar lavages (BALs) were performed with 2 × 0.75 ml aliquots of PBS. Total and individual leukocyte counts were performed on the BAL fluid as described.17 To measure active TGF-β1, BALs were performed with Dulbecco’s Modification of Eagles Medium (DMEM) (in the absence of serum) instead of PBS. After clearing cells from the DMEM/BAL fluid by centrifugation, aliquots were used in a bioassay to quantify active TGF-β1. In brief, 200 μl of BAL fluid or standard concentrations of TGF-β1 (Peprotech Inc, Rocky Hill, NJ) were applied to 1.6 × 104 mink lung epithelial cells stably transfected with a plasminogen activator inhibitor-1 promoter–luciferase reporter construct (a generous gift from Dr. Daniel Rifkin, NYU Medical Center). After culturing cells for 18 hours, luciferase activity in cell lysates was measured using a luciferase substrate (Promega) and luminometer (EG&G Berthold, Oak Ridge, TN); BAL fluid TGF-β1 concentrations were then quantified from a standard curve of luciferase activity created using standard concentrations of TGF-β1.
Lung Fibroblast Isolation and Culture
Each culture of lung fibroblasts was isolated from two mice as described.18 Briefly, ACLP+/+ or ACLP−/− mice were euthanized and, after perfusion with PBS, their lungs minced and treated with 0.25% trypsin for 45 minutes at 37°C. Primary cells were then separated from lung tissue using a 100-μm filter and cultured in DMEM with 10% FBS. Fibroblasts were used in experiments only between passages 3 and 6. Human IMR-90 lung fibroblasts (ATCC, Manassas, VA) were cultured in DMEM with 10% FBS. Cells were treated with TGF-β1 and proteins were analyzed as described in Figure legends.
Collagen Gel Contraction Assay
Collagen gel matrices were prepared by adding 250 μl of a neutralized collagen (Vitrogen, Angiotech, Palo Alto, CA) solution [8 ml collagen (3 mg/ml), 1 ml 10× DMEM, 1 ml 200 mmol/L HEPES, pH 8.5], to a 24-well tissue culture well as described.19,20 After incubating cells on the restrained gels as indicated in the Figure legends, gels were released and their two longest diameters measured with calipers at various time points. Gel size was defined as the sum of the two gel diameters and gel contraction expressed as a percentage of the original gel size.21 Where indicated, cells were extracted from collagen gels using 2 mg/ml bacterial collagenase for 1 hour at 37°C.
Lung Fibroblast Adhesion Assays
Lung fibroblasts (3 × 104) were seeded in serum-free media onto 96-well dishes coated with 10 mg/ml bovine serum albumin or 0.5 mg/ml type I collagen. After 30, 60, 120, and 180 minutes, cells were washed to remove non-adherent cells, fixed in 1% glutaraldehyde, and then stained with 0.1% crystal violet. Adherent cells were washed extensively and cell adhesion quantified by measuring the OD550 for each well as described.22
ECM Protein Extraction
After lung fibroblasts (2 × 105) were cultured on collagen gels for 18 hours, total protein or protein subfractions were extracted as described.10,13 Briefly, cell and ECM protein fractions were sequentially extracted as follows: (1) with buffered 0.5% Triton-X 100 to solubilize cytosol and membrane associated cellular proteins, (2) with 25 mmol/L ammonium acetate pH 9.0 to extract nuclear and cytoskeletal associated proteins, and finally with (3) SDS-containing buffer to extract ECM-retained proteins. Protein fractions were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot analysis was performed for ACLP.
Western Blot Analysis
Total protein from lungs and cultured cells was extracted and analyzed by Western blot as described.10 Antibodies were used, as indicated in the Figure legends, against ACLP,10 smooth muscle α-actin (Sigma, 1A4), α-tubulin (Sigma, DM1A), β-actin (Sigma), total myosin light chain (MLC), and di-phosphorylated MLC (Santa Cruz Biotechnology, Santa Cruz, CA).23 Immunoblots were quantified by measuring the density of scanned images using ImageJ software.
ACLP DLD Recombinant Expression and Purification
The discoidin domain of mouse ACLP (DLD) was obtained using PCR with Pfu Turbo (Stratagene) and primers containing 5′ BamHI and 3′ EcoRI sites respectively. The PCR product was ligated into pRSET B (Invitrogen) and expressed in the bacterial strain BL21(DE3)pLysS (Stratagene) as an N-terminal His-tagged protein. Bacterial pellets were sonicated in lysis buffer [50 mmol/L sodium phosphate, 10 mmol/L Tris (pH 8.0), 8 M/L urea, 100 mmol/L NaCl, 10 mmol/L imidazole, 1% Tween-20, and 10 mmol/L β-mercaptoethanol], and the protein was purified using TALON metal affinity resin (Clontech). DLD was eluted by three washes with lysis buffer containing 250 mmol/L imidizole and dialyzed overnight first in 1 M/L urea, 50 mmol/L Tris (pH 8.0), 0.005% Tween-20, 2 mmol/L reduced glutathione, 0.02 mmol/L oxidized glutathione, and 10% glycerol, and then in the same buffer without urea in a 10,000 molecular weight cutoff Slide-A-Lyzer cassette (Pierce). Finally, DLD was dialyzed against PBS containing 10% glycerol. Protein concentration was determined by spectrophotometry at OD280 using the extinction coefficient of 36,900 M−1cm−1. Protein concentration and purity were verified by gel electrophoresis (Novex, Invitrogen) and stained with SimplyBlue SafeStain (Invitrogen) and estimated to be >95% pure.
Statistical Analysis
Data are presented as mean values ± SEM. Statistical significance was determined by the Student’s t-test for comparisons between two groups and defined as a P value <0.05.
Results
ACLP Is Highly Expressed in Mouse Fibrotic Lung in a Pattern Similar to Collagen
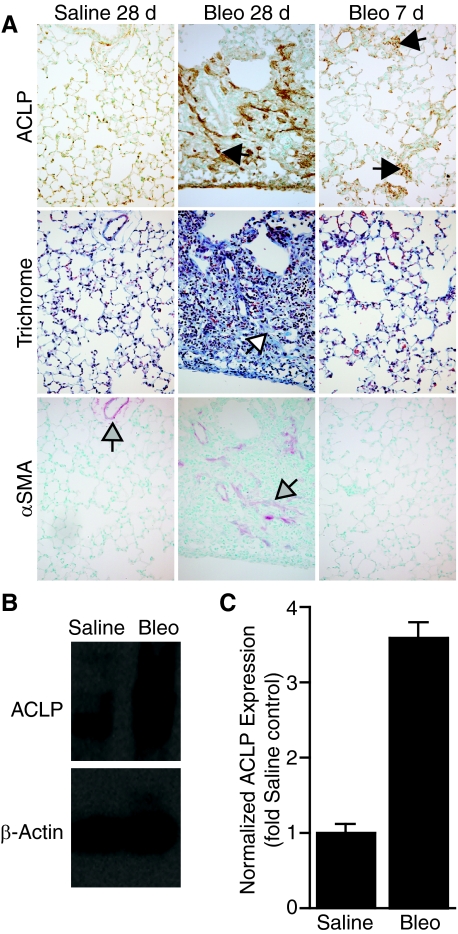
ACLP and collagen expression are closely associated in normal tissues and ACLP expression markedly increases during wound repair,11,12,13 a process with features similar to pulmonary fibrosis. To better define the relationship between ACLP expression and the onset of lung fibrosis, we studied ACLP expression in the well-characterized bleomycin model of mouse lung fibrosis. In saline-treated control mice, little ACLP is seen in the lung parenchyma, and expression is mostly confined to the walls of blood vessels and large airways (Figure 1A, and data not shown), a finding consistent with our prior observations of ACLP expression in normal developing lung tissue.12 In contrast, after bleomycin injury, mice had a marked increase in lung ACLP expression by 28 days (Figure 1A), the time of peak bleomycin-induced fibrosis.2,24 The increases in expression and distribution of ACLP in fibrotic lung lesions were very similar to trichrome-stained collagen (Figure 1A), indicating a likely association between ACLP and collagen in the ECM. Moreover, ACLP expression increases by just 7 days after bleomycin injury, a time when relatively few myofibroblasts are present as indicated by the low numbers of α-SMA positive cells at 7 days, as compared with the increased number at 28 days (Figure 1A). Although ACLP is present in areas of α-SMA staining 28 days after bleomycin injury, ACLP staining more closely associates with collagen (Figure 1A). Consistent with our immunostaining results, Western blot analysis on lung extracts also revealed a substantial increase in ACLP expression (>threefold) at 28 days in bleomycin treated mice (Figure 1, B and C).
Figure 1.
ACLP levels increase in mouse lungs after bleomycin injury. A: Wild-type mice (n = 5 each group) were treated with either 50 μl of intratracheal normal saline or 0.0011 U/g body weight of bleomycin. Lungs were harvested after 7 or 28 days, fixed, and immunostained for ACLP (brown, black arrows), stained with Masson’s trichrome for collagen (Trichrome, blue, white arrow), or immunostained for α-SMA (red, gray arrows). Data are representative of parallel 5 μm sections for each condition. Arrows indicate areas of specific immunostaining (magnification = original ×200). B: ACLP and β-actin Western blots of whole-lung protein extracts from wild-type mice 28 days after treatment with saline or bleomycin (Bleo). C: Densitometric quantitiation of ACLP Western blots of whole-lung protein extracts from wild-type mice 28 days after treatment with saline or bleomycin (n = 4 each group).
ACLP−/− Mice Are Protected from Bleomycin-Induced Lung Fibrosis
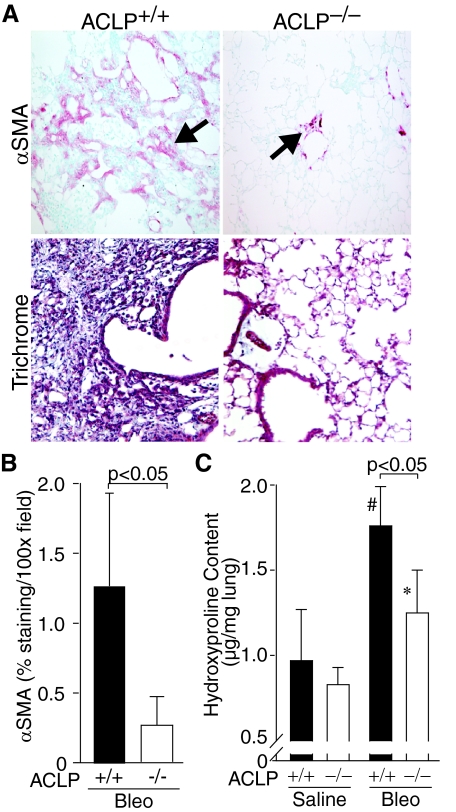
To investigate the role of ACLP in lung fibrosis, we compared the responses of ACLP+/+ and ACLP−/− mice to bleomycin lung injury. ACLP+/+ mice developed areas of dense lung fibrosis 28 days after bleomycin injury characterized by accumulation of α-SMA-positive fibroblasts (Figure 2A) and collagen, demonstrated by trichrome collagen staining (Figure 2A) and quantified by measuring whole-lung hydroxyproline (Figure 2C). In contrast, lungs from bleomycin-injured ACLP−/− mice had markedly fewer α-SMA-positive fibroblasts (4.8-fold less α-SMA immunostaining, Figure 2, A and B) and significantly less collagen staining (Figure 2A) and 28% less hydroxyproline content (Figure 2C) than ACLP+/+ mice, indicating they were protected from developing significant fibrosis.
Figure 2.
ACLP−/− mice accumulate fewer α-SMA-positive fibroblasts and less collagen in the lung after bleomycin injury compared with wild-type mice. A: Wild-type (ACLP+/+) and ACLP-deficient (ACLP−/−) mice were treated with intratracheal bleomycin (n = 6 each group). After 28 days harvested lungs were immunostained for α-SMA (red indicated by arrows) or stained with Masson’s trichrome (Trichrome, blue) to detect collagen. Data are representative of 5 μm sections for each condition. B: Wild-type (+/+, filled bar) and ACLP-deficient (−/−, open bar) mice were treated with bleomycin (Bleo) (n = 6 each group). After 28 days, harvested lungs were immunostained for α-SMA and the staining quantified by measuring the area of red staining per ×100 field; the data are expressed as % staining per ×100 field. C: Wild-type (+/+, filled bars) and ACLP-deficient (−/−, open bars) mice were treated with either saline or bleomycin (Bleo) (n = 6 each group). After 28 days harvested lungs were assayed for whole-lung hydroxyproline content and expressed as hydroxyproline (μg/mg lung weight). Data are mean values ± SE (#P < 0.05 for bleomycin-treated compared with saline-treated ACLP+/+ mice and *P = NS for bleomycin-treated compared with saline-treated ACLP−/− mice).
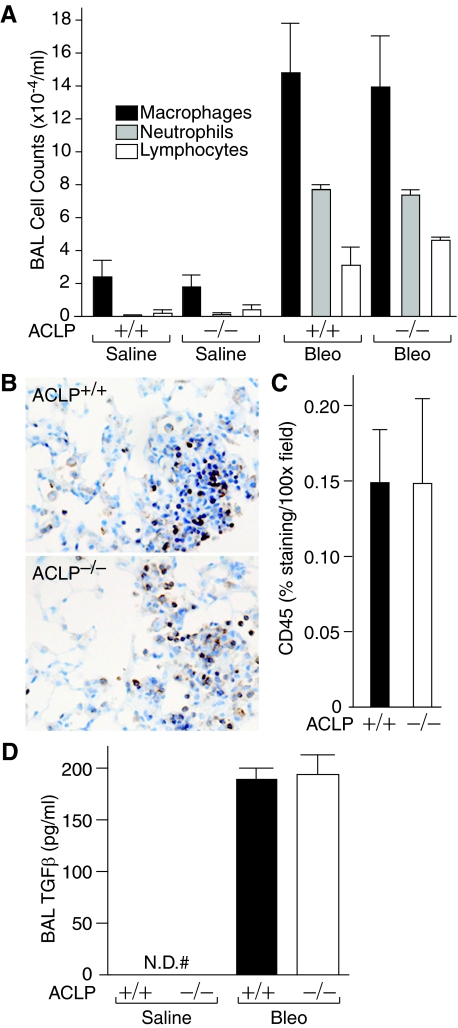
Since early inflammation and production of active TGF-β1 are important events for the development of fibrosis in the bleomycin model,2,25,26 we studied whether these early inflammatory and pro-fibrotic responses to bleomycin were different in ACLP+/+ and ACLP−/− mice. First, the marked increases in macrophages, neutrophils, and lymphocytes in BAL fluid 7 days after bleomycin injury, as compared with saline-treated controls, were not different between ACLP+/+ and ACLP−/− mice (Figure 3A). Moreover, ACLP+/+ and ACLP−/− mice developed an equally robust pneumonitis 7 days after bleomycin-injury and contained similar numbers of cells staining positive for the leukocyte common antigen, CD45 (Figure 3, B and C). Finally, when we compared the active TGF-β1 concentrations in BAL fluid between ACLP+/+ and ACLP−/− mice 7 days after treatment with saline or bleomycin, we found marked and equivalent increases in BAL fluid TGF-β1 in bleomycin treated mice (Figure 3D).
Figure 3.
ACLP+/+ and ACLP−/− mice have similar early inflammatory responses to bleomycin injury. A: Wild-type (+/+) and ACLP-deficient (−/−) mice were treated with either saline or bleomycin (Bleo) (n = 3 each group). After 7 days BALs were performed on anesthetized mice using 1.5 ml of PBS. After staining, BAL macrophages (black bars), neutrophils (gray bars), and lymphocytes (open bars) were counted and the cell counts expressed per ml of recovered BAL fluid. B: Wild-type (ACLP+/+) and ACLP-deficient (ACLP−/−) mice were treated with bleomycin (n = 4 each group). After 7 days, harvested lungs were immunostained for CD45 (brown) and the staining quantified by measuring the area of brown staining per ×100 microscope field; the data are expressed as % staining per ×100 field (C). Wild-type (+/+) and ACLP-deficient (−/−) mice were treated with either saline or bleomycin (Bleo) (n = 3 each group). After 7 days, BALs were performed on anesthetized mice using 1.5 ml of DMEM. Active TGF-β1 in the BAL fluid was assayed and expressed as ng/ml of BAL fluid (D) (#N.D. = none detected). All data are mean values ± SE.
ACLP Is Retained on Collagen and ACLP−/− Lung Fibroblasts Have a Defect in Cell Spreading and Contraction on Collagen Matrix
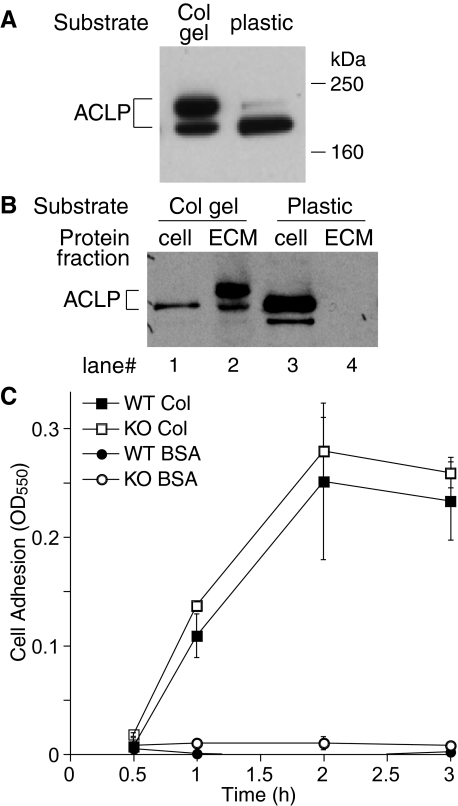
Since ACLP is secreted by fibroblasts, co-localized with collagen in vivo, and expressed early in the fibrotic phase of lung injury (see Figure 1A, column 3), we reasoned that ACLP might promote fibrosis by regulating the interaction between fibroblasts and collagen matrix. Thus, we compared primary lung fibroblast ACLP expression on a collagen-poor substrate, tissue culture plastic, to type I collagen gels. Consistent with our prior studies of smooth muscle cells,10 lung fibroblasts cultured on tissue culture plastic express ACLP in a form that migrates at ∼170 kDa on SDS-PAGE, with a second faint band at ∼195 kDa (Figure 4A). In contrast, fibroblasts cultured on fibrillar collagen gels express increased amounts of the ∼195-kDa form of ACLP (Figure 4A), a pattern of ACLP expression more consistent with what we observe in bleomycin-treated fibrotic lung (Figure 1B). In separate experiments we determined that the 195-kDa ACLP band was due to glycosylation (data not shown). Next, we examined whether the 170-kDa, 195-kDa, or both forms of ACLP associate with collagen. After culture for 18 hours on either tissue culture plastic or collagen gel, fibroblast extracts containing total protein, soluble cell-associated protein, or ECM-associated proteins were analyzed for ACLP content using Western blotting.13 Interestingly, the 195-kDa form of ACLP was present in the ECM fraction when cells are cultured on collagen (Figure 4B). In contrast, essentially no ACLP accumulated in the ECM fraction when lung fibroblasts were cultured on tissue culture plastic (Figure 4B). Importantly, Western blot analysis of our starting collagen solution revealed it contained no detectable ACLP (data not shown). We next measured wild-type and ACLP−/− fibroblast adhesion to plastic wells coated with either bovine serum albumin as a control or type I collagen as a function of time. Whereas no lung fibroblasts adhered to bovine serum albumin, equal quantities of wild-type and ACLP−/− lung fibroblasts adhered with similar kinetics to type I collagen (Figure 4C). In addition, there were no differences between wild-type and ACLP−/− lung fibroblasts in their kinetics of adhesion to several other ECM substrates, including types III and V collagen and fibronectin, or their expression of the collagen-binding α2β1 integrin (data not shown).
Figure 4.
ACLP is retained on collagen and ACLP−/− lung fibroblasts have normal adhesion to collagen. A: ACLP Western blot of total proteins extracted from wild-type lung fibroblasts cultured for 18 hours on either collagen gels (Col gel) or tissue culture plastic (plastic). B: Lung fibroblasts were cultured for 18 hours on either collagen gels (Col gel) or tissue culture plastic (plastic) followed by extraction of cell and ECM protein fractions as indicated in the Materials and Methods section. ACLP Western blot of cell and ECM fractions: lanes 1 and 2 cell and ECM proteins, respectively, from fibroblasts cultured on collagen; lanes 3 and 4 cell and ECM proteins, respectively, from fibroblasts cultured on tissue culture plastic. C: Adhesion of ACLP+/+ and ACLP−/− lung fibroblasts was measured as described in Materials and Methods. ACLP+/+ on Col I (closed square), ACLP−/− on Col I (open square), ACLP+/+ on BSA (closed circle), ACLP−/− on BSA (open circle).
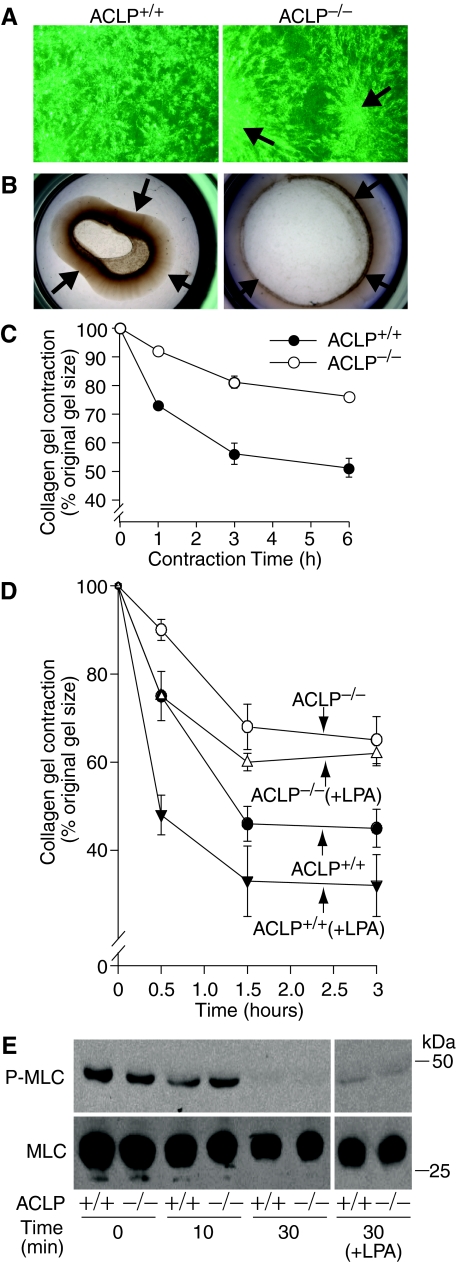
To determine whether ACLP mediates fibroblast function on collagen gels, we compared ACLP+/+ and ACLP−/− lung fibroblast morphology on purified type I collagen gels and their abilities to contract collagen matrix.19,21 After culture for 24 hours on restrained collagen gels, ACLP+/+ primary lung fibroblasts dispersed evenly across the matrix and had numerous cell membrane projections (Figure 5A). In contrast, ACLP−/− lung fibroblasts remained grouped (Figure 5A), leaving acellular gaps in the matrix, and had few membrane projections (Figure 5A). Importantly, these morphological differences were unique to collagen matrix, since both ACLP+/+ and ACLP−/− lung fibroblasts spread evenly with normal appearing cell membrane processes on either tissue culture plastic or fibronectin (data not shown). To examine the potential functional consequences of these morphological findings, we compared ACLP+/+ and ACLP−/− lung fibroblast collagen contraction by culturing the cells on tethered collagen gels for 18 hours and then gently releasing them to allow contraction. By 6 hours after gel release, ACLP+/+ fibroblasts contracted the gels to 51 ± 3% of their original size whereas ACLP−/− fibroblasts contracted the gels to only 78 ± 0.2% of their original size (Figure 5, B and C). In addition, treatment with the contractile agonist lysophosphatidic acid (LPA) augmented collagen contraction by ACLP+/+ fibroblasts but had a minimal effect on collagen contraction by ACLP−/− fibroblasts (Figure 5D).
Figure 5.
ACLP−/− lung fibroblasts have a defect in cell spreading and contraction of a collagen matrix. A: Phase contrast photomicrographs of wild-type (ACLP+/+) and ACLP-deficient (ACLP−/−) primary lung fibroblasts cultured on restrained collagen gels for 24 hours at a density of 2 × 105 cells/gel. Clustering of ACLP−/− fibroblasts is marked by arrows; viewed at ×40. B: Wild-type (ACLP+/+) and ACLP-deficient (ACLP−/−) primary lung fibroblasts were cultured on restrained collagen gels for 24 hours at a density of 2 × 105 cells/gel. The gels were then released, allowed to contract for 6 hours and photographed; arrows mark gel edges. C: Time course of collagen gel contraction by wild-type (closed circle) and ACLP−/− (open circle) lung fibroblasts. Cells were cultured and collagen gels released and allowed to contract as in panel (B); gel contraction is expressed as a percentage of the original gel size. D: Primary lung fibroblasts were cultured and collagen gels released and allowed to contract as in panel (B). Twenty min before releasing gels, fibroblasts were cultured in the absence [wild-type (ACLP+/+, closed circle); ACLP-deficient (ACLP−/−, open circle)] or presence [wild-type (ACLP+/+ + LPA, closed inverted triangle); ACLP-deficient (ACLP−/− + LPA, open triangle)] of 10 μm lysophosphatidic acid. Gel contraction is expressed as a percentage of the original gel size. E: Wild-type (+/+) and ACLP-deficient (−/−) primary lung fibroblasts (2 × 105) were cultured on restrained collagen gels for 18 hours. Twenty minutes before releasing gels, cells were cultured in the absence or presence (+LPA) of 10 μm lysophosphatidic acid. After gel release and contraction for the indicated time points [time (min)], total cell proteins were immediately extracted and equal amounts subjected to SDS-PAGE followed by Western blotting using antibodies to phosphorylated myosin light chain (P-MLC) and total myosin light chain (MLC); data shown are from one of two experiments showing similar results.
Although the lack of cell spreading and dispersion across the collagen matrix likely account for the matrix contraction defect in ACLP−/− fibroblasts, additional processes necessary for collagen contraction by fibroblasts, including expression and activation of cytoskeletal contractile proteins may be involved. We next examined the expression and phosphorylation of the key contraction regulatory protein MLC to determine whether ACLP−/− fibroblasts have an additional defect in basic cellular contractile function.23,27,28 After culture on restrained collagen gels for 18 hours, ACLP+/+ and ACLP−/− lung fibroblasts exhibited similar levels of phosphorylated MLC (P-MLC) (Figure 5E, Time 0), potentially suggesting equivalent cellular force generation before release of the restrained gels. After its release, gel contraction begins immediately and P-MLC de-phosphorylates rapidly but similarly in ACLP+/+ and ACLP−/− lung fibroblasts (Figure 5E). Moreover, while de-phosphorylation is complete by 30 minutes after gel release, treatment with LPA preserves P-MLC to a similar degree in ACLP+/+ and ACLP−/− lung fibroblasts (Figure 5E), an event that does not markedly augment collagen contraction by ACLP−/− fibroblasts (Figure 5D).
The DLD of ACLP Promotes Fibroblast Spreading on Collagen and Increases Collagen Matrix Contraction by Fibroblasts
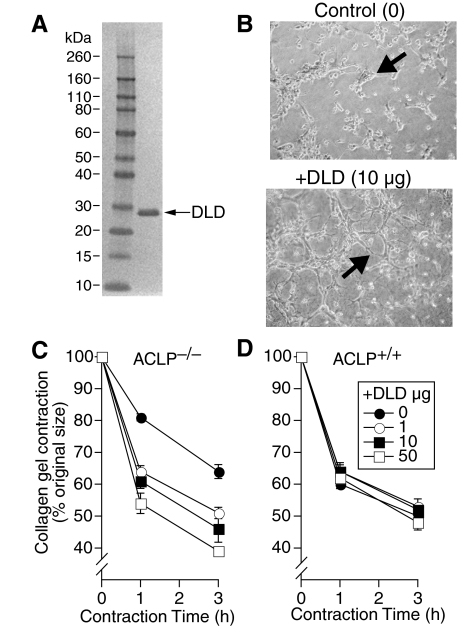
Other discoidin domain containing proteins interact with fibrillar collagens and have a diverse and extensive role in cell adhesion, membrane extension, and migration.29 To investigate whether the DLD of ACLP might be sufficient to mediate the collagen contraction phenotype, we generated and purified recombinant His-tagged DLD (Figure 6A). After culture for 18 hours on collagen matrix, ACLP−/− lung fibroblasts form clusters of rounded cells with relatively few membrane extensions (lamellipodia-like structures, Figure 6B). Interestingly, ACLP−/− fibroblasts cultured in the presence of 10 μg/ml of the DLD demonstrated more extensive cell spreading and branching membrane extensions consistent with increased lamellipodia formation (Figure 6B). Importantly, although the DLD had little effect on collagen matrix contraction by wild-type lung fibroblasts, it increased collagen contraction by ACLP−/− fibroblasts up to 39% in a dose-dependent manner (Figure 6, C and D). To control for a possible effect of the DLD’s his tag on collagen contraction, we treated ACLP−/− fibroblasts with an unrelated His-tagged protein with a similar molecular weight as DLD (His-tagged core protein from heptatitis B virus) and found no effect on collagen gel contraction (data not shown). In addition, the minimal effect of His-tagged DLD on wild-type fibroblast contraction (Figure 6D) further indicates the His tag likely has no additional effect on collagen gel contraction.
Figure 6.
The discoidin-like domain of ACLP promotes fibroblast spreading on collagen and increases collagen matrix contraction by fibroblasts. A: Purified His-tagged DLD protein was analyzed by SDS-PAGE and stained as described in the Materials and Methods. Lane 1, MW marker. Lane 2, 1 μg aliquot of purified DLD. B: Phase contrast photomicrographs of ACLP-deficient primary lung fibroblasts cultured under serum-free conditions on restrained collagen gels at a density of 1 × 105 cells/gel for 24 hours in the presence of 10 μg/ml BSA (Control) or 10 μg/ml of the discoidin-like domain (DLD) of ACLP (+DLD). The arrows indicate cells with enhanced spreading and membrane extensions; viewed at ×100. C: ACLP-deficient (ACLP−/−) and (D) wild-type (ACLP+/+) primary lung fibroblasts were cultured under serum-free conditions on restrained collagen gels at a density of 2 × 105 cells/gel for 24 hours in the presence of 0 (closed circle), 1 (open circle), 10 (closed box), or 50 (open box) μg/ml DLD. The collagen gels were then released and allowed to contract for the indicated amount of time. Collagen gel contraction is expressed as percent original gel size. All data are mean values ± SE. Data are from one of three experiments all showing similar results.
ACLP−/− Lung Fibroblasts Have a Defect in Cell Proliferation on Collagen Matrix
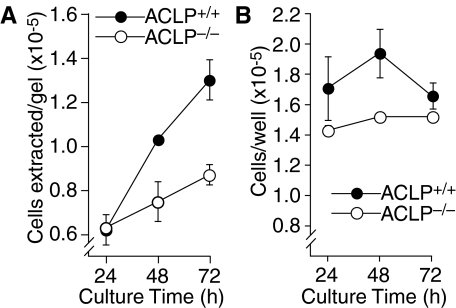
Although the collagen contraction defect in ACLP−/− fibroblasts indicates a role for ACLP in the matrix remodeling characteristic of fibrotic lung, this cellular phenotype may not be sufficient to explain the paucity of myofibroblasts and collagen in the lungs of bleomycin-treated ACLP−/− mice (Figure 2). Thus, we explored whether the altered cell–collagen interaction in ACLP−/− fibroblasts may also affect fibroblast proliferation. After culture for 24 hours equal numbers of wild-type and ACLP−/− lung fibroblasts were present on restrained collagen gels (Figure 7A), a time when there are clear differences between wild-type and ACLP−/− fibroblasts in cell spreading and dispersion on the collagen matrix (Figure 5). However, by 72 hours of culture there are 1.5-fold more wild-type than ACLP−/− fibroblasts present on the collagen matrix (Figure 7A). Wild-type fibroblasts do proliferate more than ACLP−/− fibroblasts on a plastic substrate (Figure 7B), but the difference in proliferation is not as great for cells cultured on collagen. Importantly, although an increased rate of apoptosis in ACLP−/− fibroblasts could account for the differences in cell number, we observed no difference between the number of apoptotic wild-type and ACLP−/− fibroblasts present on collagen gels (data not shown).
Figure 7.
ACLP−/− lung fibroblasts have a defect in cell proliferation on collagen matrix. Wild-type (closed circle) and ACLP-deficient (open circle) primary lung fibroblasts were cultured under serum-free conditions on restrained collagen gels (A) or tissue culture plastic (B) in 24-well dishes at a starting density of 1 × 105 cells/well. At the indicated time points, cells were extracted from collagen gels by collagenase digestion (A) or removed from plastic wells using trypsin (B). Cells were then counted using a Coulter particle counter and expressed as cells extracted/gel (A) or cells removed/well (B). All data are mean values ± SE. All experiments were performed at least three times with different primary cell cultures.
ACLP Expression in Human Fibroblasts and in Fibrotic Human Lungs
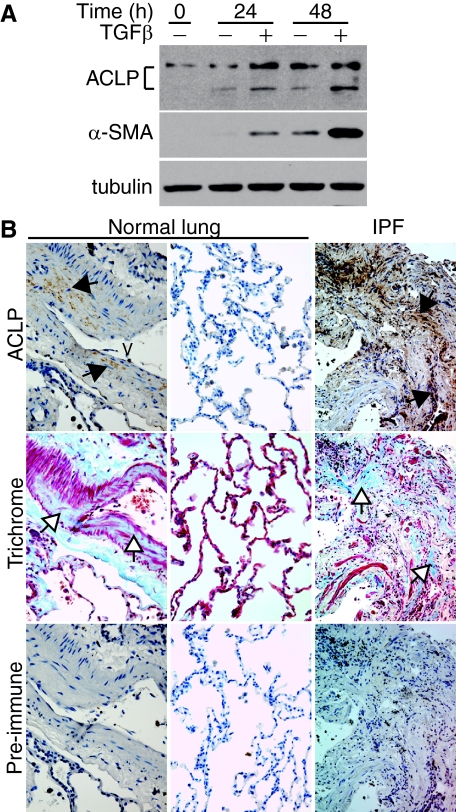
Although the mouse bleomycin model of lung fibrosis shares many characteristics with the human disease, nothing is currently known about ACLP expression by human lung fibroblasts or in human pulmonary fibrosis. We first measured ACLP expression in IMR-90 cells, a human fibroblast cell line, in the absence or presence of 10 ng/ml TGF-β1. At baseline, IMR-90 cells expressed ACLP but little α-SMA (Figure 8A, Time 0). Treatment with TGF-β induced expression of both ACLP and α-SMA by 24 and 48 hours as compared with untreated control cells (Figure 8A). Of note, the control cells also had increased expression of both ACLP and α-SMA with time in culture (Figure 8A, compare control cells at Time 0, 24, and 48 hours). We next investigated whether ACLP expression is increased in fibrotic lung tissue from patients with IPF. ACLP expression in normal human lung is modest and limited primarily to the collagen-rich areas in the media of large blood vessels (Figure 8B, column 1) and absent from lung parenchyma (Figure 8B, column 2). In contrast, lung specimens from IPF patients demonstrate high levels of ACLP immunostaining in areas of dense collagen deposition (Figure 8B, column 3). ACLP immunostaining in human lung tissue was specific since we did not detect significant staining on parallel sections incubated with control (pre-immune) serum (Figure 8B, pre-immune). Our IPF lung specimens were from patients with advanced lung disease and demonstrated similar ACLP staining in areas of dense collagen deposition. In the few areas of relatively normal lung in these samples we did not find significant ACLP immunostaining (data not shown). Collectively, these results indicate that ACLP is expressed and induced by TGF-β in human lung fibroblasts and up-regulated in fibrotic human lung tissue in areas of collagen deposition.
Figure 8.
ACLP is expressed by human fibroblasts and levels are increased in human fibrotic lung tissue. A: IMR-90 cells were cultured in the absence (−) or presence (+) of 10 ng/ml TGF-β. After culture for the indicated amount of time, cell proteins were extracted, subjected to SDS-PAGE and Western blots were performed to detect ACLP, α-SMA, and tubulin. B: Surgical specimens from normal human lung (n = 6) and from patients with idiopathic pulmonary fibrosis [IPF] (n = 6) were immunostained with either control (pre-immune) rabbit serum or anti-ACLP rabbit serum (brown) or stained with Masson’s trichrome (Trichrome, blue) to detect collagen. Data are representative serial 5-μm sections for each condition. Black arrows indicate regions of ACLP expression; white arrows indicate collagen rich areas. V: blood vessel.
Discussion
In the current study we demonstrate that ACLP, a collagen-matrix-associated protein, is expressed in fibrotic lung tissue from IPF patients and is necessary for the development of bleomycin-induced lung fibrosis in mice. Importantly, bleomycin-injured ACLP−/− mice do not accumulate a significant number of lung myofibroblasts even in the presence of preserved bleomycin-induced lung inflammation and TGF-β1 activity. These observations and the tight association between ACLP and fibrillar collagen in fibrous tissue indicate ACLP may regulate myofibroblast retention, proliferation, or differentiation on collagen-rich matrix either in conjunction with or down-stream of TGF-β1. Interestingly, ACLP is retained on fibrillar collagen and is important for lung fibroblast spreading, formation of membrane extensions, proliferation on collagen, and for collagen matrix contraction. Furthermore, the DLD of ACLP is sufficient to induce formation of cell membrane extensions and augment collagen matrix contraction in ACLP−/− lung fibroblasts.
Our findings have important implications for the molecular pathogenesis of pulmonary fibrosis and possibly the molecular mechanisms of wound healing in general. For example, collagen matrix contraction and remodeling by myofibroblasts is a central feature of both wound healing and lung fibrosis. Moreover, increased mechanical tension in collagen-rich tissue is sufficient to stimulate myofibroblast contraction, proliferation and differentiation in vivo.30 Although collagen-binding integrins certainly have a role in these processes,31 ACLP may be a novel, collagen-specific cofactor that regulates signals from collagen matrix to fibroblasts and myofibroblasts. Moreover, the ability of ACLP’s DLD to induce cell spreading on collagen and to augment collagen contraction by myofibroblasts suggests a novel role for discoidin domain proteins in fibrosis and wound healing. The discoidin domain receptor 1, for example, is a receptor tyrosine kinase necessary for bleomycin-induced lung fibrosis but acts by promoting the inflammatory phase of bleomycin lung injury.32 In contrast, ACLP is the first known discoidin protein critical for the fibrotic phase of bleomycin lung injury suggesting the discoidin motif may be a target for anti-myofibroblast and anti-fibrosis therapies. Currently little is known about the molecular events that mediate re-epithelialization of injured and fibrotic lung, a key final step in lung repair. It is likely that several ECM-associated proteins are critical in this process and our future studies will test a potential role for ACLP in promoting lung epithelial repair.
These findings also provide new insight into the mechanisms of fibroblast and myofibroblast retention, cell-spreading, and proliferation on collagen matrix. Myofibroblast remodeling of collagen requires cell adhesion, extension of cell membrane processes (eg, lamellipodia), and formation of mature focal adhesions followed by cell contraction and collagen shortening. Myofibroblasts stabilize the shortened matrix by re-extending cell membrane processes onto newly synthesized collagen and further contracting the matrix.5,7 These events and myofibroblast proliferation account for the collagen deposition, volume loss, and distorted architecture of fibrotic lung in IPF. ACLP has an important role in these processes since ACLP-null myofibroblasts have a defect in cell spreading and formation of cell membrane extensions on collagen, leading to reduced collagen matrix contraction. Although we do not yet know the detailed molecular events associated with this phenotype, ACLP-null fibroblasts, as compared with wild-type, when cultured on collagen have a marked decrease in focal adhesions within peripheral cell-membrane extensions as measured by paxillin immunocytochemistry, and altered cortical actin re-organization as indicated by decreased phosphorylation of the actin regulatory proteins moesin and cortactin (unpublished observations). Moreover, although the molecular interactions between the discoidin domain of ACLP and the fibroblast cell surface that direct membrane extensions are unknown, the discoidin domain of other proteins, including coagulation factor VIII and retinoschisin, may act by directly binding membrane phospholipids.29 Finally, the proliferative effect of ACLP on lung fibroblasts in vitro is likely key in promoting fibroblast and myofibroblast accumulation in fibrotic lung in vivo. Since ACLP null fibroblasts express α-SMA in vitro (data not shown), it is not clear if ACLP has an additional role in regulating fibroblast to myofibroblast differentiation in vivo. At least in the collagen contraction assays, the DLD did not influence cell number (data not shown), thus our future studies are aimed at identifying the domain or domains of ACLP necessary for inducing fibroblast proliferation and possibly differentiation to myofibroblasts.
In summary, ACLP is a collagen-associated protein highly expressed in fibrotic lung from IPF patients and is necessary for bleomycin-induced lung fibrosis in mice. ACLP regulates fibroblast and myofibroblast cell spreading and proliferation on collagen and mediates myofibroblast collagen matrix contraction. ACLP and its discoidin domain may be novel targets for future IPF therapies.
Acknowledgments
We thank Don Ingber and Shaw-Fang Yet for helpful suggestions, Ronald D. Brown and Bonna Ith for expert technical assistance, and Carol Feghali-Bostwick and Augustine Choi for providing the human lung specimens.
Footnotes
Address reprint requests to Matthew D. Layne, Boston University School of Medicine, Department of Biochemistry, 72 E. Concord St, K221, Boston MA 02118. E-mail: mlayne@bu.edu.
Supported in part by National Institutes of Health grants K08 HL088492 (to S.L.S.), HL060788 and GM053249 (to M.A.P.), and HL078869 (to M.D.L.), and a grant from the Pulmonary Fibrosis Foundation and Leverone Family (to S.L.S.).
References
- Mannino DM, Etzel RA, Parrish RG. Pulmonary fibrosis deaths in the United States, 1979–1991. An analysis of multiple-cause mortality data. Am J Respir Crit Care Med. 1996;153:1548–1552. doi: 10.1164/ajrccm.153.5.8630600. [DOI] [PubMed] [Google Scholar]
- Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991;138:1257–1265. [PMC free article] [PubMed] [Google Scholar]
- Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, Bankert RB, Weber L. Integrin alpha 2 beta 1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991;115:1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne MD, Endege WO, Jain MK, Yet SF, Hsieh CM, Chin MT, Perrella MA, Blanar MA, Haber E, Lee ME. Aortic carboxypeptidase-like protein, a novel protein with discoidin and carboxypeptidase-like domains, is up-regulated during vascular smooth muscle cell differentiation. J Biol Chem. 1998;273:15654–15660. doi: 10.1074/jbc.273.25.15654. [DOI] [PubMed] [Google Scholar]
- Layne MD, Yet SF, Maemura K, Hsieh CM, Liu X, Ith B, Lee ME, Perrella MA. Characterization of the mouse aortic carboxypeptidase-like protein promoter reveals activity in differentiated and dedifferentiated vascular smooth muscle cells. Circ Res. 2002;90:728–736. doi: 10.1161/01.res.0000013289.97650.c8. [DOI] [PubMed] [Google Scholar]
- Ith B, Wei J, Yet SF, Perrella MA, Layne MD. Aortic carboxypeptidase-like protein is expressed in collagen-rich tissues during mouse embryonic development. Gene Expr Patterns. 2005;5:533–537. doi: 10.1016/j.modgep.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Layne MD, Yet SF, Maemura K, Hsieh CM, Bernfield M, Perrella MA, Lee ME. Impaired abdominal wall development and deficient wound healing in mice lacking aortic carboxypeptidase-like protein. Mol Cell Biol. 2001;21:5256–5261. doi: 10.1128/MCB.21.15.5256-5261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandjord TP, Madtes DK, Weiss DJ, Sage EH. Collagen accumulation is decreased in SPARC-null mice with bleomycin-induced pulmonary fibrosis. Am J Physiol. 1999;277:L628–L635. doi: 10.1152/ajplung.1999.277.3.L628. [DOI] [PubMed] [Google Scholar]
- Wei J, Gorman TE, Liu X, Ith B, Tseng A, Chen Z, Simon DI, Layne MD, Yet SF. Increased neointima formation in cysteine-rich protein 2-deficient mice in response to vascular injury. Circ Res. 2005;97:1323–1331. doi: 10.1161/01.RES.0000194331.76925.5c. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Ingenito EP, Mora R, Cullivan M, Marzan Y, Haley K, Mark L, Sonna LA. Decreased surfactant protein-B expression and surfactant dysfunction in a murine model of acute lung injury. Am J Respir Cell Mol Biol. 2001;25:35–44. doi: 10.1165/ajrcmb.25.1.4021. [DOI] [PubMed] [Google Scholar]
- Foster JA, Rich CB, Miller MF. Pulmonary fibroblasts: an in vitro model of emphysema. Regulation of elastin gene expression. J Biol Chem. 1990;265:15544–15549. [PubMed] [Google Scholar]
- Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92:1795–1804. doi: 10.1172/JCI116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Ho CH, Lin YC, Skuta G. Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J Biol Chem. 1999;274:918–923. doi: 10.1074/jbc.274.2.918. [DOI] [PubMed] [Google Scholar]
- Yakuwa N, Inoue T, Watanabe T, Takahashi K, Sendo F. A novel neutrophil adherence test effectively reflects the activated state of neutrophils. Microbiol Immunol. 1989;33:843–852. doi: 10.1111/j.1348-0421.1989.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Abe M, Ho CH, Kamm KE, Grinnell F. Different molecular motors mediate platelet-derived growth factor and lysophosphatidic acid-stimulated floating collagen matrix contraction. J Biol Chem. 2003;278:47707–47712. doi: 10.1074/jbc.M306228200. [DOI] [PubMed] [Google Scholar]
- Zhang K, Flanders KC, Phan SH. Cellular localization of transforming growth factor-beta expression in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1995;147:352–361. [PMC free article] [PubMed] [Google Scholar]
- Katsuma S, Nishi K, Tanigawara K, Ikawa H, Shiojima S, Takagaki K, Kaminishi Y, Suzuki Y, Hirasawa A, Ohgi T, Yano J, Murakami Y, Tsujimoto G. Molecular monitoring of bleomycin-induced pulmonary fibrosis by cDNA microarray-based gene expression profiling. Biochem Biophys Res Commun. 2001;288:747–751. doi: 10.1006/bbrc.2001.5853. [DOI] [PubMed] [Google Scholar]
- Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med. 2006;173:769–776. doi: 10.1164/rccm.200505-717OC. [DOI] [PubMed] [Google Scholar]
- Polte TR, Eichler GS, Wang N, Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am J Physiol Cell Physiol. 2004;286:C518–C528. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ, Ito M, Erdodi F. Myosin light chain phosphatase: subunit composition, interactions and regulation. J Muscle Res Cell Motil. 1998;19:325–341. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- Kiedzierska A, Smietana K, Czepczynska H, Otlewski J. Structural similarities and functional diversity of eukaryotic discoidin-like domains. Biochim Biophys Acta. 2007;1774:1069–1078. doi: 10.1016/j.bbapap.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avivi-Green C, Singal M, Vogel WF. Discoidin domain receptor 1-deficient mice are resistant to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2006;174:420–427. doi: 10.1164/rccm.200603-333OC. [DOI] [PubMed] [Google Scholar]