Abstract
The E693Δ mutation within the amyloid precursor protein (APP) has been suggested to cause dementia via the enhanced formation of synaptotoxic amyloid β (Aβ) oligomers. However, this mutation markedly decreases Aβ secretion, implying the existence of an additional mechanism of neuronal dysfunction that is independent of extracellular Aβ. We therefore examined the effects of this mutation on both APP processing to produce Aβ as well as subcellular localization and accumulation of Aβ in transfected HEK293 and COS-7 cells. Both β- and γ-cleavage of mutant APP increased, indicating a lack of inhibition in Aβ production. Instead, this mutation promoted Aβ accumulation within cells, including the endoplasmic reticulum (ER), Golgi apparatus, early and late endosomes, lysosomes, and autophagosomes, all of which have been proposed as intracellular sites of Aβ generation and/or degradation, suggesting impairment of APP/Aβ trafficking. Notably, the intracellular mutant Aβ was found to predominantly form oligomers. Concomitant with this accumulation, the ER stress markers Grp78 and phosphorylated eIF2α were both strongly induced. Furthermore, the activation of caspase-4 and -3 as well as DNA fragmentation were detected in these cells. These results suggest that mutant Aβ induces alteration of Aβ trafficking and subsequent ER stress-induced apoptosis via enhancement of its intracellular oligomerization. Our findings suggest that Aβ oligomers exhibit toxicity in the extracellular space and within the cells themselves.
Soluble oligomers of amyloid β (Aβ) peptide are believed to cause synaptic and cognitive dysfunction in the early stages of Alzheimer’s disease (AD).1,2 Natural low-n Aβ oligomers, such as dimers and trimers, have been shown to inhibit hippocampal long-term potentiation (LTP)3,4 and memory4,5 when injected into rat cerebral ventricle. Synthetic and natural larger-size Aβ oligomers, such as 12-mers termed Aβ-derived diffusible ligands6,7 and Aβ*56,8 have also been demonstrated to inhibit LTP in rat hippocampal slices6 and disrupt memory when administered into rat cerebral ventricle.8 Both low-n oligomers and Aβ-derived diffusible ligands have been shown to induce loss of synapses when applied exogenously in hippocampal slices and neurons.9,10 In addition to direct evidence for the synaptotoxicity of Aβ oligomers, many correlative studies between soluble Aβ and synaptic and cognitive dysfunction have been reported.7,11,12,13,14,15,16 Taken together, these findings have established the so-called oligomer hypothesis that AD begins with synaptic dysfunction caused by diffusible, extracellular Aβ oligomers.
Nevertheless, it is still unclear whether this mechanism is actually responsible for AD in humans. We previously identified an amyloid precursor protein (APP) mutation, E693Δ, in Japanese pedigrees exhibiting AD and Alzheimer’s-like dementia.17 This mutation is located within the Aβ sequence and produces variant Aβ lacking glutamate-22 (E22Δ). Aggregation studies using synthetic peptides demonstrated that the mutant Aβ exhibited a unique property of enhanced oligomerization but no fibrillization. Amyloid imaging of patient’s brains using Pittsburgh compound-B revealed few amyloid plaques. In line with the oligomer hypothesis, this mutant peptide inhibited hippocampal LTP more potently than wild-type peptide when injected into rat cerebral ventricle. In addition, this mutant peptide induced loss of synapses more potently than wild-type peptide in mouse hippocampal slices.18 These findings suggest that the E693Δ mutation causes dementia by enhanced formation of synaptotoxic Aβ oligomers, which may provide genetic validation in humans for the oligomer hypothesis.
However, this mutation caused a marked reduction in Aβ40 and Aβ42 secretion from transfected cells,17 a finding that appears incompatible with a pathological mutation. This observation led us to speculate that the E693Δ mutation may disturb neuronal function not only by forming extracellular Aβ oligomers but also by an additional, intracellular mechanism independent of extracellular Aβ. To test this possibility, we examined the effects of this mutation on APP processing to produce Aβ and on subcellular localization and accumulation of Aβ in transfected cells. The E693Δ mutation exhibited no inhibitory effects on β- and γ-cleavage of the mutant APP, and instead enhanced them. This mutation thus increased Aβ accumulation within cells. Immunocytochemical analyses suggested that the E693Δ mutation affects Aβ trafficking and induces endoplasmic reticulum (ER) stress-mediated apoptosis probably via enhancement of Aβ oligomerization. Such toxic effects of intracellular Aβ are probably not restricted to the E693Δ mutation and appear instead to be a common mechanism by which Aβ oligomers cause neuronal dysfunction.
Materials and Methods
Antibodies
Monoclonal antibodies specific to Aβ42 (11C)19 and to Aβ oligomers (NU-1)20 and polyclonal antibodies to the N-terminal region of Aβ (β001)19 and to the C-terminal region of APP (C40)21 were prepared in our laboratories. A monoclonal antibody, 6E10, to residues 3 to 8 of Aβ (Signet Laboratories, Inc., Dedham, MA), a polyclonal antibody to actin (Sigma-Aldrich, Inc., St Louis, MO), and polyclonal antibodies to organelle markers were purchased, including anti-calnexin antibody (Stressgen Bioreagents Corp., Ann Arbor, MI) for ER, anti-furin antibody (Affinity Bioreagents, Golden, CO) for Golgi apparatus, anti-early endosome antigen-1 (EEA1) antibody (Upstate, Lake Placid, NY) for early endosomes, anti-mannose 6 phosphate receptor (M6PR) antibody (Abcam, Inc., Cambridge, MA) for late endosomes, anti-lysosome-associated membrane protein-2 (LAMP-2) antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for lysosomes, and anti-microtubule-associated protein-1 light chain 3 (LC3) antibody (MBL, Nagoya, Japan) for autophagosomes. A polyclonal antibody to Grp78 (BiP) was obtained from Stressgen Bioreagents Corp., and polyclonal antibodies to the eukaryotic initiation factor 2α subunit (eIF2α) and to phosphorylated eIF2α were from Cell Signaling Technology, Inc. (Beverly, MA). Monoclonal antibodies to caspase-4 (4B9; MBL) and to cleaved caspase-3 (5A1, Cell Signaling Technology) were also purchased.
APP and C99 Constructs
Wild-type human APP695 (APPWT) cDNA was amplified by polymerase chain reaction (PCR) from pooled human cDNA, and cloned into a pCI mammalian expression vector (Promega Corp., Madison, WI) at the NheI and NotI sites. Mutant APP cDNAs with the E693Δ and Swedish (K670N/M671L) mutations (APPE693Δ and APPSW, respectively) were prepared by site-directed mutagenesis and cloned into pCI vector at the same sites. Wild-type and mutant C99 cDNAs were amplified by PCR from these APP constructs. To express C99 on cellular membranes, we prepared a PCR primer overlapping the APP leader sequence (corresponding to the first 17 amino acids of APP) and the N-terminal region of C99. The APP leader sequence-C99 fusion cDNAs were cloned into pCI vector at the NheI and NotI sites. To prepare molecular size markers in Western blotting, C59 and C50 cDNAs were also amplified by PCR from the APPWT construct using PCR primers containing the start codon (ATG), and cloned into pCI vector at the same sites.
Western Blotting to Measure β-Cleavage Products
HEK293 cells were transfected with APPWT and APPE693Δ constructs using the Lipofectamine Plus reagent (Invitrogen Corp., Carlsbad, CA). The cells were cultured overnight in OPTI-MEM I (Gibco BRL, Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS), and media were replaced every day with serum-free OPTI-MEM I containing 1 μmol/L γ-secretase inhibitor L-685,458 (Peptide Institute, Osaka, Japan). Three days after transfection, the conditioned media were harvested and subjected to Aβ enzyme-linked immunosorbent assay (BioSource International, Inc., Camarillo, CA) to confirm that L-685,458 sufficiently inhibited γ-secretase activity. The cells were washed with phosphate-buffered saline (PBS), harvested using a cell scraper, and homogenized by sonication in 1% Triton X-100/Tris-buffered saline (100 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl) containing protease inhibitor cocktail P8340 (Sigma). After agitation at 4°C for 1 hour, the cell homogenates were centrifuged at 1000 × g for 10 minutes at 4°C to remove cell debris and insoluble materials. The supernatants were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 12% NuPage Bis-Tris gels (Invitrogen), and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). APP and its products C-terminal fragment (CTF)α and CTFβ were probed with C40 followed by horseradish peroxidase-labeled anti-rabbit antibody (Bio-Rad Laboratories, Inc., Hercules, CA) and the chemiluminescent substrate ECL Plus (Amersham, GE Health care, Buckinghamshire, UK). Signals were visualized and quantified using a LAS-3000 luminescent image analyzer (Fujifilm, Tokyo, Japan).
Western Blotting to Measure γ-Cleavage Products
HEK293 cells were transfected with C99WT and C99E693Δ constructs. The cells were cultured overnight in 10% FCS/OPTI-MEM I, and the media were replaced with serum-free OPTI-MEM I. Three days after transfection, the cells were harvested and homogenized as described above. After centrifugation at 1000 × g for 10 minutes at 4°C, the supernatants were subjected to SDS-PAGE and transferred to PVDF membranes. For molecular size markers, SDS-PAGE samples were also prepared from C59 and C50 transfectants and loaded on the gels. C99 and its product APP intracellular domain (AICD) were probed with C40 and quantified as described above.
Immunoprecipitation/Western Blotting of Intracellular Aβ
HEK293 cells were transfected with APPWT, APPE693Δ, APPSW, and APPSW/E693Δ constructs. The cells were cultured overnight in 10% FCS/OPTI-MEM I, and media were replaced with serum-free OPTI-MEM I. Two and three days after transfection, the cells were harvested and homogenized as described above. The cell homogenates were centrifuged at 14,000 × g for 15 minutes at 4°C. Aliquots of the supernatants were subjected to SDS-PAGE followed by Western blotting with C40 for quantification of APP levels. The remaining portions of the supernatants were combined into one tube to combine cell extracts from five culture dishes (10 cm diameter). APP and CTFs in the samples were precleared by immunodepletion with C40 and protein A Sepharose (Pharmacia, Piscataway, NJ) at 4°C overnight. Aβ in the samples was then immunoprecipitated with 6E10 and protein A Sepharose at 4°C overnight. The precipitates were washed three times with 1% Triton X-100/Tris-buffered saline, once with Tris-buffered saline, and boiled for 5 minutes in SDS sample buffer to elute Aβ. The eluates were subjected to SDS-PAGE with 12% Bis-Tris gels, and transferred to PVDF membranes. The membranes were boiled in PBS for 10 minutes to enhance signals, and Aβ was probed with β001 and visualized as described above.
Immunocytochemistry
COS-7 cells grown on poly-l-lysine-coated coverslips were transfected with APPWT and APPE693Δ constructs, as described above. The cells were cultured overnight in 10% FCS/OPTI-MEM I, and the media were replaced with serum-free OPTI-MEM I. Two days after transfection, the cells were fixed with 4% paraformaldehyde in PBS at room temperature for 30 minutes and permeabilized with 1% saponin in PBS for 10 minutes. In the experiment on endocytic inhibition, transfected cells were treated with 25 μg/ml of the clathrin-dependent endocytosis inhibitor chlorpromazine (Sigma) and 25 μg/ml of the clathrin-independent endocytosis inhibitor nystatin (Sigma) for 15 minutes at 37°C on day 2, and then fixed. After washing with PBS, the cells were incubated with blocking buffer containing 20% calf serum in PBS overnight at 4°C. The cells were then incubated with the primary antibodies followed by rhodamine (TRITC)- and fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). The primary antibodies included 11C (1:5), NU-1 (1:1000), β001 (1:5000), anti-calnexin antibody (1:300), anti-furin antibody (1:500), anti-EEA1 antibody (1:500), anti-M6PR antibody (1:300), anti-LAMP2 antibody (1:500), anti-LC3 antibody (1:200), anti-Grp78 antibody (1:1000), and anti-phosphorylated eIF2α antibody (1:500). Antibodies were diluted with 10% calf serum in PBS. The stained specimens were mounted with Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA) and examined under a LSM 510 confocal laser microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany).
To compare intracellular Aβ oligomerization between APPWT and APPE693Δ, 10 β001-positive cells were randomly selected from each specimen, and relative fluorescence intensities in regions of interest were quantified using ImageJ software (National Institutes of Health, Bethesda, MD). The ratio of oligomers (NU-1-positive staining) to total Aβ (β001-positive staining) in each cell was calculated from the relative fluorescence intensities with NU-1 and β001.
Western Blotting of eIF2α, Phosphorylated eIF2α, and Caspase-4
COS-7 cells grown in culture dishes (10 cm diameter) were transfected with APPWT and APPE693Δ constructs, as described above. The cells were cultured overnight in 10% FCS/OPTI-MEM I, and media were replaced with serum-free OPTI-MEM I. In some experiments, cells were cultured in serum-free OPTI-MEM I containing 1 μmol/L γ-secretase inhibitor L-685,458. Two days after transfection, cells were harvested and homogenized by sonication in 1% Triton X-100/0.5% sodium deoxycholate/0.1% SDS/Tris-buffered saline containing protease inhibitor cocktail at 4°C. After centrifugation at 1000 × g for 10 minutes at 4°C, the supernatants were subjected to SDS-PAGE and transferred to PVDF membranes. The eIF2α, phosphorylated eIF2α, and caspase-4 were probed with corresponding antibodies. The protein contents of cell lysates were normalized to actin.
Caspase-3 Assay
Activation of caspase-3 was assessed by Western blotting to detect cleaved caspase-3 fragments and enzyme assay to measure caspase-3 activity. COS-7 cells grown in 96-well culture plates (5000 cells/100 μl/well) were transfected with APPWT and APPE693Δ constructs. The cells were cultured overnight in 10% FCS/OPTI-MEM I, and media were replaced with serum-free OPTI-MEM I. Two days after transfection, staurosporine (Sigma) was added to some wells of mock transfection at a concentration of 1 μmol/L and incubated for 4 hours at 37°C to make positive control for apoptosis. For Western blotting, culture media were removed from four wells of each transfectant and SDS sample buffer was directly added to the wells (50 μl/well) to lyse cells. Cell lysates from these wells were combined into one tube, homogenized by sonication, and boiled. The samples were subjected to SDS-PAGE, transferred to PVDF membranes, and probed with an antibody to cleaved caspase-3. The protein contents of cell lysates were normalized to actin. In enzyme assay, we used the Caspase-Glo 3/7 assay kit (Promega). The luminogenic caspase-3/7 substrate/luciferase mixture was added to another four wells (100 μl/well) of each transfectant and incubated for 1 hour at room temperature, which resulted in cell lysis, caspase cleavage of the substrate, and generation of luminescent signal produced by luciferase. Cell lysates were transferred to white-walled 96-well plates and luminescence was measured using a Wallac 1420 ARVO SX multilabel counter (Wallac Oy, Turku, Finland). Values were normalized to the number of cells, which was determined by counting cells grown in the other two wells of culture plates.
Terminal dUTP Nick-End Labeling (TUNEL) Assay
COS-7 cells grown on coverslips were transfected with APPWT and APPE693Δ constructs, as described above. After fixation as described above, the cells were washed twice with PBS and incubated with 50 μl of TUNEL label mix containing TUNEL enzyme (both from Roche Diagnostic GmbH, Mannheim, Germany) for 60 minutes at 37°C. Subsequently the cells were washed three times and stained with NU-1, as described above. The specimens were examined under a confocal microscope. Five fields were randomly selected, and NU-1-positive and TUNEL-/NU-1-positive cells were counted. The experiment was repeated three times, and the mean ratio of TUNEL-/NU-1-positive cells to NU-1-positive cells (∼300 cells in each experiment) was calculated.
Results
Effects of the E693Δ Mutation on β-Cleavage of APP
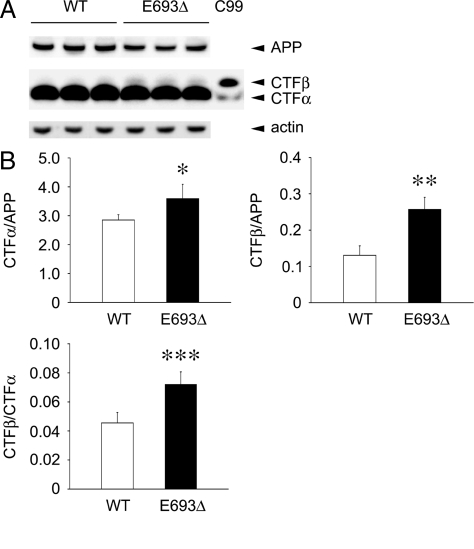
We previously showed that the E693Δ mutation markedly reduced both Aβ40 and Aβ42 secretion from transfected HEK293 cells.17 This reduction may reflect low efficiency of β- and/or γ-cleavage of the mutant APP. To address this question, we studied the effects of this mutation on APP processing to produce Aβ. The β-cleavage of APP was examined by measuring the levels of CTFβ, a β-cleavage product, in HEK293 cells transfected with APPWT and APPE693Δ constructs. To prevent further cleavage of newly generated CTFβ, the cells were cultured in the presence of 1 μmol/L L-685,458, a γ-secretase inhibitor. The levels of Aβ in conditioned media were decreased to levels similar those with mock transfection, indicating that this inhibitor sufficiently inhibited γ-secretase at the concentration used (data not shown). APP and its product CTFs in cell lysates were analyzed by Western blotting with C40, a polyclonal antibody to the C-terminal region of APP. CTFα/APP and CTFβ/APP ratios were both increased by the presence of the E693Δ mutation (Figure 1, A and B). The CTFβ/CTFα ratio was higher in the mutant APP than wild-type APP. Thus, this mutation increased cleavages of the ectodomain of APP, particularly at the β-cleavage site.
Figure 1.
Increased β-cleavage of APP in the presence of E693Δ mutation. HEK293 cells were transfected with APPWT and APPE693Δ constructs and cultured for 3 days in the presence of 1 μmol/L γ-secretase inhibitor L-685,458. A: Cell lysates were subjected to Western blotting with C40, a polyclonal antibody to the C-terminal region of APP. A sample prepared from cells transfected with C99 construct was also loaded on the gels as a molecular size marker. CTFα in the C99 lane was probably generated from transfected C99 and/or endogenous APP by α-secretase. B: Signals for APP, CTFα, and CTFβ were quantified using a LAS-3000 luminescent image analyzer, and CTFα/APP, CTFβ/APP, and CTFβ/CTFα ratios were calculated. The columns and bars represent the means ± SD for five transfectants. *P = 0.0145, **P = 0.0002, and ***P = 0.0007 versus wild-type (WT) by unpaired Student’s t-test. The E693Δ mutation increased both α- and β-cleavage of APP, particularly β-cleavage.
Effects of the E693Δ Mutation on γ-Cleavage of APP
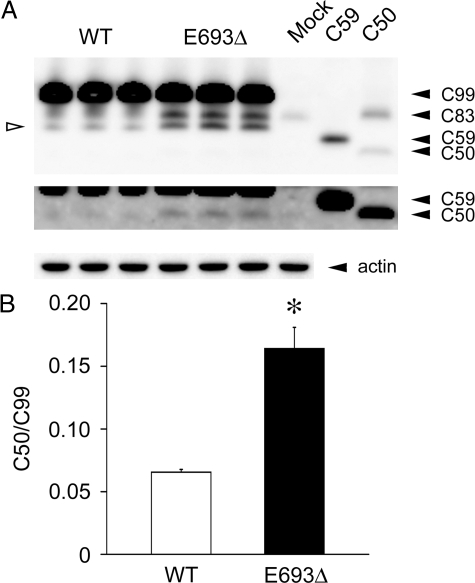
γ-Cleavage of APP was evaluated by measuring the levels of AICD, a γ-cleavage product. For this purpose, we used C99 (equivalent to CTFβ) constructs to avoid effects of β-cleavage. C99 and its product AICD in cell lysates were analyzed by Western blotting with C40. For molecular size markers, samples were also prepared from C59 and C50 transfectants and loaded on the gels. C59 and C50 are thought to be generated from CTFβ by function of γ-secretase at the γ40- and ε-cleavage sites, respectively.22 Again, the C50/C99 ratio was increased by the presence of the mutation (Figure 2, A and B), indicating that this mutation enhanced the γ-cleavage of APP. We could not detect signals corresponding to C59 in this assay. Taken together, these findings showed that the E693Δ mutation did not inhibit Aβ production, and instead increased both β- and γ-cleavage of APP.
Figure 2.
Increased γ-cleavage of C99 in the presence of E693Δ mutation. HEK293 cells were transfected with C99WT and C99E693Δ constructs and cultured for 3 days. A: Cell lysates were subjected to Western blotting with C40. For molecular size markers, samples prepared from cells transfected with C59 and C50 constructs were also loaded on the gels. C83, equivalent to CTFα, was probably generated from transfected C99 and/or endogenous APP. Open arrowhead indicates an unidentified fragment of C99. B: Signals for C99 and C50 were quantified using a LAS-3000, and C50/C99 ratios were calculated. The columns and bars represent the means ± SD for three transfectants. *P = 0.0111 versus wild-type (WT) by unpaired Student’s t-test. The E693Δ mutation increased γ-cleavage of C99. Taken together with the results in Figure 1, this mutation was shown to increase Aβ production.
Effects of the E693Δ Mutation on Intracellular Aβ Accumulation
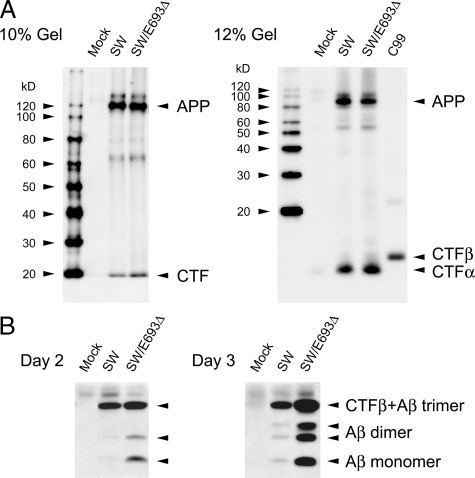
Despite the enhanced processing of the mutant APP to produce Aβ, extracellular Aβ40 and Aβ42 levels were lower in APPE693Δ-transfected cells than APPWT-transfected cells,17 suggesting that this mutation causes increased intracellular accumulation or accelerated degradation of Aβ in the extracellular space. The latter possibility is unlikely because the mutant Aβ was shown to be more resistant to proteolytic degradation.17 We therefore examined the levels of intracellular Aβ in HEK293 cells transfected with APPWT, APPE693Δ, APPSW, and APPSW/E693Δ constructs. Levels of expression of APP were confirmed to be nearly identical among different transfectants (Figure 3A). After preclearing of APP and CTFs from cell lysates with C40, Aβ was immunoprecipitated with the anti-Aβ monoclonal antibody 6E10, and detected by Western blotting with β001, a polyclonal antibody to the N-terminal region of Aβ. Compared with APPSW and APPSW/E693Δ, the mutant Aβ was shown to accumulate more abundantly than wild-type Aβ (Figure 3B). Similar results were obtained with APPWT and APPE693Δ, although the amounts of intracellular Aβ were much lower (data not shown). Notably, the intracellular Aβ appeared to form SDS-stable low-n oligomers, primarily dimers. It is possible that Aβ trimers also accumulated, although we could not distinguish Aβ trimers from CTFβ in this assay, in which CTFβ could not be completely precleared with C40 and could be co-immunoprecipitated with 6E10 and stained with β001. The results obtained suggest that the reduction in Aβ secretion caused by the E693Δ mutation is attributable to increased intracellular accumulation of Aβ.
Figure 3.
Increased intracellular accumulation of the mutant Aβ. HEK293 cells were transfected with APPSW and APPsw/E693Δ constructs and cultured for 2 and 3 days. A: Cell lysates were subjected to Western blotting with C40. Levels of expression of APP were confirmed to be nearly identical among different transfectants. No significant increase or appearance of APP-related fragments other than CTFs was observed in APPsw/E693Δ-transfected cells compared with APPSW-transfected cells. B: Cell lysates from five dishes were combined, and intracellular Aβ was immunoprecipitated with 6E10, an anti-Aβ monoclonal antibody. The eluates from the immunoprecipitates were subjected to Western blotting with β001, a polyclonal antibody to the N-terminal region of Aβ. E693Δ mutation increased intracellular accumulation of Aβ. Notably, the intracellular Aβ appeared to form SDS-stable low-n oligomers, primarily dimers and possibly trimers.
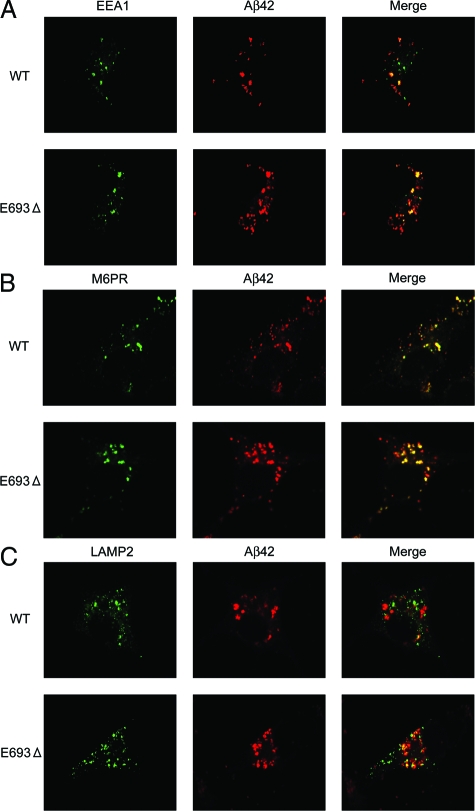
Subcellular Localization and Accumulation of Aβ
We next examined the subcellular localization of Aβ by immunocytochemistry to identify intracellular sites of Aβ accumulation. We used COS-7 cells for this, because the cell bodies of COS-7 cells are larger than those of HEK293 cells, making this cell line more suitable for examination of localization of Aβ. Cells were transfected with APPWT and APPE693Δ constructs and stained with the anti-Aβ42 monoclonal antibody 11C in combination with polyclonal antibodies to organelles, including ER (calnexin), Golgi apparatus (Furin), early endosomes (EEA1), late endosomes (M6PR), lysosomes (LAMP-2), and autophagosomes (LC3), all of which have been suggested to be intracellular sites of Aβ generation and/or degradation.23,24,25,26,27,28 No difference in level of APP expression was observed between APPWT- and APPE693Δ-transfected cells on Western blotting (data not shown). In both APPWT- and APPE693Δ-transfected cells, Aβ immunoreactivities were detected in all organelles tested, with preferential localization in late endosomes. Consistent with our immunoprecipitation/Western blotting results, the mutant Aβ was found to accumulate more abundantly than wild-type Aβ within cells.
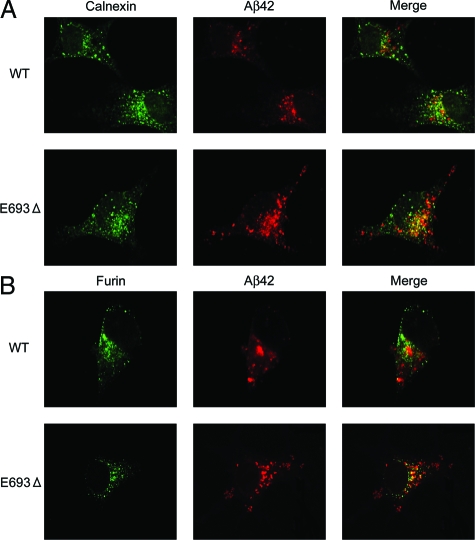
Aβ Accumulation in the Secretory Pathway
Higher accumulation of the mutant Aβ was observed in ER (Figure 4A) and the Golgi apparatus (Figure 4B). These organelles are involved in control of protein folding, modifications, and sorting in the secretory pathway. Despite the increased accumulation of the mutant Aβ in ER and Golgi apparatus, its secretion from cells was markedly reduced,17 implying impairment of APP/Aβ trafficking in this pathway. Reduced trafficking of APP to the plasma membrane has also been suggested in another APP mutation, the Arctic (E693G) mutation, which increased intracellular Aβ levels in transfected cells.29
Figure 4.
Increased accumulation of the mutant Aβ in the secretory pathway. COS-7 cells were transfected with APPWT and APPE693Δ constructs and cultured for 2 days. The cells were fixed with 4% paraformaldehyde in PBS and permeabilized with 1% saponin in PBS. After blocking with 20% calf serum in PBS, the cells were stained with the anti-Aβ42 monoclonal antibody 11C (red) in combination with an anti-calnexin antibody for ER (green) (A) or anti-furin antibody for Golgi apparatus (green) (B). The mutant Aβ was shown to accumulate in ER and Golgi apparatus more abundantly than wild-type (WT) Aβ.
Aβ Accumulation in the Endocytic Pathway
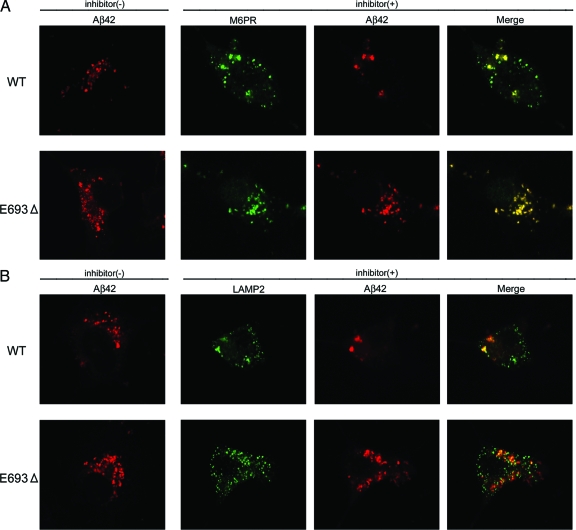
Higher accumulation of the mutant Aβ was also observed in early (Figure 5A) and late endosomes (Figure 5B) and lysosomes (Figure 5C). Of all organelles we tested, Aβ accumulation was most prominent in late endosomes. Enhanced accumulation of the mutant Aβ in endosomes/lysosomes suggests impaired sorting of Aβ in endosomal vesicles to lysosomes, which may have been caused by insufficient degradation of the mutant Aβ in lysosomes. Alternatively, endocytosis of the mutant APP may be increased, as suggested in the Arctic mutation,29 which would result in increased Aβ production in these vesicles. To test the latter possibility, endocytosis was halted by treating cells with the endocytosis inhibitors chlorpromazine and nystatin. Endosomal/lysosomal accumulation of wild-type Aβ was markedly attenuated by this treatment, whereas that of mutant Aβ was not significantly affected (Figure 6, A and B). This result suggests that the mutant Aβ accumulated in these vesicles have been primarily generated via pathways other than endocytosis, such as the secretory and autophagic pathways, and transported into endosomal/lysosomal vesicles beyond their capacity to dispose it. However, we cannot exclude the possibility that the difference in effect of treatment between APPWT- and APPE693Δ-transfected cells may have just reflected the difference in amount of Aβ accumulated intracellularly and that the time of treatment we used (15 minutes) was not enough to clear the mutant Aβ from these vesicles.
Figure 5.
Increased accumulation of the mutant Aβ in the endocytic pathway. COS-7 cells were transfected with APPWT and APPE693Δ constructs and cultured for 2 days. The cells were fixed, permeabilized, and blocked as described in Figure 4, and then stained with 11C (red) in combination with an anti-EEA1 antibody for early endosomes (green) (A), anti-M6PR antibody for late endosomes (green) (B), or anti-LAMP2 antibody for lysosomes (green) (C). The mutant Aβ was shown to accumulate in early and late endosomes and lysosomes more abundantly than wild-type (WT) Aβ.
Figure 6.
Effects of endocytic inhibition on endosomal/lysosomal accumulation of the mutant Aβ. COS-7 cells were transfected with APPWT and APPE693Δ constructs and cultured for 2 days. On day 2, the cells were treated with 25 μg/ml of the clathrin-dependent endocytosis inhibitor chlorpromazine and 25 μg/ml of the clathrin-independent endocytosis inhibitor nystatin for 15 minutes at 37°C. Soon after this treatment, the cells were fixed, permeabilized, and blocked as described in Figure 4, and then stained with 11C (red) in combination with an anti-M6PR antibody (green) (A) or anti-LAMP2 antibody (green) (B). The endosomal/lysosomal accumulation of wild-type (WT) Aβ was markedly attenuated by this treatment, whereas that of the mutant Aβ was not significantly affected.
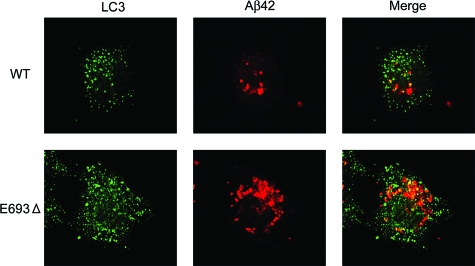
Aβ Accumulation in the Autophagic Pathway
Impairment of APP/Aβ trafficking and abnormal accumulation of Aβ in organelles may elicit the induction of autophagy, by which aged and dysfunctioning organelles are transported to late endosomes and lysosomes to be degraded. In support of this speculation, autophagosomes were much more strongly induced in APPE693Δ-transfected cells (Figure 7). In addition, higher immunoreactivity of the mutant Aβ was observed in these vesicles. Such an activation of the autophagic pathway should provide a certain amount of Aβ to endosomes/lysosomes, which may account for the steady accumulation of Aβ in endosomes/lysosomes in APPE693Δ-transfected cells regardless of inhibition of endocytosis (Figure 6).
Figure 7.
Increased accumulation of mutant Aβ in the autophagic pathway. COS-7 cells were transfected with APPWT and APPE693Δ constructs and cultured for 2 days. The cells were fixed, permeabilized, and blocked as described in Figure 4, and then stained with 11C (red) in combination with an anti-LC3 antibody for autophagosomes (green). Autophagy was substantially induced in APPE693Δ-transfected cells, and higher immunoreactivity of the mutant Aβ was observed in the autophagosomes.
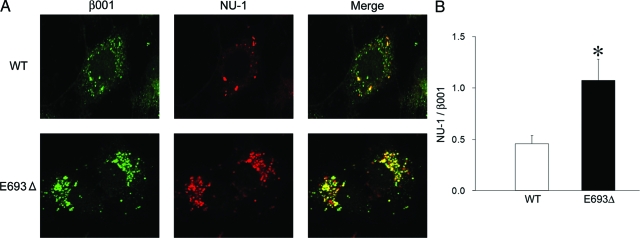
Intracellular Oligomerization of the Mutant Aβ
Although impairment of APP/Aβ trafficking was suggested in APPE693Δ-transfected cells, the cause of such impairment is unclear. We speculated that it was induced by abnormal oligomeric assembly of the mutant Aβ. We therefore examined the oligomerization of intracellular Aβ using a well-characterized anti-oligomer monoclonal antibody, NU-1.20 Notably, the intracellular mutant Aβ predominantly formed oligomers (Figure 8A). The ratio of oligomers (NU-1-positive staining) to total Aβ (β001-positive staining) was higher in APPE693Δ-transfected cells than APPWT-transfected cells (Figure 8B).
Figure 8.
Increased oligomerization of the intracellular mutant Aβ. A: COS-7 cells were transfected with APPWT and APPE693Δ constructs and cultured for 2 days. The cells were fixed, permeabilized, and blocked as described in Figure 4, and then stained with a monoclonal antibody NU-1 specific to Aβ oligomers (red) in combination with the polyclonal antibody β001 to the N-terminus of Aβ (green). B: The ratio of oligomers (NU-1-positive staining) to total Aβ (β001-positive staining) was calculated. The columns and bars represent the means ± SD for 10 transfectants. *P < 0.0001 versus wild-type (WT) by unpaired Student’s t-test. Increased oligomerization was observed in APPE693Δ-transfected cells.
ER Stress by Mutant Aβ
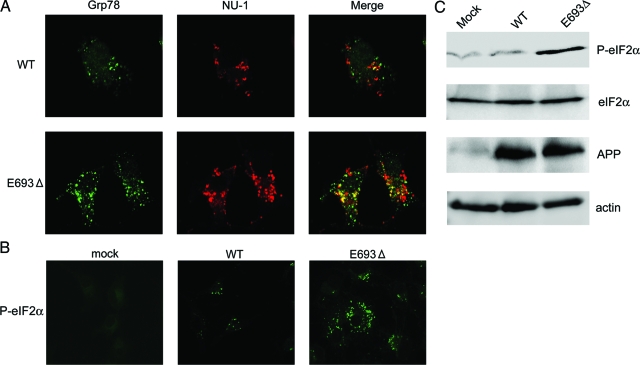
It is known that accumulation of abnormally assembled proteins in ER often induces ER stress in cells.30,31 ER stress has been shown to be associated with neurodegenerative disorders including AD.32 We therefore examined whether ER stress responses are induced in APPE693Δ-transfected cells. Two ER stress markers, Grp78 and phosphorylated eIF2α, were examined. Grp78 (also known as BiP) is an ER resident molecular chaperone that facilitates the proper folding and assembly of membrane-bound and secreted proteins and is up-regulated during ER stress.30,31 Eukaryotic initiation factor 2 (eIF2) plays a role in regulation of translation via its reversible phosphorylation. Phosphorylation of the α subunit of eIF2 immediately reduces the level of functional eIF2 and limits translation initiation events within the cell to down-regulate protein synthesis.30,31 In parallel with the increased accumulation of Aβ oligomers, Grp78 was found to be expressed more abundantly in APPE693Δ-transfected cells (Figure 9A). In addition, phosphorylated eIF2α was highly induced in these cells (Figure 9B), as confirmed on Western blotting for phosphorylated eIF2α (Figure 9C).
Figure 9.
Increased ER stress by the mutant Aβ. COS-7 cells were transfected with APPWT and APPE693Δ constructs and cultured for 2 days. A: The cells were fixed, permeabilized, and blocked as described in Figure 4, and then stained with NU-1 (red) in combination with a polyclonal antibody to Grp78 (green), a molecular chaperone that is up-regulated during ER stress. B: Cells were also stained with an antibody to phosphorylated eIF2α, an ER stress marker. C: Cell lysates were subjected to Western blotting with C40, anti-eIF2α, and anti-phosphorylated eIF2α antibodies. In parallel with the increased accumulation of Aβ oligomers, ER stress was prominently induced in APPE693Δ-transfected cells.
Apoptosis by Mutant Aβ
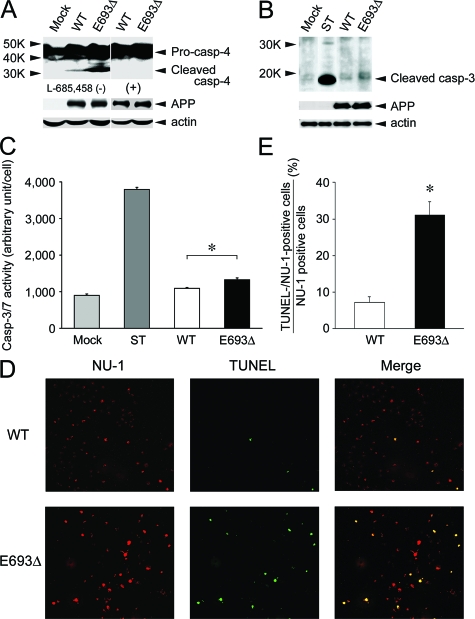
Although the ER stress response provides cells the opportunity to correct the environment within the ER, if the damage is too strong, the response initiates apoptosis.30,31 Caspase-12 is involved in signaling pathway specific to this ER stress-induced apoptosis in mice.31,33 In humans, caspase-4, which was identified as the most homologous gene to mouse caspase-12, has been shown to be specifically activated in ER stress-induced apoptosis.34 The increased ER stress in APPE693Δ-transfected cells led us to examine whether these cells exhibit activation of caspase-4 and undergo apoptosis. Caspase-4 activation was judged by the appearance of cleaved fragments of caspase-4 in Western blotting. Apoptosis was assessed by activation of caspase-3, which was determined by the appearance of cleaved fragments of caspase-3 in Western blotting and by increase in caspase-3 activity in enzyme assay using luminogenic substrate. As another sign of apoptosis, DNA fragmentation was also tested by the TUNEL method. APPE693Δ-transfected cells exhibited higher degrees of caspase-4 activation than APPWT-transfected cells (Figure 10A). These signals were completely abolished by the treatment of cells with 1 μmol/L γ-secretase inhibitor L-685,458 to inhibit Aβ generation, suggesting that the observed ER stress-induced apoptosis was caused by intracellular accumulation of Aβ but not the expression of the mutant APP or its metabolites such as CTFβ. APPE693Δ-transfected cells also demonstrated higher degrees of caspase-3 activation than APPWT-transfected cells in both Western blotting (Figure 10B) and enzyme assay (Figure 10C). Furthermore, DNA fragmentation was induced more potently in APPE693Δ-transfected cells than APPWT-transfected cells. In parallel with the increased accumulation of Aβ oligomers, more abundant TUNEL-positive staining was observed in APPE693Δ-transfected cells (Figure 10D). The TUNEL-/NU-1-positive cell to NU-1-positive cell ratio was higher in APPE693Δ-transfected cells (Figure 10E). We did not observe TUNEL-positive but NU-1-negative cells. Taken together, our findings suggest that the E693Δ mutation causes impairment of Aβ trafficking, ER stress, and apoptosis probably via enhanced formation of intracellular Aβ oligomers.
Figure 10.
Increased apoptosis by the mutant Aβ. COS-7 cells were transfected with APPWT and APPE693Δ constructs and cultured for 2 days. A: Cell lysates were subjected to Western blotting with anti-caspase-4 antibody, in which the appearance of cleaved fragments of caspase-4 represents activation of caspase-4. Higher degrees of caspase-4 activation were observed in APPE693Δ-transfected cells, signals of which were completely abolished by the treatment of cells with 1 μmol/L γ-secretase inhibitor L-685,458. B: Cell lysates were subjected to Western blotting with an antibody to cleaved caspase-3, in which the appearance of the specific bands represents activation of caspase-3. As a positive control for apoptosis, mock-transfected cells were treated with 1 μmol/L staurosporine (ST) for 4 hours at 37°C. Higher degrees of caspase-3 activation were observed in APPE693Δ-transfected cells. C: Caspase-3 activity was measured in cells using the Caspase-Glo 3/7 assay kit, which includes luminogenic substrate for caspase-3/7. Again, higher luminescence was detected in APPE693Δ-transfected cells, indicating increased apoptosis of these cells. The columns and bars represent the means ± SD for four transfectants. *P = 0.0002 by unpaired Student’s t-test. D: Cells were fixed, permeabilized, and blocked as described in Figure 4, and then incubated with TUNEL label mix containing TUNEL enzyme (green). After washing, the cells were stained with NU-1 (red). E: The ratio of TUNEL-/NU-1-positive cells to NU-1-positive cells was calculated. The columns and bars represent the means ± SD for three experiments. *P = 0.0005 versus wild-type (WT) by unpaired Student’s t-test. In parallel with the increased accumulation of Aβ oligomers, stronger TUNEL-positive staining was observed in APPE693Δ-transfected cells, indicating increased DNA fragmentation, another sign of apoptosis, of these cells. Taken together, it was shown that the mutant Aβ causes ER stress-induced apoptosis.
Discussion
In the present study, we examined the effects of the E693Δ mutation on APP processing to produce Aβ and on subcellular localization and accumulation of Aβ in transfected cells. This mutation exhibited no inhibitory effects on β- or γ-cleavage of the mutant APP, and instead enhanced them. Nevertheless, this mutation markedly decreased both Aβ40 and Aβ42 secretion from cells.17 We found that this occurred because the E693Δ mutation increases Aβ accumulation within cells. It is thought that Aβ is generated in several intracellular pathways, in addition to at the plasma membrane.35,36 In the secretory pathway, Aβ is generated in ER and Golgi apparatus and transported to the cell surface to be secreted from cells. In the endocytic pathway, Aβ is generated in endosomes or taken up from the extracellular space and sorted to lysosomes to be degraded, or released from cells by exocytosis or in association with exosomes.37 Lastly, in the autophagic pathway, Aβ is generated in autophagosomes and delivered to late endosomes and lysosomes.28 Increased accumulation of the mutant Aβ was observed in all organelles involved in these pathways, especially in late endosomes. This abnormal accumulation and reduced secretion of Aβ suggest impairment of APP/Aβ trafficking. The increased production and intracellular accumulation of Aβ have also been demonstrated in another APP mutation, the Arctic (E693G) mutation.29 This mutation decreases cell surface expression of APP by reduced trafficking to the plasma membrane and/or increased endocytosis of APP and thereby reduces availability for α-cleavage, resulting in increased extracellular and intracellular levels of Aβ. Although the E693Δ mutation did not increase extracellular Aβ40 and Aβ42 levels, both the Arctic and E693Δ mutations exhibit similar effects on Aβ production and intracellular accumulation. Our immunocytochemical findings revealed that such altered trafficking of APP/Aβ is probably attributable to enhanced intracellular oligomerization of the mutant Aβ.
It is currently believed that Aβ oligomers attack neurons from the extracellular space. Aβ oligomers bound to synapses,38 inhibited hippocampal LTP,3,4,6 disrupted memory,5,8 and caused synapse loss9,10 when applied exogenously in vivo and in vitro. It would be useful to determine whether mutant Aβs have activities similar to those of wild-type Aβ. As we previously reported, the mutant Aβ42 E22Δ peptide potently inhibited hippocampal LTP when injected into rat cerebral ventricle17 and induced dose-dependent loss of synapses in mouse hippocampal slices when added to culture medium.18 These findings led us to speculate that synaptic deficits in patients with the E693Δ mutation are probably caused by extracellular Aβ E22Δ oligomers.
On the other hand, several reports have suggested that synaptic dysfunction and alteration are associated with intraneuronal accumulation of Aβ.39,40,41 In AD brain, Aβ42 immunoreactivity was first detected within neurons in brain regions affected early in AD, preceding both plaque and tangle formation.42 This intraneuronal Aβ42 was predominantly located in multivesicular bodies, a type of endosomal vesicle, within synaptic compartments and was associated with abnormal synaptic morphology.43 Furthermore, the intraneuronal Aβ42 was shown to aggregate into oligomers.44 We also detected intraneuronal Aβ oligomers in AD brain and found that synaptophysin immunoreactivity was absent around neurons bearing Aβ oligomers.45 In the triple transgenic 3xTg-AD mice, synaptic and cognitive dysfunction were shown to correlate with the accumulation of intraneuronal Aβ, which appeared before plaque and tangles.46,47 The intraneuronal Aβ in these mice was also shown to form SDS-stable oligomers in an age-dependent manner.48 Many other studies on patients with AD49 and Down syndrome50,51 and on transgenic mouse models of AD52,53,54,55 including those with the Arctic mutation have demonstrated that intraneuronal accumulation of Aβ is an early pathological change before the onset of amyloid plaque formation, although it is not clear whether those intracellular Aβs form oligomers. In the present study, the E693Δ mutation increased intracellular accumulation of Aβ oligomers and caused ER stress and apoptosis in transfected cells, suggesting that neuronal dysfunction in patients with this mutation may be attributable to intracellular accumulation of Aβ oligomers.
Our findings may provide new insights into the mechanisms underlying the greater virulence of familial AD, which develops early and progresses rapidly. It has been shown that Aβ oligomerization initiates within cells rather than in the extracellular space.3 In familial cases, mutation-induced increase in Aβ production (particularly Aβ42) or acceleration of Aβ aggregation56 would result in more rapid and enhanced oligomerization of Aβ within the cells. Such an increased oligomerization may disturb Aβ trafficking and induce intracellular accumulation of Aβ oligomers, which causes cellular dysfunction. By strongly eliciting these intracellular mechanisms in addition to extracellular mechanisms, familial mutations would presumably lead to early onset and accelerated progression of the disease.
The mechanism by which intracellular Aβ causes neuronal dysfunction is still primarily unclear. It has been suggested that intracellular Aβ disrupts the impermeability of endosomal/lysosomal membranes to induce lysosomal leakage, which results in cell death.57,58,59,60,61 Such membrane disruption may be caused by oligomeric forms of Aβ.44,62,63 It remains to be determined whether the mutant Aβ we isolated causes lysosomal damage via its oligomerization. Another possible mechanism of neuronal dysfunction is ER stress, as proposed in the present study. ER stress is induced when abnormally folded proteins accumulate in the ER beyond the capacity of the ER to correct their conformation.30,31 In such conditions, a cellular response termed the unfolded protein response is activated to protect the cell against the toxic buildup of misfolded proteins. Molecular chaperones, such as Grp78, are up-regulated to assist appropriate refolding of misfolded proteins, and translation initiation factors such as eIF2 are suppressed to halt further protein synthesis. However, when severe and prolonged ER stress extensively impairs ER function, the unfolded protein response ultimately initiates apoptosis.30,31 We previously showed that a missense mutation in cartilage oligomeric matrix protein (COMP) linked to pseudoachondroplasia and multiple epiphyseal dysplasia caused an abnormal accumulation of COMP in ER and subsequent ER stress-induced apoptosis in transfected COS-7 cells.64 In these cells, secretion of the mutant COMP was dramatically decreased. Such toxic effects probably result in degeneration of chondrocytes and skeletal dysplasia in these diseases. In the present study, we demonstrated that the E693Δ mutation increased ER stress and apoptosis in parallel to increased intracellular accumulation of Aβ oligomers. Analogous to the mutation of COMP, the E693Δ mutation may cause degeneration of neurons and dementia by inducing impaired trafficking of the mutant Aβ and subsequent ER stress-mediated apoptosis. It remains to be studied whether Aβ oligomerization affects secretion of other proteins or solely Aβ.
Regarding the molecular sizes of Aβ oligomers, it is unclear which size oligomers, low-n, Aβ-derived diffusible ligand, or Aβ*56, were formed intracellularly to cause ER stress-induced apoptosis. We detected at least dimers on immunoprecipitation/Western blotting analysis, although these dimers may have been derived from larger-size oligomers by boiling the immunoprecipitates in the presence of detergent (SDS). In our immunocytochemical studies, we used NU-1 to detect oligomers, which has been shown to recognize Aβ-derived diffusible ligand in dot blot assay but also to react with trimers and tetramers in Western blotting.20 This issue requires further study.
In summary, we examined the cellular metabolism of APP with or without the E693Δ mutation in transfected cells and showed that this mutation affects Aβ trafficking and causes ER stress-induced apoptosis in transfected cells probably via enhanced Aβ oligomerization. Our findings suggest an additional mechanism of Aβ oligomer-induced neuronal dysfunction, in which Aβ oligomers exhibit toxicity from within the cell.
Acknowledgments
We thank Drs. Takashi Hosono and Cha Gyun Jung, National Center for Geriatrics and Gerontology, Japan, for helpful discussions.
Footnotes
Address reprint requests to Takami Tomiyama, Ph.D., Department of Neuroscience, Osaka City University Graduate School of Medicine, 1-4-3 Asahimachi, Abeno-ku, Osaka 545-8585, Japan. E-mail: tomi@med.osaka-cu.ac.jp.
Supported in part by Ministry of Education, Culture, Sports, Science, and Technology of Japan (grants-in-aid for scientific research on priority areas, research on pathomechanisms of brain disorders, 17300114, 18023033, 20023026, and 20023026).
References
- Klein WL, Krafft GA, Finch CE. Targeting small Aβ oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen W, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer’s disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Sanz Clemente A, Velasco PT, Wood M, Viola KL, Klein WL. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y-M, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE. Water-soluble Aβ (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem. 1996;271:4077–4081. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of Aβ1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Green SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Teraoka R, Mori H, Tomiyama T. Inverse correlation between amyloid precursor protein and synaptic plasticity in transgenic mice. Neuroreport. 2007;18:1083–1087. doi: 10.1097/WNR.0b013e3281e72b18. [DOI] [PubMed] [Google Scholar]
- Tomiyama T, Nagata T, Shimada H, Teraoka R, Fukushima A, Kanemitsu H, Takuma H, Kuwano R, Imagawa M, Ataka S, Wada Y, Yoshioka E, Nishizaki T, Watanabe Y, Mori H. A new amyloid β variant favoring oligomerization in Alzheimer’s-type dementia. Ann Neurol. 2008;63:377–387. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- Takuma H, Teraoka R, Mori H, Tomiyama T. Amyloid β E22Δ variant induces synapse alteration in mouse hippocampal slices. Neuroreport. 2007;19:615–619. doi: 10.1097/WNR.0b013e3282fb78c4. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Ozawa K, Mann DMA, Smith TW, Arawaka S, Mori H. Deposition of β-amyloid subtypes 40 and 42 differentiates dementia with Lewy bodies from Alzheimer disease. Arch Neurol. 1999;56:1111–1118. doi: 10.1001/archneur.56.9.1111. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN, Khuon D, Gong Y, Bigio EH, Shaw P, De Felice FG, Krafft GA, Klein WL. Monoclonal antibodies that target pathological assemblies of Aβ. J Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- Suga K, Tomiyama T, Mori H, Akagawa K. Syntaxin 5 interacts with presenilin holoproteins, but not with their N- or C-terminal fragments, and affects β-amyloid peptide production. Biochem J. 2004;381:619–628. doi: 10.1042/BJ20040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Eggert S, Reinhard FBM, Vogel M, Paliga K, Baier G, Masters CL, Beyreuther K, Evin G. A novel ε-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with Notch processing. Biochemistry. 2002;41:2825–2835. doi: 10.1021/bi015794o. [DOI] [PubMed] [Google Scholar]
- Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992;357:500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL. Evidence that production and release of amyloid β-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- Cook DG, Forman MS, Sung JC, Leight S, Kolson DL, Iwatsubo T, Lee VM, Doms RW. Alzheimer’s Aβ1-42 is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat Med. 1997;3:1021–1023. doi: 10.1038/nm0997-1021. [DOI] [PubMed] [Google Scholar]
- Hartmann T, Bieger SC, Bruhl B, Tienari PJ, Ida N, Allsop D, Roberts GW, Masters CL, Dotti CG, Unsicker K, Beyreuther K. Distinct sites of intracellular production for Alzheimer’s disease Aβ40/42 amyloid peptides. Nat Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- Xu H, Sweeney D, Wang R, Thinakaran G, Lo ACY, Sisodia SS, Greengard P, Gandy S. Generation of Alzheimer β-amyloid protein in the trans-Golgi network in the apparent absence of vesicle formation. Proc Natl Acad Sci USA. 1997;94:3748–3752. doi: 10.1073/pnas.94.8.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Naslund J, Mathews PM, Cataldo AM, Nixon RA. Macroautophagy—a novel β-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin C, Lord A, Magnusson K, Englund H, Almeida CG, Greengard P, Nyberg F, Gouras GK, Lannfelt L, Nilsson LNG. The Arctic Alzheimer mutation favors intracellular amyloid-β production by making amyloid precursor protein less available to α-secretase. J Neurochem. 2007;101:854–862. doi: 10.1111/j.1471-4159.2006.04443.x. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Cataldo AM, Methews PM. The endosomal-lysosomal system of neurons in Alzheimer’s disease pathogenesis: a review. Neurochem Res. 2000;25:1161–1172. doi: 10.1023/a:1007675508413. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Thinakaran G. Amyloidogenic processing of β-amyloid precursor protein in intracellular compartments. Neurology. 2006;66(Suppl 1):S69–S73. doi: 10.1212/01.wnl.0000192107.17175.39. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease β-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci USA. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s-related amyloid β oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Bayer TA. A modified β-amyloid hypothesis: intraneuronal accumulation of the β-amyloid peptide—the first step of a fatal cascade. J Neurochem. 2004;91:513–520. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Aβ accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Aβ42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Almeida CG, Kearney PF, Yu F, Lin MT, Milner TA, Gouras GK. Oligomerization of Alzheimer’s β-amyloid within processes and synapses of cultured neurons and brain. J Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi K, Tomiyama T, Nishitsuji K, Hara M, Mori H. Absence of synaptophysin near cortical neurons containing oligomer Aβ in Alzheimer’s disease brain. J Neurosci Res. 2006;84:632–636. doi: 10.1002/jnr.20952. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Aβ causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL, LaFerla FM. Temporal profile of amyloid-β (Aβ) oligomerization in an in vivo model of Alzheimer disease: a link between Aβ and tau pathology. J Biol Chem. 2006;281:1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- Fernández-Vizarra P, Fernández AP, Castro-Blanco S, Serrano J, Bentura ML, Martínez-Murillo R, Martínez A, Rodrigo J. Intra- and extracellular Aβ and PHF in clinically evaluated cases of Alzheimer’s disease. Histol Histopathol. 2004;19:823–844. doi: 10.14670/HH-19.823. [DOI] [PubMed] [Google Scholar]
- Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC. Intraneuronal Aβ-amyloid precedes development of amyloid plaques in Down syndrome. Arch Pathol Lab Med. 2001;125:489–492. doi: 10.5858/2001-125-0489-IAAPDO. [DOI] [PubMed] [Google Scholar]
- Mori C, Spooner ET, Wisniewsk KE, Wisniewski TM, Yamaguch H, Saido TC, Tolan DR, Selkoe DJ, Lemere CA. Intraneuronal Aβ42 accumulation in Down syndrome brain. Amyloid. 2002;9:88–102. [PubMed] [Google Scholar]
- Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Aβ42 accumulation in a novel Alzheimer transgenic model. Am J Pathol. 2004;165:1289–1300. doi: 10.1016/s0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Eldik LV, Berry R, Vassar R. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord A, Kalimo H, Eckman C, Zhang XQ, Lannfelt L, Nilsson LNG. The Arctic Alzheimer mutation facilitates early intraneuronal Aβ aggregation and senile plaque formation in transgenic mice. Neurobiol Aging. 2006;27:67–77. doi: 10.1016/j.neurobiolaging.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Knobloch M, Konietzko U, Krebs DC, Nitsch RM. Intracellular Aβ and cognitive deficits precede β-amyloid deposition in transgenic arcAβ mice. Neurobiol Aging. 2007;28:1297–1306. doi: 10.1016/j.neurobiolaging.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Murakami K, Irie K, Morimoto A, Ohigashi H, Shindo M, Nagao M, Shimizu T, Shirasawa T. Neurotoxicity and physicochemical properties of Aβ mutant peptides from cerebral amyloid angiopathy. J Biol Chem. 2003;278:46179–46187. doi: 10.1074/jbc.M301874200. [DOI] [PubMed] [Google Scholar]
- Yang AJ, Chandswangbhuvana D, Margol L, Glabe CG. Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Aβ1-42 pathogenesis. J Neurosci Res. 1998;52:691–698. doi: 10.1002/(SICI)1097-4547(19980615)52:6<691::AID-JNR8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ditaranto K, Tekirian TL, Yang AJ. Lysosomal membrane damage in soluble Aβ-mediated cell death in Alzheimer’s disease. Neurobiol Dis. 2001;8:19–31. doi: 10.1006/nbdi.2000.0364. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH. Evidence that neurons accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer’s disease. Histopathology. 2001;38:120–134. doi: 10.1046/j.1365-2559.2001.01082.x. [DOI] [PubMed] [Google Scholar]
- Ji ZS, Miranda RD, Newhouse YM, Weisgraber KH, Huang Y, Mahley RW. Apolipoprotein E4 potentiates amyloid β peptide-induced lysosomal leakage and apoptosis in neuronal cells. J Biol Chem. 2002;277:21821–21828. doi: 10.1074/jbc.M112109200. [DOI] [PubMed] [Google Scholar]
- Ji ZS, Mullendorff K, Cheng IH, Miranda RD, Huang Y, Mahley RW. Reactivity of apolipoprotein E4 and amyloid β peptide: lysosomal stability and neurodegeneration. J Biol Chem. 2006;281:2683–2692. doi: 10.1074/jbc.M506646200. [DOI] [PubMed] [Google Scholar]
- Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding disease. J Biol Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Tomiyama T, Yamano Y, Mori H. Mutation (D472Y) in the type 3 repeat domain of cartilage oligomeric matrix protein affects its early vesicle trafficking in endoplasmic reticulum and induces apoptosis. Am J Pathol. 2003;163:101–110. doi: 10.1016/S0002-9440(10)63634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]