Abstract
Galectin-3 belongs to a family of β-galactoside-binding animal lectins expressed in several cell types, including epithelial and immune cells. To establish the role of galectin-3 in the development of allergic skin inflammation, we compared inflammatory skin responses of galectin-3-deficient (gal3−/−) and wild-type (gal3+/+) mice to epicutaneous sensitization with ovalbumin (OVA). OVA-treated gal3−/− mice exhibited markedly reduced epidermal thickening, lower eosinophil infiltration, and lower serum IgE levels compared with gal3+/+ mice. The former evoked lower interleukin-4, but higher interferon-γ, mRNA expression at OVA-treated skin sites. Moreover, gal3−/− splenocytes from OVA-sensitized mice secreted more interleukin-12 compared with gal3+/+ splenocytes. In addition, antigen presentation by gal3−/− dendritic cells to T cells in vitro were T helper cell (Th1)-polarized relative to presentation by gal3+/+ dendritic cells. When exposed to OVA, recipients engrafted with T cells from gal3−/− OVA-specific T cell receptor transgenic mice developed significantly reduced dermatitis and a markedly lower Th2 response compared with recipients of comparable gal3+/+ T cells. We conclude that galectin-3 is critical for the development of inflammatory Th2 responses to epicutaneously administered antigens; in its absence, mice develop a Th1-polarized response. This regulatory effect of galectin-3 on Th development is exerted at both the dendritic cell and T cell levels. Our studies suggest that galectin-3 may play an important role in the acute phase of human atopic dermatitis.
Atopic dermatitis (AD) is a chronic, or chronically relapsing, inflammatory skin disease characterized by pruritic and eczematous skin lesions. Various studies indicate that AD has a complex etiology, with activation of multiple immunological and inflammatory pathways. Following antigen stimulation, T helper (Th) cells can develop into Th1 cells that secrete interleukin (IL)-2 and interferon (IFN)-γ, or Th2 cells that secrete IL-4, IL-5, and IL-13. It has been proposed that Th2 cells play a key pathogenic role in AD, which is supported by the presence of peripheral blood eosinophilia and enhanced serum IgE levels in a majority of AD patients. In acute lesions of AD, there is a significant increase in the number of cells expressing IL-4, IL-5, and IL-13 mRNA and proteins, suggesting preferential accumulation of Th2 cells. Our current understanding of AD is that there is a predominant Th2 cytokine milieu in the initiating stages or acute lesions of AD and a mixed Th1 and Th2 pattern in chronic lesions.1
Galectins are a family of animal lectins defined by shared consensus amino acid sequences in the carbohydrate-recognition domain and affinity for β-galactosides.2,3 Galectin-3 is the only chimeric galectin that consists of a C-terminal carbohydrate-recognition domain linked to a flexible non-lectin domain made of tandem repeats. It is abundantly present in the epithelia of several organs, and is also expressed by a number of immune and inflammatory cells, including T cells and dendritic cells (DCs).4 The expression of galectin-3 is known to be dependent on cell differentiation and activation.5 Extracellular galectin-3 has been shown to activate a variety of cells and plays an important role in cell-cell and cell-extracellular matrix interactions, and these are related to its carbohydrate-binding properties.6,7 On the other hand, intracellular galectin-3 plays a role in regulation of cell growth, cell cycle progression, and apoptosis, in a carbohydrate-independent manner.8,9
With regard to the immune and inflammatory responses, galectin-3 is known to play a role in regulation of survival of immune cells. Endogenous galectin-3 has been shown to be anti-apoptotic in human T cell Jurkat line transfected to ectopically express galectin-3 and in mouse macrophages.10,11 In contrast, exogenously added galectin-3 was reported to induce apoptosis in human T cells lines by binding to cell surface glycoproteins.12,13 In addition, extracellular galectin-3 has been shown to activate various rodent and human immune cells4 and function as a chemoattractant for human monocytes, as well as human peripheral blood and alveolar macrophages.14 Recent reports from studying galectin-3-deficient (gal3−/−) mice have indicated that endogenous galectin-3 plays a critical role in phagocytosis by macrophages15 and in the mast cell response16 by functioning intracellularly. In addition, galectin-3 was found to function as a cell surface adhesion molecule on eosinophils to support cell rolling on and adhesion to endothelium.17 These findings suggest that galectin-3 can promote the inflammatory response.
We have previously demonstrated that gal3−/− mice exhibited a lower degree of allergic airway inflammation compared with wild-type mice in a model of asthma.18 The cellular basis for the role of galectin-3 in this model of allergic inflammation has not been elucidated. Moreover, the role of galectin-3 in allergic skin inflammation is unknown. In the present study, we have investigated the role of galectin-3 in a mouse model of the acute phase of AD elicited by repeated epicutaneous sensitization with ovalbumin (OVA). These mice display many features of human AD, including disease characterized by acanthosis and eosinophil infiltration in the dermis, as well as pronounced local and systemic Th2 responses.19,20 We show galectin-3 expression in leukocytes infiltrating the skin after epicutaneous sensitization. Gal3−/− mice developed significantly diminished skin inflammation and exhibited a lower Th2 response but a higher Th1 response compared with similarly treated gal3+/+ mice. In addition, presentation of OVA peptide to T cells by galectin-3-deficient DCs resulted in a Th1-polarized response in vitro. Moreover, mice receiving T cells from gal3−/− OVA-specific T cell receptor transgenic (OVA-TCR tg) mice developed a markedly lower Th2 response and a significantly lower degree of dermatitis after exposure to OVA when compared with those receiving corresponding gal3+/+ cells. These results suggest that galectin-3 is critical for the development of the Th2 inflammatory response to epicutaneously introduced antigens, and the regulatory effect of galectin-3 on Th development is exerted at both DC and T cell levels.
Materials and Methods
Animals
Gal3−/− mice were developed as described11 and were backcrossed to BALB/c and C57BL/6 mice for nine generations. OT-II mice, OVA323–339-specific TCR tg mice in the C57BL/6 background,21 were obtained from the Jackson Laboratory (Bar Harbor, ME). Gal3−/− mice in the C57BL/6 background were crossed to OT-II mice to obtain gal3−/−/OVA-TCR tg mice. BALB/c mice were used in the existing AD model with three 1-week periods of sensitization because this is the most commonly used strain for this model. C57BL/6 mice were used in the adoptive transfer experiments, because our gal3−/− and gal3+/+/OVA-TCR tg mice are in the C57BL/6 background. It has been demonstrated that skin lesions developed in BALB/c and C57BL/6 mice are comparable.22 All mice were kept in a pathogen-free environment. All experiments with mice were approved by the Institutional Animal Care and Use Committee of the University of California, Davis (Sacramento, CA).
Sensitization Protocol
Epicutaneous sensitization of 6- to 10-week-old female BALB/c mice was performed as described previously.20 Briefly, an area on the trunk of mice was shaved and tape-stripped six times. One hundred μg of endotoxin-free OVA (prepared by collecting chicken albumin aseptically and freeze-drying it in pyrogen-free vials) in 100 μl of PBS or 100 μl of PBS alone was placed on a patch of sterile gauze (1 × 1 cm). The gauze was secured to the back with Tegaderm (3M Health Care Ltd, St. Paul, MN). Each mouse was sensitized by three 1-week periods of epicutaneous application of OVA under occlusion, at 2-week intervals.
Adoptive Transfer of OVA-Specific T Cells
Spleens were harvested from gal3−/−/OVA-TCR tg mice and gal3+/+/OVA-TCR tg mice, and single-cell suspensions of splenocytes were prepared (1 × 106 cells/ml). The cells were then cultured together with wild-type irradiated syngeneic spleen cells (1 × 106 cells/ml) as antigen-presenting cells (APCs) in the presence of OVA (100 μg/ml) and IL-2 (10 ng/ml) in RPMI 1640 medium containing 10% fetal bovine serum at 37°C for 5 days. EasySep magnetic selection kits (StemCell Technologies, Vancouver, BC, Canada) were used to isolate CD4+ T cells by negative selection (purity >90% was confirmed by staining with CD4 Ab, coupled with fluorescence-activated cell sorting analysis). The resulting cells were injected intravenously into wild-type C57BL/6 mice (5 × 106 cells per mouse). Twenty-four hours after the injection, an area on the trunk of the recipients was shaved, tape-stripped, and sensitized with OVA (100 μg in 100 μl of PBS) or PBS (100 μl) placed on a patch of sterile gauze, as described above. The patches were placed for 1 week and then removed.
Histology
Human Tissues
Skin biopsies were obtained under institutionally approved guidelines from five volunteers diagnosed with AD, at lesional and nonlesional sites, and two individuals without AD and other apparent skin diseases. Tissues were processed for embedding in paraffin or frozen in optimal cutting temperature compound (Sakura Finetek, Torrance, CA). Cryosections were fixed in 95% ethanol immediately after sectioning, and before immunolabeling, re-fixed in fresh paraformaldehyde for 2 minutes. Five-μm sections were processed for detection of galectin-3 with goat antibody,23 or anti-CD4 (clone 1F6, AbD Serotec, Raleigh, NC) as instructed by manufacturer, and developed with peroxidase-conjugated second antibodies and diaminobenzidine. Double-staining was performed with CD4 antibody and the alkaline phosphatase UltraVision LP Detection System (Labvision, Fremont, CA) with fast red substrate, and biotinylated goat anti-galectin-3, followed by streptavidin-horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA) and diaminobenzidine/Ni (Vector Laboratories, Burlingame, CA) according to the manufacturer’s recommendations. No reactivities were observed with control first antibodies, and under the conditions used for double staining, no staining was observed with unmatched second reagent (not shown). Micrographs were obtained with a DP71 camera on an Olympus IX81 microscope with ×10 (NA 0.3) and ×60 (NA 1.42) objectives.
Mouse Tissues
Skin specimens were obtained from OVA-treated sites 24 hours after removal of the skin patch. Specimens were embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA) on dry ice. Multiple 4-μm sections were obtained. The epidermal thickness was determined by averaging at least 10 random locations on each slide. Cells were counted in a blind fashion in 20 high-power fields at a magnification of ×1,000 and are expressed as mean cell numbers per high-power field per sample.
The sections were also processed for immunohistochemical analysis. For immunoperoxidase staining of galectin-3, the samples were incubated with anti-galectin-3 monoclonal antibody (Ab) A3A1224 as described previously.23 Bound antibody was detected by an avidin-biotin-immunoperoxidase method according to the manufacturer’s instructions (DakoCytomation, Inc. Carpinteria, CA). For double immunofluorescent staining for CD4 and galectin-3, an anti-CD4 monoclonal antibody (clone GK1.5, eBioscience, San Diego, CA) was applied according to the suggested protocol to cryosections, followed by Alexa488-conjugated second antibody (Invitrogen, Carlsbad, CA), and a goat antibody against galectin-3 was applied, followed by rhodamine-conjugated second antibody (Jackson ImmunoResearch, West Grove, PA).
Quantitation of IgE, IgG1 and IgG2a in the Serum
The standard protocol for sandwich enzyme-linked immunosorbent assay (ELISA) was used to quantify the total amount of antibodies in the sera. Total IgE levels were measured by ELISA as described previously.25 OVA-specific IgG1 and IgG2a Abs were also measured by ELISA as described previously.18 OVA-specific IgE was quantitated by using rat anti-IgE as capture Ab and horseradish peroxidase-conjugated rabbit anti-OVA Abs (United States Biological, Swampscott, MA) for detection.20
Analysis of in Vitro Cytokine Synthesis
Single-cell suspensions of spleen and draining (axillary) lymph nodes were prepared in RPMI 1640 supplemented with 10% fetal bovine serum. Cells were cultured in the above medium at 4 × 106 cells/ml in 24-well plates in the presence of OVA (50 μg/ml). After 72 hours, supernatants were collected and assayed for cytokines by standard sandwich ELISA, following the manufacturer’s instructions (IL-4, IFN- γ, and IL-5, eBiosciences, San Diego, CA; IL-12, BD Pharmingen, San Diego, CA).
RNA Preparation, cDNA Synthesis, and Real-Time PCR
Skin biopsies were obtained 24 hours after the third sensitization period and total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA). cDNA was synthesized from 10 μg of total RNA in a 50-μl reaction mix through use of Superscript II (Invitrogen). Real-time PCR was performed by using the TaqMan probes for mouse IL-4, IFN-γ, and glyceraldehyde-3-phosphate dehydrogenase26 and the iCycler iQ system (Bio Rad laboratories, Hercules, CA).
DCs Derived from Bone Marrow
DCs were generated from bone marrow cells, as described.27 Cells were cultured in RPMI medium supplemented with 20 ng/ml GM-CSF. Cells were harvested on day 10.
Cytokine Secretion by T Cells from OVA-Specific TCR Transgenic Mice
CD4+ T cells from spleens of OVA-TCR tg mice were purified as described above. DCs from gal3−/− and gal3+/+ mice were pulsed with OVA323–339 peptide (10 μg/ml) for 3 hours. OVA-pulsed DCs were harvested, washed, and mixed with purified naïve T cells from OVA-TCR tg mice, suspended in RPMI containing 10% fetal bovine serum and 50 μmol/L 2-mercaptoethanol. DCs (2 × 104) and T cells (2 × 105) were co-cultured for 72 hours. Expanded T cells (5 × 105) were restimulated with plate-bound anti-CD3 (10 μg/ml) plus anti-CD28 Ab (2 μg/ml) for an additional 48 hours. Supernatants were collected before and after stimulation and assayed for IFN-γ and IL-4.
Statistical Analysis
Statistical analysis was accomplished by Student’s t-test using the software GraphPad Prism ver 4. Values of P < 0.05 were considered significant.
Results
Galectin-3 Expression in Human and Mouse Skin Is Up-Regulated in AD
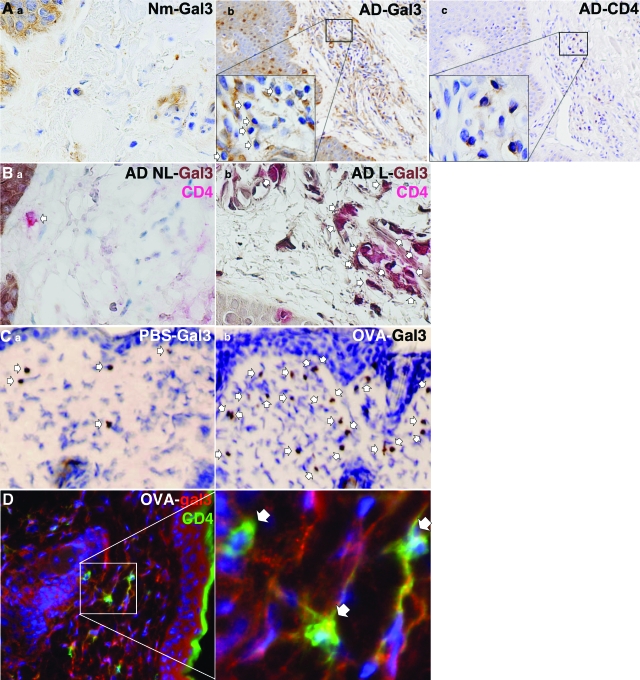
In normal human skin, we observed that galectin-3 was present predominantly in the epidermis, as described previously, with disperse cytoplasmic and nuclear localization28 (light brown staining, Figure 1, Ai). In contrast, galectin-3 expression was up-regulated in the dermis in lesional skin of patients with AD and was located in infiltrating lymphohistiocytic cells, some of which display characteristic morphologies of lymphocytes (arrows, Figure 1, Aii). CD4+ T cells were found to be present in the same vicinity as galectin-3-positive lymphoid cells in these regions of the skin in serial sections (Figure 1, Aiii), suggesting that galectin-3 may be expressed in CD4+ T cells. To firmly demonstrate localization of galectin-3 in CD4+ T cells, we performed double immunohistochemistry. In nonlesional skin from patients with AD, there were scattered CD4+ (red) T cells that do not express galectin-3 (arrow, Figure 1, Bi). In lesional skin, many cells stained positively for both CD4+ and galectin-3 (dark brown). In these cells, the reddish-brown coloration indicates colocalization of these proteins (arrows, Figure 1, Bii), The presence of galectin-3 in CD4+ T cells is consistent with the report that this protein is expressed in activated CD4+ lymphocytes.29 A few CD4+ cells were observed in biopsies from individuals without skin disease, and, like nonlesional skin in AD patients, these cells did not express galectin-3 (not shown).
Figure 1.
Galectin-3 expression is up-regulated in inflammatory cells in the skin in atopic dermatitis. A: a) Immunohistochemical staining for galectin-3 in normal skin, ×60 objective. (b, c) Immunohistochemical staining for galectin-3 and CD4 in lesional skin from AD patients. Presence of galectin-3+ lymphocytes (arrows, b) in the same vicinity as CD4+ lymphocytes (c) in serial sections was noted. Counterstained with hematoxylin. Magnification = ×10. Magnification inset ×60 objective. B: Double immunohistochemical staining for galectin-3 and CD4 in nonlesional (a) and lesional (b) skin from the same AD patient. Scanty CD4+ lymphocytes (red) were present in nonlesional skin (a) that were galectin-3-negative (arrow). In contrast, large numbers of CD4+ cells were found in lesional skin (arrows, b), and many cells express galectin-3 (brown). Cells co-expressing CD4 and galectin-3 appear reddish-brown (arrows); ×60 objective. Results are representative of five AD patients. C: Immunohistochemical staining for galectin-3 in skin from PBS controls (a) and OVA-treated (b) mice. Significantly larger numbers of galectin-3+ cells (arrows, brown) were present at the OVA-sensitized skin sites compared with PBS-treated sites. Counterstained with hematoxylin, ×10 objective. D: Double immunofluorescence staining for galectin-3 and CD4+ in mouse skin treated with OVA. Increased numbers of infiltrating CD4+ (green) lymphocytes were present in the dermis. Cells co-expressing galectin-3 (red) and CD4 appear partially yellow (arrows). An expanded region of the boxed area in the left panel is shown in the right panel. Nuclei visualized with Hoechst dye 33342 appear blue, ×20 objective.
In a mouse model of acute AD, epicutaneous sensitization with OVA in the BALB/c strain induced significant thickening of the epidermis and prominent inflammatory cell infiltrations (Figure 1C). Immunohistochemical analysis revealed that only a few cells in the dermis expressed galectin-3 in the skin from PBS-sensitized mice (Figure 1Ci). However, there was a significant increase in the number of cells staining positively for galectin-3 (brown, arrows) at OVA-sensitized skin sites (Figure 1Cii). Increased expression of galectin-3 was also observed by double immunofluorescence staining (Figure 1D). Cells staining positively for both galectin-3 (red) and CD4+ (green) appear at least partially yellow (arrows, Figure 1D). A few CD4+ T cells were observed in regions of mouse skin treated with PBS and these did not express galectin-3 (not shown).
Gal3−/− Mice Develop a Lower Degree of Acanthosis after Epicutaneous Sensitization
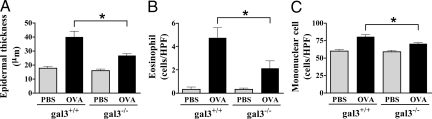
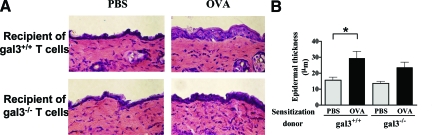
To further examine the role of galectin-3 in skin allergic inflammation, we compared gal3−/−with gal3+/+ mice. Epicutaneous OVA sensitization induced significant thickening of the epidermis in both gal3−/− and gal3+/+ mice. However, the degree of thickening was significantly lower in gal3−/− mice compared with gal3+/+ mice (Figure 2A).
Figure 2.
OVA-sensitized skin sites of gal3−−/−- mice show significantly reduced inflammation. A: Measurements of epidermal thickness in H&E-stained tissues. Quantification of the skin cellular infiltrates after Giemsa staining for (B) eosinophils and (C) mononuclear cells. Bars represent mean cell numbers ± SEM (n = 6 gal3+/+, n = 5 gal3−/−). *P < 0.05.
Gal3−/− Mice Develop Lower Levels of Eosinophil and Mononuclear Cell Infiltrations at the OVA-Sensitized Skin Sites
Dermal infiltration with eosinophils is an important feature of AD. Eosinophils were barely detectable at the PBS-sensitized skin sites, but their numbers dramatically increased following epicutaneous sensitization with OVA in gal3+/+ mice. In contrast, significantly lower amounts of eosinophils were detected at similarly treated skin sites of gal3−/− mice (Figure 2B). In addition, the numbers of dermal mononuclear cells were lower in gal3−/− mice (Figure 2C). There were no statistically significant differences in the numbers of circulating eosinophils and mononuclear cells between gal3−/− and gal3+/+ mice (data not shown).
Gal3−/− Mice Exhibit a Lower Th2 Response but a Higher Th1 Response to Epicutaneous OVA Sensitization
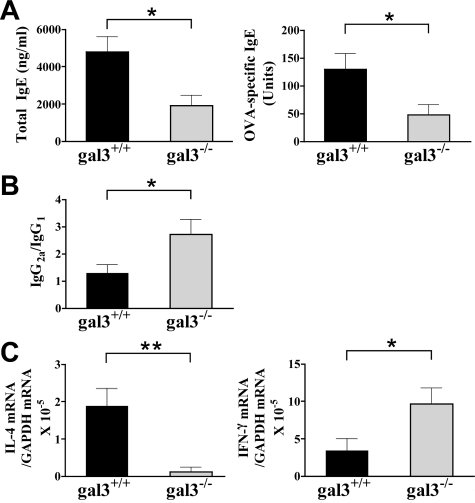
The mouse model of AD we used is characterized by a Th2-dominated systemic response including elevated serum IgE levels. We then investigated the total and antigen-specific IgE response. Gal3−/− mice sensitized with OVA exhibited strikingly lower serum IgE levels compared with similarly treated gal3+/+ mice. In addition, the levels of OVA-specific IgE were also significantly lower in gal3−/− mice (Figure 3A). Th2 cytokines play a critical role in isotype switching to IgG1, while Th1 cytokines play a role in isotype switching to IgG2a. We measured the OVA-specific serum IgG1 and IgG2a levels, and calculated the ratio as an indicator of the Th1 response. Gal3−/− mice showed significantly higher IgG2a/IgG1 ratios compared with gal3+/+ mice (Figure 3B). These results suggest that galectin-3 deficiency results in a Th1-polarized systemic response to epicutaneously introduced protein antigen.
Figure 3.
Gal3−/− mice develop a lower cytokine Th2 response, but a higher Th1 response. A: Total IgE and OVA-specific IgE in sera were determined by ELISA. B: The OVA-specific IgG1 and IgG2a were determined by ELISA, and ratios of IgG2a to IgG1 were calculated. C: Real-time PCR was performed to quantify mRNA. The results were normalized to glyceraldehyde-3-phosphate dehydrogenase. Bars represent mean ± SEM (n = 6 gal3+/+, n = 5 gal3−/−). *P < 0.05; **P < 0.01.
Skin lesions of AD are characterized by increased expression of IL-4 mRNA, although IFN-γ mRNA tends to increase in chronic lesions.30 We next examined the expression of IL-4 and IFN-γ mRNA at the skin sites. Low levels of IL-4 and IFN-γ mRNA were detected in the PBS-sensitized skin sites and the levels were comparable between gal3−/− and gal3+/+ mice. Expression of IL-4 mRNA, but not IFN-γ mRNA markedly increased at the OVA-sensitized skin sites of gal3+/+ mice, suggesting the presence of Th2 cells in the skin. In contrast, IL-4 mRNA did not significantly increase in OVA-sensitized skin of gal3−/− mice, whereas IFN-γ mRNA was significantly up-regulated (Figure 3C). These results suggest that following epicutaneous sensitization, galectin-3 deficiency skews the cytokine profile of infiltrating T cells toward Th1 and away from Th2.
Gal3−/− APCs Secrete More IL-12 and Induce a Th1-Polarized Response
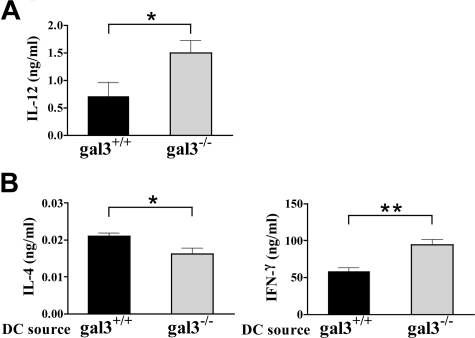
We next examined cytokine production by splenocytes from OVA-sensitized mice in response to in vitro restimulation with OVA. OVA stimulation of splenocytes from epicutaneously sensitized gal3−/− mice induced the secretion of IL-4 in amounts comparable to those secreted by gal3+/+ mice. The treatment caused higher IFN-γ production in gal3−/− mice compared with gal3+/+ mice, but the difference was not statistically significant (data not shown). However, OVA-stimulated splenocytes from gal3−/− mice secreted significantly more IL-12 than those from gal3+/+ mice (Figure 4A).
Figure 4.
Gal3−/− APCs exhibit a Th1-polarized response. A: Splenocytes from gal3+/+ and gal3−/− mice sensitized with OVA as described in Figure 2 were cultured in the presence of OVA (50 μg/ml) for 72 hours. The concentration of IL-12 in the supernatants was determined by ELISA (n = 6 gal3+/+, n = 5 gal3−/−). B: CD4+ T cells from OVA-TCR tg mice were co-cultured with bone marrow-derived DCs from gal3+/+ and gal3−/− mice in the presence of OVA323–339 peptide for 3 days. The same number of T cells (5 × 105) from the two groups was further stimulated with anti-CD3 and anti-CD28 Ab for 48 hours. The concentrations of cytokines were measured by ELISA. Results are representative of three experiments. Bars represent mean ± SEM. *P < 0.05; **P < 0.01.
IL-12 is a potent immunoregulatory cytokine that promotes Th1 differentiation and is mainly produced by macrophages and DCs. Therefore, to determine the role of galectin-3 in DCs, we examined the effect of galectin-3 deficiency on the ability of DCs to drive Th cell differentiation in vitro. Presentation of OVA323–339 peptide by gal3−/− DCs to T cells from OVA-TCR tg mice resulted in significantly higher IFN-γ secretion but lower IL-4 secretion, compared with presentation by gal3+/+ DCs (Figure 4B). These results strongly suggest that the effect of galectin-3 on Th cell polarization is exerted, at least in part, at the level of DCs as APCs.
A 1-Week Epicutaneous Sensitization with OVA Induces Significant Thickening of Epidermis at Treated Sites in Recipients of T Cells from OVA-TCR tg Mice
To further determine the role of galectin-3 in regulation of the immune response in AD, we studied mice that receive T cells expanded in vitro from gal3−/− or gal3+/+/OVA-TCR tg mice in the C57BL/6 background. Splenocytes were activated with APCs, OVA, and IL-2 in vitro, then injected intravenously into recipient wild-type C57BL/6 mice. Flow cytometric analysis showed that more than 80% of the cells transferred were CD4+ T cells (data not shown). One day after injection, mice were epicutaneously sensitized with OVA for 1 week, and biopsies were taken from the OVA-sensitized skin sites 1 day after completion of the sensitization. Remarkably, a dermatitis response similar to that observed in a documented model using three 1-week sensitizations was obtained. Histological examination indicated that a 1-week exposure to OVA in these recipients induced epidermal and dermal thickening and a dense dermal infiltration; there were focal epidermal acanthosis and spongiosis in the epidermis (Figure 5A). Recipients of gal3−/− cells exhibited less marked acanthosis in the skin as compared with recipients of gal3+/+ cells. However, this difference was not statistically significant under this experimental protocol (Figure 5B). To rule out the possible effects of other cell types (eg, APCs) transferred, we transferred purified CD4+ T cells (purity >90%), and obtained nearly identical results (data not shown).
Figure 5.
A 1-week OVA sensitization induces significant thickening of epidermis in recipients of T cells from OVA-TCR tg mice. OVA-specific gal3+/+ and gal3−/− T cell-enriched populations generated in vitro were adoptively transferred into wild-type mice (5 × 106 cells/mouse) and recipient mice were exposed to OVA for 1 week. A: H&E staining of sensitized skin sites (magnification = original ×200). B: Epidermal thickness measurements. Bars represent mean ± SEM (n = 5 animals per group). *P < 0.05. Similar results were obtained in a separate experiment.
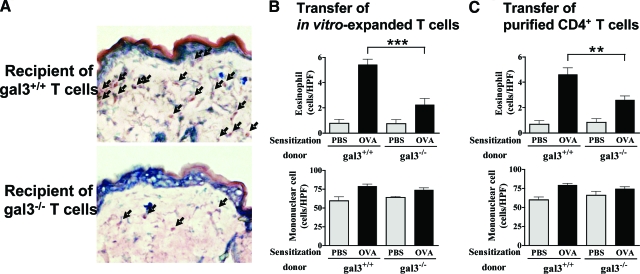
Recipients of gal3−/− OVA-Specific T Cells Show Significantly Lower Eosinophil Infiltration at OVA-Sensitized Skin Sites
We next quantified the dermal cellular infiltrates in this new AD model. We observed a dense dermal infiltration in recipients after the 1-week sensitization (Figure 6A). Importantly, eosinophil infiltration was significantly lower in recipients of gal3−/− cells. There was no significant difference in mononuclear cell infiltration between recipients of gal3−/− and gal3+/+ cells (Figure 6B). Nearly identical results were obtained when purified CD4+ T cells were transferred (Figure 6C).
Figure 6.
Skin sites of recipients of gal3−/− T cells have markedly decreased eosinophils in the dermis. In vitro-generated OVA-specific gal3+/+ and gal3−/− T cell enriched populations (A and B) or purified CD4+ T cells (C) were adoptively transferred into wild-type mice (5 × 106 cells/mouse) and recipient mice were exposed to OVA for 1 week. A: Giemsa staining of sensitized skin sites (magnification = original ×400). Arrows indicate eosinophils. B and C: Number of infiltrating cells in sensitized skin sites. Bars represent mean ± SEM (n = 5 animals per group). **P < 0.01; ***P < 0.001. Similar results were obtained in a separate experiment.
To determine whether the difference in the response in mice receiving gal3+/+ and gal3−/− CD4+ T cells is due to their differential migration, we labeled cells with carboxyl fluorescein succinimidyl ester, engrafted them into wild-type mice, and enumerated gal3+/+ and ga3−/− CD4+ T cells in the lymph nodes 7 days later. We did not notice a significant difference (data not shown).
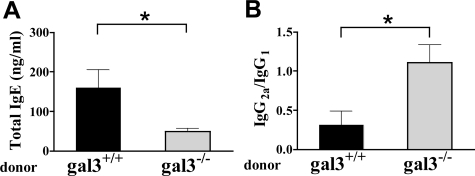
Recipients of gal3−/− OVA-Specific T Cells Exhibit a Markedly Lower Th2 Response but an Exaggerated Th1 Response
We then measured the serum IgE levels in recipients after the 1-week sensitization. While it is known that serum IgE levels did not increase after 1 week of epicutaneous OVA exposure,19 in our AD model comprising engraftment of enriched T cells, however, significantly higher levels of total IgE were detected in the recipients of gal3+/+ cells after this 1-week treatment. In contrast, there was no increase in the serum IgE levels in mice that received gal3−/− cells, and their serum IgE levels were significantly lower than those receiving gal3+/+ cells (Figure 7A). Furthermore, recipients of gal3−/− cells had significantly lower levels of OVA-specific IgG1, but significantly higher levels of IgG2a (data not shown). Thus, recipients of gal3−/− cells showed significantly higher IgG2a/IgG1 ratios (Figure 7B). Additionally, we examined cytokine production in splenocytes and draining (axillary) lymph node cells following in vitro stimulation with OVA antigen. We found that cells from mice receiving gal3−/− T cells secreted less IL-4 and IL-5 but more IFN-γ (Table 1). Nearly identical results were obtained when purified CD4+ T cells were transferred (Table 1).
Figure 7.
Recipients of gal3−/− T cells exhibit lower IgE levels but a higher IgG2a/IgG1 ratio in the serum. Adoptive transfer was conducted as in Figure 5. A: Total IgE levels in sera were determined by ELISA. B: The OVA-specific IgG1 and IgG2a were determined by ELISA, and ratios of OVA-specific IgG2a to IgG1 were calculated. Bars represent mean ± SEM (n = 5 animals per group). *P < 0.05. Similar results were obtained in a separate experiment.
Table 1.
Analysis of in Vitro Cytokine Responses from Recipient Mice
| Donor cells: In vitro-expanded T cells
| ||||
|---|---|---|---|---|
| Spleen
|
DLN
|
|||
| gal3+/+ | gal3−/− | gal3+/+ | gal3−/− | |
| IL-4 (ng/ml) | 0.251 ± 0.006 | 0.219 ± 0.011* | 0.210 ± 0.006 | 0.186 ± 0.003* |
| IFN-γ (ng/ml) | 78.1 ± 9.1 | 178.1 ± 29.4* | 154.5 ± 19.3 | 217.3 ± 9.1* |
| IL-5 (ng/ml) | 1.13 ± 0.16 | 0.54 ± 0.16* | 1.09 ± 0.07 | 0.49 ± 0.11** |
| Donor cells: Purified CD4+ T cells
| ||||
|---|---|---|---|---|
| Spleen
|
DLN
|
|||
| gal3+/+ | gal3−/− | gal3+/+ | gal3−/− | |
| IL-4 (ng/ml) | 0.222 ± 0.009 | 0.191 ± 0.009* | 0.248 ± 0.013 | 0.212 ± 0.004* |
| IFN- γ (ng/ml) | 72.8 ± 11.4 | 169.9 ± 23.9** | 122.6 ± 17.9 | 222.43 ± 29.4* |
| IL-5 (ng/ml) | 0.98 ± 0.19 | 0.46 ± 0.12* | 1.29 ± 0.17 | 0.63 ± 0.13* |
Enriched populations (upper panel) of OVA-specific gal3+/+ and gal3−/− T cells generated in vitro or purified CD4+ T cells (lower panel) were adoptively transferred into wild-type mice (5 × 106 cells/mouse), and recipients were exposed to OVA for 1 week. Spleen and draining lymph nodes (DLN) were harvested from recipient mice and restimulated as described in Figure 3. Mean values ± SEM are shown (n = 5 animals per group).
P < 0.05;
P < 0.01 vs. similarly treated gal3+/+ controls. Similar results were obtained in a separate experiment.
Taken together, these results suggest that, in addition to the effect on DCs, galectin-3 deficiency in T cells causes the development of a Th1 polarized response to epicutaneously introduced protein antigen.
Discussion
We have demonstrated in this study that there is increased galectin-3 expression in inflammatory cells at lesional sites of human patients with AD, but not in nonlesional sites of the same patients. We have also observed that galectin-3 expression is up-regulated at skin sites of allergic inflammation induced by protein antigens administered epicutaneously in mice. In mice, we have found galectin-3 deficiency results in significantly reduced allergic skin inflammation, as measured by the number of infiltrating eosinophils and mononuclear cells. In addition, galectin-3 deficiency results in the development of a Th1-polarized response. The results suggest that endogenous galectin-3 promotes allergic skin inflammation and drives a Th2-polarized response. We have also provided evidence that the effect of galectin-3 on Th development is exerted at both DC and T cell levels.
A remarkable aspect of the present study is the demonstration that Th2-mediated dermatitis is induced by epicutaneous sensitization in mice that receive T cells from OVA-specific TCR tg mice. In contrast to a previously described model in which three 1-week periods of sensitization are required, separated by 2-week intervals, eosinophilic dermatitis and IgE response develop in this new model after just 1 week of antigen exposure. It has been reported previously that RAG2−/− mice, which lack both B and T cells, failed to develop dermatitis, while IgH−/− mice, which lack mature B cells, developed dermatitis equivalent to those observed in wild-type controls after epicutaneous exposure to OVA.31 Together with these earlier studies, our findings suggest that T cells play a critical role in the development of allergic skin inflammation. Our observations of similar numbers of mononuclear cells in the skin of recipients engrafted with gal3−/− and gal3+/+ T cells, respectively, suggest that the influence of galectin-3 in T cells does not extend to this aspect of skin inflammation.
Since the skin inflammation model we used is Th2 cell-driven, the lower levels of dermatitis in gal3−/− mice may be explained by a Th1 deviation of the immune response. These results are consistent with our previous studies of a mouse model of asthma, where gal3−/− mice exhibited reduced airway inflammation and lower IgE levels in the serum.18 Our T cell engraftment experiments (Figure 6 and 7) provided evidence that galectin-3 exerts its influence in T cells, ie, endogenous galectin-3 in T cells promotes the differentiation of naïve T cells into Th2 cells. The expression of galectin-3 in Th1 and Th2 cells remains to be determined. However, galectin-3 was observed in CD4+ T cells in affected regions of human and mouse skin as determined by immunohistochemistry and immunofluorescence, respectively, consistent with the activated nature of these cells, as galectin-3 was observed in T cells when activated, but not in resting T cells.29
It has been demonstrated that T cells deficient in β1,6-N-acetyl-glucosaminyltransferase V (Mgat5), which is essential for synthesis of N-glycans carrying carbohydrate ligands of galectin-3, exhibit a higher TCR response.32 The authors attributed this to the inefficient formation of lattices made of galectin-3 and N-glycans in the TCR complexes. That study implies that by interacting with TCR, galectin-3 increases the threshold of TCR signaling, and in its absence there is an increased T cell response to antigen. Whether this mechanism accounts for our findings remains to be determined.
We have previously shown that galectin-3 is anti-apoptotic in the human Jurkat T cell line transfected to express this protein.10 Subsequent studies by this and other groups established that endogenous galectin-3 exerts this function in various cell types against different apoptotic stimuli through an intracellular mechanism by interacting with molecules in the apoptosis-regulation pathways.8,33 On the other hand, Fukumori et al12 and Stillman et al13 more recently reported that exogenous galectin-3 can induce apoptosis in T cells, probably through binding to cell surface glycoproteins. Thus, a possibility exists that galectin-3 regulates apoptosis in Th1 and Th2 differentially, and its expression favors the higher Th2/Th1 ratio. However, we did not notice a significant difference in the number of CD4+ T cells obtained from gal3+/+ and gal3−/− mice, after the culturing and isolation procedures used in the adoptive transfer experiments. In addition, when carboxyl fluorescein succinimidyl ester-labeled T cells from these two genotypes of animals were separately engrafted, similar amounts of cells were recovered in lymph nodes of respective recipients (data not shown). These data suggest that the differences in the responses we observed are not due to the effect of galectin-3 on cell survival.
A significant reduction in the number of eosinophils was observed in wild-type mice receiving gal3−/− cells expanded in vitro. Since transferred cells did not contain eosinophils, in both groups of recipients eosinophils are derived from the host, and should not differ in terms of their biological properties. Thus, our results suggest that galectin-3 controls the eosinophil response through T cells in allergic skin inflammation. In this regard, we found that both splenocytes and draining lymph node cells from recipients of gal3−/− cells had a decreased capacity to produce IL-5, which is known to prolong survival, differentiation, and activation of eosinophils. A previous report has shown that eosinophils were virtually absent in OVA-sensitized skin sites of IL-5−/− mice.22 Therefore, the decreased capacity to produce IL-5 in gal3−/− T cells may contribute to the lower eosinophil infiltration in gal3−/− mice. Epidermal thickening was observed in gal3−/− mice, although at significantly reduced degrees in comparison with gal3+/+ animals. The results suggest that molecules overexpressed in atopic dermatitis other than galectin-3 also play a role. Similar observations were made in studies of IL5−/− and IFNγ−/− mice treated to develop allergic skin inflammation, in that these mice developed skin thickening, but to lower degrees compared with wild-type mice.22
It should be mentioned that Lahoz and colleagues showed that exogenous galectin-3 suppressed the production of IL-5 in eosinophils and T cells.34 They subsequently reported that administration of plasmid-encoding galectin-3 resulted in the improvement of cellular and functional parameters of a mouse model of asthma by suppressing IL-5.35,36 These contrasting results suggest that exogenously added galectin-3 may not reproduce the function of endogenous galectin-3. This is probably because the location, amount, and intracellular versus extracellular functions of the transgenic protein derived from the plasmid may be different from those of endogenous galectin-3.
APCs are known to play an important role in Th cell polarization. We noted that splenocytes from gal3−/− mice produced higher levels of IL-12, a cytokine known to be a potent inducer of Th1 responses mainly produced by macrophages and DCs after epicutaneous OVA sensitization. This result is consistent with a recent report describing the production of larger amounts of IL-12 in galectin-3-deficient DCs.37 Here we have further directly demonstrated that presentation of OVA peptide to T cells by gal3−/− DCs resulted in significantly higher IFN-γ secretion, but lower IL-4 secretion compared with presentation by gal3+/+ DCs. Thus, galectin-3 also exerts its effect on Th cell polarization through DCs. In contrast to our results, a recent report showed that gal3−/− DCs induced an enhanced production of both IFN-γ and IL-4 by T cells.38 There are several differences in the experimental design between the two studies. Firstly, there are differences in the mouse strains and the antigen used, which might contribute to the discrepancy. Breuilh et al generated DCs from 129 mice and T cells from BALB/c mice, whereas we purified both DCs and T cells from mice in the C57BL/6 background. In addition, they stimulated DCs with schistosome eggs or Toll-like receptor agonists, whereas we pulsed DCs with OVA peptide. Finally, and, most notably, the discrepancy could be attributed to the difference in the number of T cells stimulated in the assay. After co-culturing naïve T cells with gal3+/+ or gal3−/− DCs, Breuilh et al further stimulated the resultant bulk T cell populations, while we stimulated equal numbers of T cells from the two groups, with anti-CD3 plus anti-CD28 Ab. Since they also found that gal3−/− DCs induced a higher proliferative response in T cells compared with gal3+/+ DCs, there are likely a larger number of T cells in the cultures exposed to gal3−/− DCs as compared with those exposed to gal3+/+ DCs in their study (and thus the higher cytokine production in the former could be partly due to the larger number of T cells).
In summary, endogenous galectin-3 is a pro-inflammatory molecule and a potentiator of skin inflammation in a mouse model of AD. Galectin-3 can contribute to allergic inflammation by promoting polarization toward a Th2 immune response. The regulatory effect of galectin-3 on Th development is exerted at both DC and T cell levels. Additional studies will be required to further elucidate the mechanisms and determine whether galectin-3 similarly contributes to human AD.
Footnotes
Address reprint requests to Dr. Fu-Tong Liu, Department of Dermatology, University of California, Davis, School of Medicine, 3301 C Street, Suite 1400, Sacramento, CA 95816. E-mail: fliu@ucdavis.edu.
Supported by the National Institutes of Health grants RO1 AI20958 and RO1 AI39620.
Current address for J.S. is Department of Rheumatology and Clinical Immunology, Kobe University Graduate School of Medicine, 7–5-1 Kusunoki-cho, Chuo-ku, Kobe, 650-0017, Japan.
References
- Grewe M, Bruijnzeel-Koomen CA, Schopf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998;19:359–361. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19:433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- Kasai K, Hirabayashi J. Galectins: a family of animal lectins that decipher glycocodes. J Biochem (Tokyo) 1996;119:1–8. doi: 10.1093/oxfordjournals.jbchem.a021192. [DOI] [PubMed] [Google Scholar]
- Liu FT. Regulatory roles of galectins in the immune response. Int Arch Allergy Immunol. 2005;136:385–400. doi: 10.1159/000084545. [DOI] [PubMed] [Google Scholar]
- Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophage. Am J Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- Almkvist J, Karlsson A. Galectins as inflammatory mediators. Glycoconj J. 2004;19:575–581. doi: 10.1023/B:GLYC.0000014088.21242.e0. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–273. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Nakahara S, Raz A. On the role of galectins in signal transduction. Methods Enzymol. 2006;417:273–289. doi: 10.1016/S0076-6879(06)17019-6. [DOI] [PubMed] [Google Scholar]
- Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–1083. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, Kagawa S, Raz A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302–8311. [PubMed] [Google Scholar]
- Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, Hirashima M, Liu FT. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000;165:2156–2164. doi: 10.4049/jimmunol.165.4.2156. [DOI] [PubMed] [Google Scholar]
- Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu FT. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003;112:389–397. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Sharma BB, Yu L, Zuberi R, Weng IC, Kawakami Y, Kawakami T, Hsu DK, Liu FT. Role of galectin-3 in mast cell functions: galectin-3-deficient mast cells exhibit impaired mediator release and defective JNK expression. J Immunol. 2006;177:4991–4997. doi: 10.4049/jimmunol.177.8.4991. [DOI] [PubMed] [Google Scholar]
- Rao SP, Wang Z, Zuberi RI, Sikora L, Bahaie NS, Zuraw BL, Liu FT, Sriramarao P. Galectin-3 functions as an adhesion molecule to support eosinophil rolling and adhesion under conditions of flow. J Immunol. 2007;179:7800–7807. doi: 10.4049/jimmunol.179.11.7800. [DOI] [PubMed] [Google Scholar]
- Zuberi RI, Hsu DK, Kalayci O, Chen HY, Sheldon HK, Yu L, Apgar JR, Kawakami T, Lilly CM, Liu FT. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am J Pathol. 2004;165:2045–2053. doi: 10.1016/S0002-9440(10)63255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LF, Lin JY, Hsieh KH, Lin RH. Epicutaneous exposure of protein antigen induces a predominant Th2-like response with high IgE production in mice. J Immunol. 1996;156:4077–4082. [PubMed] [Google Scholar]
- Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DK, Dowling CA, Jeng KC, Chen JT, Yang RY, Liu FT. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999;81:519–526. doi: 10.1002/(sici)1097-0215(19990517)81:4<519::aid-ijc3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Liu FT, Hsu DK, Zuberi RI, Hill PN, Shenhav A, Kuwabara I, Chen SS. Modulation of functional properties of galectin-3 by monoclonal antibodies binding to the non-lectin domains. Biochemistry. 1996;35:6073–6079. doi: 10.1021/bi952716q. [DOI] [PubMed] [Google Scholar]
- Liu FT, Bohn JW, Ferry EL, Yamamoto H, Molinaro CA, Sherman LA, Klinman NR, Katz DH. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980;124:2728–2737. [PubMed] [Google Scholar]
- Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech. 2003;14:33–43. [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinov KN, Shames B, Izuno G, Liu FT. Expression of epsilon BP, a beta-galactoside-binding soluble lectin, in normal and neoplastic epidermis. Exp Dermatol. 1994;3:9–16. doi: 10.1111/j.1600-0625.1994.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001;69:555–564. [PubMed] [Google Scholar]
- Leung DY. Pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 1999;104:S99–S108. doi: 10.1016/s0091-6749(99)70051-5. [DOI] [PubMed] [Google Scholar]
- Woodward AL, Spergel JM, Alenius H, Mizoguchi E, Bhan AK, Castigli E, Brodeur SR, Oettgen HC, Geha RS. An obligate role for T-cell receptor alphabeta+ T cells but not T-cell receptor gammadelta+ T cells. B cells, or CD40/CD40L interactions in a mouse model of atopic dermatitis. J Allergy Clin Immunol. 2001;107:359–366. doi: 10.1067/mai.2001.112695. [DOI] [PubMed] [Google Scholar]
- Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- Hsu DK, Yang RY, Liu FT. Galectins in apoptosis. Methods Enzymol. 2006;417:256–273. doi: 10.1016/S0076-6879(06)17018-4. [DOI] [PubMed] [Google Scholar]
- Cortegano I, del Pozo V, Cardaba B, de Andres B, Gallardo S, del Amo A, Arrieta I, Jurado A, Palomino P, Liu FT, Lahoz C. Galectin-3 down-regulates IL-5 gene expression on different cell types. J Immunol. 1998;161:385–389. [PubMed] [Google Scholar]
- del Pozo V, Rojo M, Rubio ML, Cortegano I, Cardaba B, Gallardo S, Ortega M, Civantos E, Lopez E, Martin-Mosquero C, Peces-Barba G, Palomino P, Gonzalez-Mangado N, Lahoz C. Gene therapy with galectin-3 inhibits bronchial obstruction and inflammation in antigen-challenged rats through interleukin-5 gene downregulation. Am J Respir Crit Care Med. 2002;166:732–737. doi: 10.1164/rccm.2111031. [DOI] [PubMed] [Google Scholar]
- Lopez E, del Pozo V, Miguel T, Sastre B, Seoane C, Civantos E, Llanes E, Baeza ML, Palomino P, Cardaba B, Gallardo S, Manzarbeitia F, Zubeldia JM, Lahoz C. Inhibition of chronic airway inflammation and remodeling by galectin-3 gene therapy in a murine model. J Immunol. 2006;176:1943–1950. doi: 10.4049/jimmunol.176.3.1943. [DOI] [PubMed] [Google Scholar]
- Bernardes ES, Silva NM, Ruas LP, Mineo JR, Loyola AM, Hsu DK, Liu FT, Chammas R, Roque-Barreira MC. Toxoplasma gondii infection reveals a novel regulatory role for galectin-3 in the interface of innate and adaptive immunity. Am J Pathol. 2006;168:1910–1920. doi: 10.2353/ajpath.2006.050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuilh L, Vanhoutte F, Fontaine J, van Stijn CM, Tillie-Leblond I, Capron M, Faveeuw C, Jouault T, van Die I, Gosset P, Trottein F. Galectin-3 modulates immune and inflammatory responses during helminthic infection: impact of galectin-3 deficiency on the functions of dendritic cells. Infect Immun. 2007;75:5148–5157. doi: 10.1128/IAI.02006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]