Abstract
Matrix metalloproteinase-9 (MMP-9) is an important enzyme in tumor invasion and metastasis in malignant tumors, including cholangiocarcinoma (CC). Tumor necrosis factor-α (TNF-α), a proinflammatory cytokine, was recently reported to induce the up-regulation of MMP-9 in cultured CC cells. We examined whether cyclooxygenase-2 (COX-2) and prostaglandin-E2 (PGE2), another endogenous tumor promoter, are involved in the up-regulation of MMP-9 in CC using CC tissue specimens and a CC cell line, HuCCT-1. MMP-9 and COX-2 were immunohistochemically expressed in 58% and 89% of 110 CC cases, respectively; the expression of MMP-9 and COX-2 was correlated (r = 0.32, P = 0.00072). Using zymography, latent MMP-9 was detectable in all cases and active MMP-9 was detected in 24% of cases of the CC specimens. The TNF-α/TNF-receptor 1 (TNF-R1) interaction induced MMP-9 production and activation, as well as COX-2 overexpression and PGE2 production, and increased the migration of CC cells. MMP-9 up-regulation was inhibited by COX inhibitors, antagonists of EP2/4 (receptors of PGE2), and COX-1 and COX-2 siRNAs. Inhibitors of both MMP-9 and MMP-9 siRNA treatment abrogated the increase in the migration of CC cells induced by TNF-α. In conclusion, we propose a novel signaling pathway of MMP-9 up-regulation in CC cells such that TNF-α induces the activation of COX-2 and PGE2 via TNF-R1 followed by the up-regulation of MMP-9 via the PGE2 (EP2/4) receptor.
Cholangiocarcinoma (CC) arising from the intrahepatic, hilar, and extrahepatic bile ducts shows a dismal prognosis even after a complete surgical resection,1,2,3 and the early invasion and metastasis of CC limit the efficacy of surgery. There have been many reports regarding the pathological factors that relate to the prognosis of CC patients, such as the TNM stage, and papillary phenotype and histological grade of the CC.1,2,3,4,5,6
Recently, much attention has been given to the endogenous factors within malignant tumors, which are directly or indirectly responsible for tumor progression.7,8,9,10 Among them, matrix metalloproteinase (MMP), cyclooxygenase (COX), and prostaglandin E2 (PGE2) are representative endogenous factors. The MMPs, a family of zinc-dependent proteinases, have been shown to dissolve various components of the extracellular matrix. In particular, MMP-9 plays an important and necessary role in the catalytic activity of tumor cell invasion and metastasis.11,12 Latent MMP-9 (92 kDa) is a proenzyme form, and the active form of MMP-9 (82 kDa) has full catalytic activity for the extracellular matrix.8,9,10,11 COX is a rate-limiting enzyme that catalyzes the conversion from arachidonic acid to prostaglandins, including PGE2.13,14,15 In contrast to COX-1, which is constitutively expressed in various organ tissues, COX-2 is induced by a variety of stimuli.13,14,15 COX-2 expression in many malignant tumors is associated with tumor growth and invasion.13,16,17 PGE2 has many biological activities such as cell proliferation, cell invasion, and angiogenesis of malignant tumors.13,18,19
MMP, COX-2, and PGE2 are thought to play an important role in the tumor invasion and metastasis of CC.7,8,11,12,13,20 MMP-9 is regarded as a prognostic factor in intrahepatic CC.7 COX-2 is reportedly overexpressed in CC and plays an important role in the development and progression of CC.9,16,21 PGE2 is also known to be involved in the progression of CC.17
Evidence supports the premise that inflammation is a crucial component of tumor progression.22,23,24 As for the CCs, long-standing inflammation, injury, and reparative biliary epithelial proliferation, such as primary sclerosing cholangitis (PSC) and hepatolithiasis,20,21,24 are reported to be background conditions.1,20,21,24,25 The tumor microenvironment is primarily orchestrated by cytokines that play an indispensable role during tumor progression.22,23,26 Tumor necrosis factor (TNF)-α, a proinflammatory cytokine, seems to belong to such cytokines and is also an important endogenous tumor promoter.27,28,29 As for the roles of TNF-α in CC, we previously showed, using a cell culture study and human CC tissue specimens, that TNF-α in proximity to the invasive front of CC is at least partly responsible for the increased migration of CC cells28; that is, the interaction of stromal cell-derived factor (SDF)-1 released from fibroblasts and CXCR4 expressed on intrahepatic cholangiocarcinoma (ICC) cells may be actively involved in ICC migration, and TNF-α may enhance ICC cell migration by increasing the CXCR4 expression on the CC cells. Furthermore, TNF-α is a well-known molecule that induces MMP-9 up-regulation in cultured CC cells,10,11,12,27 and COX-2 expression is also known to be induced by TNF-α, and its expression in malignant tumors is associated with tumor growth and invasion.13,16,17 Although there have been many studies on the roles of MMP-9 or COX-2 in the development of malignant tumors, there are only a few studies about the relationship between MMP-9 and COX-2.30,31,32 In particular, there have so far been no reports about the relationship of these two molecules in CC with respect to TNF-α.
In this study, we evaluated the roles of COX and PGE2, with respect to the production and activation of MMP-9 in CC cells induced by TNF-α, using human CC tissues and also a human CC cell line, HuCCT-1. This study for the first time showed that MMP-9 up-regulated by TNF-α is mediated by COX-2/PGE2, which is also involved in the increased tumor cell invasion in CC.
Materials and Methods
Classification of the Biliary Tree
The biliary tree is divided into the intrahepatic, hilar (right and left hepatic ducts), and extrahepatic bile ducts.33 The intrahepatic bile duct is a branch of the right or left hepatic bile duct, and the first to third branches of both hepatic ducts are termed the intrahepatic large bile duct. The extrahepatic bile duct, hilar bile duct, and intrahepatic large bile duct are referred to collectively as the large bile duct in this study. The intrahepatic small bile ducts are branches of the intrahepatic large bile duct and are classified into septal and interlobular bile ducts. The bile ductules are located at the periphery of the portal tracts.
Patient Selection and Preparation of Tissue Specimens of CC
We examined 110 cases of CC, including 75 cases of CC arising from the large bile duct (including hilar and extrahepatic bile ducts but excluding the ampulla of Vater and the gallbladder),34 and 35 cases of intrahepatic CC (all of the mass-forming type),35 from the surgical files in our laboratory and affiliated hospitals. Table 1 shows the clinicopathological features including age, gender, tumor type, histological grade of the main tumor, and TNM stage.36 The tissue specimens of CC obtained surgically or at autopsy were fixed in 10% neutral-buffered formalin and embedded in paraffin, and more than 20 serial 3-μm-thin sections were cut from each paraffin block. We chose one representative lesion from each case for the following evaluations. In addition, 21 frozen specimens of intrahepatic CC (15 surgically resected cases and 6 autopsy cases) were used for gelatin zymography and reverse transcription-polymerase chain reaction (RT-PCR) analysis. The samples for zymography and RNA isolation were extracted from the CC tissues.
Table 1.
Clinicopathological Features of Cholangiocarcinoma Cases Examined
| IHC study | Zymography and PCR study | |
|---|---|---|
| Number of CC cases examined | 110* | 21 |
| Age (years); mean ± SD (range) | 67.1 ± 11.3 (35 to 85) | 66.8 ± 9.9 (35 to 80) |
| Gender (male/female) | 67/43 | 12/9 |
| Tissue specimen (surgical resection/autopsy) | 110/0 | 15/6 |
| Tumor type (ICC/CC in LBDs) | 35/75 | 21/0 |
| Histological grade (wel/mod/por/sq) | 48/46/16/0 | 4/14/2/1 |
| Lymph node metastasis (negative/positive) | 71/39 | UA |
| UICC stage | ||
| CC in LBDs (n = 75) | ||
| Pathological T factor (Tis/T1/T2/T3) | 10/7/30/28 | * |
| Pathological N factor (N0/N1) | 47/28 | * |
| Clinicopathological M factor (M0/M1) | 72/3 | * |
| Final stage (0/IA/IB/IIA/IIB/III/IV) | 11/7/15/14/25/0/3 | * |
| ICC (n = 35) | ||
| Pathological T factor (T1/T2/T3/T4) | 0/19/12/4 | * |
| Pathological N factor (N0/N1) | 24/11 | * |
| Clinicopathological M factor (M0/M1) | 34/1 | * |
| Final stage (I/II/IIIA/IIIB/IIIC/IV) | 0/15/7/2/10/1 | * |
IHC, immunohistochemistry; PCR, polymerase chain reaction; CC, cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; LBDs, large bile ducts; wel, well-differentiated adenocarcinoma; mod, moderately-differentiated adenocarcinoma; por, poorly-differentiated adenocarcinoma; sq, squamous cell carcinoma; UA, unavailable.
Number of cases.
Antibodies and Reagents
Anti-MMP-9 antibody (56-2A4, mouse monoclonal IgG1, 1:200), which is known to detect both the latent and active forms of MMP-9, was purchased from Daiichi Fine Chemical (Takaoka, Japan). Anti-COX-1 antibody (M-20, goat polyclonal IgG; 1:400), anti-COX-2 (C-20, goat polyclonal IgG; 1:400), and normal goat IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa Fluor 488-labeled goat anti-mouse IgG (A11029, 1:200), and Alexa Fluor 488-labeled rabbit anti-goat IgG (A11078, 1:200) were purchased from Molecular Probes (Tokyo, Japan). Control mouse IgG1, IgG2, and protein block serum were purchased from DAKO Cytomation (Glostrup, Denmark). Anti-COX-1 (mouse monoclonal IgG2b, 1:100), anti-COX-2 (mouse monoclonal IgG2b, 1:500), and PGE2 were purchased from Cayman Chemical (Ann Arbor, MI). β-Actin (AC15, mouse monoclonal IgG1; 1:5000) was purchased from Abcam (Cambridge, UK). Recombinant human TNF-α, interleukin (IL)-1β, and IL-6 were purchased from Peprotech (London, UK). Recombinant human SDF-1α, epidermal growth factor, hepatocyte growth factor (HGF), neutralizing antibodies against TNF-α (MAB210, mouse monoclonal IgG1; 4 μg/ml), TNF-receptor 1 (TNF-R1, MAB625, mouse monoclonal IgG1; 20 μg/ml), and TNF-receptor 2 (TNF-R2) antibody (MAB226, mouse monoclonal IgG2a; 20 μg/ml) were purchased from R&D Systems (Minneapolis, MN). SC560 (selective COX-1 inhibitor, 100 μmol/L), NS398 (selective COX-2 inhibitor, 100 μmol/L), and MMP-9 inhibitor I (50 nmol/L) were purchased from Calbiochem (San Diego, CA). The applied concentration of SC560 and NS398 applied show selective inhibition of COX-1 and COX-2. The SP600125 (SAPK/JNK inhibitor, 50 μmol/L) was purchased from Biomol (Philadelphia, PA). Indomethacin (nonselective COX inhibitor, 100 mmol/L), SC19220 (antagonist of EP1 receptor, 100 μmol/L), AH6809 (antagonist of EP 1 and 2 receptors, 100 μmol/L), AH23848 (antagonist of EP4 receptor, 100 μmol/L), and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO). GM6001 (MMP inhibitor, 10 mmol/L) was purchased from Chemicon (Temecula, CA). Validated small interfering RNA (siRNA) for MMP-9, COX-1, and COX2 and negative control siRNA were purchased from Santa-Cruz Biotechnology and Qiagen (Hilden, Germany), respectively.
Immunohistochemistry
Immunostaining
Immunostaining was performed using an EnVision+ system (Dako Cytomation). Briefly, the deparaffinized sections were microwaved in citrate buffer (pH 6.0), followed by pretreatment with 1% H2O2 in methanol to block endogenous peroxidase activity and then with protein block serum to block nonspecific reactions; the sections were incubated overnight at 4°C with each primary antibody at the optimized dilutions. Thereafter, the sections were incubated with the secondary antibodies conjugated to a peroxidase-labeled polymer, the EnVision+ system (Dako Cytomation), or Histofine Simple Stain MAX PO (G) (Nichirei, Tokyo, Japan) at room temperature for 1 hour. Diaminobenzidine tetrahydrochloride solution containing 0.03% hydrogen peroxide was used as the chromogen. For negative control the primary antibody was substituted with equivalent concentrations of normal immunoglobulin.
Double immunostaining of MMP-9 and COX-2 was performed for the human CC tissues that were positive for MMP-9 and COX-2. Antigen retrieval was performed by microwaving, and then incubation with anti-MMP-9 and anti-COX-2 antibodies. Color was developed with a Vector Red Alkaline Phosphatase Substrate Kit I (Vector Laboratories, Burlingame, CA) for MMP-9 and with Alexa Fluor 488-labeled rabbit anti-goat IgG for COX-2.
Evaluation of Immunohistochemistry
The expressions of MMP-9, COX-1, and COX-2 were evaluated semiquantitatively according to the percentage of positive cells: 0 (negative); +1 (focal, 1 to 10% CC cells in the lesion were positive; +2 (moderate, 11 to 50% CC cells in the lesion were positive; and +3 (marked, >50% CC cells in the lesion were positive.7,12
Cell Culture
A CC cell line, HuCCT-1 (Cell Resource Center for Biochemical Research, Tohoku University, Sendai, Japan), was used. The HuCCT-1 cells were maintained in RPMI 1640 medium (Life Technologies, Inc., Grand Island, NY) and Dulbecco’s modified Eagle’s medium/F-12 (Life Technologies, Inc.) with 10% fetal bovine serum (Life Technologies, Inc.) and 1% penicillin-streptomycin-glutamine (Life Technologies, Inc.), respectively, at 37°C in a 5% CO2 atmosphere with constant humidity.
In the cytokine stimulation study, stimulants such as TNF-α were added to the serum-free medium. When using the inhibitors and neutralizing antibodies, they were added 1 hour before TNF-α treatment. The knockdown of MMP-9, COX-1, and COX-2 was performed as described previously.37 In brief, the HuCCT-1 cells were plated in 35-mm dishes (5 × 105 cells) 1 day before transfection, and then the cells were transiently transfected with either MMP-9, COX-1, and COX-2 or control siRNA (100 nmol/L) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. All in vitro experiments were performed three times and the data from three experiments were expressed as the mean ± SD.
Zymography
The MMP-9 expression was analyzed by gelatin zymography using Alexa Fluor 680-labeled gelatin.38 This fluoro-zymography system can analyze not only conditioned medium but also cell lysates and fresh tissue sections at high sensitivity. Briefly, after 5 × 105/ml of HuCTT-1 cells were seeded on 12-well plates for 48 hours, these CC cells were treated with TNF-α for 24 hours in serum-free medium with pre-incubation of the neutralizing antibody, inhibitors, or vehicle for 1 hour. The conditioned medium or treated cells were incubated with sodium dodecyl sulfate sample buffer and incubated for 30 minutes at 37°C. The samples were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis containing 0.005% Alexa-labeled gelatin. After electrophoresis, the gels were washed in 2.5% Triton X-100 for 2 hours at room temperature to remove sodium dodecyl sulfate, and then incubated in substrate buffer overnight at 37°C. The gel was scanned by an Odyssey IR imaging system (LI-COR, Lincoln, NE). The cell lysate of HT1080 (fibrosarcoma cell line) was used as a positive control of latent and active MMP-9.10,39
Extraction of RNA, RT-PCR, and Quantitative Real-Time PCR
Total RNA was isolated from HuCCT-1 and 21 human intrahepatic CC specimens using the Qiagen RNAeasy kit (Qiagen, Tokyo, Japan). Then, 1 μg of RNA was used to synthesize first-strand cDNA. The cDNA was amplified by PCR using specific primers (Table 2). Aliquots of the products were electrophoresed on a 1.5 or 2.0% agarose gel with ethidium bromide. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The negative controls replaced the reverse transcriptase with RNase- and DNase-free water.
Table 2.
Primer Sequences Used in This Study
| Target gene | Forward | Reverse | Product size | Anealing (°C) | Reference |
|---|---|---|---|---|---|
| TNF-α | 5′-CAATGTAGGAGCTGCCTTGG-3′ | 5′-CAGAGGCTCAGCAATGAGTG-3′ | 186 | 60 | |
| TNF-R1 | 5′-GAGAGGCCATAGCTGTCTGG-3′ | 5′-GTTTTCTGAAGCGGTGAAGG-3′ | 303 | 55 | 44 |
| TNF-R2 | 5′-GGATGAAGCCCAGTTAACCA-3′ | 5′-GTGTCCTGTCTTCATGGGTGA-3′ | 501 | 58 | 44 |
| COX-1 | * | * | 91 | 60 | |
| COX-2 | 5′-TGCATTCTTTGCCCAGCACT-3′ | 5′-AAGGCGCAGTTTACGCTGTCT-3′ | 145 | 60 | 31 |
| MMP-9 | * | * | 115 | 60 | |
| EP1 | 5′-CGCGCTGCCCATCTTCTCCAT-3′ | 5′-CCCAGGCCGATGAAGCACCAC-3′ | 471 | 55 | 25 |
| EP2 | 5′-GCTGCTGCTTCTCATTGTCTCG-3′ | 5′-TCCGACAACAGAGGACTGAACG-3′ | 427 | 55 | 25 |
| EP3 | 5′-GGCACGTGGTGCTTCATC-3′ | 5′-GGGTCCAGGATCTGGTTC-3′ | 427 | 55 | 25 |
| EP4 | 5′-ATCTTACTCATTGCCACC-3′ | 5′-TCTATTGCTTTACTGAGCAC-3′ | 249 | 55 | 25 |
| GAPDH | ** | ** | 143 | 60 |
TNF-α, tumor necrosis factor α; TNF-R1, TNF receptor 1; TNF-R2, TNF receptor 2; COX-1, cyclooxygenase-1; COX-2, cyclooxygenase-2; MMP-9, matrix metalloproteinase-9; EP1, EP2, EP3, and EP4, receptors of prostaglandin E2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
purchased from Qiagen (Wien, Austria) and Takara (Ohtsu, Japan), respectively.
Quantitative real-time PCR was performed to measure COX-1, COX-2, and GAPDH mRNA according to a standard protocol using the SYBR Green PCR Master Mix (Toyobo, Tokyo, Japan) and an ABI Prism 7900 sequence detection system (Applied Biosystems, Tokyo, Japan). The fold difference in comparison with the control was calculated. Each experiment was performed in triplicate, and the mean was adopted in each experiment.
Extraction of Whole Cell Protein
Whole cell proteins were extracted from the cultured cells using a tissue protein extraction reagent (Pierce Chemical Co., Rockford, IL) according to the manufacturer’s instruction. The total protein concentration was measured spectrometrically.
Western Blot Analysis
Forty μg of whole cell protein or 3 μg of nuclear protein was loaded on 10 or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis it was transferred onto a nitrocellulose membrane. The membrane was incubated with the primary antibody overnight, and then it was visualized with an enhanced chemiluminescence system.
Enzyme-Linked Immunosorbent Assay of PGE2
After 5 × 105 HuCTT-1 cells were seeded on 12-well plates for 48 hours, they were treated with TNF-α (25 to 100 ng/ml) in serum-free medium for 3 to 24 hours. The concentration of PGE2 in the supernatant was measured by enzyme-linked immunosorbent assay using a prostaglandin E2 immunoassay kit (R&D Systems), according to the manufacturer’s instructions.
Migration Assays of Cultured ICC Cells
The migration assay was performed as previously described.28 Briefly, the migration of cultured CC cells was assayed using a BD BioCoat Matrigel invasion chamber (24-well plate, 8-μm pore, growth factor-reduced type; BD Biosciences, Bedford, MA). In the upper chamber, 5 × 105 cells in 0.5 ml of serum-free medium were treated with TNF-α with pretreatment of an inhibitor, vehicle, or control IgG for 1 hour. After 24 hours at 37°C, the cultured CC cells on the upper surface of the filter were removed using a cotton-wool swab. After fixation with 100% methanol and staining using 1% toluidine blue, the number of cells migrating to the lower surface was counted in 10 fields (×200). The migration index (the ratio of migrated cells in the experimental groups/in control groups) was calculated in each experiment. Each experiment was conducted in triplicate, and the mean was adopted in this experiment.
Statistics
All statistical analyses were performed using the Dr SPSS II software program (version 11.01 J; SPSS Japan Inc., Tokyo, Japan). In the immunohistochemical analysis, differences in the expression frequency of MMP-9, COX-1, and COX-2 were evaluated using the Mann-Whitney U-test. The correlation coefficient of 2 factors was evaluated using Spearman’s rank correlation test. Differences between the two groups of individual in vitro experiments were analyzed using Student’s t-test. When the P value was <0.05, the difference was regarded as significant.
Results
Immunohistochemistry of MMP-9, COX-1, and COX-2 in CC
MMP-9 and COX-1 were expressed in the cytoplasm of the CC cells as a diffuse and granular pattern (Figure 1A, a–c), whereas COX-2 was expressed in the CC cells as granular cytoplasmic and also membrane patterns (Figure 1Ad). The expression of MMP-9, COX-1, and COX-2 was either faint or absent in the nonneoplastic bile ducts in the adjacent normal tissue (Figure 1A, e–g). MMP-9, COX-1, and COX-2 were expressed in the CC cells in 58%, 55%, and 89% of 110 CC cases, respectively, and the degree of expression of COX-2 was more extensive than that of COX-1 (Figure 1B). As shown in Table 3, the expression of COX-2 was significantly correlated with that of MMP-9 (r = 0.32, P = 0.00072), although no such relation was evident between the expressions of MMP-9 and COX-1. By double-immunofluorescent staining from the same sample, MMP-9 and COX-2 showed a similar stain pattern and they were frequently co-expressed in the same CC cells (Figure 1C, a–c), whereas no such topological relation was evident between the MMP-9 and COX-1 expressions in CC tissues (Figure 1C, d–f).
Figure 1.
Expression of MMP-9, COX-1, and COX-2 in human CC tissues. A: Representative histology of CC (a) and the immunohistochemical staining of MMP-9 (b and e), COX-1 (c and f), and COX-2 (d and g). a: A representative histology of CC. H&E. b: MMP-9 is strongly and diffusely expressed in the cytoplasm of almost all CC cells. Immunohistochemistry of MMP-9. c: COX-1 is expressed diffusely in the cytoplasm of CC cells. Immunohistochemistry of COX-1. d: COX-2 is expressed strongly and diffusely in the cytoplasm and membrane of almost all CC cells. Immunohistochemistry of COX-2. e: MMP-9 is not expressed in the nonneoplastic bile ducts in the adjacent normal tissue. f: COX-1 is faintly expressed in the nonneoplastic bile ducts in the adjacent normal tissue. g: COX-2 is faintly expressed in the nonneoplastic bile ducts in the adjacent normal tissue. B: Frequency and degree of immunohistochemical expression of MMP-9, COX-1, and COX-2 in CC tissues (110 cases of CC). C: Double-immunofluorescent staining of MMP-9 (a) (Alexa Fluor 594, red) and COX-2 (b) (Alexa Fluor 488, green) and their merged images (c) and that of MMP-9 (d) (Alexa Fluor 594, red) and COX-1 (e) (Alexa Fluor 488, green) and their merged images (f) in CC. A subset of CC cells are positive for COX-1 alone, whereas another subset of CC cells are positive for both MMP-9 and COX-1. The majority of CC cells are positive for MMP-9 and COX-2, and the merging of double-positive tumor cells gives a yellow color (c), whereas the expression of MMP-9 and COX-1 are dissimilar in CC; some cells expressed MMP-P and the others expressed both proteins. Original magnifications, ×400 (A).
Table 3.
Correlation Coefficient of Matrix Metalloproteinase-9 (MMP-9) and Cyclooxygenase (COX)-1) and COX-2 Expression in Cholangiocarcinoma
| MMP-9
|
r-value | ||||
|---|---|---|---|---|---|
| 0 | +1 | +2 | +3 | P value | |
| COX-1 | |||||
| 0 | 22* | 9 | 15 | 3 | r = 0.12 |
| +1 | 12 | 5 | 3 | 4 | P = 0.21 |
| +2 | 8 | 6 | 6 | 8 | |
| +3 | 4 | 2 | 2 | 1 | |
| COX-2 | |||||
| 0 | 16 | 3 | 1 | 1 | r = 0.32 |
| +1 | 7 | 3 | 6 | 1 | P = 0.00072 |
| +2 | 10 | 5 | 7 | 4 | |
| +3 | 13 | 11 | 12 | 10 | |
Number of case(s) of cholangiocarcinoma; 0 (negative); +1 (focal, 1 to 10% CC cells are positive); +2 (moderate, 11 to 50% CC cells are positive); and + 3 (marked, >50% CC cells are positive).
Detection of Latent and Active MMP-9 in CC by Zymography
Gelatin zymography using frozen specimens of 21 cases of intrahepatic CCs showed that all cases expressed latent MMP-9 and 5 (24%) additionally expressed active MMP-9 (Figure 2A). Gelatin zymography using frozen normal control samples (n = 5) showed that all cases expressed faintly latent MMP-9, but none of cases expressed active MMP-9 (Figure 2A).
Figure 2.
Detection of MMP-9, TNF-α, COX-1, and COX-2 protein and mRNA in frozen tissue specimens of ICC. A: Gelatin zymography of MMP-9 in 21 cases of ICC and 5 normal control samples. Latent MMP-9 (92 kDa) and active MMP-9 (82 kDa) were observed in all cases (1 to 21) and in 5 cases (1 to 5) of ICC, respectively. Latent MMP-9 (92 kDa) was observed faintly in all of normal control samples (N1 to N5). HT1080 (human fibrosarcoma cell line) was used as a positive control of latent and active MMP-9. B: TNF-α, MMP-9, COX-1, and COX-2 mRNAs were detected in all 21 cases of ICC examined. TNF-α, MMP-9, COX-1, and COX-2 mRNA were also detected in one, four, five, and four of five frozen control normal samples, respectively. GAPDH was the internal control. RT-PCR study. C: The correlation of the mRNA expression of TNF-α, MMP-9, COX-1, and COX-2 in 21 CC tissues and 5 control tissues. TNF-α mRNA level was correlated positively with the mRNA level of MMP-9, COX-1, and COX-2, respectively. MMP-9 was correlated with COX-1. CC case, closed diamond; normal control case, half-tone triangle. Expression of mRNA was quantified with real-time PCR. The expression was normalized as a ratio using GAPDH as an internal control.
Detection of TNF-α, MMP-9, COX-1, and COX-2 mRNA in CC by RT-PCR
TNF-α, MMP-9, COX-1, and COX-2 mRNA were detected in the frozen CC tissue of all 21 cases of intrahepatic CC (Figure 2B). TNF-α, MMP-9, COX-1, and COX-2 mRNA was also detected in one, four, five, and four of five frozen control normal samples, respectively. We assessed the mRNA expression level of TNF-α, MMP-9, COX-1, and COX-2 by quantitative real-time PCR and we evaluated the correlation of the mRNA level of TNF-α, MMP-9, COX-1, and COX-2 in 21 CC tissues and 5 control tissues, and plotted them in Figure 2C. It was found that TNF-α mRNA level was correlated positively with the mRNA level of MMP-9, COX-1, and COX-2, respectively, in 21 CC tissues. MMP-9 mRNA level was correlated with COX-1 and tended to correlate with COX-2 (statistically not significant) in 21 CC tissues. In contrast, the mRNA level of TNF-α, MMP-9, COX-1, and COX-2 was low in five normal control tissues (Figure 2C) and the correlation among the mRNA expression of these proteins was not statistically significant.
TNF-α/TNF-R1 Interaction Induces MMP-9 Production and Activation
Zymography showed that TNF-α (10 to 100 ng/ml, 24 hours) treatment induced latent MMP-9 production in the conditioned medium and cell lysate, and active MMP-9 in the cell lysate of cultured CC cells of HuCCT-1 (Figure 3A). The MMP-9 production and activation by TNF-α treatment increased in a dose-dependent matter. HGF (100 ng/ml) and epidermal growth factor (100 ng/ml) mildly increased the latent MMP-9 production, whereas SDF-1α (100 ng/ml), IL-1β (100 ng/ml), and IL-6 (100 ng/ml) failed to induce MMP-9 production. The latent MMP-2 used as an internal control was not affected by any of these stimulations. TNF-R1, TNF-R2, and MMP-9 mRNA were detected in the cultured CC cells (HuCTT-1) by a RT-PCR analysis (Figure 3B). The anti-TNF-α (4 μg/ml) neutralizing antibodies, anti-TNF-R1 neutralizing antibodies (20 μg/ml), and anti-TNF-R1 neutralizing antibodies (20 μg/ml) + anti-TNF-R2 neutralizing antibodies (20 μg/ml) inhibited the TNF-α-induced (100 ng/ml, 24 hours) MMP-9 production and activation, whereas the anti-TNF-R2 neutralizing antibodies alone (20 μg/ml) failed to inhibit the TNF-α-induced MMP-9 up-regulation as shown by a RT-PCR analysis and gelatin zymography (Figure 3C).
Figure 3.
TNF-α-induced MMP-9 up-regulation was mediated by TNF-receptor 1 (TNF-R1) in cultured CC cells of HuCCT-1. A: TNF-α (24 hours) induced latent MMP-9 in the cell lysate and conditioned medium in a dose-dependent manner and induced active MMP-9 in the cell lysate of cultured CC cells of HuCCT-1. HGF and epidermal growth factor mildly increased only latent MMP-9 in the cell lysate, whereas SDF-1α, IL-1β, and IL-6 did not induce MMP-9. MMP-2 used as an internal control was not affected by any of these stimulations. Gelatin zymography of MMP-9 and MMP-2. B: TNF-R1, TNF-R2, and MMP-9 mRNA were detected in the cultured CC cells of HuCCT-1. GAPDH was used as the internal control. PCR study. C: Inhibition study using neutralizing antibodies against TNF-α and its receptors in TNF-α-induced MMP-9 up-regulation. A PCR study for MMP-9 mRNA expression (a) and gelatin zymography of MMP-9 and MMP-2 (b). a: Anti-TNF-R1 neutralizing antibodies (AR1, 20 μg/ml) clearly inhibited the TNF-α (100 ng/ml, 12 hours)-induced up-regulation of MMP-9 mRNA expression, whereas anti-TNF-R2 neutralizing antibody alone (AR2, 20 μg/ml) failed to inhibit such TNF-α-induced MMP-9 up-regulation. Experiments were performed in triplicate and the data are the mean ± SD. *P < 0.05 versus control group; **P < 0.05 versus TNF-α group. b: Anti-TNF-α neutralizing antibodies (AT, 4 μg/ml), anti-TNF-R1 neutralizing antibodies (AR1, 20 μg/ml), and anti-TNF-R1 neutralizing antibody (20 μg/ml) + anti-TNF-R2 neutralizing antibody (AR2, 20 μg/ml), clearly inhibited TNF-α (100 ng/ml, 24 hours)-induced up-regulation of MMP-9 production and activation, whereas anti-TNF-R2 neutralizing antibody alone (20 μg/ml) failed to inhibit such TNF-α-induced MMP-9 up-regulation. MMP-2 used as an internal control was not affected by any of these stimulations. Gelatin zymography of MMP-9 and MMP-2.
TNF-α Induces COX-2 Up-Regulation
RT-PCR showed that COX-1 and COX-2 mRNA were expressed in the cultured cells of HuCCT-1 without treatment (Figure 4A). Real-time PCR showed that TNF-α treatment (100 ng/ml, 6 hours) increased the COX-2 mRNA expression, but not COX-1 mRNA (Figure 4B), and that the expression of COX-2 mRNA by TNF-α treatment increased in a dose-dependent manner. Western blot showed that TNF-α (100 ng/ml, 24 hours) treatment increased the expression of COX-2 protein in the cultured CC cells, whereas the COX-1 protein expression was not affected by the TNF-α treatment (Figure 4C).
Figure 4.
Induction of mRNA and protein of COX-2 and PGE2 by TNF-α treatment with respect to MMP-9 up-regulation in cultured CC cells of HuCCT-1. Participation of COX-1, COX-2, and PGE2, and PGE2 receptor (EP1, EP2, EP3, and EP4) in this process was examined. A: COX-1, COX-2, and receptors of PGE2 (EP1, EP2, and EP) mRNA were detected in cultured CC cells of HuCCT-1, although EP3 mRNA was not detectable. GAPDH was used as an internal control. RT-PCR study. B: TNF-α treatment (100 ng/ml, 6 hours) increased COX-2 mRNA 5.6-fold in cultured CC cells of HuCCT-1, although no such increment was evident in COX-1 mRNA expression. This increase was dose-dependent. Experiments were performed in triplicate and the data are the mean ± SD. *P < 0.005 versus control. Real-time PCR study. C: TNF-α treatment (100 ng/ml, 24 hours) increased COX-2 protein concentration in the supernatant of cultured CC cells of HuCCT-1, but not COX-2 protein. β-Actin was used as the internal control. Western blot study. D: Gelatin zymography for latent and active MMP-9 and MMP-2 after treatment by TNF-α alone or TNF-α + either COX inhibitor or either EP blocking peptide. Indomethacin (Indo, nonselective COX inhibitor) and NS398 (NS, selective COX-2 inhibitor) inhibited the TNF-α-induced MMP-9 production and activation in the cell lysate of cultured CC cells of HuCCT-1, whereas SC560 (SC, selective COX1 inhibitor) partially inhibited the production in HuCCT-1. AH6809 (BP1, blocking peptide of EP1) and SC19220 (BP1/2, blocking peptide of EP1/2) also inhibited completely, whereas AH23848 (BP4 blocking peptide of EP4) did not suppress it. MMP-2 used as an internal control was not affected by any of these stimulations. E: MMP-9 mRNA expression was increased by TNF-α treatment (100 ng/ml, 24 hours). Indomethacin (Indo, nonselective COX inhibitor; 100 μmol/L), SC560 (SC, selective COX1 inhibitor; 100 μmol/L), and NS398 (NC, selective COX-2 inhibitor; 100 μmol/L) reduced TNF-α (100 ng/ml, 24 hours)-induced increased MMP-9 promoter activity in cultured CC cells of HuCCT-1. Expression of mRNA was quantified with real-time PCR. The expression was normalized as a ratio using GAPDH as an internal control. The experiments were performed in triplicate and the data are the mean ± SD. *P < 0.01 versus control group; **P < 0.01 versus TNF-α group. F: TNF-α treatment (25 ng/ml and 100 ng/ml) significantly enhanced PGE2 protein production in the conditioned medium in a dose- and time-dependent manner in both cultured CC cells of HuCCT-1. The experiments were performed in triplicate and the data are the mean ± SD. *P < 0.05 versus control. G: Indomethacin (Indo, nonselective COX inhibitor), SC560 (C, selective COX1 inhibitor), and NS398 (NS, selective COX-2 inhibitor) inhibited TNF-α-induced PGE2 production. The experiments were performed in triplicate and the data are the mean ± SD. *P < 0.05 versus control, TNF-α + indomethacin, TNF-α + SC560, TNF-α + NS398. H: Gelatin zymography for latent and active MMP-9 and MMP-2 after treatment by PGE2 alone or PGE2 + TNF-α. PGE2 alone did not induce MMP-9 production and activation and the administration of PGE2 in addition to TNF-α (100 ng/ml) did not affect TNF-α-induced MMP-9 production and activation. MMP-2 used as an internal control was not affected by any of these stimulations.
COX-2 Inhibitors Inhibit TNF-α-Induced MMP-9 Up-Regulation
Gelatin zymography using indomethacin (nonselective COX inhibitor, 100 μmol/L), SC560 (selective COX-1 inhibitor, 100 μmol/L), and NS398 (selective COX-2 inhibitor, 100 μmol/L) showed that indomethacin completely inhibited TNF-α-induced (100 ng/ml, 24 hours) MMP-9 up-regulation in the cell lysate of HuCCT-1. NS398 also almost inhibited the TNF-α-induced MMP-9 production and activation, whereas SC560 inhibited it only partially and active MMP-9 was still detected (Figure 4D). Real-time PCR showed that TNF-α (100 ng/ml, 24 hours) treatment increased the MMP-9 mRNA expression and indomethacin, SC560, and NS398 significantly reduced the increased the MMP-9 mRNA expression induced by TNF-α (Figure 4E).
PGE2 Up-Regulation by TNF-α Is Inhibited by COX Inhibitors
Enzyme-linked immunosorbent assay showed that TNF-α treatment increased the PGE2 production in the supernatant of the cultured CC cells in a time- and dose-dependent manner (Figure 4F), and three kinds of COX inhibitors (indomethacin, NS398, and SC560) inhibited such TNF-α-induced PGE2 hypersecretion (Figure 4G), suggesting that COX actively participates in the secretion process of PGE2 in cultured CC cells by TNF-α treatment.
Inhibition of TNF-α Induced MMP-9 Up-Regulation by Blocking of PGE2 Receptors EP1, EP2, and EP4
RT-PCR showed PGE2 receptor EP1, EP2, and EP4 mRNA expression in the HuCCT-1 cells, whereas the PGE2 receptor EP3 was not expressed (Figure 4A). Gelatin zymography showed that pretreatment with SC19220 (blocking peptide of EP1 and EP2) and AH23848 (blocking peptide of EP4) clearly reduced the TNF-α-induced MMP-9 production and activation, whereas AH6809 (BP1, blocking peptide of EP1) failed to show such inhibition (Figure 4D). Gelatin zymography showed that the administration of PGE2 alone did not induce MMP-9 production and activation (Figure 4H).
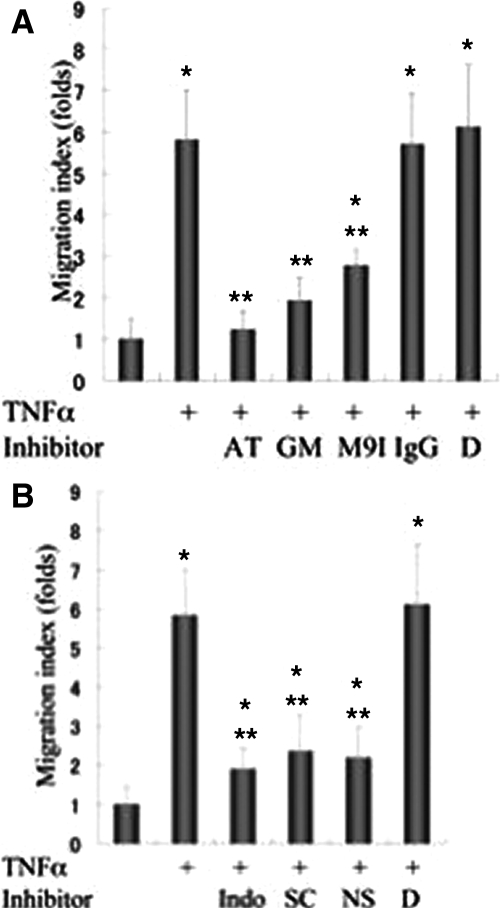
TNF-α Treatment Increases Migration of Cultured CC Cells via MMP-9 Production
By the TNF-α treatment (100 ng/ml, 24 hours) the migration index of the cultured CC cells increased by 5.8-fold (Figure 5A). Such increased CC cell migration was significantly inhibited by pretreatment with anti-TNF-α neutralizing antibodies (4 ng/ml), GM6001 (MMP inhibitor; 10 μmol/L), and MMP-9 inhibitor I (50 nmol/L). The migration index of the cultured CC cells by the GM6001 group (MMP inhibitor) and MMP-9 inhibitor I group was reduced by almost the same degree, suggesting that MMP-9 produced and activated by TNF-α was mainly responsible for this increased migration of CC cells. As for the influence of the COX inhibitors, TNF-α-induced tumor cell migration was significantly inhibited by one of three kinds of COX inhibitors (indomethacin, 100 μmol/L; SC560, 100 μmol/L; and NS398, 100 μmol/L), thus suggesting that COX is involved in the production of MMP-9 and also the increased migration of the CC cells by TNF-α (Figure 5B). DMSO (negative vehicle control) failed to show such inhibition.
Figure 5.
Migration study of cultured CC cells of HuCTT-1 after TNF-α treatment with respect to MMP-9 up-regulation. A: Migration assay of cultured CC cells. TNF-α (100 ng/ml, 24 hours) increased tumor cell migration 5.8-fold in cultured CC cells. Pretreatment by anti-TNF-α neutralization antibodies (4 μg/ml), GM6001 (nonselective MMP inhibitor; 10 μmol/L), and MMP-9 inhibitor I (50 nmol/L) significantly inhibited such increased tumor cell migration. The migration index of CC cells by treatment with control IgG and vehicle DMSO (D) was similar to that by TNF-α treatment alone. The experiments were performed in triplicate and the data are the mean ± SD. *P < 0.005 versus control, **P < 0.005 versus TNF-α (100 ng/ml) alone. AT, anti-TNF-α neutralization antibody; GM, GM 6001; M9I, MMP-9 inhibitor I; IgG, IgG; D, vehicle DMSO. B: Migration assay of cultured CC cells. Indomethacin (nonselective COX inhibitor), SC560 (selective COX1 inhibitor), NS398 (selective COX-2 inhibitor) inhibited TNF-α-induced migration of cultured CC cells, significantly. The migration index of CC cells by vehicle DMSO (D) was similar to that by TNF-α treatment alone. The experiments were performed in triplicate and the data are the mean ± SD. *P < 0.01 versus control group; **P < 0.005 versus TNF-α group.
The Knockdown of MMP-9, COX-1, and COX-2 by siRNA Inhibits TNF-α-Induced MMP-9 Up-Regulation and Cell Migration
Gelatin zymography using MMP-9, COX-1, and COX-2 siRNA showed that MMP-9 and COX-1 siRNA treatment inhibited the TNF-α-induced MMP-9 up-regulation effectively in the cell lysate of HuCCT-1 (Figure 6Aa). MMP-9 siRNA and COX-1 siRNA produced ∼60% of the down-regulation of MMP-9 activity, when determined by a LI-COR Odyssey IR imaging system (Figure 6Ab). COX-2 siRNA also showed a significant inhibitory effect on the TNF-α-induced MMP-9 up-regulation (Figure 6A). COX-1 and COX-2 siRNA inhibited the protein expression of COX-1 and COX-2, respectively, and MMP-9 siRNA did not inhibit the expression of COX-1 and COX-2 (Figure 6B). The TNF-α-induced CC cell migration was significantly inhibited by MMP-9, COX-1, and COX-2 siRNA treatment (Figure 6C). The level of MMP-9 activity might have been decreased under the threshold level required for invasion by MMP-9, COX-1, and COX-2 siRNA, followed by a significant decrease in migration. A threshold level of active MMPs is known to be critical for the activation of other MMPs and the capacity of invasion in carcinoma cells.40 Taken together, these findings also suggest that COX-1 and COX-2 are involved in the TNF-α-induced MMP-9 up-regulation and cell migration.
Figure 6.
The knockdown of MMP-9, COX-1, and COX-2 by siRNA inhibited TNF-α-induced MMP-9 up-regulation and cell migration. A: Gelatin zymography (a) and a quantification by an LI-COR Odyssey IR imaging system (b) for latent and active MMP-9 after treatment by TNF-α alone or TNF-α + either of MMP-9, COX-1, and COX-2 siRNA. MMP-9 and COX-1 siRNA treatment inhibited TNF-α-induced MMP-9 up-regulation effectively in the cell lysate of HuCCT-1. COX-2 siRNA also showed a partial inhibitory effect on TNF-α-induced MMP-9 up-regulation. The experiments were performed in triplicate and the data are the mean ± SD. *P < 0.01 versus control. B: COX-1 and COX-2 siRNA inhibit the protein expression of COX-1 and COX-2, respectively, and MMP-9 inhibition did not affect on the expression of COX-1 and COX-2 in HuCCT-1. β-Actin was used as the internal control. Western blot study. C: A migration assay of HuCTT-1. TNF-α (100 ng/ml, 24 hours) increased tumor cell migration 5.2-fold in cultured CC cells. Pretreatment by MMP-9, COX-1, and COX-2 siRNA significantly inhibited TNF-α-induced increase of tumor cell migration. The experiments were performed in triplicates and the data are the mean ± SD. *P < 0.05 versus control, **P < 0.05 versus TNF-α (100 ng/ml) alone.
Discussion
Recent studies showed that MMPs and COX are overexpressed in CCs and play an important role in the progression of CCs,7,8,9,11,12,16,17,20 and the overexpression of MMP-7, MMP-9, and COX-2 is regarded as a prognostic factor in the CCs.6,7,9,16 In this study, the overexpression of MMP-9, COX-1, and COX-2 protein and mRNA was shown in human CC tissues. MMP-9 and COX-2 were overexpressed in CC cells in 58% and 89% of 110 cases with CC, respectively, and the degree of COX-2 expression was more extensive than that of COX-1. Although mRNA level of COX-2 and more significantly that of COX-1 seemed to correlate with that of MMP-9 in CC tissues, COX-2 was more closely and significantly related to MMP-9 than COX-1 at the protein level. In fact, MMP-9 and COX-2 were frequently co-expressed in the same CC cells, suggesting that the overexpression of MMP-9 and COX-2 is significantly involved in the tumorigenesis of CC. Furthermore, gelatin zymography showed that CC expressed latent MMP-9 in all cases examined and active MMP-9 in one-fourth. The presence of active MMP-9 in CC tissue, a previously unreported finding, suggests further that MMP-9 activation thus plays an important role in CC progression.
Thus, we examined the significance of the production and activation of MMP-9 and the overexpression of COX-2 in CC by using cultured CC cells (HuCCT-1), with respect to TNF-α. The tumor microenvironment, which plays an indispensable role during tumor progression, is primarily orchestrated by cytokines.7,22,26 TNF-α, a proinflammatory cytokine, is reportedly an important endogenous tumor promoter11,12,27,28,29; that is, the production of TNF-α is elevated in various types of human cancer, has a positive correlation with tumor grade, and is associated with poor prognosis, although the exact roles of TNF-α remain primarily speculative in CC. Recently, Tanimura and colleagues27 reported that TNF-α increased the MMP-9 production in cultured CC cells (CCKS-1), and TNF-α is also a well-known molecule inducing COX-2 up-regulation.11,12,29
It was found in this study that TNF-α treatment induced MMP-9 production and activation in cultured CC cells. Furthermore, inhibition studies, using neutralizing antibodies, confirmed that the TNF-α/TNF-R1 interaction is mainly involved in this process; however, other cytokines such as HGF, epidermal growth factor, IL-1β, and IL-6, did not up-regulate MMP-9 significantly. Furthermore, TNF-α treatment increased COX-2 mRNA and protein expression in cultured CC cells in a dose-dependent manner. Interestingly, zymography showed that indomethacin (nonselective COX inhibitor) and NS398 (selective COX-2 inhibitor) almost completely inhibited the TNF-α-induced MMP-9 production and activation in the cell lysate of cultured CC cells, whereas SC560 (selective COX-1 inhibition) showed partial inhibition, thus suggesting that COX, particularly COX-2, is significantly involved in the MMP-9 up-regulation by TNF-α treatment. An inhibitory effect of COX-2 siRNA treatment on MMP-9 production and cell migration also suggests the involvement of COX-2 in this pathway. Because the COX-1 inhibitor and the COX-1 siRNA treatment inhibited a part of the TNF-α-induced MMP-9 production and cell migration, it is plausible that COX-1 is also involved in this pathway. As described above, MMP-9 and COX-2 were frequently expressed, and both the latent and active MMP-9 were evidently detectable in the human CC specimens. Taken together, it seems likely that TNF-α secreted in tumor tissue, mainly from infiltrating macrophages,28 may be involved in the up-regulation of MMP-9 and COX-2 in human CC tissues.
COX is the enzyme responsible for the generation of PGE2.13,14,15,16,24 It was found that TNF-α treatment increased PGE2 secretion in the supernatant of the cultured CC cells in a time- and dose-dependent manner. We therefore examined whether COX is involved in this increased secretion of PGE2 in cultured CC cells. Interestingly, the COX inhibitors inhibited the TNF-α-induced PGE2 secretion in the cultured CC cells, suggesting that COX is involved in the increased secretion of PGE2 in cultured CC cells by TNF-α treatment.
We examined the roles of PGE2 and its receptors in the up-regulation of MMP-9 by TNF-α treatment. It was found that pretreatment with SC19220 (blocking peptide of EP1 and EP2) and AH23848 (blocking peptide of EP4) clearly reduced the TNF-α-induced MMP-9 up-regulation in cultured CC cells, whereas AH6809 (blocking peptide of EP1) did not. SC19220 and AH23848 may have an inhibitory effect on the posttranscriptional pathway in the TNF-α-induced MMP-9 production and activation, because they did not affect the TNF-α-induced MMP-9 promoter activity despite its inhibitory effect on the TNF-α-induced MMP-9 production and activation. This suggested that TNF-α/TNF-R1 induces COX-2 overexpression, which increases PGE2 secretion, followed by MMP-9 production and activation via the receptor of PGE-2 (EP2 and EP4). This hypothetical scenario is shown in Figure 7. The role of nuclear factor-κB in MMP-9 gene regulation is well established.10 Although Mon and colleagues27,41 reported that TNF-α-induced phosphorylated focal adhesion kinase (FAK) accumulated at focal adhesions, which gave rise to an increased production of MMP-9 and increased the tumor cell migration in cultured CC cells (CCKS-1). Such a signaling pathway involving COX-2 and PGE2 for the increased production and activation of MMP-9 by TNF-α treatment has not been reported so far; therefore, there are at least two intracellular signaling pathways for MMP-9 up-regulation in CC after TNF-α treatment.
Figure 7.
Schema of TNF-α-induced up-regulation of COX-2 and MMP-9, and their interaction. Activated MMP-9 induced the migration of CC cells. In addition to active participation of COX-2, COX-1 may be also partly involved in this process. TNF-R1, TNF receptor 1; EP2/4, PGE2 receptors EP2 and EP4; MMP-9.
It was found in this study that TNF-α treatment promoted the migration of cultured CC cells (HuCCT-1); the migration index of the CC cells was increased by 5.8-fold. Such an increased tumor cell migration was inhibited by a pretreatment of anti-TNF-α neutralizing antibodies, thus suggesting that a TNF-α/TNF-R interaction is involved in this increased migration. Interestingly, such increased migration by TNF-α treatment was also inhibited by GM6001 (nonselective MMP inhibitor), and also by MMP-9 inhibitor I. In addition, the degree of migration inhibition by these two inhibitors was almost identical, suggesting that MMP-9 up-regulated by TNF-α was mainly responsible for this increased migration of the CC cells. The TNF-α-induced tumor cell migration was also significantly inhibited by three COX inhibitors (indomethacin, SC560, and NS398). Taken together, it seems likely that TNF-α/TNF-R1 up-regulates COX-2 expression followed by MMP-9 overproduction and activation, and in turn by the increased migration of the CC cells.
It seems, therefore, likely that overexpressed and secreted COX-2 and PGE2 in human CC tissues, probably induced by a locally high level of TNF-α secreted locally at the boundaries of the CC,28 may be involved in the up-regulation of MMP-9 followed by the increased migration of tumor cells, and then the invasion by the CC. Although TNF-α treatment failed to induce up-regulation and overproduction of COX-1 in CC culture studies, the expression of COX-1 mRNA and that of MMP-9 mRNA was correlated in CC tissues and inhibitor of COX-1 (SC560) reduced PGE2 production and also migration of CC cells induced by TNF-α. So, it seems likely that COX-2 is mainly involved in the TNF-α-induced migration of CC cells, although COX-1 may be at least partly involved in this migration process. This hypothetical scenario is shown in Figure 7.
Accumulating evidence strongly suggests that inflammation is a crucial component of tumor progression, and inflammatory cytokines/chemical mediators play an important role in tumorigenesis.22,23 It was found in this study that TNF-α, a proinflammatory cytokine, and COX-2/PGE2, chemical mediators, were significantly involved in the production and activation of MMP-9, and such up-regulated MMP-9 is involved in the increased migration of cultured CC cells and may relate at least partly to the tumor invasion of CC. So far, many biological functions related to carcinogenesis have been found to be induced by the COX-2/PGE2 signaling pathway, which leads to the up-regulation of HGF and IL-6, followed by activation of PI3-k/Akt and MAPK signaling, leading to the promotion of cholangiocarcinogenesis.17,18,19,42,43,44,45 The present study revealed novel COX-2/PGE2 signaling that is activated by the TNF-α/TNF-R1 interaction and is actively involved in the up-regulation of MMP-9 followed by the increased migration of CC.
Identifying and clarifying the endogenous factors such as MMPs, COX-2, and PGE2 within malignant tumors should lead not only to an understanding of the processes of tumor progression and metastasis, but should also provide new strategies for developing agents that specifically suppress these processes.17 From a therapeutic aspect, it has been established that the single use of MMP inhibitors is not particularly effective in the later stages, as seen in the results of randomized control trials, even though survival benefits are reported in some cancers.11 COX inhibitors are thought to function as anti-tumor agents by the inhibition of COX-2 and PGE2.13,14,15 COX inhibitor may also have anti-tumor effects.13,14,15 Recently, however, several studies showed that the nonsteroidal anti-inflammatory drug-mediated anti-tumor effect is complicated by the presence of COX-independent effects.15,41 This study proposes a combination therapy of MMP and COX-2 inhibitors or an EP antagonist, as a challenging therapeutic approach.
If NS398 inhibits COX-2 superselectively, the addition of PGE2 is thought to reverse MMP-9 production. Another study also reported that the indomethacin-mediated prevention of lipopolysaccharide-induced MMP-9 production was not reversed by PGE2 addition, whereas that of MMP-1 was completely reversed by PGE2.42 They suggested that the COX inhibitors also function by a PGE2-independent mechanism in MMP-9 up-regulation.42 The administration of PGE2 alone did not induce MMP-9 production and activation in this study, despite the significant effect of the COX inhibitors and blocking peptides for the PGE2 receptors. This finding also supports the effect of the COX inhibitors on a PGE2-independent mechanism.
In conclusion, culture studies using HuCTT-1 suggest that up-regulation of COX-2 by TNF-α/TNF-R1 interaction may lead to the up-regulation of PGE-2 to then up-regulate MMP-9 via EP2/4. Up-regulated MMP-9 may lead to increased migration of CC cells and then tumor invasion.
Footnotes
Address reprint requests to Yasuni Nakanuma, M.D., Department of Human Pathology, Kanazawa University Graduate School of Medicine, Kanazawa 920-8640, Japan. E-mail: pbcpsc@kenroku.kanazawa-u.ac.jp.
Supported in part by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports and Science and Technology of Japan (19390098).
References
- Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: recent progress. Part 2: molecular pathology and treatment. J Gastroenterol Hepatol. 2002;17:1056–1063. doi: 10.1046/j.1440-1746.2002.02780.x. [DOI] [PubMed] [Google Scholar]
- Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, Sano T, Yamamoto H, Hayakawa N. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155–162. doi: 10.1007/s005340050170. [DOI] [PubMed] [Google Scholar]
- Nagakawa T, Kayahara M, Ikeda S, Futakawa S, Kakita A, Kawarada H, Matsuno M, Takada T, Takasaki K, Tanimura H, Tashiro S, Yamaoka Y. Biliary tract cancer treatment: results from the Biliary Tract Cancer Statistics Registry in Japan. J Hepatobiliary Pancreat Surg. 2002;9:569–575. doi: 10.1007/s005340200076. [DOI] [PubMed] [Google Scholar]
- Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S, Niwa H, Mitsui T, Asada Y, Miura S, Ohta T, Nakanuma Y. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333–1343. doi: 10.1002/hep.21387. [DOI] [PubMed] [Google Scholar]
- Itatsu K, Zen Y, Ohira S, Ishikawa A, Sato Y, Harada K, Ikeda H, Sasaki M, Nimura Y, Nakanuma Y. Immunohistochemical analysis of the progression of flat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int. 2007;27:1174–1184. doi: 10.1111/j.1478-3231.2007.01577.x. [DOI] [PubMed] [Google Scholar]
- Itatsu K, Zen Y, Yamaguchi J, Ohira S, Ishikawa A, Sato Y, Harada K, Sasaki M, Sakamoto H, Nagino M, Nimura Y, Ohta T, Nakanuma Y. Expression of matrix metalloproteinase 7 is an unfavorable post-operative prognostic factor in cholangiocarcinoma of perihilar, hilar and extrahepatic bile ducts. Hum Pathol. 2008;39:710–719. doi: 10.1016/j.humpath.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Shirabe K, Shimada M, Kajiyama K, Hasegawa H, Gion T, Ikeda Y, Takenaka K, Sugimachi K. Expression of matrix metalloproteinase-9 in surgically resected intrahepatic cholangiocarcinoma. Surgery. 1999;126:842–846. [PubMed] [Google Scholar]
- Miwa S, Miyagawa S, Soeda J, Kawasaki S. Matrix metalloproteinase-7 expression and biologic aggressiveness of cholangiocellular carcinoma. Cancer. 2002;94:428–434. doi: 10.1002/cncr.10235. [DOI] [PubMed] [Google Scholar]
- Schmitz KJ, Lang H, Wohlschlaeger J, Reis H, Sotiropoulos GC, Schmid KW, Baba HA. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for overall survival in intrahepatic cholangiocarcinoma. Virchows Arch. 2007;450:135–141. doi: 10.1007/s00428-006-0355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- Björklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochem Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- Taketo MM. Cyclooxgenase-2 inhibitors in tumorigenesis (part II). J Natl Cancer Inst. 1998;90:1609–1620. doi: 10.1093/jnci/90.21.1609. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, DuBois RN. COX-2: a target for colon cancer prevention. Annu Rev Pharmacol Toxicol. 2002;42:55–80. doi: 10.1146/annurev.pharmtox.42.082301.164620. [DOI] [PubMed] [Google Scholar]
- Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- Wu T. Cyclooxygenase-2 and prostaglandin signaling in cholangiocarcinoma. Biochim Biophys Acta. 2005;1755:135–150. doi: 10.1016/j.bbcan.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- Terada T, Okada Y, Nakanuma Y. Expression of immunoreactive matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human normal livers and primary liver tumors. Hepatology. 1996;23:1341–1344. doi: 10.1053/jhep.1996.v23.pm0008675149. [DOI] [PubMed] [Google Scholar]
- Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–969. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Kokuryo T, Yamamoto T, Oda K, Kamiya J, Nimura Y, Senga T, Yasuda Y, Ohno Y, Nakanuma Y, Chen MF, Jan YY, Yeh TS, Chiu CT, Hsieh LL, Hamaguchi M. Profiling of gene expression associated with hepatolithiasis by complementary DNA expression array. Int J Oncol. 2003;22:175–179. [PubMed] [Google Scholar]
- Sirica AE, Lai G-H, Zhang Z. Biliary cancer growth factor pathways, cyclo-oxygenase-2 and potential therapeutic strategies. J Gastroenterol Hepatol. 2001;16:363–372. doi: 10.1046/j.1440-1746.2001.02438.x. [DOI] [PubMed] [Google Scholar]
- Nakanuma Y, Terada T, Tanaka Y, Ohta G. Are hepatolithiasis and cholangiocarcinoma aetiologically related? A morphological study of 12 cases of hepatolithiasis associated with cholangiocarcinoma. Virchows Arch A Pathol Anat Histopathol. 1985;406:45–58. doi: 10.1007/BF00710556. [DOI] [PubMed] [Google Scholar]
- Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- Tanimura Y, Kokuryo T, Tsunoda N, Yamazaki Y, Oda K, Nimura Y, Naing Mon N, Huang P, Nakanuma Y, Chen MF, Jan YY, Yeh TS, Chiu CT, Hsieh LL, Hamaguchi M. Tumor necrosis factor alpha promotes invasiveness of cholangiocarcinoma cells via its receptor, TNFR2. Cancer Lett. 2005;219:205–213. doi: 10.1016/j.canlet.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Ohira S, Sasaki M, Harada K, Sato Y, Zen Y, Isse K, Kozaka K, Ishikawa A, Oda K, Nimura Y, Nakanuma Y. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor α and stromal derived facor-1 released in stroma. Am J Pathol. 2006;168:1155–1168. doi: 10.2353/ajpath.2006.050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Katsumata K, Tsuchida A, Wada T, Mori Y, Hisada M, Kawakita H, Aoki T. Etodolac, a selective cyclooxygenase-2 inhibitor, inhibits liver metastasis of colorectal cancer cells via the suppression of MMP-9 activity. Int J Mol Med. 2006;17:357–362. [PubMed] [Google Scholar]
- Yao M, Lam EC, Kelly CR, Zhou W, Wolfe MM. Cyclooxygenase-2 selective inhibition with NS-398 suppresses proliferation and invasiveness and delays liver metastasis in colorectal cancer. Br J Cancer. 2004;90:712–719. doi: 10.1038/sj.bjc.6601489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic S, Du B, Sakamoto K, Khan KM, Natarajan C, Breyer RM, Dannenberg AJ, Falcone DJ. Targeting prostaglandin E2 receptors as an alternative strategy to block cyclooxygenase-2-dependent extracellular matrix-induced matrix metalloproteinase-9 expression by macrophages. J Biol Chem. 2006;281:3321–3328. doi: 10.1074/jbc.M506846200. [DOI] [PubMed] [Google Scholar]
- Nakanuma Y, Hoso M, Sanzen T, Sasaki M. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech. 1997;38:552–570. doi: 10.1002/(SICI)1097-0029(19970915)38:6<552::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Albores-Saavedra J, Menck HR, Scoazec JC, Soehendra N, Wittekind C, Sriram PVJ, Stripa B B. Hamilton SR, Aaltonen LA, editors. Lyon: IARC Press,; Carcinoma of the gallbladder and extrahepatic bile ducts. World Health Organization Classification of Tumours, Pathology and Genetics: Tumours of the Digestive Systems. 2000:pp 206–214. [Google Scholar]
- Nakanuma Y, Leong AS-Y, Sripa B, Ponchon T, Vatanasapt V, Ishak KG. Intrahepatic cholangiocarcinoma. Lyon: IARC Press,; World Health Organization Classification of Tumours, Pathology and GeneticsTumours of the Digestive Systems Edited by Hamilton SR, Aaltonen LA. 2000:pp 173–180. [Google Scholar]
- Sobin LH, Wittekind C. International Union Against Cancer (UICC). New York: Wiley-Liss,; TNM Classification of Malignant Tumors, (ed 6) 2002 [Google Scholar]
- Sasaki M, Yamaguchi J, Itatsu K, Ikeda H, Nakanuma Y. Over-expression of polycomb group protein EZH2 relates to decreased expression of p16(INK4a) in cholangiocarcinogenesis in hepatolithiasis. J Pathol. 2008;215:175–183. doi: 10.1002/path.2345. [DOI] [PubMed] [Google Scholar]
- Kudo T, Takino T, Miyamori H, Thompson EW, Sato H. Substrate choice of membrane-type 1 matrix metalloproteinases is dictated by tissue inhibitor of metalloproteinase-2 levels. Cancer Sci. 2007;98:563–568. doi: 10.1111/j.1349-7006.2007.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquoi E, Munaut C, Colige A, Lambert C, Frankenne F, Noël A, Grams F, Krell HW, Foidart JM. Stimulation of matrix metalloproteinase-9 expression in human fibrosarcoma cells by synthetic matrix metalloproteinase inhibitors. Exp Cell Res. 2002;275:110–121. doi: 10.1006/excr.2002.5489. [DOI] [PubMed] [Google Scholar]
- Aznavoorian S, Moore BA, Alexander-Lister D, Hallit SL, Windsor J, Engler JA. Membrane type-I matrix metalloproteinase-mediated degradation of type I collagen by oral squamous carcinoma cells. Cancer Res. 2001;61:6264–6275. [PubMed] [Google Scholar]
- Mon NN, Hasegawa H, Thant AA, Huang P, Tanimura Y, Senga T, Hamaguchi M. A role for focal adhesion kinase signaling in tumor necrosis factor-α-dependent matrix metalloproteinase-9 production in a cholangiocarcinoma cell line, CCKS1. Cancer Res. 2006;66:6778–6784. doi: 10.1158/0008-5472.CAN-05-4159. [DOI] [PubMed] [Google Scholar]
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175–180. doi: 10.1016/s0046-8177(98)90229-5. [DOI] [PubMed] [Google Scholar]
- Han C, Leng J, Demetris AJ, Wu T. Cyclooxygenase-2 promotes human cholangiocarcinoma growth: evidence for cyclooxygenase-2-independent mechanism in celecoxib-mediated induction of p21waf1/cip1 and p27kip1 and cell cycle arrest. Cancer Res. 2004;64:1369–1376. doi: 10.1158/0008-5472.can-03-1086. [DOI] [PubMed] [Google Scholar]
- Lai WC, Zhou M, Shankavaram U, Peng G, Wahl LM. Differential regulation of lipopolysaccharide-induced monocyte matrix metalloproteinase (MMP)-1 and MMP-9 by p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Immunol. 2003;170:6244–6249. doi: 10.4049/jimmunol.170.12.6244. [DOI] [PubMed] [Google Scholar]
- Sugawara H, Yasoshima M, Katayanagi K, Kono N, Watanabe Y, Harada K, Nakanuma Y. Relationship between interleukin-6 and proliferation and differentiation in cholangiocarcinoma. Histopathology. 1998;33:145–153. doi: 10.1046/j.1365-2559.1998.00445.x. [DOI] [PubMed] [Google Scholar]