Abstract
Background
Most cured meats contain nitrites. Nitrites generate oxidative-nitrative stress and were shown in animal models to cause emphysema. Prospective epidemiologic data on cured meats and chronic obstructive pulmonary disease (COPD), however, are sparse.
Objective
We examined the relation between cured meat consumption and the prospective risk of newly diagnosed COPD in women.
Design
This was a prospective cohort study of 71 531 women from the Nurses’ Health Study who completed a validated dietary questionnaire at baseline in 1984 and had no baseline COPD or a report of asthma. Participants were aged 38–63 y in 1984 and were followed for 16 y.
Results
A total of 750 new cases of COPD were documented during the follow-up. Cured meat consumption was positively associated with COPD risk after adjustment for age, smoking, and multiple other potential confounders. The adjusted relative risks of COPD across categories of cured meat consumption (never or almost never, 1–3 servings/mo, 1 serving/wk, 2–3 servings/wk, and ≥4 servings/wk) were 1.0, 1.14 (95% CI: 0.78, 1.66), 1.15 (95% CI: 0.79, 1.69), 1.40 (95% CI: 0.96, 2.05), and 1.51 (95% CI: 1.00, 2.27), respectively, (P for trend = 0.005). This positive association was present among both past (P for trend = 0.02) and current (P for trend = 0.03) smokers. No association was observed among never smokers, probably because of the small number of COPD cases in these women.
Conclusion
Frequent cured meat consumption was associated with an increased risk of newly diagnosed COPD among women who smoke.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD), characterized by the presence of airflow limitation on spirometry that is not fully reversible, is currently the fourth-leading cause of death in the United States (1, 2). COPD prevalence and the rate of mortality continue to rise, particularly among women. By the year 2020, COPD is projected to be the third-leading cause of death worldwide (3). Despite this disease burden, preventive strategies for COPD are limited to avoidance of harmful exposures, including cigarette smoke, occupational exposures, and high amounts of ambient air pollution.
Cured meats such as bacon, hot dogs, and processed meats contain nitrites, which are added to meat products as a preservative, antimicrobial agent, and color fixative (4). Nitrites are involved in the formation of reactive nitrogen species such as nitrogen dioxide, nitrosonium, or dintrogen trioxide (5, 6) that may cause nitrative and nitrosative stress in the lung. Experiments in animal models performed almost 40 y ago showed that ingestion of sodium nitrite (1000–3000 mg/L) (7) and inhalation of nitrogen dioxide (a nitrite precursor) (8–10) cause pathologic changes to the lungs consistent with emphysema.
We recently found in a cross-sectional study of 7352 men and women from the population-based third National Health and Nutrition Examination Survey that frequent consumption of cured meats was associated with an obstructive pattern of spirometry (11). We have replicated this finding with the use of a validated definition of self-reported physician diagnosis of COPD in the Health Professionals Follow-up Study, a prospective cohort study of men (12). To our knowledge, this is the only published prospective study that has evaluated the relation between consumption of cured meats and prospective risk of COPD. We therefore further examined the association between frequency of cured meat consumption and newly diagnosed COPD in a large prospective cohort study of women, hypothesizing that frequent cured meat consumption was associated with an increased risk of newly diagnosed COPD.
SUBJECTS AND METHODS
Study population
The Nurses’ Health Study (NHS) is a cohort study of 121 700 US female registered nurses, aged 30–55 y at enrollment in 1976, who completed a mailed questionnaire about their lifestyle and medical history at baseline (13). Updated information on lifestyle, health behaviors, including smoking status, and disease status has been collected biennially by mailed questionnaires since 1976. Diet was assessed with a 116-item semiquantitative food-frequency questionnaire in 1984, and similar questionnaires were used to update dietary information every 2–4 y. The institutional review board approved the NHS protocols, and written consent was obtained from all subjects. We followed participants from 1984 to 2000, excluding women with an inadequate assessment of diet (≥70 blanks of 116 listed food items) or an implausibly high (>3500 kcal/d) or low (<500 kcal/d) total energy intake. We further excluded 3745 women with COPD at baseline in 1984, missing date of COPD diagnosis, or unconfirmed diagnosis, and 5874 with reported asthma. After these exclusions, 71 531 participants remained in the analysis.
Dietary assessment
The semiquantitative food-frequency questionnaires asked participants how often, on average, during the previous year they had consumed listed food items and beverages, with a specified commonly used unit or portion size (14). Nine frequency responses were listed, ranging from “never or almost never (less than once per month)” to “6+ per day.” We defined total cured meat consumption as the sum of the intakes for bacon (portion size: 2 slices), hog dogs (portion size: 1), and processed meats (sausage, salami, bologna, etc; portion size: 1 piece or slice), which were listed as 3 separate items. The responses for bacon, hot dogs, and processed meats on the food-frequency questionnaire were previously validated against four 1-wk diet records in a random sample of 173 women (15). Pearson’s correlation coefficients for intake measured by the food-frequency questionnaire and by four 1-wk diet records were 0.70 for bacon, 0.56 for hot dogs, and 0.55 for sausage, salami, bologna, and other processed meats, indicating the questionnaire measured cured meat consumption adequately. To reduce within-subject variation and to best represent long-term dietary intake, we used the cumulative average of cured meat intakes from the questionnaires up to the start of each 2-y follow-up interval (16). For example, 1984 intake was used for follow-up between 1984 and 1986; the average of the 1984 and 1986 intakes was used for follow-up between 1986 and 1990; and the average of the 1984, 1986, and 1990 intakes was used for follow-up between 1990 and 1994 (16).
Measurement of nondietary factors
The participants provided information about their date of birth, race-ethnicity, and spouse’s education on the baseline NHS questionnaire in 1976; exposure to secondhand smoke in 1982 (at home or work); and their smoking (never smoker, former smoker, or current smoker, including the number of cigarette smoked per day), region, menopause status, and body weight in 1976 and every 2 y during the follow-up period. Pack-years of smoking in 1976 was calculated as duration of smoking (in y) reported up to 1976 multiplied by the average packs of cigarettes smoked per day before 1976. Pack-years in subsequent years were calculated as pack-years in 1976 plus the interval smoking history, which was collected prospectively by questionnaire every 2 y. Physician visits and exposure to secondhand tobacco smoke were assessed on the 1982 questionnaire. Body mass index (BMI; in kg/m2) was calculated (2); the correlation coefficient between self-reported weight and measured weight was 0.96 (17). A physical activity score in metabolic equivalents (MET-h/wk) was derived, based on questions from the 1986, 1988, 1992, 1996, and 1998 questionnaires, including the average weekly time spent during the past year on walking, hiking, jogging, running, bicycling, lap swimming, playing tennis or squash, and participating in calisthenics. The correlation between activities reported in four 1-wk diaries and those reported on the questionnaire was 0.62 (18).
Definition of COPD
We were unable to obtain standardized, reliable spirometry on this random sample because NHS participants are geographically dispersed and are contacted by mail. All participants were asked about a new physician diagnosis of emphysema or chronic bronchitis on the biennial questionnaires. Participants who responded in the affirmative were sent supplemental questionnaires on COPD in 1998–2000. COPD was defined as a self-report of a physician diagnosis on both the original and supplemental questionnaires, plus a report of spirometry, chest radiograph or chest computed tomography at the time of diagnosis, or documentation of COPD on death records. If a physician diagnosis of chronic bronchitis only was reported, symptoms consistent with chronic bronchitis defined by the Medical Research Council were required in addition to the above criteria. This definition of COPD was previously validated in a 10% random sample of this cohort (19). The diagnosis of COPD was confirmed in 88%. Results of pulmonary function testing were available for 71%; the mean forced expiratory volume in 1 s in this group was 50% of predicted.
A supplementary questionnaire was also mailed to all participants who reported a physician diagnosis of asthma on biennial questionnaires in the NHS. Adult-onset asthma, as a control outcome in this study, was defined as self-report on original and supplemental questionnaires (20).
Statistical analysis
We divided the participants into 5 categories (never or almost never, 1–3 serving/mo, 1 serving/wk, 2–3 servings/wk, and ≥4 servings/wk) according to their frequency of cured meat consumption. These categories were consistent with prior publications from this cohort for food intake (21, 22) and also allowed each category to have an adequate number of cases for the main analysis. Means and SDs or medians and interquartile ranges (25th and 75th percentiles) and proportions were calculated, as appropriate, for selected variables by categories of cured meat consumption.
Person-time for each participant was calculated from the date of return of the 1984 questionnaire to the date of COPD diagnosis, death from any cause, or 1 June 2000, whichever came first. Relative risks (RRs) were estimated for specific categories of cured meat consumption with the lowest category as the common comparison group by Cox proportional hazards models. RR for the continuous measure of cured meat intake was also computed. In the multivariate models, we controlled for age; race-ethnicity; smoking status; pack-years; exposure to secondhand tobacco smoke; menopausal status; spouse’s education attainment (as a surrogate measure of socioeconomic status); physician visits; US region; multivitamin use; BMI; physical activity; and intakes of fish, fruit, vegetables, and total energy. Multiple categories of BMI (<20, 20–22.4, 22.5–24.9, 25.0 –27.4, 27.5–29.9, 30.0– 34.9, ≥35.0) were used in the models.
We also conducted stratified analyses to examine whether the association between cured meat consumption and COPD risk were modified by smoking status. Because tobacco smoke is another main source of nitrites in the body besides cured meats, we further examined the joint association of cured meat consumption and smoking status. Interaction terms between cured meat consumption and smoking status on the multiplicative scale were entered into the models, and tests for models with and without interaction terms were used to evaluate the significance of the interaction terms.
Tests for trend were calculated by treating the medians of the categories of cured meat consumption as a continuous variable. All P values were 2-sided. P<0.05 was considered statistically significant. Weused SAS software (SAS Institute Inc, Cary, NC) version 9 for analyses.
RESULTS
The baseline characteristics of participants, stratified by frequency of cured meat consumption, are shown in Table 1. Participants who consumed cured meats more frequently were more likely to smoke and be exposed to secondhand smoke, had a higher BMI, were less physically active, had higher total energy intake, and were less likely to use multivitamin supplements than were participants who rarely consumed cured meats. Participants who consumed cured meats more frequently had lower intakes of fruit; however, intakes of fish and vegetables were similar across categories of cured meat consumption.
TABLE 1.
Baseline characteristics according to frequency of cured meat consumption in 19841
| Never or almost never (n = 9086) |
1–3 servings/mo (n = 19 973) |
1 serving/wk (n = 19 366) |
2–3 servings/wk (n = 13 138) |
≥4 servings/wk (n = 9968) |
|
|---|---|---|---|---|---|
| Age (y) | 52.4 ± 6.872 | 51.1 ± 7.10 | 49.9 ± 7.17 | 49.8 ± 7.16 | 49.4 ± 7.16 |
| Race-ethnicity (white) (%)3 | 81.3 | 80.1 | 79.3 | 78.3 | 77.7 |
| Smoking status (%) | |||||
| Never smokers | 47.1 | 44.8 | 43.9 | 43.4 | 43.3 |
| Past smokers | 36.3 | 33.9 | 31.9 | 29.6 | 27.8 |
| Current smokers | 16.6 | 21.4 | 24.2 | 27.0 | 28.9 |
| Pack-years among ever smokers | 15.0 (5, 30)4 | 16.0 (6, 31) | 17.0 (6, 32) | 19.0 (7, 33) | 20.0 (7, 34) |
| Exposure to secondhand smoke at work or home (%) | 75.3 | 80.8 | 83.9 | 83.5 | 85.2 |
| Postmenopausal hormone use (%) | 13.8 | 12.6 | 11.8 | 11.8 | 10.9 |
| Husbands’ education attainment (%) | |||||
| High school | 34.2 | 42.5 | 47.8 | 49.5 | 54.5 |
| College | 31.3 | 29.9 | 29.7 | 29.0 | 26.7 |
| Graduate school | 34.5 | 27.6 | 22.5 | 21.5 | 18.8 |
| No. of physician visits (%) | 11.9 | 11.3 | 11.6 | 12.2 | 12.9 |
| US region (%) | |||||
| New England | 15.4 | 15.7 | 14.6 | 13.8 | 11.3 |
| Mid-Atlantic | 38.6 | 42.6 | 44.4 | 44.6 | 44.9 |
| East North Central | 15.6 | 17.8 | 20.5 | 20.3 | 22.7 |
| South Atlantic | 5.9 | 5.5 | 5.7 | 6.0 | 6.5 |
| West South Central | 4.5 | 4.5 | 4.1 | 5.3 | 6.0 |
| Pacific | 20.1 | 13.9 | 10.7 | 10.0 | 8.6 |
| Multivitamin use (%) | 45.4 | 38.4 | 35.7 | 35.1 | 32.4 |
| BMI (kg/m2) | 23.3 (21.3, 25.8) | 23.7 (21.6, 26.6) | 23.9 (21.8, 27.2) | 24.2 (21.9, 27.5) | 24.5 (22.0, 28.2) |
| Physical activity (MET/wk)5 | 10.2 (3.40, 23.2) | 8.4 (2.90, 20.2) | 7.7 (2.7, 18.4) | 6.7 (2.40, 16.5) | 5.5 (2.30, 15.2) |
| Intake | |||||
| Fish (servings/d) | 0.28 (0.14, 0.50) | 0.21 (0.14, 0.42) | 0.21 (0.14, 0.35) | 0.21 (0.14, 0.35) | 0.21 (0.14, 0.35) |
| Fruit (servings/d) | 2.20 (1.40, 3.20) | 1.93 (1.20, 2.85) | 1.85 (1.14, 2.71) | 1.85 (1.13, 2.71) | 1.85 (1.14, 2.77) |
| Vegetables (servings/d) | 3.33 (2.24, 4.73) | 3.01 (2.06, 4.26) | 2.91 (2.05, 4.04) | 2.98 (2.12, 4.11) | 3.11 (2.20, 4.26) |
| Total energy (kcal/d) | 1447 (1153, 1788) | 1540 (1244, 1882) | 1675 (1379, 2012) | 1820 (1502, 2178) | 2060 (1719, 2463) |
All tests for trends or chi-square tests across categories were significant (P < 0.001).
x̄ ± SD (all such values).
Participants were classified as white if they only reported being white (Southern European or Mediterranean origin, Scandinavian, and other whites).
Median; 25th, 75th percentiles in parentheses (all such values).
MET, metabolic equivalent.
During the 16-y (1 110 126 person-years) follow-up, we documented 750 cases of newly diagnosed COPD. Frequency of cured meat consumption was positively associated with the risk of COPD in the age-adjusted model (Table 2). The age-adjusted RR for women consuming cured meat ≥4 servings/wk was 3.00 (95% CI: 2.05, 4.41) compared with participants who rarely consumed cured meats. After controlling for smoking variables (smoking status, pack-years, pack-years squared, and exposure to secondhand tobacco smoke), the association was moderately attenuated but remained statistically significant (RR: 1.67; 95% CI: 1.13, 2.46; P for trend < 0.001). In addition, adjustment for multiple other potential confounders did not materially change the relation (Table 2). Each serving-per-week increase in cured meat intake was associated with a 4% higher risk of newly diagnosed COPD.
TABLE 2.
Relative risks (95% CI) of chronic obstructive pulmonary disease according to frequency of cumulative cured meat consumption (1984–2000)
| Never or almost never | 1–3 servings/mo | 1 serving/wk | 2–3 servings/wk | ≥4 servings/wk | P for trend1 | |
|---|---|---|---|---|---|---|
| No. of cases | 33 | 176 | 189 | 225 | 127 | |
| Person-years | 74 471 | 317 386 | 319 460 | 275 905 | 122 905 | |
| Age-adjusted | 1 (referent) | 1.47 (1.01, 2.13) | 1.72 (1.18, 2.49) | 2.42 (1.68, 3.50) | 3.00 (2.05, 4.41) | <0.001 |
| Age- and smoking-adjusted2 | 1 (referent) | 1.19 (0.82, 1.73) | 1.23 (0.85, 1.78) | 1.52 (1.05, 2.21) | 1.67 (1.13, 2.46) | <0.001 |
| Multivariate3 | 1 (referent) | 1.14 (0.78, 1.66) | 1.15 (0.79, 1.69) | 1.40 (0.96, 2.05) | 1.51 (1.00, 2.27) | 0.005 |
Tests for linear trend were calculated by assigning the medium values for each category of cured meat consumption.
Cox proportional hazard model was adjusted for age, smoking status, pack-years, pack-years squared, and exposure to secondhand tobacco.
Cox proportional hazard model was adjusted for age, smoking status, pack-years, pack-years squared, exposure to secondhand tobacco, race-ethnicity, menopausal status, postmenopausal hormone use, spouse’s educational attainment, physician visits, US region, multivitamin use, BMI, physical activity, and dietary intake of fish, fruit, vegetables, and total energy.
To examine whether smoking status modifies the relation between cured meat consumption and COPD risk, we conducted multivariate analyses within strata defined by smoking status. The RR comparing the highest with the lowest categories of cured meat consumption was 2.34 (95% CI: 0.98, 5.62; P for trend = 0.02) in past smokers and was 1.30 (95% CI: 0.77, 2.20; P for trend = 0.03) in current smokers. Cured meat consumption was not associated with COPD risk among never smokers, possibly because of the small number of COPD cases, particularly in the lowest and highest categories of cured meat consumption (Table 3). The P value for the interaction between cured meat consumption and smoking status was 0.12 (2 df).
TABLE 3.
Relative risks (95% CI) of chronic obstructive pulmonary disease according to frequency of cumulative cured meat consumption (1984 –2000), stratified by smoking status1
| Never or almost never | 1–3 servings/mo | 1 serving/wk | 2–3 servings/wk | ≥4 servings/wk | P for trend2 | |
|---|---|---|---|---|---|---|
| Never smokers | ||||||
| No. of cases (n = 84) | 7 | 29 | 21 | 21 | 6 | |
| Person-years | 36 502 | 144 025 | 141 039 | 121 015 | 52 493 | |
| Age-adjusted | 1 (referent) | 1.14 (0.50, 2.61) | 0.91 (0.39, 2.16) | 1.06 (0.45, 2.52) | 0.69 (0.23, 2.07) | 0.40 |
| Multivariate3 | 1 (referent) | 1.21 (0.52, 2.80) | 0.97 (0.40, 2.36) | 1.14 (0.46, 2.86) | 0.72 (0.22, 2.30) | 0.46 |
| Past smokers | ||||||
| No. of cases (n = 198) | 7 | 49 | 52 | 64 | 26 | |
| Person-years | 29 237 | 131 360 | 124 726 | 101 837 | 41 357 | |
| Age-adjusted | 1 (referent) | 1.73 (0.78, 3.82) | 2.14 (0.97, 4.73) | 3.28 (1.50, 7.17) | 3.31 (1.43, 7.65) | <0.001 |
| Multivariate2 | 1 (referent) | 1.54 (0.69, 3.44) | 1.77 (0.79, 3.97) | 2.46 (1.09, 5.51) | 2.34 (0.98, 5.62) | 0.02 |
| Current smokers | ||||||
| No. of cases (n = 466) | 19 | 98 | 115 | 139 | 95 | |
| Person-years | 7855 | 39 904 | 51 490 | 51 140 | 27 864 | |
| Age-adjusted | 1 (referent) | 1.13 (0.69, 1.85) | 1.18 (0.72, 1.92) | 1.49 (0.92, 2.41) | 1.85 (1.13, 3.04) | <0.001 |
| Multivariate3 | 1 (referent) | 0.98 (0.59, 1.61) | 0.98 (0.59, 1.61) | 1.12 (0.68, 1.85) | 1.30 (0.77, 2.20) | 0.03 |
P for interaction between cured meat consumption and smoking status = 0.12 (2 df).
Tests for linear trend were calculated by assigning the medium values for each category of cured meat consumption.
Cox proportional hazard model was adjusted for age, pack-years, pack-years squared, exposure to secondhand tobacco, race-ethnicity, menopausal status, postmenopausal hormone use, spouse’s educational attainment, physician visits, US region, multivitamin use, BMI, physical activity, and dietary intake of fish, fruit, vegetables, and total energy.
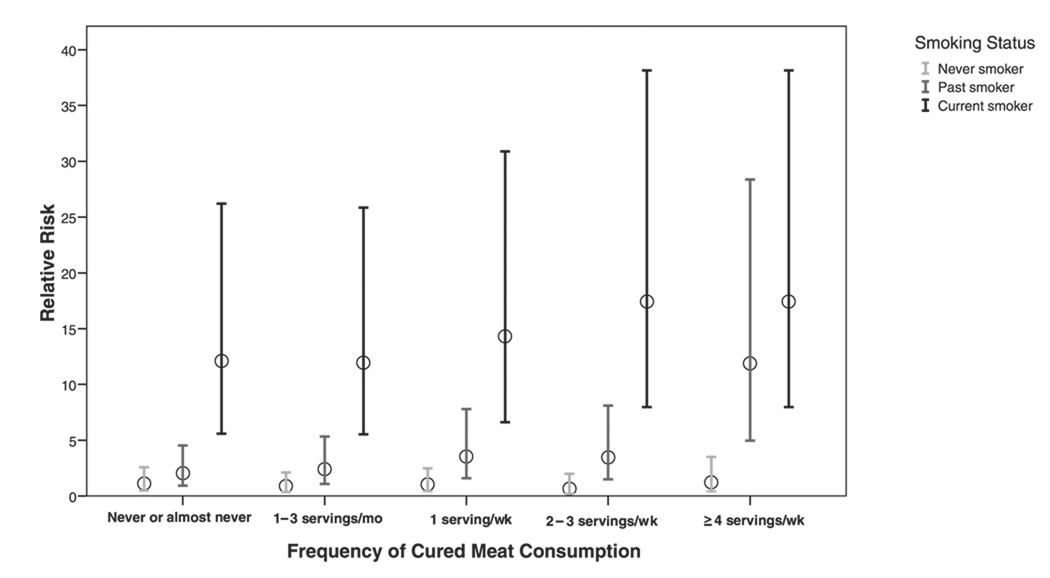
We next examined the joint association of cured meat consumption and smoking status with risk of COPD. We found that women who were current smokers and had cured meat consumption ≥4 servings/wk had the highest risk (RR: 17.4; 95% CI: 7.97, 38.1), in comparison with never smokers who rarely ate cured meats (Figure 1).
FIGURE 1.
Joint association of cured meat consumption and smoking status. Cox proportional hazard model was adjusted for age, exposure to secondhand tobacco smoke, race-ethnicity, menopausal status, postmenopausal hormone use, spouse’s educational attainment, physician visits, US region, multivitamin use, BMI, physical activity, and dietary intake of fish, fruit, vegetables, and total energy.
In the analyses of bacon, hot dogs, and processed meats separately, we combined the 2 highest categories because of the small number of cases in these categories (Table 4). The positive association was statistically significant for bacon, hot dogs, and processed meats in age-adjusted models but was not statistically significant for hot dogs and processed meats in multivariate models.
TABLE 4.
Relative risks (95% CI) of chronic obstructive pulmonary disease according to frequency of cumulative consumption of bacon, hot dogs, and processed meats (1984 –2000)
| Never or almost never | 1–3 servings/mo | 1 serving/wk | ≥2 servings/wk | P for trend1 | |
|---|---|---|---|---|---|
| Bacon | |||||
| No. of cases | 135 | 453 | 69 | 93 | |
| Person-years | 279 639 | 696 730 | 58 285 | 75 472 | |
| Age-adjusted | 1 (referent) | 1.56 (1.28, 1.89) | 2.85 (2.12, 3.82) | 2.57 (1.97, 3.35) | <0.001 |
| Multivariate2 | 1 (referent) | 1.23 (1.01, 1.50) | 1.85 (1.36, 2.51) | 1.44 (1.09, 1.91) | 0.006 |
| Hot dogs | |||||
| No. of cases | 167 | 513 | 36 | 34 | |
| Person-years | 255 541 | 766 130 | 52 782 | 35 673 | |
| Age-adjusted | 1 (referent) | 1.20 (1.01, 1.43) | 1.43 (0.99, 2.06) | 1.71 (1.18, 2.47) | 0.002 |
| Multivariate2 | 1 (referent) | 0.99 (0.83, 1.19) | 1.04 (0.71, 1.52) | 1.32 (0.90, 1.94) | 0.16 |
| Sausage, salami, bologna, and other processed meats | |||||
| No. of cases | 142 | 405 | 70 | 133 | |
| Person-years | 223 662 | 642 252 | 98 120 | 146 092 | |
| Age-adjusted | 1 (referent) | 1.22 (1.00, 1.48) | 1.48 (1.11, 1.99) | 1.80 (1.42, 2.29) | <0.001 |
| Multivariate2 | 1 (referent) | 0.99 (0.81, 1.21) | 1.01 (0.74, 1.36) | 1.13 (0.88, 1.46) | 0.22 |
Tests for linear trend were calculated by assigning the medium values for each category of cured meat consumption.
Cox proportional hazard model was adjusted for age, smoking status, pack-years, pack-years squared, exposure to secondhand tobacco, race-ethnicity, menopausal status, postmenopausal hormone use, spouse’s educational attainment, physician visits, US region, multivitamin use, BMI, physical activity, and dietary intake of fish, fruit, vegetables, and total energy.
In further analyses of cured meat consumption and risk of newly diagnosed, adult-onset asthma, no significant associations were observed. For 818 cases of newly diagnosed asthma during follow-up and the age-adjusted RR for newly diagnosed asthma that compared the highest with the lowest category of cured meat consumption was 0.85 (95% CI: 0.61, 1.19; P for trend = 0.28).
DISCUSSION
In this large prospective cohort study of women, frequency of cured meat consumption was positively associated with risk of newly diagnosed COPD. The association was stronger in past smokers than in current smokers. No association was observed in never smokers, probably because of the small number of COPD cases. The interaction between cured meat consumption and smoking was not statistically significant.
Although animal and experimental studies summarized below suggest that nitrite exposure may cause lung damage, epidemiologic studies relating cured meat consumption to lung function or risk of COPD are sparse. Aside from our cross-sectional study in the third National Health and Nutrition Examination Survey and prospective study in the Health Professionals Follow-up Study (11, 12), the only related study in the literature, to our knowledge, described a positive association between preserved foods (preserved meats, eggs, and shellfish) eaten in Singapore and the development of persistent cough with phlegm (23). No association of preserved meats was observed in that study with asthma, and neither COPD nor chronic bronchitis was assessed. These consistent epidemiologic results from a large cross-sectional study, a longitudinal study on preserved meats and incident productive cough, and 2 prospective cohort studies in women and men suggest that frequent cured meat consumption might contribute to the development of COPD.
Animal studies suggest a causal effect. In 1968, a rodent model of experimental emphysema was described in which rats exposed to 10–25 ppm of ambient nitrogen dioxide developed emphysematous changes in their lungs (8 –10). In 1972, Shuval and Gruener (7) showed that rats fed sodium nitrite in their drinking water developed pulmonary emphysema.
Cured meats may cause lung tissue damage through the effects of nitrite on connective tissue protein collagen and elastin in the lung. The integrity of elastin and collagen is principally responsible for the maintenance of alveolar air space size. In vitro experimental studies have shown that primary nitrite modification of extracellular matrix proteins produces effects that mimic age-related damage, including elastin fragmentation (24), non-enzymatic collagen cross-linking (25), and lens crystallin cross-linking (26). Furthermore, reactions of nitrite with hydrogen peroxide-myeloperoxidase (6), hydrogen peroxide-iron ions (27), and hydrogen peroxide-hypochlorous acid (28) all result in protein tyrosine nitration. 3-Nitro-tyrosine is elevated in the inflammatory cells (29) and bronchial submucosa (30) of patients with COPD, suggesting that nitrating and nitrosating reactions may be increased in COPD.
Cured meats are the principal source of dietary nitrites. The contribution of other sources of dietary nitrites, such as contaminated drinking water and certain fish and vegetables, are thought to contribute less to dietary nitrite exposure. It has been estimated that, in the US diet, a high cured meat diet (120 g cured meats/d) can more than double a person’s nitrite intake from 1.2 to 2.6 mg/d (31). If we take the 2.6 mg nitrate/d from cured meats, then the total exposure for 1-y duration will be 0.949 g, 10-y exposure is 9.49 g, 20-y exposure is 18.98 g, 30-y exposure is 28.47g, and so forth. Such cumulative human exposures from cured meat consumption are comparable to deleterious exposures suggested by previous animal studies. Thus, at 20 y, a high cured meat human diet could produce a level of exposure sufficient to cause emphysema in the rat.
Nitrites are also byproducts of tobacco smoke. Of the ≈4000 different compounds in tobacco smoke, carbon monoxide, nicotine, and nitrogen oxides are present in relatively high amounts (32). Nitrogen oxide comprises mainly nitric oxide and nitrogen dioxide, and almost all of the nitrogen oxide inhaled in cigarette smoke is retained in the body (33). Nitrogen oxide gas is converted exclusively to nitrite as it enters the pulmonary circulation (34), and inhaled nitric oxide results in marked increases in serum nitrite (35, 36). Nitrite intake may therefore be one of the mechanisms by which tobacco smoke causes COPD; our data suggest that smokers with higher cured meat consumption have the highest risk of newly diagnosed COPD.
This study has a number of strengths, including its prospective ascertainment of diet and COPD, repeated measures of potential confounders, including smoking history, and large size of the cohort. We considered the possibility of confounding by tobacco smoking, in addition to dietary factors such as fish (which is high in n−3 fatty acids) and fruit and vegetables (high in antioxidant vitamins) that were reported to be associated with lung function or COPD in previous epidemiologic studies. For smoking, we adjusted not only for self-report of smoking status and pack-years updated biennially from 1976 to 2000 but also for exposure to environmental tobacco smoke in the home and at work. In this and other observational studies, we could not rule out the possibility of residual confounding by smoking and other unmeasured confounders. The correlation, however, of cured meat consumption and pack-years among ever smokers was weak (r = 0.08), suggesting that residual confounding by smoking was probably small. Of note, other foods that were equally or more correlated with total pack-years among smokers (eg, vegetables, r = −0.07; fruit, r = −0.16) were not predictors of COPD risk after adjusting for pack-years (P for trend = 0.79 for vegetables; P for trend = 0.41 for fruit). Because few never smokers develop COPD, we did not have sufficient power to examine the association among never smokers.
Another concern is the possibility for inaccurate assessment of cured meat consumption and COPD. Cured meat consumption was shown previously to be reported on dietary questionnaires with reasonable accuracy (15). Grouping cured meats (bacon, hot dogs, sausage, and other processed meats) with varying nitrite contents may not be ranked based on their nitrite intake. COPD is defined by postbronchodilator spirometric measures (37), repeated measures of which were not feasible in this large study. We therefore relied on a definition of physician diagnosis of COPD that included participants’ self-report of the diagnosis on 2 separate occasions plus report of confirmatory tests. The validation of this definition showed it to be much more accurate than a one-time self-report of COPD in the general population (19, 38). Nonetheless, some misclassification of COPD probably occurred because of underdiagnosis and misdiagnosis with asthma. Such diagnostic misclassification may have biased our results toward the null (because no association was observed between cured meats and newly diagnosed asthma in this cohort) but could potentially have introduced a nonconservative bias also. The COPD incidence rate in the NHS was close to but slightly lower than the rates forwomenreported in other studies (39–42). Several factors may be responsible for the relatively lower incidence in the NHS, such as the exclusion of asthma from our analyses, the study population (all women in this cohort are nurses who in general have better health, health behaviors, and social economic status than women in the general population), and the more restricted definition of COPD in the present study.
In conclusion, frequent consumption of cured meats was associated with an increased risk of newly diagnosed COPD among women smokers. Although repeated measures of postbronchodilator lung function would be preferred to define incident COPD and a randomized clinical trial would be necessary to eliminate the possibility of residual confounding by smoking, our current findings suggest that frequent cured meat consumption might increase the risk of newly diagnosed COPD in women who smoke.
Acknowledgments
The author’s responsibilities were as follows—RJ, CAC, RV, DCP, WCW, and RGB: provided study concept and design and analyzed and interpreted data; CAC, WCW, and RGB: acquisition of data; RJ: drafted the manuscript; CAC, RV, DCP, WCW, and RGB: performed critical revision of the manuscript for important intellectual content; RJ, CAC, WCW, and RGB: provided statistical expertise; CAC, WCW, and RGB: obtained funding.
Footnotes
Supported by research grants HL75476, HL77612, CA87969, HL63841, HL60712, and AI52338 from the National Institutes of Health, Bethesda, MD. RV was supported by grants from the Société Française de Nutrition (Paris, France) and the Société de Pneumologie de Langue Française (Paris, France).
None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Hurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest. 2000;117 suppl:1S–4S. doi: 10.1378/chest.117.2_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 2.Hoyert DL, Kung HC, Smith BL. Deaths: preliminary data for 2003. Natl Vital Stat Rep. 2005;53:1–48. [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.Walters CL. Nitrate and nitrite in food. In: Hill MJ, editor. Nitrates and nitrites in food and water. West Sussex, England: Ellis Horwood Limited; 1991. pp. 93–112. [Google Scholar]
- 5.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eiserich JP, Hristova M, Cross CE, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 7.Shuval HI, Gruener N. Epidemiological and toxicological aspects of nitrates and nitrites in the environment. Am J Public Health. 1972;62:1045–1052. doi: 10.2105/ajph.62.8.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman G, Haydon GB. Emphysema after low-level exposure to NO2. Arch Environ Health. 1964;8:125–128. doi: 10.1080/00039896.1964.10663640. [DOI] [PubMed] [Google Scholar]
- 9.Haydon GB, Freeman G, Furiosi NJ. Covert pathogenesis of NO2 induced emphysema in the rat. Arch Environ Health. 1965;11:776–783. doi: 10.1080/00039896.1965.10664300. [DOI] [PubMed] [Google Scholar]
- 10.Freeman G, Crane SC, Stephens RJ, Furiosi NJ. Pathogenesis of the nitrogen dioxide-induced lesion in the rat lung: a review and presentation of new observations. Am Rev Respir Dis. 1968;98:429–443. doi: 10.1164/arrd.1968.98.3.429. [DOI] [PubMed] [Google Scholar]
- 11.Jiang R, Paik DC, Hankinson JL, Barr RG. Cured meat consumption, lung function, and chronic obstructive pulmonary disease among United States adults. Am J Respir Crit Care Med. 2007;175:798–804. doi: 10.1164/rccm.200607-969OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varraso R, Jiang R, Barr RG, Willett WC, Camargo CA., Jr Prospective study of cured meats consumption and risk of chronic obstructive pulmonary disease in men. Am J Epidemiol. 2007;166:1438–1445. doi: 10.1093/aje/kwm235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 14.Willett WC. Nutritional epidemiology. 2nd ed. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 15.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 17.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Stampfer MJ, Solomon C, et al. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med. 2001;134:96–105. doi: 10.7326/0003-4819-134-2-200101160-00009. [DOI] [PubMed] [Google Scholar]
- 19.Barr RG, Herbstman J, Speizer FE, Camargo CA., Jr Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol. 2002;155:965–971. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]
- 20.Barr RG, Somers SC, Speizer FE, Camargo CA., Jr Patient factors and medication guideline adherence among older women with asthma. Arch Intern Med. 2002;162:1761–1768. doi: 10.1001/archinte.162.15.1761. [DOI] [PubMed] [Google Scholar]
- 21.He K, Rimm EB, Merchant A, et al. Fish consumption and risk of stroke in men. JAMA. 2002;288:3130–3136. doi: 10.1001/jama.288.24.3130. [DOI] [PubMed] [Google Scholar]
- 22.Tsai CJ, Leitzmann MF, Hu FB, Willett WC, Giovannucci EL. A prospective cohort study of nut consumption and the risk of gallstone disease in men. Am J Epidemiol. 2004;160:961–968. doi: 10.1093/aje/kwh302. [DOI] [PubMed] [Google Scholar]
- 23.Butler LM, Koh WP, Lee HP, Tseng M, Yu MC, London SJ. Prospective study of dietary patterns and persistent cough with phlegm among Chinese Singaporeans. Am J Respir Crit Care Med. 2006;173:264–270. doi: 10.1164/rccm.200506-901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik DC, Ramey WG, Dillon J, Tilson MD. The nitrite/elastin reaction: implications for in vivo degenerative effects. Connect Tissue Res. 1997;36:241–251. doi: 10.3109/03008209709160224. [DOI] [PubMed] [Google Scholar]
- 25.Paik DC, Dillon J, Galicia E, Tilson MD. The nitrite/collagen reaction: non-enzymatic nitration as a model system for age-related damage. Connect Tissue Res. 2001;42:111–122. doi: 10.3109/03008200109014253. [DOI] [PubMed] [Google Scholar]
- 26.Paik DC, Dillon J. The nitrite/alpha crystallin reaction: a possible mechanism in lens matrix damage. Exp Eye Res. 2000;70:73–80. doi: 10.1006/exer.1999.0761. [DOI] [PubMed] [Google Scholar]
- 27.Thomas DD, Espey MG, Vitek MP, Miranda KM, Wink DA. Protein nitration is mediated by heme and free metals through Fenton-type chemistry: an alternative to the NO/O2- reaction. Proc Natl Acad Sci U S A. 2002;99:12691–12696. doi: 10.1073/pnas.202312699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eiserich JP, Cross CE, Jones AD, Halliwell B, van der Vliet A. Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric oxide-mediated protein modification. J Biol Chem. 1996;271:19199–19208. doi: 10.1074/jbc.271.32.19199. [DOI] [PubMed] [Google Scholar]
- 29.Ichinose M, Sugiura H, Yamagata S, Koarai A, Shirato K. Increase in reactive nitrogen species production in chronic obstructive pulmonary disease airways. Am J Respir Crit Care Med. 2000;162:701–706. doi: 10.1164/ajrccm.162.2.9908132. [DOI] [PubMed] [Google Scholar]
- 30.Di Stefano A, Caramori G, Ricciardolo FL, Capelli A, Adcock IM, Donner CF. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy. 2004;34:1156–1167. doi: 10.1111/j.1365-2222.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- 31.Walker R. Nitrates, nitrites and N-nitrosocompounds: a review of the occurrence in food and diet and the toxicological implications. Food Addit Contam. 1990;7:717–768. doi: 10.1080/02652039009373938. [DOI] [PubMed] [Google Scholar]
- 32.Smith CJ, Fischer TH. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis. 2001;158:257–267. doi: 10.1016/s0021-9150(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 33.Bokhoven C, Niessen HJ. Amounts of oxides of nitrogen and carbon monoxide in cigarette smoke, with and without inhalation. Nature. 1961;192:458–459. doi: 10.1038/192458a0. [DOI] [PubMed] [Google Scholar]
- 34.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci U S A. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wennmalm A, Benthin G, Edlund A, et al. Metabolism and excretion of nitric oxide in humans. An experimental and clinical study. Circ Res. 1993;73:1121–1127. doi: 10.1161/01.res.73.6.1121. [DOI] [PubMed] [Google Scholar]
- 36.Valvini EM, Young JD. Serum nitrogen oxides during nitric oxide inhalation. Br J Anaesth. 1995;74:338–339. doi: 10.1093/bja/74.3.338. [DOI] [PubMed] [Google Scholar]
- 37.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 38.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance–United States, 1971–2000. Respir Care. 2002;47:1184–1199. [PubMed] [Google Scholar]
- 39.Johannessen A, Omenaas E, Bakke P, Gulsvik A. Incidence of GOLD-defined chronic obstructive pulmonary disease in a general adult population. Int J Tuberc Lung Dis. 2005;9:926–932. [PubMed] [Google Scholar]
- 40.Lindberg A, Jonsson AC, Ronmark E, Lundgren R, Larsson LG, Lund-back B. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest. 2005;127:1544–1552. doi: 10.1378/chest.127.5.1544. [DOI] [PubMed] [Google Scholar]
- 41.de Marco R, Accordini S, Cerveri I, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175:32–39. doi: 10.1164/rccm.200603-381OC. [DOI] [PubMed] [Google Scholar]
- 42.Kojima S, Sakakibara H, Motani S, et al. Incidence of chronic obstructive pulmonary disease, and the relationship between age and smoking in a Japanese population. J Epidemiol. 2007;17:54–60. doi: 10.2188/jea.17.54. [DOI] [PMC free article] [PubMed] [Google Scholar]