Abstract
Lysosomal acid lipase (LAL) cleaves cholesteryl esters and triglycerides to generate free fatty acids and cholesterol in lysosomes. In LAL gene-knockout (lal−/−) mice, blockage of cholesteryl ester and triglyceride metabolism led to abnormal organization of the thymus and spleen, as well as neutral lipid accumulation in these organs. LAL deficiency impaired T cell development in the thymus. Peripheral T cells were reduced dramatically in lal−/− mice, due largely to increased apoptosis and decreased proliferation of lal−/− T cells in the thymus and peripheral compartments. These lal−/− T cells lost the ability to respond to T cell receptor stimulation, including reduced expression of cell surface receptor CD69, abolishment of T cell proliferation, and decreased expression of T lymphokines after stimulation by either anti-CD3 plus anti-CD28 or phorbol-12-myristate-13-acetate and ionomycin. Differentiation of Th1 and Th2 CD4+ effector lymphocytes by T cell receptor stimulation was blocked in lal−/− mice. The ratio of CD4+CD25+FoxP3+ Tregs to CD4+ T cells was increased in lal−/− spleens. Bone marrow chimeras demonstrated retardation of T cell development and maturation in lal−/− mice due to defects in T cell precursors. Therefore, LAL, its downstream genes, and lipid mediators all play essential roles in development, homeostasis, and function of T cells. The altered development and function of lal−/− T cells contributes to disease formation in various organs during LAL deficiency.
Lysosomal acid lipase (LAL) is a lysosomal hydrolase that is synthesized in rough endoplasmic reticulum and is cotranslationally glycosylated as it emerges in the endoplasmic reticulum lumen.1 The low-density lipoprotein (LDL) receptor or other receptors on the plasma membranes of various cells can deliver low-density lipoprotein-bound cholesteryl esters and triglycerides to the lysosome. Cholesteryl esters and triglycerides are important components in neutral lipids that can be hydrolyzed by LAL in the lysosome of cells to generate free cholesterol and free fatty acids. Free cholesterol is released from lysosomes, which leads to activation of acyl-CoA:cholesterol acyltransferase and down-regulation of LDL receptor gene expression and 3-hydroxy-3-methyl-glutaryl coenzyme A reductase activity.2 LAL contributes to the homeostatic control of plasma lipoprotein levels and to the prevention of cellular lipid overload in various cells of the arterial wall. Therefore, LAL plays an essential role in modulation of cholesterol metabolism in all cells.
In humans, LAL deficiency produces two human diseases, Wolman’s disease and cholesteryl ester storage disease.2,3 In vivo, lal−/− mice appeared normal at birth, survived into adulthood, and were fertile. However, many organs including the liver, lung, spleen, adrenal glands, and small intestine developed severe pathogenic phenotypes due to elevated levels of cholesteryl ester and triglycerides in adult lal−/− mice.4,5,6 These pathogenic phenotypes resulted from malformation and malfunction of both residential cells and immune cells at the molecular and cellular levels. For example, in the lung, cytokines/chemokines, endopeptidases (eg, matrix metalloproteinases), apoptosis inhibitors, transcription factors, and oncogenes were expressed aberrantly.4,7 The Affymetrix GeneChip Microarray study demonstrated a correlation between gene profile changes and pathogenic progression in the lal−/− lung in an age-dependent manner. Some of these genes are downstream targets for lipid mediators (eg, peroxisome proliferator-activated receptor gamma), which can be activated by neutral lipid metabolic derivatives (hormonal ligands).
Since lal−/− pathogenic phenotypes are highly associated with inflammation in multiple organs, immune cells, including myeloid lineage cells and lymphocyte lineage cells, must contribute significantly to disease formation. Altered differentiation and dysfunction of myeloid lineage cells (macrophages, neutrophils) have been reported previously to contribute to pathogenic progression in lal−/− mice.4,7,8 In these mice, Kupffer cells (liver macrophages), bronchoalveolar macrophages, and intestinal macrophages appeared abnormal (foamy) with neutral lipid accumulation and aberrant cytokine/chemokine secretion.4,5,8 In a cell type-specific rescue experiment, a myeloid-specific doxycycline-inducible transgenic mouse system was generated to specifically express wild-type hLAL in lal−/− myeloid lineage cells under the control of the c-fms promoter. Expression of LAL in myeloid lineage cells corrected myeloid cell abnormalities. As a result, the pathogenic phenotypes in the lung, liver and small intestine of lal−/− mice were partially corrected.8 On the other hand, the function of neutral lipids in lymphocytes, especially in T cells, has not been investigated in lal−/− mice. This report will test the hypothesis that LAL deficiency influences T cell development, homeostasis and function. Limited reports have shown that triacylglycerol accumulation in T cell was associated with T cell apoptosis.9
In this report, development, maturation and function of T cells were systematically examined in lal−/− mice. The blockage of the cholesteryl ester and triglyceride metabolic pathway in lal−/− mice caused disorganization of immune tissues (eg, thymus, spleen), retardation of T cell development in the thymus, reduced T cell numbers and loss of T cell function in responding to T cell receptor (TCR) stimulation. These results support the concept that neutral lipids, their downstream genes and lipid mediators play essential roles in development and function of T cells, which contribute to abnormal inflammatory responses and disease formation in various organs in lal−/− mice.
Materials and Methods
Animal Care
All scientific protocols involving the use of animals have been approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine and followed guidelines established by the Panel on Euthanasia of the American Veterinary Medical Association. Protocols involving the use of recombinant DNA or biohazardous materials have been reviewed by the Biosafety Committee of Indiana University School of Medicine and followed guidelines established by the National Institutes of Health. Animals were housed under Institutional Animal Care and Use Committee-approved conditions in a secured animal facility at Indiana University School of Medicine.
Flow Cytometric Analysis and Cell Staining
The single-cell suspensions from the thymus and spleen of lal+/+ and lal−/− mice were obtained by grinding and filtration through nylon mesh into fluorescence-activated cell sorting (FACS) buffer (PBS, 2% fetal bovine serum, 0.01% sodium azide). Bone marrow cells were flushed from femurs and resuspended in FACS buffer as previously described.10 Peripheral blood mononuclear cells were obtained after red blood cell lysis. Lung single cell suspension was prepared as previously described.11 Briefly, the mouse chest cavity was opened using surgical dissection, and the inferior vena cava and abdominal aorta were clamped. The left atrium was opened by incision, and the right ventricle was infused with 1× PBS to remove any residual blood in the pulmonary vasculature. The lung was cut into small pieces and placed in RPMI 1640 containing 5% fetal bovine serum, collagenase (Sigma-Aldrich, St. Louis, MO), and DNase. After 40 minutes of collagenase digestion at 37°C, lungs were further disrupted by aspiration through an 18-gauge needle. Approximately 1 to 2 × 106 cells from various organs in FACS buffer were stained with isotype control or primary antibodies. The primary antibodies CD4 (GK1.5), CD8 (53–6.7), CD69 (H1.2F3), CD25 (PC61.5), CD44 (IM7), TCRβ (H57–597), FoxP3 (150D/E4), CD90.2 (thy1.2, 53–2.1), anti-B220 (RA3–6B2), and CD11b (M1/70) were purchased from eBiosciences (San Diego, CA). FoxP3 intracellular staining was performed according to the protocol from eBiosciences. Cells were analyzed on a LSRII machine (BD Biosciences, San Jose, CA). Data were analyzed using the BD FACStation Software (BD Biosciences). The total number of positive cells was calculated as gated viable cells. Quadrants were assigned using isotype control mAb. Fluorescein isothiocyanate (FITC)-, phycoerythrin-, phycoerythrin cytochrome- and allophycocyanin-conjugated mAbs with irrelevant specificities were used as isotype control.
Oil Red O Staining
Oil red O staining followed a previously published procedure.4,5 Briefly, frozen tissue sections were prepared from lal+/+ or lal−/− thymus and spleen after a standard cryostat procedure. Tissue section slides were stained with oil red O solution (0.5% in propylene glycol) in a 60°C oven for 10 minutes and placed in 85% propylene glycol for 1 minute. Slides were counterstained in hematoxylin.
Immunohistochemical Staining
The spleen and thymus from lal+/+ and lal−/− mice were washed with PBS and dehydrated by a series of increasing ethanol concentrations, followed by paraffin embedding. Five-μm tissue sections were incubated at 4°C overnight with rat anti-mouse CD3 (145–2C11, 1:500; BD Biosciences Clontech, Mountain View, CA), LAMP-2 (M3/84, 1:400; Santa Cruz Biotechnology, CA) as the primary antibody. Tissue sections were washed and treated with biotinylated secondary antibodies. The signals were detected with a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) to visualize the signals following a procedure recommended by the manufacturer.
Lymphocyte Preparation and Culture
CD4+ and CD8+ T cells from lal+/+ and lal−/− spleen were purified with an antibody-linked magnet-activated cell sorter (Miltenyi Biotec Inc.) according to the manufacturer’s instructions. For TCR stimulation studies, the sorted T cells were cultured in 96-wells coated with anti-CD3 mAb (145–2C11, 2 μg/ml) alone or in combination with anti-CD28 mAb (37.51.1, 5 μg/ml). In other experiments, T cells were cultured in the presence of phorbol-12-myristate-13-acetate (PMA) (10 nmol/L) and ionomycin (1 μg/ml).
T Cell Proliferation Assay
For in vitro proliferation, CD4+ T cells were incubated in PBS containing 1 μmol/L Carboxyfluorescein diacetate succinimidyl diester (CFSE, Molecular Probes, Eugene, Oregon) at 37°C for 15 minutes and then pelleted and resuspended in complete medium for 30 minutes. CD4+ T cells were washed twice in PBS and then cultured in 96-well round-bottom plates in complete medium alone, or with the addition of anti-CD3 plus anti-CD28 mAb. After 3 days, cells were harvested, and stained with allophycocyanin-labeled anti-CD4 mAb (eBiosciences), and analyzed using flow cytometry (BD Biosciences). Analyses were performed on 1 × 105 viable cells gated according to their forward/side scatter profile and CD4 expression. Data were analyzed using CellQuest software (BD Biosciences).
Cytokine Measurement by ELISA
After T cells were cultured in wells coated with anti-CD3 and/or anti-CD28 for 24 to 72 hours, supernatants were harvested. In certain experiment, T cells were cultured in the presence of PMA and ionomycin. Levels of interleukin (IL)-2, IL-4, and interferon (IFN)-γ in the supernatants were measured using DueSet ELISA kits for mouse IL-2, IL-4, and IFN-γ according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Reverse Transcription and Real-Time PCR
CD4+ and CD8+ T cells were sorted from the spleen in lal+/+ and lal−/− mice, and stimulated with plate-bound anti-CD3 mAb (2 μg/ml, clone 145–2C11) plus anti-CD28 mAb (5 μg/ml, clone 37.51.1) for 16 or 40 hours. Total RNA was isolated using Qiagen RNeasy Midi Kit according to the manufacturer’s instruction (Qiagen Inc., Valencia, CA). Total mRNA (750 ng) was reversely transcribed using TaqMan Reverse Transcription Reagent as recommended by the manufacturer (Applied Biosystems, Branchburg, NJ) on GeneAmp PCR system 9700 (Applied Biosystems) with a suggested cycling protocol of 25°C for 10 minutes, 48°C for 30 minutes, and 95°C for 5 minutes. For real-time PCR, a total of 5 μl of cDNA was amplified by a pair of sequence-specific DNA oligonucleotide primers (Table 1) for apoptosis molecules or cytokines in a 50 μl reaction solution containing SYBR Green PCR Master Mix (Applied Biosystems). Reactions were analyzed using the Relative Quantification Assay of 7500 System Sequence Detection Software (Applied Biosystems). The default cycling protocol for this software was 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Dissociation curves were measured to determine the quality for each pair of DNA oligonucleotide primers. All samples were measured in triplicate. Real-time PCR was normalized by glyceraldehyde-3-phosphate dehydrogenase mRNA expression. Fold changes were determined by 2(ddCt), in which ddCT = dCt (lal+/+ sample) − dCt (lal−/− sample). The expression level of lal+/+ samples was set as 1.
Table 1.
Primers for Real-Time PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| mApaf-1 | 5′-CAGTAATGGCGTCTTGTCAGTGA-3′ | 5′-CGTTGATATTGAGTGGCCTGACT-3′ |
| mBak | 5′-CAACCCCGAGATGGACAACT-3′ | 5′-GACCCACCTGACCCAAGATG-3′ |
| mBax | 5′-GGGCCCACCAGCTCTGA-3′ | 5′-TGGATGAAACCCTGTAGCAAAA-3′ |
| mBid | 5′-GGCGTCTGCGTGGTGATT-3′ | 5′-ACAAGCTGTGTAGCTCCAAGCA-3′ |
| mCasp3 | 5′-TGCTTACTCTACAGCACCTGGTTACT-3′ | 5′-TGAACCACGACCCGTCCTT-3′ |
| mCasp6 | 5′-CGGGCAGGTGAAAGTAAAACA-3′ | 5′-GGATCGAACACTTCCCTACTTTTG-3′ |
| mCasp7 | 5′-CCGTCCACAATGACTGCTCTT-3′ | 5′-GGTCCTCCTCAGAGGCTTTTC-3′ |
| mCasp8 | 5′-CGCCAAGAGAACAAGACAGTGA-3′ | 5′-CGAGGTTTGTTCTTCATTTGGTAA-3′ |
| mCasp9 | 5′-AACGACCTGACTGCCAAGAAA-3′ | 5′-GGTTCCGGTGTGCCATCTC-3′ |
| mCideA | 5′-CAGCAGCCTGCAGGAACTTAT-3′ | 5′-ACCAGGCCAGTTGTGATGACT-3′ |
| mFasL | 5′-CGAGGAGTGTGGCCCATT T-3′ | 5′-TCCAGAGGGATGGACCTTGA-3′ |
| mGranzyme B | 5′-AAAGCTGAAGAGTAAGGCCAAGAG-3′ | 5′-CGCCTGGGCAGGTTGAG-3′ |
| mIL-1β | 5′-TTGACGGACCCCAAAAGATG- 3′ | 5′-CAGGACAGCCCAGGTCAAA-3′ |
| mIL-2 | 5′-AAACTAAAGGGCTCTGACAACACA-3′ | 5′-CACCACAGTTGCTGACTCATCA-3′ |
| mIL-4 | 5′-TTGAACGAGGTCACAGGAGAAG-3′ | 5′-AGGACGTTTGGCACATCCA-3′ |
| mIL-6 | 5′-GAGGCTTAATTACACATGTTC-3′ | 5′-TGCCATTGCACAACTCTTTTCT-3′ |
| mIL-10 | 5′-GCTGCGGACTGCCTTCA-3′ | 5′-TCCAGCTGGTCCTTTGTTTGA-3′ |
| mIL-13 | 5′-AGCAACATCACACAAGACCAGACT-3′ | 5′-CCAGGTCCACACTCCATACCA-3′ |
| mIL-16 | 5′-GCTCCCTGCATGGTGACAA-3′ | 5′-CACCCTGTTCTGTCCCTTTGA-3′ |
| mIL-17 | 5′-TCTGTGTCTCTGATGCTGTTGCT-3′ | 5′-CGCTGCTGCCTTCACTGTAG-3′ |
| mIFNγ | 5′-CAAGGCGAAAAAGGATGCAT-3′ | 5′-CTGGACCTGTGGGTTGTTGAC-3′ |
| mTNFα | 5′-CCCCAAAGGGATGAGAAGTTC-3′ | 5′-TGAGGGTCTGGGCCATAGAA-3′ |
| GAPDH | 5′-GGCCATCAAGCCAGAGCTT-3′ | 5′-CCAAACCATCACTGACACTCAGA-3′ |
Annexin V Binding
Dual staining with FITC-annexinV and propidium iodide (PI) was performed to detect cells undergoing apoptosis using an Annexin V-FITC kit (BD Biosciences, Bedford, MA). T cells from different tissues of lal+/+ and lal−/− mice were stained with surface markers and washed twice with PBS. After resuspension of labeled cells in annexin V-binding buffer containing FITC-conjugated annexin V, PI was added into samples and incubated on ice for 10 minutes. Cells were analyzed on LSRII within 1 hour. Viable cells were defined by FITC negative (FITC−) and PI negative (PI−) population. Early apoptotic cells were defined by FITC positive (FITC+) and PI− population. Nonspecific binding was blocked by pre-incubating the cells with rat IgG (10 μg/ml) and anti-FcII/III.
In Vitro Suppression Assay
CD4+CD25+ regulatory T cells (Treg) from lal+/+ and lal−/− mice (CD45.2 mice) were sorted using negative selection with the anti-CD4 MACS system, followed by positive selection with an anti-CD25 MACS column (Miltenyi Biotech, Anburn, CA). CD4+CD25− responder cells from CD45.1+ mice were purified from the spleen and labeled with CFSE (2.5 μmol/L). To assess Treg suppressor activity, freshly isolated Tregs were co-cultured with CD4+CD25− T cells at different ratios in the presence of 5 × 105 irradiated (3000 rad) T cell-depleted autologous splenocytes. Cells were incubated at 37°C with 5% CO2 for 4 days with stimulation of anti-CD3 antibody (2 μg/ml) plus anti-CD28 antibody (2 μg/ml) or PBS (control). Responder cells, gated with CD45.1 antibody staining, were plotted for proliferation analysis. Cell proliferation was measured by CFSE dilution. The acquired FACS data were analyzed with the CellQuest program.
Bone Marrow Chimera
Bone marrow was flushed from femurs and tibias of 8 to 10-week-old donor lal−/− or lal+/+ mice. Mature lymphocytes were depleted from the bone marrow cell preparation by using CD4 and CD8 antibody-linked magnet-activated cell sorting (Miltenyi Biotech, Auburn, CA). These cells were referred to as T cell-depleted bone marrow cells. Three-month old lal−/− or lal+/+ recipient mice were lethally irradiated with 1000 rad of γ-irradiation and rested 1 day before receiving cells. Recipient mice were injected with 2.5 to 5 × 106 T cell-depleted bone marrow cells in 500 μl 1× PBS via tail vein. Reconstituted mice were analyzed 10 weeks later.
T Cell Activation in Vivo with Anti-CD3 mAb
Three-month-old lal+/+ and lal−/− mice were injected i.p. with 20 μg of either control hamster IgG or anti-CD3ε mAb (2C11, BD Bioscience). After 3 hours, spleen cells were isolated from animals as described above and stained with FITC- or phycoerythrin-conjugated anti-CD4 (GK1.5), anti-CD8 (53–6.7), and anti-CD69 (H1.2F3) for flow cytometry analysis.
LDL-Cholesterol Measurement
Blood samples were collected from lal+/+ and lal−/− mice after 3 hours i.p. injection of anti-CD3ε mAb or control hamster IgG. Plasma was isolated by centrifugation at 5000 rpm for 10 minutes at 4°C. LDL-cholesterol was measured using N-GENEOUS LDL-cholesterol reagent (Genzyme, Cambridge, Massachusetts) follow by manufactured protocol. Briefly, 150 μl of Reagent 1 was added to 3 μl of plasma and incubated in room temperature for 10 minutes. Detergent in Reagent 1 solubilized non-LDL lipoprotein particles. Cholesteryl esters released free cholesterol that reacted with cholesterol oxidase to generate hydrogen peroxide. The latter was consumed by horseradish peroxidase in the presence of 4-aminoantipyrine to generate a colorless solution. The addition of Reagent 2 (50 μl) solubilized LDL particles and produced a color proportional to the amount of cholesterol present in the sample. The color was measured at 660 nm. LDL-cholesterol calibrator (Genzyme) (concentration ranged from 175 to 886 mg/dL) was used as standard.
Oxidized LDL-C Determined by TBARS Assay
Lipoprotein (d <1.21) was isolated from lal+/+ and lal−/− mice 3 hours post-injection of anti-CD3 antibody or control IgG. Lipoprotein (50 μg) in final volume of 0.33 ml of 1 × PBS was incubated with TBARS reagent (0.67 ml; 15% trichloroacetic acid, 0.375% thiobarbituric acid, 0.25 N hydrochloric acid) at 95°C for 30 minutes. Samples were centrifuged to remove precipitate (10,000 × g for 15 minutes at room temperature), and read absorbance at 535 nm. Diluted maloaldehyde (0 to 10 nmol) was used as standard.
Statistical Analysis
A paired Student’s t-test or analysis of variance was used to evaluate the significance of the differences. Statistical analysis was performed with SPSS version 11.5, with a value of P < 0.05 regarded as statistically significant.
Results
LAL Deficiency Caused Disorganization of the Thymus
The thymus is divided into two main compartments, the cortex and the medulla. H&E staining revealed that the lal−/− mouse thymic architecture appeared to be disturbed histologically, lacking the distinct cortical and medullary structures (Figure 1A). Oil red O staining identified massive neutral lipid accumulation in lal−/− thymi with age progression. Biochemical measurement showed increased levels of triglycerides and cholesterol in lal−/− thymi at various age groups (Figure 1B). As we reported previously, macrophages are the major cells for lipid storage due to LAL deficiency.4,8 Indeed, Mac3 antibody (a macrophage marker) staining showed massive macrophage infiltration in the lal−/− thymus, which was colocalized in the same area of lipid accumulation (Figure 1C). In general, the size of thymus and mean thymocyte weight were significantly reduced in lal−/− mice compared with age-matched lal+/+ control mice (Table 2). This thymic hypoplasia became more evident with age progression. The ratio between thymus weight versus body weight was severely reduced in lal−/− mice as well. These pathogenic results suggest a correlation between LAL deficiency and T cell development in the thymus of lal−/− mice.
Figure 1.
Pathogenic disorganization of the thymus in lal−/− mice. A: H&E staining and oil red O staining of the thymus in 3-month-old lal+/+ and lal−/− mice. Original magnifications were ×40 and ×200. B: Total cholesterol (both cholesteryl esters and free cholesterol) and triglycerides concentrations in the thymus of lal+/+ and lal−/− mice at different ages. *P < 0.05; **P < 0.01, (n = 3 to 5 mice). C: Macrophage infiltration in the lal−/− thymus by Mac3 antibody immunostaining. Dark dots represent Mac3 positive macrophages. Original magnification was ×200.
Table 2.
Thymus Weight (TW) and TW/BW from lal+/+ and lal−/− Mice*
| Mice | Thymus weight (mg)
|
TW/BW (%)
|
||||
|---|---|---|---|---|---|---|
| 3 mo | 5 mo | 7 mo | 3 mo | 5 mo | 7 mo | |
| Lal+/+ | 66.17 ± 6.38 | 76.67 ± 19.61 | 53.52 ± 12.40 | 0.20 ± 0.02 | 0.26 ± 0.07 | 0.14 ± 0.03 |
| lal−/ − | 39.87 ± 7.00†‡ | 41.63 ± 10.64†‡ | 28.67 ± 3.57†‡ | 0.16 ± 0.03 | 0.14 ± 0.04†‡ | 0.09 ± 0.01†‡ |
TW, thymus weight; BW, body weight; 3 mol/L, 5 mol/L, 7 mol/L: 3, 5, 7-month-old; Shown are combined data from four independent experiments (3 mice per group per experiment).
Significant difference between lal+/+ and lal−/− mice. The P values were determined by student’s t-test. Changes were significant with
P < 0.05 as compared with lal+/+ mice.
LAL is Essential for T Cell Development in the Thymus
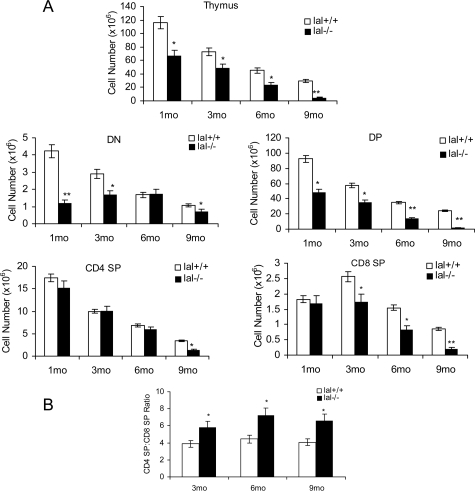
The thymus is the most important organ for T cell development. The total cellular numbers in the lal−/− thymus were significantly reduced compared with those in age-matched lal+/+ mice (Figure 2A). Next, T cells at different developmental stages were evaluated in lal+/+ and lal−/− mice. T cell development in the thymus can be divided into various stages, including CD4−CD8− double-negative (DN), CD4+CD8+ double-positive (DP), and CD4+CD8−/CD4−CD8+ single-positive (SP) stages. T cells in almost all these stages were significantly reduced in lal−/− mice compared with those in lal+/+ mice, except that decrease of CD4 SP cells was only observed at 9 months of age (Figure 2A). At 9 months old, the DP thymocytes were almost absent. Therefore, the blockage of T cell development occurred before the DP stage. Importantly, the CD4:CD8 ratio was significantly increased in the lal−/− thymus (Figure 2B). To determine possible alterations at the earliest stages of intrathymic T cell development, the earliest stages of thymocyte subsets were analyzed as defined by CD44 and CD25 markers, including DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−). The DN subpopulation was gated with CD90.2 for CD44 and CD25 analysis. In 1-month old lal−/− mice, the DN1 (11.25% vs 12.17%), DN2 (5.69% vs 5.35%), and DN3 (67.06% vs 53.02%) subsets were relatively unchanged or higher, whereas the DN4 subset was significantly lower (16.06% vs 29.94%) in the lal−/− thymus compared with those in the lal+/+ thymus (Table 3). This suggests that the blockage of T cell development initially occurred at the DN3 to DN4 transition.
Figure 2.
T cell developmental defect in the lal−/− thymus. A: Total, DN, DP, CD4 SP, and CD8 SP thymocytes were defined by the expression of CD4/CD8 cell markers from the thymus of lal+/+ and lal−/− mice at different ages (1, 3, 6, and 9 months old). B: Comparison of the CD4 SP/CD8 SP ratio in the thymus of lal+/+ and lal−/− mice at different ages (3, 6, and 9 months old). *P < 0.05; **P < 0.01.
Table 3.
Altered Populations of DN Thymocytes in lal−/− Thymus*
| Mice | DN1 | DN2 | DN3 | DN4 |
|---|---|---|---|---|
| lal+/+ | 12.17 ± 5.20 | 5.35 ± 1.78 | 53.02 ± 3.02 | 29.94 ± 2.53 |
| lal−/ − | 11.25 ± 5.79 | 5.69 ± 2.81 | 67.06 ± 8.64† | 16.01 ± 0.85† |
Represents the average percentage numbers of DN thymocytes ± SD in thymus. DN, double-negative T cells. Values are given as average ± SD for 4 independent experiments with multiple 1-month-old lal+/+ and lal−/− mice per group.
P < 0.05 compared with lal+/+ mice.
LAL Was Essential for Peripheral T Cell Maturation
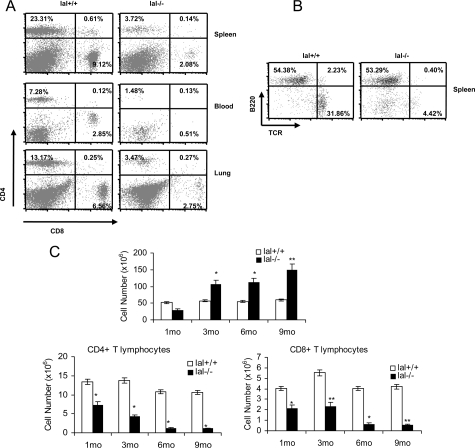
To investigate the intrinsic function of LAL in T cell maturation and proliferation in vivo, we analyzed the number of T cells from various tissues in lal+/+ and lal−/− mice. Compared with lal+/+ mice, CD4+CD8− T cells were reduced in the 3-month lal−/− spleen (from 23.31% to 3.72%), blood (from 7.28% to 1.48%) and lung (from 13.17% to 3.47%) of the gated areas, respectively (Figure 3A). CD4−CD8+ T cells were also reduced in the 3-month lal−/− spleen (from 9.12% to 2.08%), blood (from 2.85% to 0.51%), and lung (from 6.56% to 2.75%) (Figure 3A). In contrast, B220+ B cell numbers were less affected in these organs. An example in the spleen is presented in Figure 3B. Since the spleen is the organ for T cell maturation, an age-dependent study was performed to evaluate progressive maturation of T cells. Both CD4+ and CD8+ T cells were significantly decreased in the 1, 3, 6, and 9 month-old lal−/− spleens, as compared with those in the lal+/+ spleens with age progression, even though the total number of lal−/− splenocytes was increased with age progression (Figure 3C). The cellular architecture of the spleen (white pulp and red pulp) was disorganized or not detected in lal−/− mice. At the cellular level, oil red O staining identified massive neutral lipid accumulation surrounding CD3+ T cells in the lal−/− spleen (Figure 4A). Biochemical measurement showed increased levels of triglycerides and cholesterol in the spleen at various age groups (Figure 4B). Similar to the thymus, Mac3 antibody staining showed massive macrophage infiltration in the lal−/− spleen, which was colocalized in the same area of lipid accumulation (Figure 4C). This has been confirmed by flow cytometry analysis (Figure 4D).
Figure 3.
Defective peripheral T cell maturation in lal−/− mice. A: CD4+ and CD8+ profiles of the spleen, blood and lung in 3 month-old lal+/+ and lal−/− mice. B: B220+ and CD3+ profiles of the spleen in 3 month-old lal+/+ and lal−/− mice. C: Numbers of total, CD4+ and CD8+ T cells from the spleen in lal+/+ and lal−/− mice at different ages (1, 3, 6, and 9 months old). Total cellularity was determined by using a hemocytometer and trypan blue exclusion of dead cells. Absolute numbers of cells in each T cell subset were determined by multiplying the total number of cells × % live gated × % CD4+ or CD8+ subset. Data represented the mean ± SD of at least five independent animals. The significance between lal+/+ and lal−/− mice by Student’s t-test was indicated: *P < 0.05; **P < 0.01.
Figure 4.
Neutral lipid and macrophage accumulation in the spleen of lal−/− mice. A: Oil red O staining of the spleen in 3-month-old lal+/+ and lal−/− mice. Scale bars = 50.0 μm. RP: red pulp; WP: white pulp. B: Total cholesterol (both cholesteryl esters and free cholesterol) and triglycerides concentrations in the spleens of lal+/+ and lal−/− mice at different ages. *P < 0.05; **P < 0.01, (n = 3 to 5 mice). C: Macrophage infiltration in the lal−/− spleen by Mac3 antibody immunostaining. Dark dots represent macrophages. Original magnification was ×200. D: Macrophage increase in the thymus and spleen by flow cytometry with CD11b antibody. Isotypic antibody (shaded area) served as control. Lal+/+: gray line. Lal−/−: dark line.
T Cell Decrease Was Caused by Increased Apoptosis during LAL Deficiency
To evaluate the contribution of apoptosis in T cell decrease, Annexin V staining was performed in the lal−/− thymus and spleen. In the thymus, Annexin V staining of thymocytes was increased at the DN, DP, and SP stages of T cell development in the lal−/− mice (Figure 5A and Table 4). In the spleen, Annexin V staining was also significantly increased in CD4+ and CD8+ T cells in lal−/− mice (Figure 5B). Furthermore, expression of pro-apoptotic signaling molecules including intrinsic molecules (eg, Apaf-1, Bid, Bax, Bak,Casp9), extrinsic molecules (Fas, Casp8), executioner caspases (Casp3, Casp6, and Casp7) and DNA fragmentation enzyme (CideA) in splenic CD4+ and CD8+ T cells of lal−/− mice were all increased by quantitative real-time PCR analysis (Figure 6A and B). These data strongly indicate that LAL deficiency triggers T cell apoptosis through both apoptotic intrinsic and extrinsic pathways.
Figure 5.
Apoptosis increases of T cells in the lal−/− and lal+/+ mice. A: Annexin V-PI staining of apoptotic cells in total thymocytes of 3-month old lal−/− and lal+/+ mice. B: Annexin V staining in combination with CD4 and CD8 expression in the spleen of 1, 3, and 6-month old lal−/− and lal+/+ mice. The increased apoptotic activity of T lineage splenocytes was monitored at various ages. *P < 0.05; **P < 0.01.
Table 4.
Comparison of Annexin V-Positive Cells inlal+/+ and lal−/− Thymus*
| 3 mo
|
6 mo
|
9 mo
|
||||
|---|---|---|---|---|---|---|
| lal+/+ | lal−/ − | lal+/+ | lal−/ − | lal+/+ | lal−/ − | |
| Total cells | 3.64 ± 0.82 | 8.47 ± 1.51† | 3.94 ± 1.73 | 15.39 ± 3.07† | 7.14 ± 1.99 | 35.59 ± 5.63† |
| DN | 5.18 ± 0.98 | 10.80 ± 1.73† | 4.40 ± 0.55 | 17.88 ± 2.76† | 8.34 ± 1.37 | 26.78 ± 4.88† |
| DP | 3.03 ± 0.52 | 9.28 ± 2.05† | 3.18 ± 1.04 | 17.01 ± 1.69† | 7.27 ± 2.31 | 50.72 ± 7.60† |
| CD8 SP | 3.46 ± 1.27 | 4.34 ± 1.32 | 2.17 ± 0.42 | 14.76 ± 1.57† | 4.66 ± 0.87 | 23.29 ± 2.01† |
| CD4 SP | 5.10 ± 1.19 | 6.15 ± 1.34 | 3.46 ± 0.74 | 19.44 ± 2.69† | 7.15 ± 1.85 | 34.81 ± 6.77† |
Thymocytes were stained with CD4, CD8 and annexin V/PI as described in Materials and Methods. Apoptotic cells were defined as Annexin V+PI−. Percentages of apoptotic cells represent annexin V-positive cells from three independent experiments.
P < 0.05 compared with lal+/+ mice.
Figure 6.
Expression of pro-apoptosis molecules in cultured T cells from the lal−/− and lal+/+ spleen. A: Real-time PCR analyses of pro-apoptosis molecules in CD4+ sorted T cells after TCR stimulation for 48 hours. B: Real-time PCR analyses of pro-apoptosis molecules in CD8+ sorted T cells after TCR stimulation for 48 hours. The expression levels of each gene were normalized by the expression level of the glyceraldehyde-3-phosphate dehydrogenase gene. Data were represented as means ± SD from three independent experiments, P < 0.05.
In Vitro Function of T Cells Was Compromised in the lal−/− Spleen
To assess the LAL role in T cell proliferation, we compared lal+/+ and lal−/− splenic T cells in vitro for their CD69 expression on activation by anti-CD3 Ab (at a suboptimal concentration) and anti-CD28 Ab. In a representative in vitro study (Figure 7A), anti-CD3 Ab alone stimulated CD69 expression in T cells to 44.72% in 1-month old lal+/+ mice. In combination with anti-CD28 Abs, CD69 expression was further elevated to 90.93%. However, only 40.25% of T cells expressed CD69 when stimulated with anti-CD3 Ab plus CD28 Ab in age-matched lal−/− mice, a significant drop from lal+/+ mice. Figure 7B is a summary study of three independent in vitro experiments of the CD69 expression assay. In a separate experiment, T cells isolated from lal−/− mice almost completely lost the response to PMA and ionomycin stimulation compared with those isolated from lal+/+ mice (55.17% vs 3.98%), indicating that lal−/− T lymphocytes lacked the response to the protein kinase C and Ca2+ flux signaling during LAL deficiency (Figure 7, A and B, last column). These functional analyses suggest that LAL is essential for T cells to express cell surface proliferating markers such as CD69. To measure T cell proliferation, CD4+ splenic T cells from lal+/+ and lal−/− mice were stimulated with anti-CD3 Ab plus anti-CD28 Ab for 72 hours. T cell proliferation was measured by CFSE labeling dilution. As demonstrated in Figure 7C, TCR stimulated T cell proliferation in lal+/+ mice (represented by multiple peaks), whereas no proliferation was observed in lal−/− mice. In the PBS control group, no proliferation was observed in both lal+/+ and lal−/− mice (data not shown). Thus, LAL deficiency resulted in a profound defect in T cell proliferation and survival.
Figure 7.
Compromised in vitro function of lal−/− T cells. A and B: Purified splenic CD4+T cells from lal+/+ or lal−/− mice were cultured in wells precoated with PBS, anti-CD3 mAb (suboptimal), and anti-CD3 mAb (suboptimal), plus anti-CD28 mAb, or PMA plus ionomycin (iono), as indicated. After 24 hours culturing, the expression of CD69 was assessed by two-color flow cytometry. The percentages represented positive CD69 populations among Thy1.2+ cells after deduction of background staining. The histogram of a representative experiment is presented in (A). Isotype control, gray line; CD69 staining, black line. The summary of 3 independent experiments is presented in (B). *P < 0.05. C: For in vitro proliferation experiments, CFSE-labeled CD4+ T cells were stimulated by plate-bound anti-CD3 (2 μg/ml) + anti-CD28 (5 μg/ml). Cell division of CD4+ T cell subpopulations was measured by CFSE dilution on day 4 by flow cytometric analyses of CD4+ stained cells. Isotype control, gray line; CD69 staining, black line.
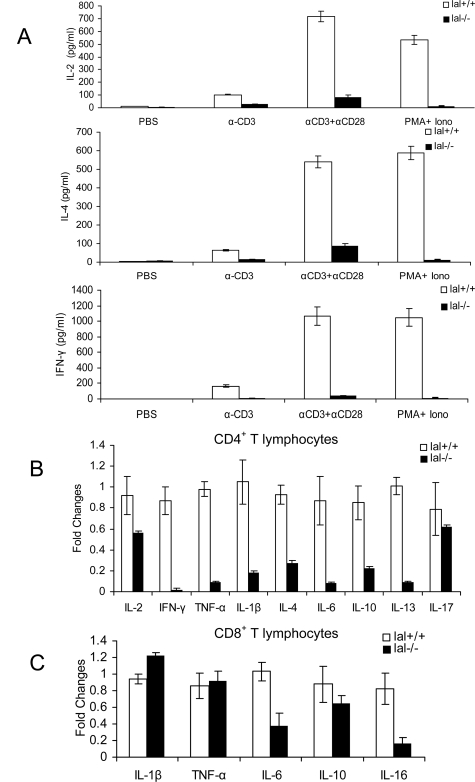
LAL Deficiency Reduced Lymphokine Expression in T Cells
To further evaluate T cell function, secretion of lymphokines by lal+/+ and lal−/− splenic T cells was assessed by ELISA. To mimic the physiological condition of T cell activation, a suboptimal concentration of anti-CD3 along with an optimal concentration of anti-CD28 was used to stimulate IL-2, IL-4, and IFN-γ production by lal+/+ and lal−/− T cells in vitro. Under this condition, lal−/− T cells showed dramatically reduced secretion of IL-2, IL-4, and IFN-γ after TCR activation compared with those by lal+/+ T cells (Figure 8A). In addition, PMA and ionomycin also failed to stimulate lymphokine secretion in lal−/− T cells (Figure 8A). To broaden investigation, expression of multiple lymphokines associated with T helper (Th) cells was measured by real-time PCR using sequence-specific oligonucleotide primers after in vitro stimulation with anti-CD3 plus anti-CD28 antibodies. Compared with lal+/+ controls, CD4+ T splenocytes in the lal−/− mice showed significantly lower mRNA levels of Th1 (IL-1β and TNF-α) and Th2 (IL-4, IL-6, IL-10, and IL-13) cytokines. The expression of Th17 cytokine IL-17 remained relatively unchanged (Figure 8B). CD8+ T splenocytes in lal−/− mice showed significantly lower mRNA levels of IL-6, IL-10, and IL-16, and relatively unchanged mRNA levels of IL-1β and TNF-α (Figure 8C).
Figure 8.
Cytokine expression by splenic CD4+ and CD8+ T cells after TCR stimulation in lal−/− and lal+/+ mice. A: Purified CD4+ T splenocytes from lal+/+ or lal−/− mice were cultured in wells precoated with PBS, anti-CD3 mAb (suboptimal), and anti-CD3 mAb (suboptimal), plus anti-CD28 mAb, or PMA plus ionomycin (iono). After culturing, supernatants were collected after 48 hours. IL-2, IL-4, and IFN-γ in the supernatants were measured by ELISA. All experiments were performed at least three times (n = 3). B: Real-time PCR analyses of Th1, Th2, and Th17 cytokines on CD4+ sorted T splenocyte from lal+/+ and lal−/− mice after stimulation by anti-CD3/anti-CD28 antibodies for 48 hours. C: Real-time PCR analyses of cytokines on CD8+ sorted T splenocyte from lal+/+ and lal−/− mice after stimulation with anti-CD3/anti-CD28 antibodies for 48 hours. The expression levels of each gene were normalized by the expression level of the glyceraldehyde-3-phosphate dehydrogenase gene. Data are represented as means ± SD from three independent experiments.
In Vivo Function of T Cells Was Compromised in the lal−/− Spleen
To see if above observed defect of T cell proliferation and survival in lal−/− mice also take place in vivo, lal+/+ and lal−/− 3-month-old mice were injected with control IgG or anti-CD3 mAb, and analyzed by CD69 expression in CD4 T lymphocytes after isolation. In a representative in vivo study, anti-CD3 Ab stimulated CD69 expression in CD4+ T cells by flow cytometry with 15.62% in lal+/+ mice, as compared with 1.15% in lal−/− mice (Figure 9A). There was no change of CD69 expression in control anti-IgG injected lal+/+ and lal−/− mice (0.05% vs 0.05%). Figure 9B is a summary study of three independent in vivo experiments. Importantly, decreased CD4+ T cell activation was correlated with the increased LDL-cholesterol level and the oxidation level of LDL in serum of lal−/− mice (Figure 9C). Anti-CD3 mAb injection had no effect on LDL-cholesterol and LDL oxidation levels in serum of lal−/− mice. These analyses suggest that LAL and neutral lipids are essential for CD4+ T cell function in vivo.
Figure 9.
Compromised in vivo function of lal−/− T cells. A and B: Splenic CD4+ T cells were isolated from lal+/+ or lal−/− mice that were injected with anti-CD3 mAb, or control mIgG. Expression of CD69 was assessed 3 hours post-injection by two-color flow cytometry. The percentages represent positive CD69 populations among CD4+ T cells. A representative experiment is presented in (A). The summary of three independent experiments is presented in (B). **P < 0.01. C: The LDL-cholesterol and oxidized LDL-cholesterol (oxLDL-C) levels in plasma of lal+/+ and lal−/− mice that were injected with anti-CD3 mAb, or control mIgG. Plasma samples were collected 3 hours post-injection and LDL-cholesterol level were measured. Data are mean ± SD. *P < 0.05. n = 3 to 5.
LAL Deficiency Increased Foxp3+Treg Ratio
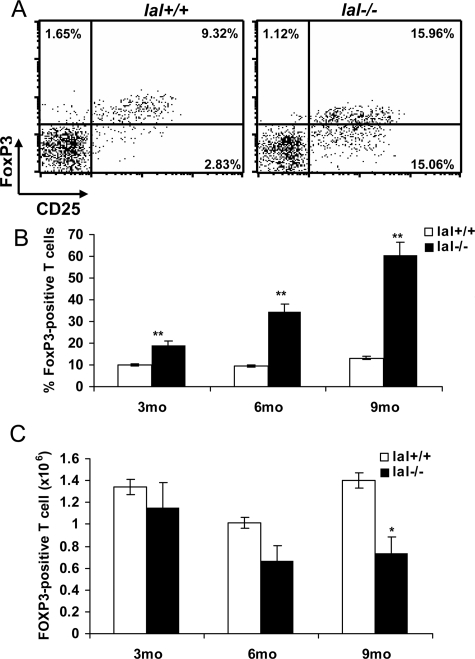
CD4+CD25+ regulatory cells (Tregs) play important roles in maintaining T-cell homeostasis and modulating immune responses by inhibiting effector T cell proliferation.12 Foxp3 is a master control gene for the development and function of natural CD4+CD25+ Tregs, and its expression serves as a specific and stable marker for Tregs. Therefore, FoxP3 expression by CD4+CD25+ Tregs between the lal+/+ and lal−/−spleen was compared. FACS analysis showed an increased percentage number of CD4+CD25+FoxP3+ Tregs in the lal−/− spleen compared with that in the lal+/+ spleen (9.32% vs 15.96%) (Figure 10A). With age progression, the percentage number of CD4+CD25+Foxp3+ cells among total CD4+ T cells was increased to as high as 60% in 9-month old lal−/− mice (Figure 10B). This was largely due to a differential age-associated reduction between CD4+CD25+Foxp3+ Tregs and other T cells (Figure 3C and 10C) in lal−/− mice compared with lal+/+ mice. CD4+CD25+Foxp3+ Tregs decreased less than other types of T cells (especially CD4+CD25− effector T cells) in lal−/− mice, resulting in a relative increase of CD4+CD25+Foxp3+ Tregs percentage in lal−/− T cells. In lal+/+ control, the percentage number of CD4+CD25+Foxp3+ Tregs among total CD4+ T cells remained at approximately 10% throughout age progression. This higher ratio of CD4+CD25+ FoxP3+Tregs to CD4+ T cells in lal−/− mice can be detrimental to the immune responses and contribute to pathogenesis in various organ tissues as we previously reported.4,5
Figure 10.
Treg development in lal−/− mice. A: The FoxP3 and CD25 profile of the spleen in 3-month-old lal+/+ and lal−/− mice in a representative experiment. B: The percentage numbers of FoxP3+ Treg among total CD4+ T cells from the spleen of lal−/− and lal+/+ mice at different ages. C: Cell numbers of FoxP3+ Tregs among peripheral CD4+ T splenocytes of lal−/− and lal+/+ mice at different ages. Data were mean ± SD of the results from three independent experiments. *P < 0.05, **P < 0.01, n = 3 to 5.
Suppressive Function of CD4+CD25+Foxp3+ Treg in lal−/− Mice
To determine whether CD4+CD25+ FoxP3+Treg population in lal−/− mice retain the suppressive function, CD4+CD25+ T cells (CD45.2+) from lal+/+ or lal−/− mice were FACS-sorted according to CD4 and CD25 expression, and mixed with CFSE labeled wild-type responding CD4 effector (E) T cells (CD4+CD25−, CD45.1+). In the presence of CD4+CD25+ Treg (S), CD4 effector T cell recovery and proliferation were monitored to evaluate the suppressive activity. At the E:S ratio of 1:1, CD4+CD25+Tregs from both lal+/+ and lal−/− mice inhibited the proliferation of effector T cells in vitro as shown by CFSE labeling (Figure 11A). This inhibitory effect was clearly dose-dependent (Figure 11B). This study suggests that LAL deficiency has little effect on CD4+CD25+ Treg suppressive function. In an additional study, CFSE-labeled CD4+CD25− effector T cells from lal−/− mice were unable to survive in vitro culturing conditions regardless addition of CD4+CD25+ Treg, suggesting that LAL plays an essential role for CD4+CD25− effector T cell survival.
Figure 11.
Suppressive function of CD4+CD25+ FoxP3+ Treg. A: A representative experiment of the CFSE profile of lal+/+ CD4 effector T cells in the absence (NO Tregs) or presence of lal+/+ (lal+/+ Treg), or lal−/− (lal−/− Treg) CD4+CD25+ Tregs. The ratio of Tregs (S):effector cells (E) was 1:1. B: Suppression of CD4 effector T cells by CD4+CD25+ Tregs at various S:E ratios (0:1, 1:25, 1:5, and 1:1). Data are mean ± SD of the results from three independent experiments.
T Cell Precursor Defect in lal−/− Mice
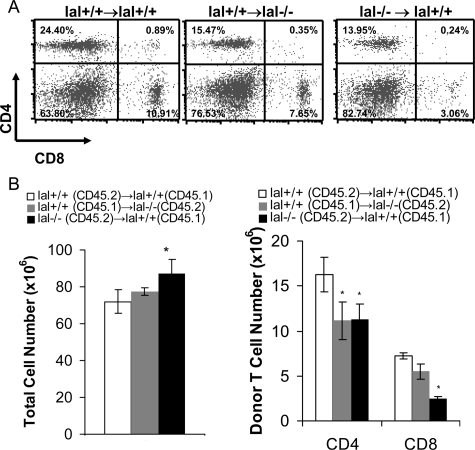
To determine whether the retardation of T cell development in lal−/− mice is due to the defects in T cell precursors, T cell development in bone marrow chimeras was examined. Bone marrow cells from lal−/− and lal+/+ mice (both CD45.2+) were transplanted into lal+/+ or lal−/− recipients (CD45.1+) that were lethally irradiated to generate bone marrow chimeric mice. Recipient cells were excluded from analysis by gating on CD45.2+ cells that were derived from donor bone marrow cells by flow cytometry. The analysis of DN thymocyte subpopulations in lal−/− bone marrow-transplanted recipient mice again revealed that the DN1 and DN2 subsets were relatively unchanged, the DN3 subset (77.83% ± 4.26% vs 51.7% ± 9.06%) is higher, whereas the DN4 (16.01% ± 3.06% vs 43.95% ± 7.66%) subset was significantly lower in the thymus of lal−/− bone marrow-transplanted recipient mice compared with those in lal+/+ bone marrow-transplanted recipient mice (Figure 12A). Total thymic cellularity and donor thymocytes (Figure 12C) were drastically reduced in lal+/+ recipient mice that were transplanted with lal−/− bone marrow cells with significant increase of the CD4SP/CD8SP ratio (Figure 12, B and D). These observations were similar to that listed in Table 3 and Figure 2. Interestingly, the donor CD4SP/CD8SP ratio in lal−/− recipient mice that were transplanted with lal+/+ bone marrow cells was also increased, suggesting that the microenvironment in the thymus of lal−/− mice also has defect that determines T cell development. In the spleen, donor CD4+ and CD8+ T cell numbers were significantly decreased despite total cell number was increased in lal−/− bone marrow-transplanted lal+/+ recipient mice (Figure 13 A and B), similar to that observed in the lal−/− spleen (Figure 3, A and C). Since a similar result was observed in lal−/− recipient mice that were transplanted with lal+/+ bone marrow cells, it suggests that the microenvironment in the spleen of lal−/− mice determines T cell maturation as well. Together, these bone marrow chimeric results suggest defects of T cell precursors and microenvironment (cells responsible for the T cell selection) in lal−/− mice.
Figure 12.
Thymocyte defect in bone marrow chimeric mice. A: Altered populations of DN1, DN2, DN3, and DN4 thymocytes in lal−/− and lal+/+ bone marrow-transplanted recipient thymus. B: Altered populations of CD4+/CD8+ thymocytes in lal−/− and lal+/+ bone marrow-transplanted recipient thymus. C: Total, donor DP thymocytes in lal−/− and lal+/+ bone marrow-transplanted recipient thymus. D: Comparison of the CD4 SP/CD8 SP ratio in lal−/− and lal+/+ bone marrow-transplanted recipient thymus. *P < 0.05, n = 3.
Figure 13.
Splenic T cell defect in bone marrow chimeric mice. A: Altered populations of CD4+/CD8+ T cells in lal−/− and lal+/+ bone marrow-transplanted recipient spleens. B: The numbers of total T cells and donor CD4+ and CD8+ T cells in lal−/− and lal+/+ bone marrow-transplanted recipient spleens; *P < 0.05, n = 3.
Discussion
Previously, we reported that LAL deficiency caused severe tissue inflammation and damage in multiple organs. These observations made us speculating that there was a systemic malformation and malfunction in lal−/− immune cells. While the functional role of LAL in myeloid cells have been reported previously,4,7,8 this report emphasizes characterizing the LAL functional roles in development, maturation, homeostasis, and function of T cells. The thymus and spleen are important immune organs for T cell development and maturation. Histological analyses showed abnormal structures in the thymus and spleen of lal−/− mice. This was the first sign for deficiency of T cell production and function. Blockage of the LAL activity was evidenced by massive neutral lipid accumulation as assessed by oil red O staining and biochemical measurement (Figure 1 and 4). This is consistent with our previous observations made in other organs.4,6,8 Further analyses by flow cytometry showed systemic reduction of CD4+ and CD8+ populations in multiple lal−/− organs, including the thymus, spleen, blood, and lung (Figure 2 and 3) and lymph node (data not shown). Therefore, LAL and downstream lipid hormonal derivatives play a key role in maintaining T cell homeostasis.
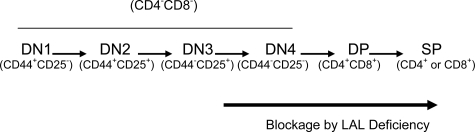
With age progression, the severity of T cell deficiency was getting worse in the lal−/− mice (Figure 3C), suggesting that development and maturation processes for T cells were blocked by LAL deficiency. Since the thymus is the organ for T cell development, T cells at different developmental stages (including DN1, DN2, DN3, DN4, DP, and SP) were investigated. The earliest stage for thymocyte paucity appeared at the DN4 (CD25−CD44−) stage in the lal−/− thymus (Table 3). After this developmental point, thymocytes were declining at all stages as illustrated in Figure 14. One mechanism to explain this reduction is the increased apoptotic activity in thymocytes. Indeed, the increased apoptotic activity was monitored in various thymocyte developmental stages (Figure 5A and Table 4). With age progression, apoptosis in DN and DP thymocytes was steadily increased (Table 4). Therefore, increased apoptosis was likely responsible for the severely reduced thymocyte numbers in the lal−/− thymus.
Figure 14.
LAL deficiency blocks T cell development in the thymus.
In peripheral lymphoid tissues, apoptosis continues to be a factor for reduced T cells. In the spleen, the apoptotic activity by annexin V staining was increased in lal−/− CD4+ and CD8+ T cells compared with those in lal+/+ mice (Figure 5B). A direct link between LAL and various apoptotic pathways has not been reported before. Therefore, it is highly desirable to understand the underlying molecular mechanisms for LAL-induced apoptosis in T cells. By quantitative real-time PCR analysis, mRNA levels of many pro-apoptotic molecules in intrinsic (Apaf-1, Bid, Bax, Bak, Casp9) and extrinsic (Fas, Casp8) pathways were elevated in lal−/− CD4+ and CD8+ T cells in the spleen (Figure 6). It has been well established that LAL downstream metabolites serve as hormonal ligands for various nuclear receptors (eg, peroxisome proliferator-activated receptor gamma), which negatively control gene expression. One mechanism to explain up-regulation of apoptotic genes is that blockage of hormone production in lal−/− mice resulted in inactivation of peroxisome proliferator-activated receptor gamma-negative gene regulation function.
LAL deficiency not only affected development, maturation and homeostasis of T cells in the thymical and peripheral lymphoid organs, but also affected critical T cell functions. T cell activation via CD69 usually results in up-regulation of cytokines (IL-2, TNFα, and IFN-γ), which trigger T cell proliferation.13 We found that after TCR stimulation, numbers of CD69+ T cells in culture were significantly lower (Figure 7A and B) and the T cell proliferative activity was almost lost (Figure 7C) in lal−/− mice. In addition, production of IL-2, IL-4 and IFN-γ in T cells was dramatically lower (Figure 8A). It has been shown that mildly oxidized LDL inhibited the proliferation of cells stimulated by lectins.14 Lal−/− mice has an elevated level of LDL-cholesterol although the oxidative level of LDL in lal−/− mice has not been evaluated before.5 By generation of free intracellular cholesterol, LAL contributes to homeostasis of plasma lipoprotein levels and to prevention of cellular lipid overload in the spleen.12 Thus, one mechanism for LAL deficiency to inhibit the activation of T cells is to disturb cellular lipid and lipoprotein metabolism. This possibility has been proven in Figure 9, in which elevated LDL-cholesterol and LDL oxidation levels are tightly associated with inhibition of the CD4+ T cell activation in vivo. Protein kinase C and Ca2+ influx play a critical role in T cell activation. In addition to interfering with T cell apoptosis, it is possible that LAL deficiency causes T cell defects by blocking this pathway. Indeed, inhibition of T cell proliferation and lymphokine production became more severe after PMA/ionomycin stimulation in lal−/− mice. PMA is an agonist to activate protein kinase C. Ionomycin is a calcium ionophore, which can increase the intracellular calcium content directly. Both compounds are known for T cell proliferation and lymphokine production.15,16 These compounds can bypass the requirement for lectin-induced signal at the onset of cell activation. Furthermore, when CD4+ T cell differentiation was investigated after TCR stimulation, both Th1 and Th2 signature lymphokines were compromised in lal−/− mice (Figure 8B). LAL deficiency could not drive effector T cells into either Th1 or Th2 status compared with lal+/+ mice. Expression of many CD8+ lymphokines was also responded poorly to TCR stimulation in lal−/− mice (Figure 8 C). Therefore, inhibition of T cell proliferation also contributes to the reduced numbers of CD4+ and CD8+ T cells in the lal−/− mice due to reduced production of lymphokines.
Compared with other CD4+CD25− T splenocytes, the FoxP3+ Treg subpopulation was reduced relatively slower in the lal−/− spleen (Figure 10C). This resulted in a steady increase of the ratio of CD4+CD25+FoxP3+ Treg to CD4+ T cells(from 30% to 60% in the lal−/− spleen vs 10% in the lal+/+ spleen) with age progression among the total T cell population (Figure 10, A and B). In vitro functional analysis showed that lal−/− CD4+CD25+FoxP3+ Tregs still possessed suppressive function on CD4 effector T cells (Figure 11). This imbalance of CD4+CD25+FoxP3+ Treg can impede host protective immunity in cancer.17 In the lal−/− lung, hypercellularity is one of the major manifestation of pathogenic phenotypes.4 It is known that Foxp3 in association with other transcription factors (Foxp3/NFAT/Runx1 complex) represses IL-2 expression.12,17
All above T cell malformation and malfunction are resulted from the defects in T cell precursors. This has been proved by the bone marrow transplantation experiment. In this study, lal+/+ recipient mice that were transplanted with lal−/− bone marrow cells showed similar T cell deficiency to those observed in lal−/− mice, including the blockage in DN3 to DN4 transition, reduced total thymic cellularity with increase of the donor CD4SP/CD8SP ratio, and reduced CD4+ and CD8+ T splenocyte population etc. On the other hand, lal−/− recipient mice that were transplanted with lal+/+ bone marrow cells still retained certain T cell defects, such as the higher CD4SP/CD8SP ratio in the thymus and reduced CD4+ and CD8+ T cells in the spleen, suggesting that the microenvironment in lal−/− mice also influence T cell development, maturation, homeostasis, and function.
Taken together, LAL and neutral lipid metabolism are absolutely required for T cell development, maturation, homeostasis, and function. T cells in lal−/− mice lack the ability to make normal contributions to organ function and to prevent the processes of pathogenesis. Further studies are needed to identify which LAL-derived hormonal ligands, nuclear receptors, and downstream genes are required for development, homeostasis, and function of T cells. Characterizing interaction and interference between the LAL pathway and other well-defined pathways for T cell development and activation will facilitate elucidating LAL pathogenesis in future studies, and will significantly enhance our understanding of neutral lipid functions in various disease states.
Acknowledgments
We thank Jennifer Roberts for assisting real-time RT-PCR analysis, Marjorie E. Albrecht and Huimin Li for assisting animal maintenance, tissue collection, and treatment. The authors have no conflicting financial interests.
Footnotes
Address reprint requests to Cong Yan, The Center for Immunobiology, Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, 635 Barnhill Dr., Indianapolis, IN 46202. E-mail: coyan@iupui.edu, or, Hong Du, Division of Human Genetics, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., Cincinnati, OH 45229-3039. E-mail: hong.du@cchmc.org.
Supported by National Institute of Health Grants HL-061803 (C. Yan), HL-067862 (C. Yan and H. Du) and HL087001 (H. Du).
References
- Du H, Witte DP, Grabowski GA. Tissue and cellular specific expression of murine lysosomal acid lipase mRNA and protein. J Lipid Res. 1996;37:937–949. [PubMed] [Google Scholar]
- Assmann G, Seedorf U. Scriber CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzle KW, editors. New York,: McGraw-Hill,; Metabolic and Molecular Bases of Inherited Diseases. 2001:3551–3572. [Google Scholar]
- Aslanidis C, Ries S, Fehringer P, Buchler C, Klima H, Schmitz G. Genetic and biochemical evidence that CESD and Wolman disease are distinguished by residual lysosomal acid lipase activity. Genomics. 1996;33:85–93. doi: 10.1006/geno.1996.0162. [DOI] [PubMed] [Google Scholar]
- Lian X, Yan C, Yang L, Xu Y, Du H. Lysosomal acid lipase deficiency causes respiratory inflammation and destruction in the lung. Am J Physiol Lung Cell Mol Physiol. 2004;286:L801–L807. doi: 10.1152/ajplung.00335.2003. [DOI] [PubMed] [Google Scholar]
- Du H, Heur M, Duanmu M, Grabowski GA, Hui DY, Witte DP, Mishra J. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J Lipid Res. 2001;42:489–500. [PubMed] [Google Scholar]
- Du H, Duanmu M, Witte D, Grabowski GA. Targeted disruption of the mouse lysosomal acid lipase gene: long-term survival with massive cholesteryl ester and triglyceride storage. Hum Mol Genet. 1998;7:1347–1354. doi: 10.1093/hmg/7.9.1347. [DOI] [PubMed] [Google Scholar]
- Lian X, Yan C, Qin Y, Knox L, Li T, Du H. Neutral lipids and peroxisome proliferator-activated receptor-{gamma} control pulmonary gene expression and inflammation-triggered pathogenesis in lysosomal acid lipase knockout mice. Am J Pathol. 2005;167:813–821. doi: 10.1016/s0002-9440(10)62053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Lian X, Li Y, Dai Y, White A, Qin Y, Li H, Hume DA, Du H. Macrophage-specific expression of human lysosomal acid lipase corrects inflammation and pathogenic phenotypes in lal-/- mice. Am J Pathol. 2006;169:916–926. doi: 10.2353/ajpath.2006.051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saffar NM, Titley JC, Robertson D, Clarke PA, Jackson LE, Leach MO, Ronen SM. Apoptosis is associated with triacylglycerol accumulation in Jurkat T-cells. Br J Cancer. 2002;86:963–970. doi: 10.1038/sj.bjc.6600188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Lian X, Dai Y, Wang X, Qu P, White A, Qin Y, Du H. Gene delivery by the hSP-B promoter to lung alveolar type II epithelial cells in LAL-knockout mice through bone marrow mesenchymal stem cells. Gene Ther. 2007;14:1461–1470. doi: 10.1038/sj.gt.3303006. [DOI] [PubMed] [Google Scholar]
- Li Y, Du H, Qin Y, Roberts J, Cummings OW, Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;67:8494–8503. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–310. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- Rutella S, Rumi C, Lucia MB, Barberi T, Puggioni PL, Lai M, Romano A, Cauda R, Leone G. Induction of CD69 antigen on normal CD4+ and CD8+ lymphocyte subsets and its relationship with the phenotype of responding T-cells. Cytometry. 1999;38:95–101. [PubMed] [Google Scholar]
- Caspar-Bauguil S, Saadawi M, Negre-Salvayre A, Thomsen M, Salvayre R, Benoist H. Mildly oxidized low-density lipoproteins suppress the proliferation of activated CD4+ T-lymphocytes and their interleukin 2 receptor expression in vitro. Biochem J. 1998;330 (Pt 2):659–666. doi: 10.1042/bj3300659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger B, Weiss A, Imboden J, Laing T, Stobo JD. The role of protein kinase C in transmembrane signaling by the T cell antigen receptor complex. Effects of stimulation with soluble or immobilized CD3 antibodies. J Immunol. 1987;139:2755–2760. [PubMed] [Google Scholar]
- Stoffel B, Bauer P, Nix M, Deres K, Stoffel W. Ceramide-independent CD28 and TCR signaling but reduced IL-2 secretion in T cells of acid sphingomyelinase-deficient mice. Eur J Immunol. 1998;28:874–880. doi: 10.1002/(SICI)1521-4141(199803)28:03<874::AID-IMMU874>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]