Abstract
The growth factor proepithelin has recently emerged as an important regulator of transformation in several physiological and pathological systems. In this study, we determined the biological roles of proepithelin in prostate cancer cells using purified human recombinant proepithelin as well as proepithelin-depletion strategies. Proepithelin promoted the migration of androgen-dependent and -independent human prostate cancer cells; androgen-independent DU145 cells were the more responsive. In these cells, proepithelin additionally stimulated wound closure, invasion, and promotion of cell growth in vitro. These effects required the activation of both the Akt and mitogen-activated protein kinase pathways. We have analyzed proepithelin expression levels in different available prostate cancer microarray studies using the Oncomine database and found a statistically significant increase in proepithelin mRNA expression levels in prostate cancers compared with nonneoplastic controls. Notably, depletion of endogenous proepithelin by siRNA and antisense strategies impaired the ability of DU145 cells to grow and migrate after serum withdrawal and inhibited anchorage-independent growth. Our results provide the first evidence for a role of proepithelin in stimulating the migration, invasion, proliferation, and anchorage-independent growth of prostate cancer cells. This study supports the hypothesis that proepithelin may play a critical role as an autocrine growth factor in the establishment and initial progression of prostate cancer. Furthermore, proepithelin may prove to be a useful clinical marker for the diagnosis of prostate tumors.
Prostate cancer is the most frequently diagnosed cancer in the United States. Approximately 186,320 new cases will be diagnosed and an estimated 28,660 deaths will occur in the current year.1 Despite extensive experimental studies throughout the past decades, the pathogenesis of prostate cancer remains primarily unknown. In addition, the genetic and molecular mechanisms responsible for the development and progression of malignant prostate cells are very poorly characterized.
The growth factor proepithelin, also known as granulin-epithelin precursor (GEP1), progranulin, PC cell-derived growth factor, or acrogranin, is a member of a family of cysteine-rich polypeptides that plays a critical role in several physiological and pathological processes.2 Proepithelin, originally purified by several laboratories,3,4,5,6 is a 593-amino acid secreted protein that is heavily glycosylated and migrates on sodium dodecyl sulfate-polyacrylamide gel electrophoresis as an ∼80-kDa protein.5,7 Proepithelin undergoes proteolytic processing with the generation of small ∼6-kDa peptides, which retain biological activity8 but can exert opposite biological effects than the precursor protein.3,9
Zhu and colleagues9 have shown that the secretory leukocyte protease inhibitor interacts with proepithelin and blocks elastase-dependent proepithelin processing. Elastase digests proepithelin in the interepithelin linkers with the generation of epithelin peptides, suggesting that this protease may be a critical component of a proepithelin convertase. Secretory leukocyte protease inhibitor blocks this proteolysis by either directly binding to elastase or by sequestering epithelin peptides from the enzyme.9
The role of proepithelin in cellular proliferation has been well characterized using mouse embryonic fibroblasts derived from mice with a targeted deletion of the insulin-like growth factor receptor I (IGF-IR) gene (R− cells).10 Proepithelin is the only known growth factor able to bypass the requirement for the IGF-IR, thus promoting growth of R− cells.4,11 Although proepithelin replaces the function of the IGF-IR in cell proliferation, it does not promote the anchorage-independent growth of R− cells and does not protect R− cells from anchorage-independent apoptosis (anoikis).11 Conversely, in SW13 carcinoma cells, proepithelin protects cells from anoikis, confers anchorage-independent growth, and promotes tumor formation in nude mice.12 In several breast cancer cell lines, proepithelin expression levels correlate with an aggressive phenotype13,14 and immunoneutralization of proepithelin inhibits estrogen-dependent proliferation of MCF-7 cells.15 Targeting of proepithelin expression by an antisense strategy inhibits tumorigenicity of the human breast carcinoma cell line MDA-MB-468.14 Moreover, increased proepithelin expression levels play a significant role in teratomas, glioblastomas, multiple myelomas, uterine leiomyosarcomas, renal cell, gastric, ovarian, and adenocarcinomas.2,16,17,18,19,20,21
Data from our laboratory have demonstrated that proepithelin promotes migration and invasion of 5637 bladder cancer cells, supporting the evidence that proepithelin may play a critical role in bladder cancer as well.22 It has been more recently reported that frontotemporal dementia is caused by mutations in the proepithelin gene.23,24 These mutations create null alleles indicating that proepithelin haploinsufficiency leads to neurodegeneration, pointing out a critical function of proepithelin in regulating survival of neuronal cells.25 So far, a functional proepithelin membrane receptor has not been identified, although proepithelin26 and epithelins5 bind specifically to membrane proteins. Competitive binding experiments indicate that a long list of known growth factors and cytokines are unable to displace radiolabeled-epithelin binding to its putative receptor5 suggesting that the receptor for proepithelin is not a known tyrosine-kinase receptor.
Using prostate cancer cell lines, we provide the first evidence for a role of proepithelin in promoting proliferation, migration, invasion, and anchorage-independent growth of prostate cancer cells, supporting the hypothesis that this growth factor may play a significant role in the establishment and initial progression of the transformed phenotype in prostate cancer. Furthermore, proepithelin may prove a useful clinical marker for diagnosis of prostate tumors.
Materials and Methods
Cell Lines
PC3, LNCaP, and DU145 human prostate cancer cells were obtained from American Type Culture Collection (Rockville, MD). PC3 and LNCaP cells were maintained in RPMI medium supplemented with 10% fetal bovine serum. DU145 cells were maintained in EARL-modified minimal essential medium supplemented with 10% fetal bovine serum, 1% nonessential amino acid, and 1% vitamins. Serum-free medium (SFM) is Dulbecco’s modified Eagle’s medium containing 0.1% bovine serum albumin and 50 μg/ml of bovine transferrin (Sigma-Aldrich, St. Louis, MO).
Purification of Human Recombinant Proepithelin
Human proepithelin was purified from conditioned medium of 293-EBNA/proepithelin cells. This cell line expresses a 6His-tagged human proepithelin.7 Serum-free conditioned medium was concentrated with polyethylene glycol, dialyzed, and purified on Ni-NTA resin eluted with 250 mmol/L imidazole, as previously described.27
Migration Assay
PC3, DU145, and LNCaP cells were serum-starved for 24 hours. Cells (2 × 104 in 200 μl) were then seeded in Boyden chambers (upper chamber). Chambers contained 500 μl of SFM or purified recombinant proepithelin (240 nmol/L). After 24 hours, the cells in the upper chamber were removed, whereas the cells that migrated to the lower chamber were counted after fixation and staining with Coomassie Blue solution for 5 minutes as described.22 Cells that migrated to the lower chamber were counted under the microscope. Specific inhibitors for Akt, LY294002, or ERK1/2, U0126 (Calbiochem, La Jolla, CA) were used at a concentration of 30 μmol/L and 10 μmol/L, respectively.
Invasion and Wound Healing Assays
Cell invasion through a three-dimensional extracellular matrix was assessed by a Matrigel invasion assay using BD Matrigel Invasion Chambers (BD Biocoat; BD Biosciences, San Jose, CA) with 8.0-μm filter membranes. PC3, DU145, and LNCaP cells (2.5 × 104) in 200 μl of SFM or SFM supplemented with 240 nmol/L of recombinant proepithelin were plated onto each filter, and 750 μl of SFM supplemented placed in the lower chamber. After 24 hours filters were washed, fixed, and stained with Coomassie Brilliant Blue. Cells on the upper surface of the filters were removed with cotton swabs. Cells that had invaded to the lower surface of the filter were counted under the microscope.
For wound-healing assays, DU145 cells were seeded onto 35-mm plates in serum-containing medium until subconfluent and then transferred to SFM. After 24 hours, the plates were scratched with a thin disposable tip to generate a wound (500 μm) in the cell’s monolayer as previously described.22,28 The cells were incubated for an additional 24 hours in SFM without or with purified proepithelin (240 nmol/L). Cells were analyzed and photographed with an Axiovert 200M cell live microscope (Zeiss, Thornwood, NY) using the Metamorph Image Acquisition and Analysis software (Universal Imaging, Sunnyvale, CA) at the Kimmel Cancer Center Confocal Microscopy Core Facility. BrdU incorporation in a wound-healing assay was determined using a BrdU cell proliferation assay, HTS kit (Calbiochem, BD Biosciences) following the manufacturers’ instructions.
Cell Growth Assay
DU145 cells were plated in triplicate at a density of 4 × 104 cells/35-mm plate in serum-supplemented medium. After 24 hours cells were washed three times in Dulbecco’s modified Eagle’s medium and transferred to SFM or SFM supplemented with 240 nmol/L of proepithelin. Cells were counted after 48 hours with a hemocytometer.
Immunoblot Detection of Activated Signaling Molecules
DU145 cells were serum-starved for 24 hours and then stimulated for 10, 30, and 120 minutes with purified proepithelin (240 nmol/L). Twenty μg of lysates were run on a 12% gel sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The activation of p90RSK, Akt, ERK1/2, and S6 ribosomal protein was analyzed by Western immunoblot using the PathScan Multiplex Western Cocktail I (Cell Signaling Technology, Beverly, MA), which provides a mix of phospho-specific antibodies for different activated protein. The anti-β-actin polyclonal antibodies are from Sigma-Aldrich (St. Louis, MO). The total level of Akt and MAPK was determined by immunoblot using anti-Akt (Cell Signaling Technology) and anti-ERK1 (Santa Cruz Biotechnology, Santa Cruz, CA) polyclonal antibodies.
Gene Silencing
Gene silencing of human ERK1/2, Akt, and proepithelin was obtained by RNA interference using small interfering RNA (siRNA). DU145 cells were transfected with vehicle (diethylpyrocarbonate-treated water), control siRNA (scrambled), or siRNA directed against ERK2, Akt, or proepithelin (200 pmol) using either TransIT-siQUEST or TransIT-siTKO reagents (Mirus Bio Corporation, Madison, WI) according to the manufacturer’s instructions. Scramble and anti-ERK2 (NM 002745, exon 3, sequences not disclosed by the vendor) or proepithelin (number 11032) silencer siRNA oligos were from Ambion (Austin, TX). Scramble and anti-Akt siRNA were from Dharmacon (Lafayette, CO). Twenty-four hours after transfection, DU145 cells were starved in SFM for 24 hours and then stimulated with 240 nmol/L of recombinant proepithelin. After 24 hours cells were processed and analyzed for migration and invasion as described above. The expression of ERK1/2 and Akt proteins was detected by immunoblot using anti-ERK1/2 polyclonal antibodies (Santa Cruz Biotechnology) or anti-Akt polyclonal antibodies (Cell Signaling Technology). Proepithelin was detected by immunoblot using anti-proepithelin polyclonal antibodies (Zymed Laboratories, South San Francisco, CA) and the amount produced by DU145 cells was determined by enzyme-linked immunosorbent assay (Alpco Diagnostics, Salem, NH).
Generation of Proepithelin-Depleted DU145 Cells
DU145 cells were transfected with either the pcDNA3 vector or a pcDNA3 vector expressing an antisense cDNA against human proepithelin (−31 bp to 374 bp)14 using the TransIt-Prostate transfection reagent (Mirus Corporation). Cells were selected in medium supplemented with 2 mg/ml of G418, pooled, and tested for proepithelin expression in cell lysates and conditioned medium by immunoblot using anti-proepithelin polyclonal antibodies (Zymed Laboratories). Conditioned medium was prepared as previously described.11
Colony Formation Assay in Soft Agar
Colony formation in soft-agar was performed as previously described.29 DU145 cells were seeded in 10% fetal bovine serum-supplemented medium in soft agar at a density of 6 × 103 cells/35-mm plate and counted after 4 weeks in culture. Colonies >100 μm were scored as positive.
Immunohistochemical Detection of Proepithelin in Prostate Tissues
Immunohistochemical analysis of proepithelin levels in prostate tissues was performed as previously described.7 Formalin-fixed, paraffin-embedded sections of two unidentified prostate carcinomas (Gleason score 7; pTNM: T3a N0 M0; stage III; not previously treated) were selected from the Pathology Tissue Bank of Thomas Jefferson University and used to analyze the expression of proepithelin in normal and adjacent cancerous prostatic glands. Slides were incubated overnight with 1:50 dilution of primary goat anti-human proepithelin (Santa Cruz Biotechnology). We have previously tested the specificity of anti-proepithelin antibody in normal bladder stained with primary antibody after affinity depletion with blocking peptide (Santa Cruz Biotechnology, data not shown).
cDNA Microarray Analysis
The Oncomine database (Compendia Bioscience, Ann Arbor, MI) and gene microarray analysis tool, a repository for published cDNA microarray data (http://www.oncomine.org),30,31 was explored (30th August 2008) for mRNA expression of proepithelin (progranulin, PC cell-derived growth factor) in nonneoplastic and primary prostate cancers. Statistical analysis of the differences in proepithelin expression between the aforementioned tissues was accomplished through use of Oncomine algorithms, which allow for multiple comparisons among different studies.30,31,32 Only studies with analysis results with P < 0.05 were considered.
Statistical Analysis
Experiments were performed in triplicate and repeated at least three times. Results are expressed as mean ± SD. All statistical analyses were performed with SigmaStat for Windows version 3.10 (Systat Software, Inc., Port Richmond, CA). Results were compared by using the two-sided Student’s t-test. Differences were considered statistically significant at P < 0.05.
Results
Proepithelin Promotes Migration and Invasion of Prostate Cancer Cells
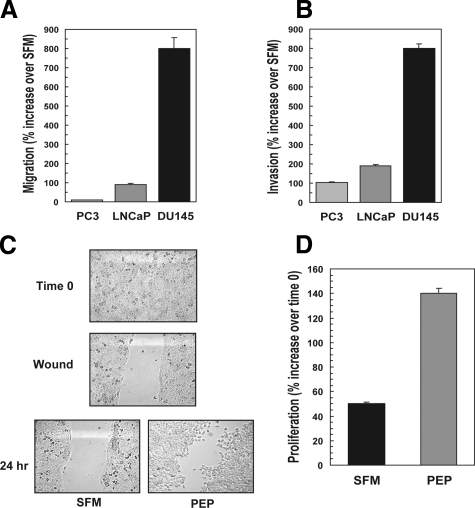
Because proepithelin is established as a growth factor that promotes cell-cycle progression and cell growth of many cellular systems,16 we sought to determine whether proepithelin played a role in transformation of prostate cancer cells. Therefore, we tested whether proepithelin may promote migration and invasion of various prostate cancer cells. As a source of proepithelin we used human proepithelin purified from conditioned medium of 293-EBNA/proepithelin cells.7 As previously shown, human proepithelin purified using this methodology is biologically active with an optimal effective dose at 240 nmol/L, as determined by migration and invasion assays on 5637 bladder cancer cells.22 Prostate cancer cell lines have different abilities to migrate: PC3 cells are highly migratory even in SFM whereas LNCaP cells show poor ability to migrate. Taking into account these differences, we have expressed the results as percent increase over SFM. As shown in Figure 1A, 240 nmol/L of purified proepithelin induced cell migration of prostate cancer cells. PC3 showed a high level of migration in SFM with only a modest increase after proepithelin stimulation, whereas in both LNCaP and DU145 cells proepithelin promoted a significant increase in migration over SFM (Figure 1A).
Figure 1.
Proepithelin induces migration, invasion, wound healing, and cell growth of prostate cancer cells. Human proepithelin was purified from conditioned medium of 293-EBNA/proepithelin cells7 as described.27 Migration (A) and invasion (B) experiments on PC3, LNCaP, and DU145 cells were performed as described in the Materials and Methods. Values are expressed as percent increase over SFM ±SD. C: The in vitro wound-healing motility assay in DU145 cells in SFM or SFM supplemented with purified proepithelin (240 nmol/L) was performed as described in Materials and Methods. Cells were analyzed with a cell live microscope using the Metamorph Image Acquisition and Analysis software (Universal Imaging). A minimum of 10 fields per plate was examined. Original magnifications, ×100. D: For the cell growth assay, DU145 cells were plated in triplicate at a density of 4 ×104 cells/35-mm plates. After 24 hours, cells were washed in Dulbecco’s modified Eagle’s medium and transferred to SFM or SFM supplemented with 240 nmol/L of proepithelin. Cells were counted after 48 hours with a hemocytometer. The data are the average of four independent experiments run in duplicates ±SD.
The acquisition by cancer cells of an invasive phenotype is a critical step for tumor progression.33 Matrigel-coated filters are widely used to examine invasive migration through a three-dimensional extracellular matrix. We tested the ability of prostate cancer cells to invade a three-dimensional extracellular matrix by performing invasion assays using Matrigel-coated trans-wells, as we have recently described.22 Proepithelin (240 nmol/L) substantially enhanced the ability of the various prostate cancer cells to invade through Matrigel (Figure 1B) indicating that proepithelin promotes invasion of prostate cancer cells through a three-dimensional matrix.
We demonstrated that lower doses of proepithelin are also effective in promoting invasion of DU145 by repeating invasion assays in SFM supplemented with 60, 120, and 240 nmol/L of recombinant proepithelin (see Supplemental Figure S1 at http://ajp.amjpathol.org). Because DU145 cells showed the strongest response to proepithelin in both migration and invasion assays, we used them as a primary model to further characterize proepithelin signaling in prostate cancer cells.
Proepithelin Promotes Wound Healing and Enhances Proliferation of DU145 Cells
We further determined the ability of proepithelin to induce motility of DU145 prostate cancer cells using an in vitro wound-healing assay.22,28 DU145 cells were plated at high density in serum-containing medium (Figure 1C, time 0). After 24 hours of starvation in SFM, confluent DU145 cells were wounded (Figure 1C, wound) and incubated for an additional 24 hours in SFM with or without proepithelin (240 nmol/L). In contrast to control, proepithelin induced a substantial migration of DU145 cells into the denuded area (Figure 1C).
Recently published data have shown that proepithelin effectively promoted migration and invasion of 5637 bladder22 and breast cancer cells.34 In contrast, it did not induce substantial mitogenic response in bladder cancer cells.22 Because proepithelin is mitogenic in other cancer cell models,15,16 we wanted to determine whether proepithelin may promote cell proliferation of DU145 cells. Proepithelin induced an increase in cell growth of DU145 compared with SFM-incubated cells (Figure 1D), indicating that proepithelin is important not only for migration and invasion of DU145 prostate cancer cells but also for promoting their proliferation.
Because proepithelin-induced proliferation of DU145 cells may contribute to the healing process of these cells in a wound healing motility assay, we tested by BrdU incorporation the proliferation level at the wound edge and we determined similar levels of BrdU incorporation between SFM and proepithelin-stimulated DU145 cells at the wound (see Supplemental Figure S2 at http://ajp.amjpathol.org). The results suggest that the ability of proepithelin to promote lateral motility (wound healing) can be separated from the capacity to induce cell proliferation. Collectively, our results indicate that proepithelin may play a critical role in prostate cancer by promoting migration, invasion, and proliferation of prostate cancer cells.
Signaling Pathways Stimulated by Proepithelin in DU145 Prostate Cancer Cells
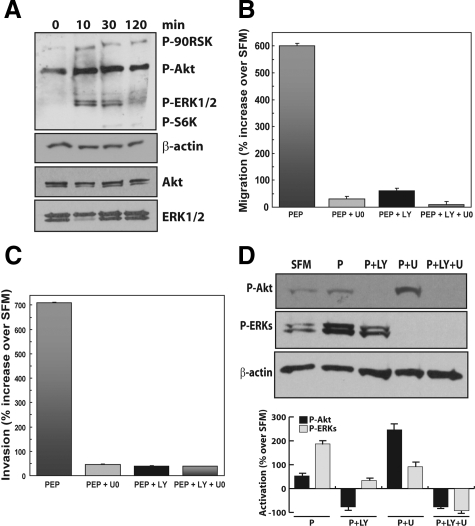
Recent data from our laboratory have shown that proepithelin promotes the activation of the MAPK pathway, which was required for migration and invasion of bladder cancer cells.22 To determine the signaling pathway(s) induced by proepithelin in DU145 prostate cancer cells, we initially tested the activation of Akt and ERK1/2 kinases using the PathScan Multiplex Western Cocktail I (Cell Signaling Technology). The PathScan cocktail offers a unique method to test the activation of multiple pathways in one blot, providing a mix of phospho-specific antibodies for different activated proteins. As shown in Figure 2A, proepithelin induced in DU145 cells sustained activation of both Akt (up to 2 hours) and ERK1/2 kinases (up to 30 minutes) compared with SFM-stimulated cells, with a subsequent stronger activation of their downstream effectors, like S6 ribosomal protein and p90RSK, respectively. The level of Akt and ERK1/2 proteins was instead not affected by proepithelin stimulation, as determined by immunoblot with anti-Akt and ERK1 antibodies (Figure 2A). These results indicate that proepithelin promotes the activation of both Akt and ERK1/2 kinase pathways in DU145 cells, suggesting that both pathways may be critical for proepithelin-induced cell proliferation, migration, and invasion of DU145 prostate cancer cells.
Figure 2.
Proepithelin-mediated activation of the Akt and MAPK pathway promotes migration and invasion of DU145 cells. A: DU145 cells were serum-starved for 24 hours and stimulated with proepithelin (240 nmol/L) for 10, 30, and 120 minutes. The activation of p90RSK, Akt, ERK1/2, and S6 ribosomal protein (top to bottom) was analyzed by Western immunoblot using phosphoro-specific antibodies as described in Materials and Methods. The experiment shown is representative of three independent experiments. Migration (B) and invasion (C) experiments were performed on DU145 cells as described in Materials and Methods. U0126 (Calbiochem) was used at 10 μmol/L concentration and LY294002 was used at 30 μmol/L concentration. Values are expressed as percent increase over SFM condition (70 ± SD). D: Top: DU145 cells were serum-starved for 24 hours and then stimulated for 10 minutes with purified proepithelin (240 nmol/L). The activation of Akt and ERK1/2 in the presence of 10 μmol/L U0126 or 30 μmol/L LY294002 or the combination of the two was detected by immunoblot with anti-phospho-specific p90RSK and ERK1/2 (Cell Signaling). Samples were normalized using anti-β-actin polyclonal antibodies (Sigma-Aldrich). Bottom: Densitometric analysis of the Akt and ERK activation levels was performed using the Image J program (National Institutes of Health). Values in arbitrary units from three independent experiments are shown.
The Activation of the Akt and MAPK Pathways Is Required for Proepithelin-Mediated Responses in DU145 Cells
To confirm the role of Akt and ERK1/2 activation in proepithelin-mediated responses in DU145 prostate cancer cells we performed proepithelin-mediated migration and invasion experiments in DU145 cells in the presence of specific inhibitors of either Akt (LY294002) or MAPK (U0126). LY294002 (30 μmol/L) and U0126 (10 μmol/L) severely reduced proepithelin-evoked migration (Figure 2B) and invasion of DU145 cells (Figure 2C), confirming that the activation of both the Akt and ERK1/2 pathways is necessary for proepithelin-mediated migration and invasion of DU145 prostate cells. The same concentration of inhibitors had no effect on DU145 cells growing in serum, indicating that the concentration of inhibitors used is not toxic for exponentially growing DU145 cells (not shown). To verify that the concentrations of LY294002 and U0126 used for these experiments are effectively inhibiting the activation of Akt and ERK1/2, we tested by Western blot the activation of both proteins after stimulation with proepithelin in the presence of the specific inhibitors. Proepithelin-mediated activation of Akt and ERK1/2 was completely abolished in the presence of LY294002 and UO126, respectively, establishing the effectiveness of both inhibitors at the concentration used in the migration and invasion assays (Figure 2D).
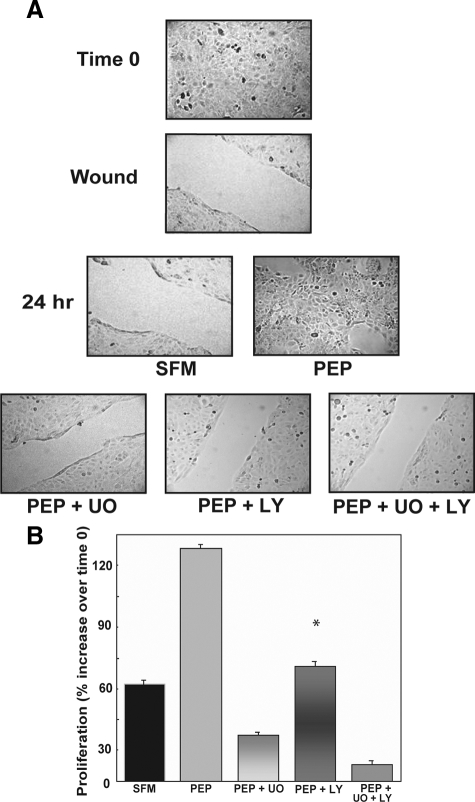
We also determined that the activation of both the Akt and MAPK pathways is necessary for proepithelin-induced wound healing and proliferation of DU145 cells by using the same concentrations of LY294002 and U0126 in wound healing and cell growth assays. Both inhibitors were very effective in inhibiting proepithelin-promoted wound repair (Figure 3A) and cell growth (Figure 3B) of DU145 cells indicating that Akt and MAPK are critical components of proepithelin-mediated biological responses in DU145 prostate cancer cells.
Figure 3.
U0126 and LY294002 inhibit in vitro closure of a wound and cell growth of DU145 cells. A: For the in vitro wound-healing motility assay, DU145 cells were incubated in SFM, SFM with purified proepithelin (240 nmol/L) supplemented or not with 10 μmol/L U0126 or 30 μmol/L LY294002, or the combination of the two. Cells were analyzed as described in the legend for Figure 1. The experiment shown is representative of three independent experiments. B: Cell proliferation experiments were performed as described in the legend for Figure 1 in the presence of the same concentration of inhibitors. Data are the average of three independent experiments run in duplicates ±SD. *P < 0.05 compared with proepithelin-stimulated cells (second column).
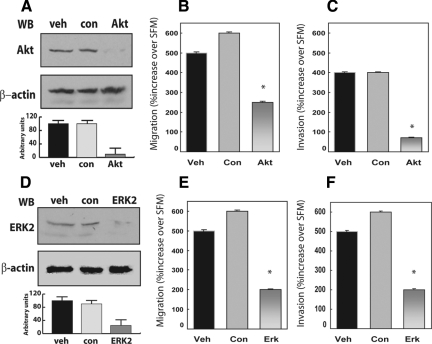
To further establish the role of Akt and MAPK activation in proepithelin-induced migration and invasion of DU145 cells, we used Akt and ERK-specific small interference RNA (siRNA). Our approach yielded almost complete suppression of endogenous Akt protein expression as compared with vehicle- or control-treated cells (Figure 4A) and a concurrent suppression of proepithelin-mediated migration (Figure 4B, P < 0.04 values) and invasion (Figure 4C, P < 0.025) through Matrigel-coated filters. Suppression of endogenous ERK expression (Figure 4D) also caused a significant reduction in the ability of DU145 cells to migrate (Figure 4E, P < 0.04) and invade (Figure 4F, P < 0.03) in response to proepithelin. Collectively, our findings demonstrate an essential role for Akt and MAPK signaling in proepithelin-dependent regulation of tumor cell motility and invasion, two key properties of aggressive cancer phenotypes.
Figure 4.
Depletion of endogenous Akt and ERK1/2 proteins effectively reduces proepithelin-mediated migration and invasion of DU145 cells. Gene knockdown for Akt and ERK1/2 was achieved by siRNA. Twenty-four hours after transfection, DU145 cells were serum-starved for 24 hours and then stimulated with 240 nmol/L of purified proepithelin. After 24 hours, cells were processed and analyzed for migration (B and E) and invasion (C and F). Values are expressed as percent increase over SFM (70 ± SD). *P < 0.05 compared with vehicle-treated control cells (first column). The level of endogenous Akt (A) and ERK1/2 proteins (D) was detected by immunoblot using anti-ERK1/2 (Santa Cruz Biotechnology) or anti-Akt polyclonal antibodies (Cell Signaling Technology). Protein loading was normalized using anti-β-actin polyclonal antibodies (Sigma-Aldrich). One representative blot is shown. Densitometric analysis of the Akt and ERKs protein levels (A and D) was performed using the Image J program (National Institutes of Health). Values in arbitrary units from three independent experiments are shown.
Proepithelin Is Overexpressed in Prostate Cancers
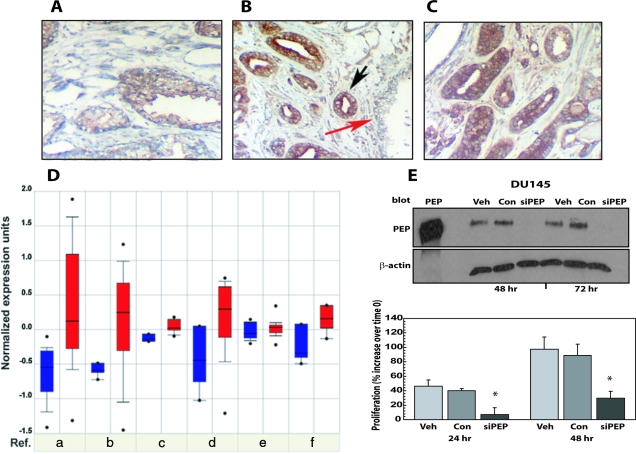
Because proepithelin is overexpressed in several cancers,16 we wanted to determine by immunohistochemical analysis the level of proepithelin expression in normal versus prostate cancer sections and showed that proepithelin is present, although at low levels, in normal prostate [Figure 5, A and B (red arrow)]. Proepithelin was instead expressed at higher levels in tumor glands [Figure 5, B (black arrow) and C] from two unidentified prostate carcinomas (Gleason score 7; pTNM: T3a N0 M0; stage III; not previously treated). These results confirmed the data reported by Pan and colleagues21 in which they measured proepithelin (GP88) in normal prostate, prostatic intraepithelial neoplasia, and prostatic adenocarcinoma tissue samples.
Figure 5.
Proepithelin is overexpressed in prostate cancer tissues and contributes in an autocrine manner to the transforming phenotype of DU145 cells. Formalin-fixed, paraffin-embedded sections of nonmalignant human prostate and prostate cancer tissue were deparaffinized in preparation for antigen retrieval by heating in a microwave oven for 10 minutes. Slides were cooled, rinsed, and immunostained using a goat anti-proepithelin antibody (Santa Cruz Biotechnology) and visualized with an avidin-biotin method. A and B, red arrow: Normal prostatic glands. B, black arrow, and C: Invasive prostate cancer. At least 10 independent fields were examined. Original magnifications, ×400. D: Expression array analysis of multiple prostate cancer microarray data sets was collected and analyzed, and statistical significance was calculated. Blue: proepithelin expression in normal tissues; red: proepithelin expression in prostate cancers. (a) Dhanasekaranet et al36 (normal/cancer = 12/25; t-test = −4.8; P = 2.9E-5); (b) Dhanasekaranet al35 (N/C = 22/59; t-test = −4.81; P = 4.3E-5); (c) Holzbeierlein et al37 (N/C = 4/23; t-test = −4.074; P = 0.01); (d) Welsh et al38 (N/C = 9/25; t-test = −3.03; P = 0.013); (e) Yu et al41 (N/C = 23/64; t-test = −2.279; P = 0.029); (f) Magee et al39 (N/C = 4/8; t-test = −3.005; P = 0.034). E: DU145 cells were transfected with a specific siRNA for human proepithelin (siPEP) and either DMSO (Veh) or control siRNA (Con) as described in Materials and Methods. Proepithelin expression in cell lysates (40 μg) was detected by immunoblot at 48 and 72 hours after transfection using anti-proepithelin polyclonal antibodies (Zymed Laboratories). One hundred ng of recombinant protein was loaded as control (PEP). Twenty-four hours after transfection, cells were washed and transferred in SFM and counted after 24 and 48 hours in a hemocytometer. The experiment is the average of three independent experiments run in duplicates ±SD. *P < 0.05 compared with control oligos-treated control cells (second and fifth columns).
We further analyzed proepithelin expression in available prostate cancer microarray studies using the Oncomine database and gene microarray data analysis tool.30 The analysis evaluated proepithelin mRNA expression levels for each of the individual studies as well as performing a summary statistic, which takes into account the significance of the gene expression across the considered studies. The meta-analysis (Figure 5D) demonstrated that proepithelin mRNA expression levels were significantly up-regulated in primary prostate cancers compared with nonneoplastic samples across six independent data sets (total normal/cancer samples = 74 of 204, P = 0.017).30,35,36,37,38,39 In one study40 higher Gleason scores correlated to higher proepithelin mRNA expression levels (correlation = 0.458 P = 0.032). No studies showed a statistical correlation between tumors stages and proepithelin mRNA expression levels. However, three independent studies35,39,41 showed decreased proepithelin levels in metastatic prostate cancers in comparison with nonmetastatic prostate carcinomas (P = 5.4E-4, P = 0.037, and P = 1.6E-4, respectively). Furthermore, proepithelin levels have been shown to be significantly decreased in treated versus nontreated tumors after hormone therapy in one study37 (treated/not-treated = 17 of 23; t = 3.201; P = 0.005). Collectively, these results strongly suggest that proepithelin may play a critical role as an autocrine growth factor in the establishment and initial progression of prostate cancer. In addition, proepithelin could represent a promising novel clinical marker for diagnosis of prostate tumors.
Proepithelin Contributes in an Autocrine Manner to the Transforming Phenotype of DU145 Cells
DU145 prostate cancer cells express considerable levels of proepithelin (250 ng/ml) and exhibit the ability to proliferate and migrate in the absence of serum. We performed a series of experiments to ask whether endogenously produced proepithelin may contribute to the transforming phenotype of DU145 cells. We initially determined the effect of transient proepithelin depletion on cell proliferation of DU145 cells in a serum-withdrawal condition. Using siRNA strategies, we achieved almost complete depletion of endogenous proepithelin levels by transiently transfecting DU145 cells with specific siRNA oligos for human proepithelin, as compared with control oligos and vehicle-transfected cells (Figure 5E, top). Proepithelin depletion induced a considerable reduction of cell proliferation in a serum-deprived condition of DU145 cells compared with control cells (Figure 5E, bottom), suggesting that endogenously produced proepithelin contributes to the ability of DU145 cells to grow in the absence of serum.
Stable Depletion of Endogenous Proepithelin by Antisense Approaches Inhibits Cell Proliferation, Wound Healing, and Anchorage-Independent Growth of DU145 Prostate Cancer Cells
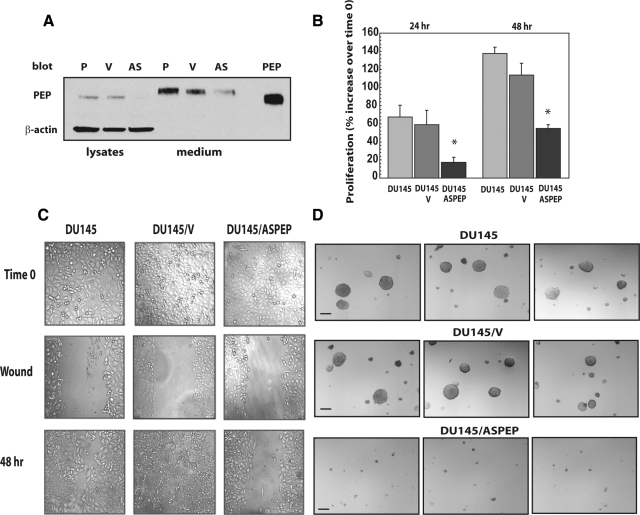
Based on the above results, we wanted to further confirm the role of endogenous proepithelin in transformation of DU145 cells by generating DU145 cells where proepithelin expression had been inhibited by stable expression of antisense proepithelin cDNA. The proepithelin antisense cDNA fragment has been previously described and inhibited proliferation and tumorigenicity of MDA-MB-468 breast cancer cells.42 Mock-, vector-, and antisense proepithelin-transfected DU145 cells were selected for 4 weeks in medium containing G418 and resistant cells were pooled and tested for proepithelin expression. Immunoblot analysis with anti-proepithelin antibodies showed that antisense proepithelin cDNA transfection dramatically reduced proepithelin protein expression in DU145 cells in both cell lysates and conditioned media (Figure 6A), compared with parental and vector-transfected DU145 cells. Inhibition of proepithelin expression determined a strong decrease in the ability of DU145 cells to grow in condition of serum deprivation (Figure 6B) confirming our previous results obtained using siRNA approaches. We next examined cellular migration of the various DU145 cells by performing wound-healing motility assays in SFM: antisense proepithelin DU145 cells were severely inhibited in their capacity to fill the denuded area (Figure 6C) whereas parental and vector-transfected DU145 cells were able to substantially close the wound after 48 hours in culture (Figure 6C). Together, these results indicate that endogenous production of proepithelin plays an important role in regulating the ability of DU145 to proliferate and migrate in the absence of serum, one of the distinctive features of transformed cells.
Figure 6.
Stable depletion of endogenous proepithelin in DU145 cells reduces growth in serum-deprived medium and inhibits anchorage-independent growth. The generation of DU145/V and DU145/ASPEP has been described in the Materials and Methods. A: Proepithelin expression in lysates and conditioned medium (medium) (40 μg) of parental (P), vector-transfected (V), and antisense proepithelin DU145 cells (AS) was detected by immunoblot. Secreted proepithelin in DU145 cells migrates on sodium dodecyl sulfate-polyacrylamide gel electrophoresis slightly slower then intracellular proepithelin and recombinant protein (PEP). B: Cell growth in SFM was determined as described in the Materials and Methods. The experiment is the average of three independent experiments run in duplicates ±SD. *P < 0.05 compared with V-transfected control cells (second and fifth columns). C: The in vitro wound-healing motility assay in untransfected and transfected DU145 cells in SFM was performed as described in the Materials and Methods. Cells were analyzed after 48 hours with a cell live microscope using the Metamorph Image Acquisition and Analysis software (Universal Imaging). A minimum of 10 fields per plate were examined. Original magnification: ×100. D: Colonies in soft agar in serum-supplemented medium were photographed using an Olympus Optic microscope. The experiment is representative of three independent experiments in duplicates. At least 10 independent fields were examined. Scale bars = 100 μm. Original magnification: ×40.
Because one critical parameter of cell transformation is also the ability of cancer cells to grow in an anchorage-independent manner,43 we wanted to determine whether proepithelin depletion of DU145 cells affected their capacity to form colonies in a soft-agar. Parental and V-transfected DU145 cells formed several colonies bigger then 100 μm, with a slight increase in number in V-transfected cells (Table 1 and Figure 6D), in soft agar plates. On the contrary, DU145/ASPEP had dramatically impaired colony formation ability (Table 1 and Figure 6D) indicating that endogenously produced proepithelin critically contributed as an autocrine growth factor in promoting anchorage-independent growth of DU145 prostate cancer cells.
Table 1.
Soft Agar Assay in Various DU145 Cell Lines
| Cell line | Number of colonies |
|---|---|
| DU145 | 74 ± 16 |
| DU145/V | 118 ± 34 |
| DU145/ASPEP | 3 ± 2 |
Cells were counted after 4 weeks. Colonies >100 μm were scored as positive. Data are the average of three independent experiments ±SD.
Discussion
In this study, we provide several lines of evidence that underscore a key role for proepithelin in prostate cancer biology. First, human recombinant proepithelin induces migration and invasion of PC3, LNCaP, and DU145 prostate cancer cells. Second, proepithelin stimulates in vitro closure of a wound and cell proliferation of DU145 prostate cancer cells. Third, proepithelin-mediated effects on DU145 cells require the activation of the Akt and MAP kinase pathway, as determined by using specific pharmacological and genetic inhibitors. Fourth, using siRNA and antisense strategies, we demonstrated that endogenously produced proepithelin by DU145 cells is important for cell growth, migration, and wound healing in serum-deprived conditions and it is critical in regulating anchorage-independent growth in the presence of serum.
Although cases of localized prostate cancer are potentially curable by surgery or radiation techniques, there are no uniformly effective treatments for hormone-refractory metastatic prostate cancer. Moreover, there is a lack of reliable prognostic markers that would identify low-or intermediate-grade prostate cancers that are likely to progress to metastatic disease after local therapy. The present study provides the first evidence that the growth factor proepithelin may play a significant role in prostate tumor formation and initial progression by promoting proliferation, migration, and anchorage-independent growth of prostate cancer cells. In addition, we show that proepithelin is overexpressed in prostate cancer compared with normal prostate, suggesting that endogenous proepithelin may contribute in an autocrine manner to the transforming phenotype.
In other cellular systems, there are reports that indicate that proepithelin is also active intracellularly, where it has been shown to interact with cyclin T144 and regulate transcriptional elongation.44,45 Co-localization experiments have shown that proepithelin co-localizes with cyclin T1 predominantly in the cytoplasm, whereas a protein encompassing epithelins CDE mostly translocates to the nucleus, where it interacts with cyclin T1.44 These data strongly suggest that intracellular proepithelin may have a biological function not only in the cytoplasm but also in the nucleus, where it might affect the transcriptional machinery.44,45
We have characterized the signaling pathways activated by proepithelin in DU145 cells and have shown that the activation of the Akt and MAPK pathway is required for proepithelin-induced migration, invasion, and growth of DU145 prostate cancer cells. This is distinct from our recently reported data that in 5637 bladder cancer cells, where proepithelin does not promote the activation of Akt, suggesting that the PI3K pathway does not play a role in migration and invasion in these bladder cancer cells.22 Although proepithelin is effective in promoting migration and invasion of 5637 cells, it is not strongly mitogenic for these cells, suggesting that proepithelin in some cell systems may play a more critical role in promoting a stimulus for migration and invasion rather than for proliferation.12,46
The ability of cancer cells to migrate and invade through extracellular matrix is a critical step for tumor metastasis to occur.33 Our results suggest that proepithelin by promoting MAPK- and Akt-dependent cell growth, migration, and invasion may play an important role in the establishment and progression of prostate cancer. Proepithelin may also determine the transition from a noninvasive to an invasive phenotype in prostate and in other solid tumors, where the role of proepithelin in promoting invasion and migration has been reported as well.12,28,34
Prostate cancer cells respond in migration and invasion to the purified proepithelin precursor. Furthermore, DU145 cells produce the 88-kDa precursor protein, which is detectable by immunoblot in lysates and conditioned medium. These results would indicate that these cells secrete the proepithelin precursor, which is likely the bioactive form in prostate cancer cells. Significantly, endogenous proepithelin is required for growth of DU145 cells in serum deprivation and it is critical for anchorage-independent growth indicating that proepithelin acts as an autocrine growth factor in promoting transformation of DU145 cells. However, we cannot totally rule out the possibility that some intracellular processing into epithelin peptides might occur and contribute to the biological response.
Antisense proepithelin-expressing DU145 cells show a growth rate in monolayer in serum-containing medium very similar to parental and vector-transfected control cells. Targeting endogenous proepithelin severely reduced but did not completely abolish the ability of DU145 cells to grow in monolayer culture without serum. In contrast, the reduction in proepithelin levels had a really dramatic effect in inhibiting growth of DU145 cells in an anchorage-independent condition in the presence of serum, a condition that is closer to the cellular environment in vivo. It is, therefore, reasonable to speculate that targeting of proepithelin may be very effective on metastases, which can be considered a model of anchorage independence, because they usually arise from single cells or small clusters of metastatic cells. These results strongly suggest that proepithelin may be a very attractive target in prostate cancer cells with little toxicity to normal cells. Although our results clearly establish a role of proepithelin in regulating proliferation and motility of prostate cancer cells in vitro, further experiments are required to address the role that proepithelin might play in regulating these processes in vivo.
In addition to DU145 cells, which are androgen-independent and originally cultured from a human prostate adenocarcinoma brain metastasis,47 we tested proepithelin activity on androgen-dependent LNCaP prostate cancer cells and, although at a reduced level, proepithelin promoted migration and invasion in these cells. In contrast, proepithelin was not effective as in DU145 cells in promoting cell proliferation of LNCaP cells (data not shown). These results suggest that proepithelin in prostate cancer may play a role in promoting the progression to the androgen-independent/hormone-refractory stage of the tumor, which is more aggressive and highly metastatic.
In conclusion, the identification of the growth factor proepithelin as a novel regulator of transformation of prostate cancer cells could represent an additional molecular target for prostate cancer and could provide new leads for developing novel tumor biomarkers and for improved therapeutic approaches against prostate cancer.
Supplementary Material
Acknowledgments
We thank Dr. Renato Baserga for the kind gift of the human proepithelin expression vector and Judy Verdone for her skillful assistance in cell culture.
Footnotes
Address reprint requests to Andrea Morrione, Department of Urology, Endocrine Mechanisms and Hormone Action Program, Kimmel Cancer Center, Thomas Jefferson University, 233 South 10th St., BLSB Room 620, Philadelphia, PA, 19107. E-mail: andrea.morrione@jefferson.edu or amorrion@mail.jci.tju.edu.
Supported by the Benjamin Perkins Bladder Cancer Fund, the Martin Greitzer Fund, and the National Institutes of Health (grants RO1 DK 068419 to A.M. and RO1 CA39481 and RO1 CA047282 to R.V.I.).
G.M. and V.E. contributed equally to this study.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas Jefferson University was involved in the peer review process or final disposition for this article.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jones MB, Spooner M, Kohn EC. The granulin-epithelin precursor: a putative new growth factor for ovarian cancer. Gynecol Oncol. 2003;88:S136–S139. doi: 10.1006/gyno.2002.6704. [DOI] [PubMed] [Google Scholar]
- Plowman GD, Green JM, Neubauer MG, Buckley SD, McDonald VL, Todaro GJ, Shoyab M. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J Biol Chem. 1992;267:13073–13078. [PubMed] [Google Scholar]
- Xu SQ, Tang D, Chamberlain S, Pronk G, Masiarz FR, Kaur S, Prisco M, Zanocco-Marani T, Baserga R. The granulin/epithelin precursor abrogates the requirement for the insulin-like growth factor 1 receptor for growth in vitro. J Biol Chem. 1998;273:20078–20083. doi: 10.1074/jbc.273.32.20078. [DOI] [PubMed] [Google Scholar]
- Bateman A, Bennett HP. Granulins: the structure and function of an emerging family of growth factors. J Endocrinol. 1998;158:145–151. doi: 10.1677/joe.0.1580145. [DOI] [PubMed] [Google Scholar]
- Baba T, Hoff HB, III, Nemoto H, Lee H, Orth J, Arai Y, Gerton GL. Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol Reprod Dev. 1993;34:233–243. doi: 10.1002/mrd.1080340302. [DOI] [PubMed] [Google Scholar]
- Gonzalez EM, Mongiat M, Slater SJ, Baffa R, Iozzo RV. A novel interaction between perlecan protein core and progranulin: potential effects on tumor growth. J Biol Chem. 2003;278:38113–38116. doi: 10.1074/jbc.C300310200. [DOI] [PubMed] [Google Scholar]
- Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S. Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun. 1990;173:1161–1168. doi: 10.1016/s0006-291x(05)80908-8. [DOI] [PubMed] [Google Scholar]
- Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl SM, Lacomis L, Erdjument-Bromage H, Tempst P, Wright CD, Ding A. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–878. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14:3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanocco-Marani T, Bateman A, Romano G, Valentinis B, He ZH, Baserga R. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999;59:5331–5340. [PubMed] [Google Scholar]
- He Z, Ismail A, Kriazhev L, Sadvakassova G, Bateman A. Progranulin (PC-cell-derived growth factor/acrogranin) regulates invasion and cell survival. Cancer Res. 2002;62:5590–5596. [PubMed] [Google Scholar]
- Lu R, Serrero G. Stimulation of PC cell-derived growth factor (epithelin/granulin precursor) expression by estradiol in human breast cancer cells. Biochem Biophys Res Commun. 1999;256:204–207. doi: 10.1006/bbrc.1999.0253. [DOI] [PubMed] [Google Scholar]
- Lu R, Serrero G. Inhibition of PC cell-derived growth factor (PCDGF, epithelin/granulin precursor) expression by antisense PCDGF cDNA transfection inhibits tumorigenicity of the human breast carcinoma cell line MDA-MB-468. Proc Natl Acad Sci USA. 2000;97:3993–3998. doi: 10.1073/pnas.97.8.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Serrero G. Mediation of estrogen mitogenic effect in human breast cancer MCF-7 cells by PC-cell-derived growth factor (PCDGF/granulin precursor). Proc Natl Acad Sci USA. 2001;98:142–147. doi: 10.1073/pnas.011525198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81:600–612. doi: 10.1007/s00109-003-0474-3. [DOI] [PubMed] [Google Scholar]
- Zhang H, Serrero G. Inhibition of tumorigenicity of the teratoma PC cell line by transfection with antisense cDNA for PC cell-derived growth factor (PCDGF, epithelin/granulin precursor). Proc Natl Acad Sci USA. 1998;95:14202–14207. doi: 10.1073/pnas.95.24.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hayashi J, Kim WE, Serrero G. PC cell-derived growth factor (granulin precursor) expression and action in human multiple myeloma. Clin Cancer Res. 2003;9:2221–2228. [PubMed] [Google Scholar]
- Wang W, Hayashi J, Serrero G. PC cell-derived growth factor confers resistance to dexamethasone and promotes tumorigenesis in human multiple myeloma. Clin Cancer Res. 2006;12:49–56. doi: 10.1158/1078-0432.CCR-05-0929. [DOI] [PubMed] [Google Scholar]
- Davidson B, Alejandro E, Florenes VA, Goderstad JM, Risberg B, Kristensen GB, Trope CG, Kohn EC. Granulin-epithelin precursor is a novel prognostic marker in epithelial ovarian carcinoma. Cancer. 2004;100:2139–2147. doi: 10.1002/cncr.20219. [DOI] [PubMed] [Google Scholar]
- Pan CX, Kinch MS, Kiener PA, Langermann S, Serrero G, Sun L, Corvera J, Sweeney CJ, Li L, Zhang S, Baldridge LA, Jones TD, Koch MO, Ulbright TM, Eble JN, Cheng L. PC cell-derived growth factor expression in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. Clin Cancer Res. 2004;10:1333–1337. doi: 10.1158/1078-0432.ccr-1123-03. [DOI] [PubMed] [Google Scholar]
- Monami G, Gonzalez EM, Hellman M, Gomella LG, Baffa R, Iozzo RV, Morrione A. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66:7103–7110. doi: 10.1158/0008-5472.CAN-06-0633. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Van Hoecke A, Lambrechts D, Vanacker P, Bogaert E, van Swieten J, Carmeliet P, Van Den Bosch L, Robberecht W. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol. 2008;181:37–41. doi: 10.1083/jcb.200712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Serrero G. Identification of cell surface binding sites for PC-cell-derived growth factor, PCDGF, (epithelin/granulin precursor) on epithelial cells and fibroblasts. Biochem Biophys Res Commun. 1998;245:539–543. doi: 10.1006/bbrc.1998.8498. [DOI] [PubMed] [Google Scholar]
- Mongiat M, Sweeney SM, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- Chen HY, Shen CH, Tsai YT, Lin FC, Huang YP, Chen RH. Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol Cell Biol. 2004;24:10558–10572. doi: 10.1128/MCB.24.24.10558-10572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrione A, Valentinis B, Resnicoff M, Xu S, Baserga R. The role of mGrb10alpha in insulin-like growth factor I-mediated growth. J Biol Chem. 1997;272:26382–26387. doi: 10.1074/jbc.272.42.26382. [DOI] [PubMed] [Google Scholar]
- Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci USA. 2004;101:9309–9314. doi: 10.1073/pnas.0401994101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, Rhodes DR, Ingold C, Chinnaiyan AM, Rubin MA. Dysregulation of the annexin family protein family is associated with prostate cancer progression. Am J Pathol. 2003;162:255–261. doi: 10.1016/S0002-9440(10)63816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Tangkeangsirisin W, Serrero G. PC cell-derived growth factor (PCDGF/GP88, progranulin) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis. 2004;25:1587–1592. doi: 10.1093/carcin/bgh171. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran SM, Dash A, Yu J, Maine IP, Laxman B, Tomlins SA, Creighton CJ, Menon A, Rubin MA, Chinnaiyan AM. Molecular profiling of human prostate tissues: insights into gene expression patterns of prostate development during puberty. FASEB J. 2005;19:243–245. doi: 10.1096/fj.04-2415fje. [DOI] [PubMed] [Google Scholar]
- Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, Reuter V, Gerald WL. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, Frierson HF, Jr, Hampton GM. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61:5974–5978. [PubMed] [Google Scholar]
- Magee JA, Araki T, Patil S, Ehrig T, True L, Humphrey PA, Catalona WJ, Watson MA, Milbrandt J. Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res. 2001;61:5692–5696. [PubMed] [Google Scholar]
- Nanni S, Priolo C, Grasselli A, D'Eletto M, Merola R, Moretti F, Gallucci M, De Carli P, Sentinelli S, Cianciulli AM, Mottolese M, Carlini P, Arcelli D, Helmer-Citterich M, Gaetano C, Loda M, Pontecorvi A, Bacchetti S, Sacchi A, Farsetti A. Epithelial-restricted gene profile of primary cultures from human prostate tumors: a molecular approach to predict clinical behavior of prostate cancer. Mol Cancer Res. 2006;4:79–92. doi: 10.1158/1541-7786.MCR-05-0098. [DOI] [PubMed] [Google Scholar]
- Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, McDonald C, Thomas R, Dhir R, Finkelstein S, Michalopoulos G, Becich M, Luo JH. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Lei K, Jin W, Longenecker G, Kulkarni AB, Greenwell-Wild T, Hale-Donze H, McGrady G, Song XY, Wahl SM. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147–1153. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- Boyd DD, Levine AE, Brattain DE, McKnight MK, Brattain MG. Comparison of growth requirements of two human intratumoral colon carcinoma cell lines in monolayer and soft agarose. Cancer Res. 1988;48:2469–2474. [PubMed] [Google Scholar]
- Hoque M, Young TM, Lee CG, Serrero G, Mathews MB, Pe'ery T. The growth factor granulin interacts with cyclin T1 and modulates P-TEFb-dependent transcription. Mol Cell Biol. 2003;23:1688–1702. doi: 10.1128/MCB.23.5.1688-1702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque M, Tian B, Mathews MB, Pe'ery T. Granulin and granulin repeats interact with the Tat: P-TEFb complex and inhibit tat transactivation. J Biol Chem. 2005;280:13648–13657. doi: 10.1074/jbc.M409575200. [DOI] [PubMed] [Google Scholar]
- He Z, Bateman A. Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer Res. 1999;59:3222–3229. [PubMed] [Google Scholar]
- Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.