Abstract
Polymyositis is a rare and severe inflammatory muscle disorder. Treatments are partially efficacious but have many side effects. New therapeutic approaches must be first tested in a relevant animal model. Regulatory CD4+CD25+ T cells (Tregs) have been rediscovered as a pivotal cell population in the control of autoimmunity, but the connection between polymyositis and Tregs is currently unknown. To develop a reproducible experimental autoimmune myositis model of polymyositis, mice were immunized once a week for 3 weeks with 1 mg of partially purified myosin emulsified in complete Freund’s adjuvant. All mice injected with myosin and complete Freund’s adjuvant developed myositis. The infiltrates were composed of CD4+ and CD8+ cells, as well as macrophages, but did not contain B lymphocytes. In mice that were depleted of Tregs, the myositis was more severe, as determined by quantitative scoring of muscle inflammation (2.36 ± 0.9 vs. 1.64 ± 0.8, P = 0.019). In contrast, injection of in vitro expanded polyclonal Tregs at the time of immunization significantly improved the disease (quantitative score of inflammation 0.87 ± 1.06 vs. 2.4 ± 0.67, P = 0.047). Transfer of sensitized or CD4+-sorted cells from the lymph nodes of experimental autoimmune myositis mice induced myositis in naïve, irradiated, recipient mice. Thus, experimental autoimmune myositis is a reproducible, transferable disease in mice, both aggravated by Treg depletion and improved by polyclonal Treg injection.
Idiopathic inflammatory myopathies can be classified into six categories based on clinical and immunohistological features1,2,3: (i) dermatomyositis; (ii) polymyositis, (iii) inclusion body myositis, (iv) non-specific myositis2; (v) immune-mediated necrotizing myopathy,2 and (vi) overlap myositis.3 Depending on the classifications,1,2,3 overlap myositis is sometimes also called polymyositis or myositis associated with specific autoantibodies (such as anti-synthetase or anti-signal recognition particle antibodies). Inclusion body myositis is the most frequent myositis in patients >50 years of age and overlap myositis represents two thirds of the remaining cases.3 Polymyositis is very rare and overdiagnosed.4
Patients with polymyositis or overlap myositis present proximal muscle weakness and may also have cardiac and/or pulmonary dysfunctions that result in substantial morbidity and mortality. The general treatment is corticosteroid and immunosuppressive drugs. The obligatory and often severe side effects of these drugs, which have to be taken for many months or years, prompted us to propose an alternative treatment, which first had to be tested in an animal model.
Myositis has been induced previously in guinea pigs, rats, mice, cats, and monkeys with a variety of agents, including syngenic and xenogenic muscle homogenates, purified muscle antigens, viruses (coxsackie virus, influenza, FIV), trypanosomes, drugs, and naked DNA constructs (for review5). The results of these experiments varied, and a generalized, reproducible myositis was rarely observed. Nevertheless, a reproducible inducible rat model (called experimental autoimmune myositis [EAM]) was obtained by immunizing Lewis rats with purified skeletal myosin or C-protein.6,7
Regulatory CD4+CD25+ T cells (Tregs) have been identified as a pivotal cell population in the control of autoimmunity.8 Mice that are rendered deficient for these cells develop multiple T-cell mediated organ specific autoimmune diseases.9 Moreover, administration of Treg to such deficient mice prevents the appearance (or re-appearance) of autoimmune diseases. The best example of the role of natural Treg in humans is a fatal autoimmune disease called IPEX [immune dysregulation (allergy), polyendocrinopathy (type 1 diabetes, thyroiditis), enteropathy (inflammatory bowel diseases), X-linked syndrome], which is due to mutations in the gene FOXP3 encoding for the forkhead/winged-helix transcription factor Scurfin.10 CD4+CD25+ natural Tregs in mice specifically express Foxp3 (the murine ortholog of human FOXP3), and ectopic or transgenic expression of Foxp3 can convert naïve T cells to Tregs that phenotypically and functionally resemble natural CD4+CD25+ Tregs.11 Finally, Foxp3-deficient mice fail to develop CD4+CD25+ Tregs, leading to the occurrence of fatal inflammatory diseases.12 Tregs represent about 10% of the normal CD4 T-cell compartment in mice and humans. To our knowledge, the role of Tregs in myositis has not been analyzed.
Our goals were i) to establish a reproducible EAM model in mice, and ii) to analyze how Tregs regulate myositis in vivo and, in particular, to provide the pre-clinical basis for future Treg therapy.
Materials and Methods
Mice
Six to twelve-week-old female BALB/c or C57Bl6 mice were purchased from Charles River Laboratories or from Iffra Credo (France). All animals were maintained under specific pathogen-free conditions. Mice were manipulated according to European Economic Community guidelines.
Evaluation of Muscle Strength
Muscle strength was evaluated using an inverted screen test as described in the literature.13 A 50-cm2 screen of wire mesh consisting of 12 mm2 of 1 mm diameter wire was used. The mouse was placed in the center of the wire mesh screen. Immediately, the screen was rotated to the inverted horizontal position over 3 s, with the mouse’s head declining first. The screen was held steadily 20 cm above a padded surface. A stop-watch was started and the time at which the mouse fell off was noted. All mice were evaluated independently by one investigator who was blinded to the immunization protocol that had been used.
Preparation of Myosin
Myosin was partially purified according to the method of Perry with few modifications.6 Rabbit or autologous mouse skeletal muscle kept at −80°C was thawed, minced, and weighed. A total of 30 ml of chilled 0.3 M/L KCl–0.15 M/L sodium phosphate buffer (pH 6.5) was added to 10 g of minced muscle tissue and kept on ice for 45 minutes. This homogenate was centrifuged at 15,000 rpm for 30 minutes at 4°C, and the supernatant collected. The filtrate was then diluted with 15 volumes of chilled Milli-Q-filtered (Millipore, France) purified water to precipitate the myosin. The precipitated myosin was collected by centrifugation at 5000 rpm, dissolved in 0.5 M/L KCl, and stored at −80°. For in vitro culture and Western blot analysis, this myosin preparation was solubilized after chymotrypsin digestion.14
Mice Immunization Protocol
Mice were immunized 1, 2, 3, or 4 times, at 1-week intervals, with 100 μl containing 1 mg myosin (or with 100 μl of phosphate-buffered saline [PBS] in control groups) emulsified with an equal volume of adjuvant. Tested adjuvants were complete Freund’s adjuvant (CFA, Sigma), incomplete Freund’s adjuvant (Sigma), Alum, CpG (ODN, Coley Pharmaceutical Group) or PBS alone. These preparations were injected on bilateral sides of the hind foot pads (first immunization), the tail base (second immunization), and flanks (third and fourth immunizations) on a weekly basis. Pertussis toxin (Sigma) was injected intraperitoneally (500 ng in 200 μl saline) during the first immunization. Two weeks after the last immunization, muscle blocks were taken for histology.
Histological Grading of Inflammatory Lesions and Immunohistochemistry
H&E-stained 5-μm paraffin sections of three muscles (gastrocnemius, quadriceps, and triceps) were examined histologically for the presence of mononuclear cell infiltration and necrosis of muscle fibers. For each sample, four groups of serial sections were cut at a 200-μm distance. From each group, one 5-μm-thick section was used for H&E staining. All fields of each section were analyzed. The histological severity of inflammation in each muscle block was graded as follows6,7: grade 1 = involvement of a single muscle fiber or <5 muscle fibers; grade 2 = a lesion involving 5 to 30 muscle fibers; grade 3 = a lesion involving a muscle fasciculus; and grade 4 = diffuse, extensive lesions. When multiple lesions with the same grade were found in a single muscle block, 0.5 points were added to the grade. All slides were evaluated independently by two investigators who were blinded to the immunization protocol that had been used. The differences in evaluation between the two observers were resolved via conference microscopy.
For immunohistological analyses, serial cryosections were fixed with cold acetone and immunostained with rat anti-mouse CD4 (clone RM4-5, Pharmingen), rat anti-mouse CD8b (clone 53-5.8, Pharmingen), rat anti-mouse CD45R/B220 (clone RA3-6B2, Pharmingen), rat anti-mouse CD11b (clone M1-70.15, Pharmingen) monoclonal antibodies (BD Biosciences), and rabbit anti-laminin (Sigma) polyclonal antibodies. AlexaFluor488-conjugated anti-rat (Molecular Probes) and Cy3-conjugated goat anti-rabbit IgG (H+L, Jackson ImmunoResearch) were used as secondary antibodies. For major histocompatibility complex (MHC) class I staining, cryosections were immunostained with biotin anti-mouse H-2Kd (SF1-1.1, Pharmingen) in combination with fluorescein isothiocyanate (FITC)-conjugated-streptavidin. Immunostaining with biotin mouse IgG 2a, κ isotype control was performed. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI) and appeared in blue. The slides were mounted and observed with a Leica TCS-SP(UV) confocal microscope at the MSSM-Microscopy Shared Resource Facility.
Lymphoproliferative Test
Lymphocytes were harvested from lymph nodes and spleen. Splenic dendritic cells were isolated from splenocyte suspensions using magnetic CD11c+ microbeads (N418, Miltenyi Biotec, Germany) and MACS Columns placed in a magnetic field, according to the manufacturer’s protocol. Three × 105 lymphocytes and 6 × 104 dendritic cells pulsed with different concentration of chymotrypsin-digested mouse myosin (2 to 20 μg/ml) per well were added in 96-well, flat bottomed plates. Cells were cultured in complete RPMI-1640 medium supplemented with 10% fetal calf serum for 96 hours at 37°C and 5% CO2, and 1μCi [3H] thymidine was added during the last 18 hours. Thymidine incorporation was measured in a β-scintillation counter (1450 PLUS Wallac). Values are expressed as cpm from triplicate wells. Anti-CD3 antibodies (1 μg/ml, BD bioscience) were used as a positive control.
Western Blot for Anti-Myosin Antibody and Autoantibody Detections
SDS-polyacrylamide gel electrophoresis of myosin, solubilized after chymotrypsin digestion, was performed on a gradient (5% to 15%) acrylamide gel with Broad Range (Bio-Rad) as a size marker. Partially purified digested myosin peptides were then blotted onto a polyvinylidene difluoride membrane, Immobilon (Millipore) using a semidry-transfer system. The transferred membrane was blocked with 5% skimmed milk and incubated with diluted sera (1/8000), followed by detection with horseradish peroxidase-conjugated anti-mouse IgG (H+L) (DAKO) and enhanced chemiluminescence (Amersham Biosciences).
Screening of autoantibodies was performed on sera (1/80 dilution) by indirect immunofluorescence using HEp-2 cells (Immuno Concepts, Sacramento, CA) according to standard clinical procedures, along with positive and negative controls. For detection of autoantibodies against Jo-1, PL-7, PL-12, Mi-2, Ku, and PM-Scl immunoblot assay was performed using the anti-Myositis Antigen kit (EUROLINE-WB, Bio Advance, Bussy Saint Martin) conforming to manufacturer’s protocol. To detect bound antibodies, enzyme-labeled anti-mouse IgG (Sigma) was used in PBS buffer (0.01 M/L, pH 7.4) at a 1:1000 dilution.
Adoptive Transfer of Mononuclear Cells
For transfer of sensitized cells, EAM mice were sacrificed 10 days after the last immunization. Draining lymph nodes (inguinal and popliteal) were collected (so called ex vivo cells). CD4+ or CD8+ lymphocytes were isolated using magnetic CD4+ (Ly-2) or CD8a+ (L3-T4) microbeads (Miltenyi Biotec, Germany) and MACS Columns according to the manufacturer’s protocol. B-cells were isolated from the remaining fraction after CD4+ and CD8+ T-cell sorting. For in vitro restimulated cells, draining lymph node cells (8 × 106/ml) were stimulated in complete RPMI-1640 medium supplemented with 10% fetal calf serum for 6 days at 37°C and 5% CO2, with 20 μg/ml of chymotrypsin-treated mouse myosin in the presence of human recombinant interleukin 2 (100 U/ml, Proleukin, Novartis Pharma). Cells (1 × 106 cells/ml) were then cultured with anti-CD3 and anti-CD28-coated 4.5-μm paramagnetic microbeads using a bead to cell ratio of 1:1 (Invitrogen) for 24 hours. Paramagnetic microbeads were removed before transfer. Before transfer, cells were characterized using fluorescence-activated cell sorting (FACS) analyses. Ex vivo or in vitro restimulated cells were transferred by retro-orbital i.v. injections into naive 9-week-old BALB/c irradiated (4 Gy, total body irradiation, 24 hours before transfer) recipient mice. The control group consisted of transferred unsorted, CD4+, CD8+, and B (ex vivo or in vitro restimulated) cells from PBS-CFA-immunized mice. Recipient mice were sacrificed on day 15 for histopathological analysis.
Adoptive Transfer of Serum
For transfer of serum, EAM mice were sacrificed and bled 10 days after the last immunization. Three hundred μl of serum were injected by retro-orbital i.v. injection into naive 9-week-old BALB/c irradiated (4 Gy, total body irradiation, 24 hours before transfer) recipient mice. The control group consisted of serum transfer from PBS-CFA-immunized mice or normal mice. Recipient mice were sacrificed on day 15 for histopathological analysis.
In Vivo Treg Depletion Protocol
For Treg cell depletion, mice were injected i.p. with 200 μl of saline buffer containing 100 μg of anti-CD25+ antibodies (PC 61 clone) 1 day before the first immunization.15
In Vitro Poly-Treg Production and in vivo Treg Injection Protocol
Treg cells were purified as previously described.15 Briefly, spleen and peripheral lymph node cells were first labeled with biotin-labeled, anti-CD25 monoclonal antibody, (7D4, Becton Dickinson, San Diego, CA) and antibiotin microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and purified using magnetic large cell selection columns (Miltenyi Biotec). CD25+ cells were then stained with FITC-labeled anti-CD4, phycoerythrine-labeled anti-CD62L and streptavidin-Cy-Chrome (Pharmingen, San Diego, CA). The CD4+CD25+CD62Lhigh T cells were sorted by flow cytometry using a FACS Aria (Becton Dickinson), yielding a purity of 99%. CD4+CD25+CD62Lhigh Tregs were cultured in the presence of anti-CD3 and anti-CD28-coated 4.5-μm paramagnetic microbeads (Invitrogen) and human IL-2 (600 U/ml).16 Before transfer, Tregs were phentoypically and functionally characterized using FACS analysis and suppression assay. For Treg injection, 2 ×106 Tregs were transferred by retro-orbital injection the day before each immunization.
Suppression Assays
Splenocytes, harvested from syngenic naive mice, were irradiated (20 Gy) and plated onto 96-well plates (105 cells/well). T effector cells (Teff, lymph node cells harvested from naïve mice) at a constant number (105 cells/well) were then added with a varying number of in vitro expanded Treg cells to provide Teff/Treg ratios of 1:0, 1:1, 2:1, 4:1, and 8:1. A combination of 2 μg/ml of soluble anti-CD3 mAb (145–2C11, PharMingen) provided the polyclonal stimulus for proliferation. Cells were incubated in RPMI with 10% fetal bovine serum in a total volume of 200 μl. At 3 days of culture, 1 μCi of 3H-thymidine (Amersham Biosciences) was added for the final 12 hours to assess proliferation.
Flow Cytometric Analysis
The following antibodies were used for flow cytometry analysis: PE-labeled anti-CD4 (RM4-5, Pharmingen), anti-CD8 (clone 53-6.7), anti-CD19 (clone 1D3, Pharmingen), anti-62L (clone MEL-14, Pharmingen), anti-FoxP3 (FJK16-second, eBioscience), FITC-labeled anti-CD4 (clone RM4-5 Pharmingen), anti-62L (clone MEL-14, Pharmingen), APC-labeled anti-CD25 (clone PC61 Pharmingen), PercP CD4 (clone RM4-5, Pharmingen), and biotinylated anti-CD25 (clone 7D4, Pharmingen) revealed with streptavidin-Cy-Chrome (Pharmingen) and appropriate isotype antibody controls. At least 105 events were acquired on a FACS LSRII (Becton Dickinson) and analyzed using FlowJo software.
Statistical Analysis
Histological scores were compared using the Mann-Whitney U-test. P values less than or equal to 0.05 were considered significant. StatView software (Abacus Concepts) was used for statistical analyses.
Results
Myosin in CFA Injection Creates an Obvious EAM
The muscle strength of BALB/c mice was evaluated 10 days after the last immunization using an inverted screen test.13 Significant loss of strength was observed as the screen test score for EAM was 50 seconds ±20 (n = 10) vs. for control mice 128 seconds ±21 (n = 10), P = 0.016.
Pathological analysis remains the cornerstone to confirm the presence of EAM. Myositis in EAM mice was characterized by both endomysial inflammation (Figure 1 A–C), scattered fibers undergoing necrosis and phagocytosis, or invaded fibers (Figure 1 D) and regenerated fibers (Figure 1E). The incidence of EAM increased significantly with the number of immunizations (table 1). Only one out of four mice developed myositis after the first myosin injection, whereas 82% (9/11; P = 0.038) developed myositis after two immunizations, and 100% (20/20; P < 0.005) after three and four immunizations. In parallel, the degree of muscle inflammation, evaluated by the score of histological inflammation,6,7 increased with the number of immunizations (from 0.5 ± 0.86 after one injection to 2.33 ± 1.10 after four immunizations, P = 0.033, Table 1) ie, going from some single sparse muscle fiber invasion to massive endomysial inflammatory infiltration in some mice (Figure 1 A–C).
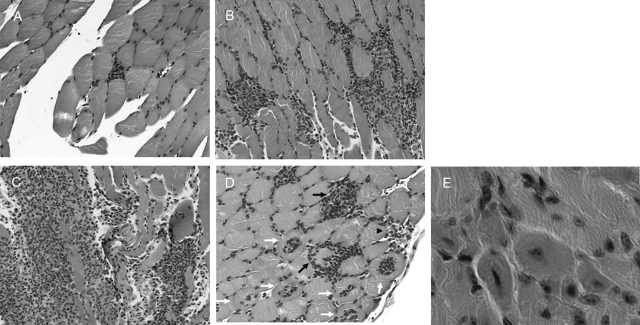
Figure 1.
Cross-sections of Gastrocnemius muscles from mice with EAM (after three immunizations with purified myosin), 10 days after the last immunization. Endomysial inflammatory infiltrate: (A) histological grade 1, (B) grade 2, and (C) grade 4. D: invaded fibers (white arrows), scattered fibers undergoing necrosis and phagocytosis (black arrows) perivascular infiltrate (black arrowhead). E: regenerated fibers. H&E staining.
Table 1.
Incidence and Severity of EAM in Gastrocnemius Muscle of BALB/c Mice after Various Numbers of Immunizations with Partially Purified Myosin
| Number of injections | PBS in CFA Incidence of EAM/n | Rabbit myosin in CFA
|
Mouse myosin in CFA
|
||
|---|---|---|---|---|---|
| Incidence of EAM/n | Histologic score mean ± SD | Incidence of EAM/n | Histologic score mean ± SD | ||
| 1 | 0/4 | 1/4 | 0.5 ± 0.86 | ND | ND |
| 2 | 0/11 | 9/11 | 1.45 ± 0.89 | ND | ND |
| 3 | 0/19 | 13/13 | 1.76 ± 0.79 | 6/6 | 2.33 ± 0.47 |
| 4 | 0/7 | 7/7 | 2.33 ± 1.10 | ND | ND |
ND: not done.
Endomysial muscle lesions in EAM mice consisted of infiltration by mononuclear cells (CD4+ and CD8+) and macrophages (CD11b+, Figure 2A–C). B cells have never been observed in inflammatory infiltrates, but were observed in lymph node sections of the same EAM mice (data not shown).
Figure 2.
Immunohistochemistry of muscular infiltrates on 3 serial sections. Muscle fiber membranes are stained with an anti-laminin antibody in red. Nuclei were counterstaining with DAPI in blue. The cells of the muscular inflammatory infiltrates are stained in green with (A) anti-CD4, (B) anti-CD8, or (C) anti-CD11b (macrophages) antibodies. Arrows highlight the same scattered fiber undergoing necrosis and phagocytosis.
MHC class I overexpression was observed in some non-atrophic or atrophic fibers, always in contact with inflammatory cells, and in regenerated fibers (Figure 3A–F). It was also observed in capillaries and inflammatory cells, as expected (Figure 3). No fluorescence was observed when serial staining was performed with control isotypic antibody (data not shown).
Figure 3.
MHC class I expression in muscle fibers of EAM mice. Muscle fiber membranes are stained with an anti-laminin antibody in red. Nuclei were counterstaining with DAPI in blue. MHC class I immunoreactivity (anti-H2kd) in green was observed on capillaries (arrows A–B), but also on inflammatory cells. A–D: Overexpression of MHC class I was observed on the surface and/or within muscle fibers of EAM mice, in non-atrophic (A–B) or atrophic (C–D) fibers, in contact with inflammatory cells, and in regenerated fibers characterized by centralized nuclei (arrows E–F). Asterisks represent inflammatory cells.
Among the different adjuvants tested (CFA, incomplete Freund’s adjuvant, Alum, CpG) or PBS alone, only CFA triggered myositis (data not shown). Similarly, EAM was induced in BALB/c (Figures 1 and 2) and C57Bl/6 mice (n = 10, data not shown). Two different sources of myosin were tested, from autologous or xenogenic (rabbit) muscle homogenates, with equivalent results (Table 1). Further experiments involving cell transfers and Treg depletion (see below) were then performed using autologous (BALB/c) myosin.
Transfer of the Disease and Myosin-Specific CD4+ T Cell Involvement
EAM could be transferred to irradiated naïve mice by injection of freshly purified unsorted lymph node cells from the EAM mice (pooled cells consisted of 42% CD4+, 20% CD8+ T-cells, and 38% CD19+ B-cells). Indeed, two out of the six recipients developed myositis with a histological grade of 1 and 2 (Table 2), while none of the recipients of cells from control mice (immunized by PBS in CFA) developed myositis (data not shown). In other transfer experiments performed with in vitro re-stimulated unsorted cells (pooled cells consisted of 67% CD4+ and 29% CD8+ T-cells), seven out of nine injected mice developed a myositis (Table 2). As shown in Figure 4, some of the infiltrates achieved grade 4 and a number of invaded fibers can be seen (Figure 4A). Furthermore, as expected, lymphoproliferative responses of these lymph node cells from EAM mice were also observed (and not from control ones) when they were exposed to progressively increasing doses of the myosin preparation, with a dose effect (Figure 4B). Using in vitro restimulated sorted CD4+ T cells from EAM mice, two out of five recipient mice developed the disease, with a histological grade of 1 and 2.5 (Table 2). In contrast, the CD8+ T cells and B cells could not transfer EAM (Table 2, purity of sorted cells was up to 95%). Finally, none of the eight sera from eight different EAM mice injected into recipient mice generated any detectable muscle infiltrate.
Table 2.
Incidence and Severity of Myositis in Recipient Irradiated Mice after Passive Transfer of Draining Lymph Node Cells from EAM Mice
| In vitro stimulation | Type of cells | Number of injected cells × (106) | Incidence of myositis | Histologic score |
|---|---|---|---|---|
| No | Unsorted cells* | 20 | 2/6 | 1 and 2 |
| No | Sorted CD4+ cells | 10 | 0/5 | 0 |
| No | Sorted CD8+ cells | 10 | 0/3 | 0 |
| No | Sorted B cells | 10 | 0/5 | 0 |
| Yes | Unsorted cells** | 10 | 7/9 | 2.4 ± 1.45 (mean ± SD) |
| Yes | Sorted CD4+ cells | 6 | 2/5 | 1 and 2.5 |
| Yes | Sorted CD8+ cells | 4 | 0/5 | 0 |
| Yes | Sorted B cells | 10 | 0/4 | 0 |
Cells were harvested from draining lymph nodes of EAM mice.
Ex vivo unsorted cells consist of 42% CD4+, 20% CD8+ T-cells and 38% CD19+ B-cells.
In vitro restimulated unsorted cells consist of 67% CD4+ and 29% CD8+ T-cells. CD4+ and CD8+ T-cells were sorted by using magnetic label. B-cells were isolated using the negative fraction after selection of CD4+ and CD8+ T-cells. Purity of sorted cells was up to 95%.
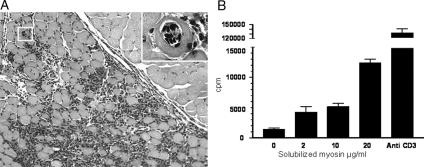
Figure 4.
Transfer of EAM. A: Cross-sections of gastrocnemius muscle were analyzed 10 days after the transfer of 10 × 106 in vitro restimulated cells from EAM mice. Endomysial inflammatory infiltrate: histological grade 4. The inset panel shows high-power magnification of an invaded fiber (boxed area). B: Specific response to myosin of lymph node cells from EAM mice. Cells were in vitro restimulated for 96 hours in the presence of increasing doses of the solubilized myosin preparation. Thymidine incorporation was measured and values are expressed as cpm from triplicate wells. Anti-CD3 antibodies (1 μg/ml, BD bioscience) were used as a positive control.
Autoantibodies in EAM Mice
Although myositis was not transferable by B cells or sera, we also looked for humoral responses against myosin in EAM mice. By Western blotting, the serum from 25 out of 25 tested EAM mice reacted to solubilized myosin (Figure 5A). The signal was detected at up to a 1:8000 serum dilution and corresponds to the electophoretic pattern of chemotrypsin-digested myosin.17 In contrast, the sera of control mice (immunized by PBS in CFA, n = 19) did not react with these extracts (Figure 5A). EAM mice had therefore produced antibodies specific to muscle proteins. Because patients with myositis may have specific autoantibodies, we also looked for their presence in EAM mice. We first screened sera by immunofluorescence on HEp-2 cells. The induction of EAM resulted in detectable antinuclear antibodies (at 1/80 dilution) in only 4 out 16 mice, without any specific pattern evocative of a particular autoantibody, consequently most of the EAM mice did not produce antinuclear antibodies (Figure 5B). Nevertheless, we tested 16 EAM and 18 control mice for the presence of autoantibodies against Jo-1, PL-7, PL-12, Mi-2, Ku, and PM-Scl. None of the EAM mice had positive immunoassay dot blot, and only one control mouse was anti-Jo-1 positive.
Figure 5.
A: Presence of antibodies against myosin in EAM mice. Western blot analyses revealed antibodies against myosin in all 25 EAM mice analyzed (3 representative samples are shown lanes 1–3), whereas they were not observed in 19 control mice immunized with PBS in CFA (3 representative samples are shown lanes 4–6). On the left side molecular weights in kDa are represented. B: Immunofluorescent staining on HEp-2 cells to detect autoantibodies from the sera (1:80 dilution) of EAM and (C) Treg depleted EAM mice. The latter shows a cytoplasmic pattern condensed around the nucleus, which diminishes toward the periphery.
Effect of Tregs in the EAM Model
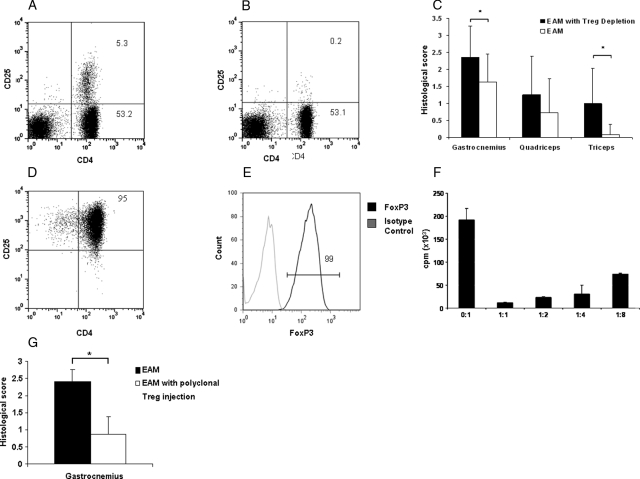
To evaluate the effect of Tregs on the severity of EAM, we first examined the effect of Treg depletion. Twelve mice per group were immunized with or without Treg cell depletion using the PC61 antibody. As we have previously shown,18 the effect of PC61 lasts for at least 30 days and as expected at day 16 after its injection, Treg cells are almost undetectable (Figure 6, A and B). Myositis was studied 10 days after the third immunization. In Treg depleted mice, we observed a significantly increased score of muscular inflammation in the gastrocnemius (2.36 ± 0.9 vs. 1.64 ± 0.8 in non-depleted animals, P = 0.019, Figure 6C). The disease was also more diffuse in Treg-depleted animals compared with non-depleted animals, ie, triceps were more frequently affected (Figure 6C). No histological abnormalities were detected in cardiac muscle, small intestine, spleen, or lungs. As a control, we checked that muscles of Treg depleted mice receiving CFA alone remained free of endomysial inflammatory infiltrates and necrosis.
Figure 6.
Role of Treg in EAM. A: CD4 CD25 expression of lymph node cells in a control and (B) a Treg depleted mouse. The analyses were performed 16 days after injection of (A) PBS or (B) anti-CD25+ monoclonal antibody (PC61 clone). C: Scores of inflammatory infiltrates in EAM mice with or without T reg depletion (n = 12 for each group, *P < 0.05). D–E: In vitro expanded Treg phenotype after 21 days of culture. F: In vitro expanded Tregs suppress lymphoproliferation of lymph nodes cells stimulated with anti-CD3 monoclonal antibody. G: Scores of inflammatory infiltrates in control EAM mice and EAM mice treated with polyclonal Tregs, intravenously injected the day before each immunization (n = 5 for each group, * P < 0.05).
EAM Treg-depleted mice were also tested for the presence of autoantibodies. Nine Treg depleted EAM mice were tested for the presence of antinuclear antibodies on HEp-2 cells. Nine out of 9 Treg depleted EAM tested mice developed autoantibodies (detectable at 1/80 dilution) with a cytoplasmic pattern on HEp-2 cells (Figure 5C), evocative of the pattern observed in the sera from patients with known autoantibodies to anti-aminoacyl-tRNA synthetases. Then, we looked for specific myositis-associated antibodies in a large panel (n = 37) of Treg-depleted EAM mice. As a control, the same antibodies were tested in 16 non-Treg depleted EAM and 18 control mice. Seven out of the 37 (19%) depleted EAM mice had positive immuno-dot blot assays (two anti-Jo-1, one anti-PL7, and four anti-Ku). Compared with control and non Treg-depleted EAM mice (one anti-Jo-1 positive mouse among 34), the difference was significant (P = 0.03).
We next examined the effect of the injection of in vitro expanded polyclonal Tregs on EAM severity. Cells expanded after 21 days of culture kept the phenotype (Figure 6, D and E) and function (Figure 6F) of Tregs. When 2 × 106 polyclonal Tregs were injected at the time of immunization (n = 5), a significant decrease of the myositis histological scores was observed compared with mice not injected with Tregs (0.87 ± 1.06 vs. 2.4 ± 0.67, P = 0.047, Figure 6G).
Finally, we looked for the persistence over time of the myositis in Treg depleted mice. Muscle sections were examined at various time points after the last immunization. We observed a spontaneous regression of muscle inflammation. The incidence of EAM was 100% on days 10 (n = 35), 35 (n = 3), and 50 (n = 3); 50% on days 70 (n = 2); and 0% on days 140 (n = 2).
Discussion
We have established a highly reproducible EAM mouse model, adapted from the rat, offering all of the advantages of this extensively studied species, in particular for the Treg analysis. This EAM was transferable, aggravated by Treg depletion, accompanied in some cases by specific autoantibodies and improved by in vitro expanded polyclonal Treg injection.
Concerning the pathological features, this EAM mouse model resembles human polymyositis in several respects. We observed necrotic and regenerating fibers and endomysial infiltrates with some invaded fibers. Immunohistochemical examination revealed that many of the infiltrating cells were CD8+ lymphocytes, with MHC class I overexpression in some fibers. In mice sensitized by C protein, called the C protein-induced myositis (CIM) model, abundant infiltration of CD8 and perforin-expressing cells in the endomysial site of injured muscle, together with MHC class I overexpression in some fibers19 is also observed, in line with our results. However, some of the pathological features of the EAM are distinct from polymyositis. The MHC class I overexpression is not diffuse, and not only CD8+ T cells, but also many CD4+ cells and CD11b+ macrophages infiltrated the muscle fibers, thus simulating more overlap myositis20 rather than pure polymyositis. Overlap myositis can also be defined by the presence of autoantibodies such as anti-synthetases or anti-Ku.3 Looking for the presence of these antibodies, we found significantly more positive mice in the Treg-depleted group. Because we did not perform IgG purification or competitive testing, the positive signal, which we observed could be due to a xenogenic reaction. Nevertheless, the aspect of anti-nuclear antibodies by immunofluorescence on HEp-2 cells is evocative of the presence of these specific antibodies. Furthermore, in one other transgenic myositis model induced by conditional up-regulation of MHC class I in muscle, the same autoantibodies were also evidenced in some mice, by a similar method.21 Treg cells are able to suppress humoral responses to systemic self-antigens such as DNA/chromatin22 and Treg-depleted mice have higher titers of autoantibodies compared with non Treg depleted ones.23 Similarly, in EAM, dendritic cells presenting self-antigens (such as t-RNA synthetases found in regenerating muscle cells24) can initiate anti-synthetase autoantibodies production, but in particular when Treg cells are depleted. The presence of such autoantibodies, together with the pathological features of the infiltrate observed in this model, are arguments for the overlap myositis nature of the disease. Another argument came from the adoptive transfer experiments: unsorted or CD4+ lymph node cells elicited myositis in some of the recipient mice. Polymyositis and inclusion body myositis are known as mainly CD8 cell-mediated intramuscular Ag-specific autoimmunity.25,26,27 However, nothing in this field is known for overlap myositis but CD4+ cells may play a role in the development of eg, autoantibodies in this condition.
An elevation in the levels of creatine kinase was found in our EAM mice (data not shown). However, since some mice, including healthy animals, also have unexpectedly high levels of creatine kinase, this elevation could be attributed to uncontrollable hemolysis, as described elsewhere.19 We observed spontaneous regression of the muscle infiltrations over time, as described in the CIM model.19 Compared with our EAM model, the spontaneous remission of CIM was more rapid since it was already complete at day 63.19 This difference in the time course may be due to intrinsic differences between the two diseases or to the Treg depletion in our model that aggravates the EAM.
Concerning the effect of treatments in EAM or CIM models, it has been reported that intravenous glucocorticoid pulse therapy caused an increase in the number of apoptotic T cells in the inflamed muscles of rat EAM.28 ICOS (a member of CD28 family costimulatory receptor) has been demonstrated to be expressed on muscle fiber-infiltrating T cells in the EAM rats, but not in normal rats.29 Treatment with anti-ICOS monoclonal antibody reduced both the incidence and the severity of myositis.29 In the CIM model,19 myositis was inducible in tumor necrosis factor α-null mutant mice but not in interleukin-1α/β-null mutant mice, but intravenous immunoglobulin therapy improved the disease.19 We demonstrated here that Treg depletion aggravated the disease, whereas injection of polyclonal Treg reduced both the incidence and the severity of myositis. Furthermore, for breaking immune self-tolerance, a strong adjuvant effect (only CFA works) and pertussis toxin are needed in our model, as previously described.6,7,19 Pertussis toxin has been reported to have adjuvant properties with Th-1 induction effects.30 But, it has also been reported that in vivo pertussis toxin injection reduces both the number and the function of Treg.31 The effects of pertussis toxin should then be additive to those of CFA and Treg depletion. There are evidences that Tregs play a major role in the regulation of many models of rodent autoimmune diseases. Adoptive transfer of Tregs into autoimmune prone animals has a profound effect on disease incidence and progression. The first experiments performed by Sagakuchi et al9 in 1995 showed that adoptive transfer of CD25+-depleted T cells resulted in the onset of various autoimmune diseases, such as gastritis, thyroiditis, or diabetes in recipient mice. Other examples include type 1 diabetes, experimental autoimmune encephalitis, gastritis, thyroïditis, oophoritis, inflammatory bowel disease, graft versus host disease, arthritis, and systemic lupus erythematosus.9,32,33,34,35,36,37 We have previously successfully tested the therapeutic effects of CD4+CD25highCD62LhighTreg in type 1 diabetes, graft versus host disease, and uveoretinitis animal models.32,38,39 We have also shown,40 as have others,41,42 that antigen specific Tregs are more effective than polyclonal Tregs. That is why we are currently looking for the effect of myosin specific Tregs in our model.
Finally, this preclinical data has encouraged us to design a clinical trial in severe myositis patients, consisting of the injection of in vitro expanded polyclonal Tregs.
Acknowledgments
We acknowledge Jean-Luc Charuel from the Service d’Immunochimie, Gisela Stoltenburg-Didinger from Institut de Myologie, Fernanda Pinto-Mariz from UMR 787S, and Brigitte Verneau, Françoise Blanchet, Valérie Thuries, and Odile Freytag from the Service de Neuropathologie, Hôpital Pitié-Salpêtrière for their advice and technical assistance.
Footnotes
Address reprint requests to Olivier Benveniste, Service de Médecine Interne 1, Groupe Hospitalier Pitié-Salpêtrière, 47-83, Boulevard de l’Hôpital, 75651 Paris Cedex 13, France. E-mail address: olivier.benveniste@psl.aphp.fr.
Supported by the Association Française contre les Myopathies to A.F.M.
Y.A. and S.S. contributed equally to this work.
References
- Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore) 1977;56:255–286. doi: 10.1097/00005792-197707000-00001. [DOI] [PubMed] [Google Scholar]
- Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, Vencovsky J, de Visser M, Hughes RA. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul Disord. 2004;14:337–345. doi: 10.1016/j.nmd.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Troyanov Y, Targoff IN, Tremblay JL, Goulet JR, Raymond Y, Senecal JL. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore) 2005;84:231–249. doi: 10.1097/01.md.0000173991.74008.b0. [DOI] [PubMed] [Google Scholar]
- van der Meulen MF, Bronner IM, Hoogendijk JE, Burger H, van Venrooij WJ, Voskuyl AE, Dinant HJ, Linssen WH, Wokke JH, de Visser M. Polymyositis: an overdiagnosed entity. Neurology. 2003;61:316–321. doi: 10.1212/wnl.61.3.316. [DOI] [PubMed] [Google Scholar]
- Nagaraju K, Plotz PH. Animal models of myositis. Rheum Dis Clin North Am. 2002;28:917–933. doi: 10.1016/s0889-857x(02)00026-1. [DOI] [PubMed] [Google Scholar]
- Kojima T, Tanuma N, Aikawa Y, Shin T, Sasaki A, Matsumoto Y. Myosin-induced autoimmune polymyositis in the rat. J Neurol Sci. 1997;151:141–148. doi: 10.1016/s0022-510x(97)00148-2. [DOI] [PubMed] [Google Scholar]
- Kohyama K, Matsumoto Y. C-protein in the skeletal muscle induces severe autoimmune polymyositis in Lewis rats. J Neuroimmunol. 1999;98:130–135. doi: 10.1016/s0165-5728(99)00087-9. [DOI] [PubMed] [Google Scholar]
- Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Contet C, Rawlins JN, Deacon RM. A comparison of 129S2/SvHsd and C57BL/6JOlaHsd mice on a test battery assessing sensorimotor, affective and cognitive behaviours: implications for the study of genetically modified mice. Behav Brain Res. 2001;124:33–46. doi: 10.1016/s0166-4328(01)00231-5. [DOI] [PubMed] [Google Scholar]
- Butler-Browne GS, Bugaisky LB, Cuenoud S, Schwartz K, Whalen RG. Denervation of newborn rat muscle does not block the appearance of adult fast myosin heavy chain. Nature. 1982;299:830–833. doi: 10.1038/299830a0. [DOI] [PubMed] [Google Scholar]
- Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen RG, Sell SM, Butler-Browne GS, Schwartz K, Bouveret P, Pinset-Harstom I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1981;292:805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]
- Darrasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102:106–109. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Sugihara T, Sekine C, Nakae T, Kohyama K, Harigai M, Iwakura Y, Matsumoto Y, Miyasaka N, Kohsaka H. A new murine model to define the critical pathologic and therapeutic mediators of polymyositis. Arthritis Rheum. 2007;56:1304–1314. doi: 10.1002/art.22521. [DOI] [PubMed] [Google Scholar]
- Mozaffar T, Pestronk A. Myopathy with anti-Jo-1 antibodies: pathology in perimysium and neighbouring muscle fibres. J Neurol Neurosurg Psychiatry. 2000;68:472–478. doi: 10.1136/jnnp.68.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraju K, Raben N, Loeffler L, Parker T, Rochon PJ, Lee E, Danning C, Wada R, Thompson C, Bahtiyar G, Craft J, Hooft Van Huijsduijnen R, Plotz P. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc Natl Acad Sci USA: 2000;97:9209–9214. doi: 10.1073/pnas.97.16.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields ML, Hondowicz BD, Metzgar MH, Nish SA, Wharton GN, Picca CC, Caton AJ, Erikson J. CD4+ CD25+ regulatory T cells inhibit the maturation but not the initiation of an autoantibody response. J Immunol. 2005;175:4255–4264. doi: 10.4049/jimmunol.175.7.4255. [DOI] [PubMed] [Google Scholar]
- Hsu WT, Suen JL, Chiang BL. The role of CD4CD25 T cells in autoantibody production in murine lupus. Clin Exp Immunol. 2006;145:513–519. doi: 10.1111/j.1365-2249.2006.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, Gabrielson E, Corse A, Rosen A. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med. 2005;201:591–601. doi: 10.1084/jem.20041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste O, Cherin P, Maisonobe T, Merat R, Chosidow O, Mouthon L, Guillevin L, Flahault A, Burland MC, Klatzmann D, Herson S, Boyer O. Severe perturbations of the blood T cell repertoire in polymyositis, but not dermatomyositis patients. J Immunol. 2001;167:3521–3529. doi: 10.4049/jimmunol.167.6.3521. [DOI] [PubMed] [Google Scholar]
- Benveniste O, Herson S, Salomon B, Dimitri D, Trebeden-Negre H, Jean L, Bon-Durand V, Antonelli D, Klatzmann D, Boyer O. Long-term persistence of clonally expanded T cells in patients with polymyositis. Ann Neurol. 2004;56:867–872. doi: 10.1002/ana.20293. [DOI] [PubMed] [Google Scholar]
- Dimitri D, Benveniste O, Dubourg O, Maisonobe T, Eymard B, Amoura Z, Jean L, Tiev K, Piette JC, Klatzmann D, Herson S, Boyer O. Shared blood and muscle CD8+ T-cell expansions in inclusion body myositis. Brain. 2006;129:986–995. doi: 10.1093/brain/awl020. [DOI] [PubMed] [Google Scholar]
- Schneider C, Matsumoto Y, Kohyama K, Toyka KV, Hartung HP, Gold R. Experimental autoimmune myositis in the lewis rat: lack of spontaneous T-cell apoptosis and therapeutic response to glucocorticosteroid application. J Neuroimmunol. 2000;107:83–87. doi: 10.1016/s0165-5728(00)00254-x. [DOI] [PubMed] [Google Scholar]
- Katsumata Y, Harigai M, Sugiura T, Kawamoto M, Kawaguchi Y, Matsumoto Y, Kohyama K, Soejima M, Kamatani N, Hara M. Attenuation of experimental autoimmune myositis by blocking ICOS-ICOS ligand interaction. J Immunol. 2007;179:3772–3779. doi: 10.4049/jimmunol.179.6.3772. [DOI] [PubMed] [Google Scholar]
- Hou W, Wu Y, Sun S, Shi M, Sun Y, Yang C, Pei G, Gu Y, Zhong C, Sun B. Pertussis toxin enhances Th1 responses by stimulation of dendritic cells. J Immunol. 2003;170:1728–1736. doi: 10.4049/jimmunol.170.4.1728. [DOI] [PubMed] [Google Scholar]
- Cassan C, Piaggio E, Zappulla JP, Mars LT, Couturier N, Bucciarelli F, Desbois S, Bauer J, Gonzalez-Dunia D, Liblau RS. Pertussis toxin reduces the number of splenic Foxp3+ regulatory T cells. J Immunol. 2006;177:1552–1560. doi: 10.4049/jimmunol.177.3.1552. [DOI] [PubMed] [Google Scholar]
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: cD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Gartner D, Hoff H, Gimsa U, Burmester GR, Brunner-Weinzierl MC. CD25 regulatory T cells determine secondary but not primary remission in EAE: impact on long-term disease progression. J Neuroimmunol. 2006;172:73–84. doi: 10.1016/j.jneuroim.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Frey O, Petrow PK, Gajda M, Siegmund K, Huehn J, Scheffold A, Hamann A, Radbruch A, Brauer R. The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4+CD25+ T cells. Arthritis Res Ther. 2005;7:R291–R301. doi: 10.1186/ar1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177:1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, Cohen JL. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrada C, Fisson S, De Kozak Y, Kaddouri M, Lehoang P, Klatzmann D, Salomon BL, Bodaghi B. Regulatory T cells control uveoretinitis induced by pathogenic Th1 cells reacting to a specific retinal neoantigen. J Immunol. 2006;176:7171–7179. doi: 10.4049/jimmunol.176.12.7171. [DOI] [PubMed] [Google Scholar]
- Fisson S, Djelti F, Trenado A, Billiard F, Liblau R, Klatzmann D, Cohen JL, Salomon BL. Therapeutic potential of self-antigen-specific CD4+ CD25+ regulatory T cells selected in vitro from a polyclonal repertoire. Eur J Immunol. 2006;36:817–827. doi: 10.1002/eji.200535445. [DOI] [PubMed] [Google Scholar]
- Tung KS, Setiady YY, Samy ET, Lewis J, Teuscher C. Autoimmune ovarian disease in day 3-thymectomized mice: the neonatal time window, antigen specificity of disease suppression, and genetic control. Curr Top Microbiol Immunol. 2005;293:209–247. doi: 10.1007/3-540-27702-1_10. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Tang Q. Therapeutic vaccination using CD4+CD25+ antigen-specific regulatory T cells, Proc Natl Acad Sci USA. 2004;101 Suppl 2:14622–14626. doi: 10.1073/pnas.0405234101. [DOI] [PMC free article] [PubMed] [Google Scholar]