Abstract
Background:
The genes coding for ethanol metabolism enzymes [alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH)] have been widely studied for their influence on the risk to develop alcohol dependence (AD). However, the relation between polymorphisms of these metabolism genes and AD in Caucasian subjects has not been clearly established. The present study examined evidence for the association of alcohol metabolism genes with AD in the Irish Affected Sib Pair Study of alcohol dependence.
Methods:
We conducted a case–control association study with 575 independent subjects who met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, AD diagnosis and 530 controls. A total of 77 single nucleotide polymorphisms (SNPs) in the seven ADH (ADH1-7) and two ALDH genes (ALDH1A1 and ALDH2) were genotyped using the Illumina GoldenGate protocols. Several statistical procedures were implemented to control for false discoveries.
Results:
All markers with minor allele frequency greater than 0.01 were in Hardy–Weinberg equilibrium. Numerous SNPs in ADH genes showed association with AD, including one marker in the coding region of ADH1C (rs1693482 in exon6, Ile271Gln). Haplotypic association was observed in the ADH5 and ADH1C genes, and in a long haplotype block formed by the ADH1A and ADH1B loci. We detected two significant interactions between pairs of markers in intron 6 of ADH6 and intron 12 of ALDH2 (p = 5 × 10−5), and 5′ of both ADH4 and ADH1A (p = 2 × 10−4).
Conclusion:
We found evidence for the association of several ADH genes with AD in a sample of Western European origin. The significant interaction effects between markers in ADH and ALDH genes suggest possible epistatic roles between alcohol metabolic enzymes in the risk for AD.
Keywords: Ethanol Metabolism Genes, Alcohol Dehydrogenase, Aldehyde Dehydrogenase, Gene Interaction, Epistasis
Alcohol dependence (AD) is a complex trait that has significant genetic influences but exhibits great clinical and etiological heterogeneity (Prescott et al., 2006a). Identification of specific genes that contribute to susceptibility to develop AD has been a focus of intense research but progress has been slow. Many candidate genes have been examined in association. The genes coding for alcohol metabolizing enzymes are clearly relevant and the most studied.
Two sets of genes code for enzymes in the oxidative pathway of ethanol metabolism: the alcohol dehydrogenases (ADH) convert ethanol into acetaldehyde and the aldehyde dehydrogenases (ALDH) transform acetaldehyde into acetic acid which can then be easily excreted. Several significant associations with AD have been reported for subsets of alcohol metabolism genes (e.g. ADH1B, ADH1C, ALDH2)†, primarily in East Asian populations (Chen et al., 1999; Cheng et al., 2004; Higuchi et al., 2004; Osier et al., 1999). A meta-analysis including different ethnic groups reported a significantly higher risk of alcoholism‡ was associated with subjects having ADH1B*1 (odds ratio, OR = 2.23), ADH1C*2 (OR = 1.91), and ALDH2*1 (OR = 4.35) alleles, but only in East Asian populations (Zintzaras et al., 2006). The roles of these genes in non-Asian populations are less clear.
There are 7 human ADH genes cluster in a small region (approximately 365 kb) on chromosome 4 q21–q24. It has been suggested that 3 genes are class I (ADH1A,1B, 1C) and 1 genes is class II (ADH4), which code for primary enzymes for conversion of ethanol to acetaldehyde are involved in ethanol oxidation in vivo (Crabb et al., 2004). The class I enzymes are mainly expressed in liver and contribute about 70% of the total ethanol oxidizing capacity (Lee et al., 2004). Most prior studies have focused on class I ADH genes, especially ADH1B and ADH1C, which have known functional polymorphisms in their coding regions.
The functional variants of allele ADH1B*2 (His47) and ADH1B*3 (Cys369) have high enzyme activity and unusually rapid conversion of ethanol to acetaldehyde. This causes facial flushing and aversive effects after consuming alcohol and is protective against AD (Osier et al., 1999). These variants have markedly different frequencies in different ethnic groups. ADH1B*2 allele is common in Asian populations (approximately 90%) (Eriksson et al., 2001) but is much less common in Caucasians. ADH1B*3 has been found in African and native American populations (Osier et al., 2002; Wall et al., 1997). Results of meta-analysis for ADH1B*2 comparing homozygous versus heterozygous genotypes of ADH1B*2 with AD exhibited an OR of 5 in Han-Chinese and Japanese, but an OR of 2 in Europeans (Whitfield, 2002). Positive association of ADH1B*3 with AD was also found in African and native American populations (Ehlers et al., 2001; Wall et al., 2003).
Three functional polymorphisms have been identified for ADH1C. The wild-type ADH1C*1 has about twice the enzyme activity of ADH1C*2 and is very common in Asian and African populations (approximately 90%), whereas the allele frequency is about 50% in Caucasians (Goedde et al., 1992; Osier et al., 2002). Some studies report protective effects of ADH1C*1 against AD in Asian samples (Chen et al., 1996; Higuchi, 1994; Osier et al., 1999) and a native American sample (Mulligan et al., 2003). Other studies report no association of ADH1C*1 with AD in European samples (Borras et al., 2000; Pares et al., 1994). Strong linkage disequilibrium (LD, i.e., the nonrandom association of alleles at two or more loci) has been observed between ADH1B*2 and ADH1C*1 (Chen et al., 1999; Osier et al., 1999), so whether there is an independent effect of ADH1C on AD is unclear.
Recently, functional polymorphisms were identified in the class II ADH4 gene; one variant in the promoter region of ADH4 (C-75A) alters expression level (Edenberg et al., 2004). Some studies reported association of ADH4 with AD in European-Americans (Luo et al., 2006a), Brazilians (Guindalini et al., 2005) and another sample of European- and African-Americans (Edenberg et al., 2006). The class IV ADH7 gene, which is mainly expressed in upper digestive tract (Farres et al., 1994; Moreno et al., 1994), codes for the enzyme with the highest activity for oxidizing retinol. Previous studies suggest that ADH7 has an epistatic effect with ADH1B for protection against AD in Taiwanese Han and European populations (Han et al., 2005; Luo et al., 2006b; Osier et al., 2004).
Genes that code for ALDH enzymes are located on several different chromosomes. Nineteen putatively functional genes and 3 pseudogenes in the ALDH gene superfamily have been identified to be encoding ALDH isozymes (Vasiliou and Nebert, 2005), but only two of them, ALDH1 (ALDH1A1, 9q21.13, cytosolic isozyme) and ALDH2 (12q24.2, mitochondrial isozyme) are thought to be significantly involved in acetaldehyde oxidation (Ramchandani et al., 2001). The ALDH2 gene is highly expressed in liver and stomach with a very high affinity for acetaldehyde and plays a central role in human acetaldehyde metabolism. The ALDH1 gene also has high affinity for acetaldehyde. A functional polymorphism of the ALDH2 gene, ALDH2*2 (Lys487RAA) has lower enzyme activity than the wild-type allele. This mutant allele is mainly present in East Asians (approximately 30%). Although homozygous individuals have no ALDH2 activity; heterozygous individuals maintain 30 to 50% activity. The deficient ALDH2*2 allele is associated with decreasing risk of AD (Chai et al., 2005; Higuchi et al., 2004) or alcoholism‡ (Zintzaras et al., 2006) in Asian populations. In addition, individuals with the combination of a fast form of ADH1B and slow form of ALDH2 had particularly reduced risk for AD in a Han-Chinese samples (Chen et al., 1999).
Two genetic variants are known in ALDH1A1, ALDH1A1*2 (a 17 bp deletion in the promoter region present in many populations) and ALDH1A1*3 (a 3 bp insertion in the promoter region present only in populations of African descent). Both have protective effects against AD in African-Americans (Spence et al., 2003), and ALDH1A1*2 was further reported to be protective in a native American sample (Ehlers et al., 2004).
Overall, both ADH and ALDH enzymes exhibit genetic polymorphism and ethnic variation. The functional polymorphisms of ADH and ALDH with protective effects against AD are mainly reported in Asian and native American populations, or in African-Americans. Several of these functional polymorphisms have extremely low minor allele frequency (MAF) in European populations (Goedde et al., 1992; Oota et al., 2004; Osier et al., 1999), making it difficult to generalize findings across populations. Consequently, the roles of ADH and ALDH genes in the development of AD are less clear in Caucasians. We are aware of only 2 studies that examined all 7 ADH genes. Edenberg et al. (2006) genotyped 110 single nucleotide polymorphisms (SNPs) in ADH genes in the Collaborative Studies on Genetics of Alcoholism samples (comprised by Americans of European and African origin) and found significant ethnic differences in allele frequency for approximately 80% of these markers (Edenberg et al., 2006). They identified associations of ADH4 SNPs with AD but only in European descended samples (results were not reported in African-American samples because of small numbers of African origin families in their study). In another study of European- and African-Americans, 23 SNPs in ADH genes plus 4 SNPs in ALDH2 were genotyped (Luo et al., 2006b). Significant associations reported with AD included ADH5 genotypes and diplotypes of ADH1A, ADH1B, ADH7, and ALDH2, although the associated genotypes or diplotypes differed in the European- and African-American samples.
In the literature, only a few studies examined the whole ADH genes cluster in relation to AD, and examination of both ADH and ALDH genes is very limited. Furthermore, the roles of these genes in non-Asian populations are less clear. The primary goal of the present study was to test for association of AD with SNPs in all 7 ADH genes as well as ALDH1A1 and ALDH2 in a sample of Western European origin that is more culturally and genetically homogeneous than samples used in many previous studies. The ADH gene cluster is of particular interest in this sample because we found a significant linkage peak for AD symptoms on a region of 4q that includes the ADH gene cluster (Prescott et al., 2006b). In addition, because of the related biological roles of ADH and ALDH enzymes in ethanol metabolism, a second goal was to test for interactions between markers in the ADH and ALDH genes.
MATERIALS AND METHODS
Subjects and Phenotype Measurement
Participants in this study were recruited in Ireland and Northern Ireland between 1998 and 2002. Details of the study design, sample ascertainment, and clinical characteristics of this sample are described elsewhere (Prescott et al., 2005). In brief, ascertainment of probands was mainly conducted in community alcoholism treatment facilities and public and private hospitals. Probands were eligible for study inclusion if they met the current DSM-IV criteria for AD and if all four grandparents had been born in Ireland, Northern Ireland, Scotland, Wales, or England. After a prospective family was identified through probands, parents and potentially affected siblings whom the probands provided permission to contact were recruited.
Probands, siblings and parents were interviewed by clinically-trained research interviewers, most of whom had extensive clinical experience with alcoholism. The assessment included demographic characteristics, lifetime history of AD and other comorbid conditions, alcohol-related traits, personality features, and clinical records. The DSM-IV AD diagnosis was assessed in probands and siblings using the SSAGA (semi-structured assessment of the genetics of alcoholism) interview (version II, Bucholz et al., 1994), modified to reduce assessment time by omitting items that address onset age of each symptom.
All participants provided informed consent. There were 1238 individuals who completed the SSAGA interview and met DSM-IV AD diagnosis, including 591 probands, 620 affected siblings, and 27 other relatives from 10 complex families. Controls were recruited in the Northern Ireland from volunteers donating at the Northern Ireland Blood Transfusion Service (n = 554) and in Ireland from the Garda Siochana (the national police force, n = 38) and the Forsa Cosanta Aituil (the army reserve, n = 34). Controls were screened and their samples excluded if they reported a history of heavy drinking or problematic alcohol use. In the present case–control study design, we included 530 controls and 575 independent AD cases (399 probands and 176 sibs) from the Irish Affected Sib Pair Study of alcohol dependence families. Samples were selected based on high yield of high quality DNA for genotyping and only one case per family was included.
Genotyping
Genotypes for a total of 77 SNPs in the seven ADH (ADH1-7)and 2 ALDH genes (ALDH1A1 and ALDH2) were obtained as part of a large candidate gene study using an Illumina (San Diego, CA) custom genotyping array designed in Dr. David Goldman's Laboratory of Neurogenetics, National Institute on Alcohol Abuse and Alcoholism. A total of 130 candidate genes were selected from multiple functional systems implicated in substance dependence phenotypes (primarily alcohol, cocaine and opiates).
A genomic region including 5 kb upstream and 1 kb downstream of each candidate gene were retrieved from NCBI Human Genome Build 35.1. Genotype data from the African population, which is the most diverse, were obtained from HapMap Project Public Release No. 18 to re-construct haplotypes for each gene using SNPHAP (http://www-gene.cimr.cam.ac.uk/clayton/software/snphap.txt). A double classification tree search algorithm (Zhang et al., 2004) was applied to select minimum index SNPs that represent maximum haplotype information for each gene. Probable functional SNPs (nonsynonymous, splice site and putative functional SNPs from the literature) were forced in during the selection process. Physically large genes were split into two or three regions, which were processed separately, if the complexity of their haplotype structures was high. Finally, the performance of the initially selected SNP set was validated by the manufacturer and replacements made where necessary.
All genotyping was conducted in Dr. David Goldman's Laboratory. Genotyping was performed using the Illumina GoldenGate genotyping protocols on 96-well format Sentrix® arrays and 500 ng of sample DNA were used per assay. All pre-PCR processing was performed using a Tecan liquid handling robot running Illumina protocols. Arrays were imaged using an Illumina Beadstation GX500 and the data analyzed using gencall Version 6.2.0.4 and gts reports software Version 5.1.2.0 (Illumina).
Statistical Methods
Case–control association analyses were conducted using PLINK (Purcell, http://pngu.mgh.harvard.edu/∼purcell/plink/) at both single marker and haplotype levels, which provides significance tests and estimated ORs for risk alleles. We explored all possible pair-wise marker-marker interactions among all genotyped SNPs in PLINK. The SNP pair-wise interactions were reported if p-values less than or equal to 0.01. Among all the examined SNPs, five of the ADH cluster SNPs were in coding regions: rs1126671 (ADH4), rs698 (ADH1C), rs1693482 (ADH1C), rs971074 (ADH7), and rs1573496 (ADH7). None of the 22 ALDH SNPs were in coding regions.
Because of prior evidence in our sample for linkage to AD symptoms on chromosome 4q22 to 4q32 (Prescott et al., 2006b) (peak multipoint logarithm of the odds score = 4.59 at D4S1611), we adopted a weighted false discovery rate (FDR) approach (Roeder et al., 2006) for associations of single markers in ADH loci. This approach weighs the association p-values using prior linkage data while applying the FDR method to obtain weighted p-values. If the linkage findings are informative, applying this procedure can increase power. Therefore, for single marker tests, we reported weighted p-values for ADH loci and unweighted p-values for ALDH loci.
To address multiple testing issues and control the risk of false discoveries in these analyses, we calculated for each p-value, a so-called q-value (Storey, 2003; Storey and Tibshirani, 2003). A q-value is an estimate of the proportion of false discoveries among all significant markers (i.e., q-values are FDRs) when the corresponding p-value is used as the threshold for declaring significance. We use a q-value threshold of 0.2 for declaring significance, meaning that we allow 20% of the significant findings to be false discoveries. Reasons for this choice are that this threshold provides a reasonable balance between the competing goals of controlling false positives versus detecting true positive associations (van den Oord and Sullivan, 2003). This FDR approach and threshold was applied for both single marker and interaction tests; q-values were calculated assuming that the proportion of markers without effects (P0) equals one. As this results in a conservative bias (i.e., q-values that are too high), we also estimated P0 from the data (for interaction tests only because we have too few data entry to get reliable P0 estimate for single marker tests) and re-calculated the q-values using that estimate. For this purpose, we selected the maximum likelihood estimator that takes advantage of the knowledge that in genetic studies P0 has to be close to one and produces the most precise estimates in this scenario (Kuo et al., in press; Meinshausen and Rice, 2006) for interaction tests.
RESULTS
Genotyping Completion
Genotyping was completed for 43 SNPs in the ADH genes cluster and 34 SNPs in the two ALDH genes. Among the 77 genotyped SNPs, we excluded 5 SNPs with low genotyping rate (<50%) and 10 with MAF less than 0.01. Sixty-two SNPs were retained in the analysis and all were in Hardy-Weinberg equilibrium (using a regular cut-off p-value of 0.001) in the overall sample and in controls alone. Among the 1105 genotyped individuals, 17 (10 cases, 7 controls) were removed from analysis as genotyping call rates were less than 80%. The sample included in the final analysis thus consisted of 565 AD cases and 523 controls. The average genotyping rate among these individuals is 92.46%.
Single Marker Association and Linkage Disequilibrium Structure
Marker information, allele frequency, and single marker association results are displayed in Table 1 for 40 SNPs in the seven ADH genes across a 364.7 kb region, 17 SNPs in ALDH1A1 in a 136.6 kb region, and 5 SNPs in the ALDH2 gene in a 35.2 kb region. Many ADH markers showed association with AD, but no markers in the ALDH genes showed association. ORs were in the range of 1.18 to 1.48 among associated markers. The SNP showing the greatest evidence of association, rs1154414 in the ADH5 intron region, yielded a weighted p-value of 0.004 (OR = 1.48). The 3 class I ADH genes (ADH1A, 2, 3) consistently exhibited association with AD, with the strongest association to marker rs1353621 in ADH1B intron 1 with a weighted p-value of 0.005 (OR = 1.27). The two significant markers, rs1154414 and rs1353621 have low q-values and pass our prespecified threshold of 0.2. Among the 5 SNP coding region in the ADH gene cluster, only marker rs1693482 (ADH1C exon6, Ile271Gln) showed association with AD (p = 0.047, OR = 1.18) in our sample.
Table 1.
Allele Frequency and Association Tests for Single Markers in the ADH and ALDH Genes
| Allele frequency |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Ch | SNP | Position (bp) | Case | Control | χ2 | p-valuea | Odds ratio (95%CI) | q-valueb |
| ADH5 | 4 | rs7684986 | 100348854 | 0.119 | 0.113 | 0.17 | 0.56 | 1.06(0.81, 1.38) | 0.859 |
| 4 | rs17595424 | 100351047 | 0.121 | 0.110 | 0.65 | 0.34 | 1.11(0.86, 1.45) | 0.669 | |
| 4 | rs1154414 | 100357314 | 0.913 | 0.876 | 7.88 | 0.004 | 1.48(1.13, 1.96) | 0.186 | |
| 4 | rs7683704 | 100361404 | 0.121 | 0.112 | 0.45 | 0.41 | 1.09(0.84, 1.42) | 0.722 | |
| 4 | rs1154405 | 100365928 | 0.300 | 0.277 | 1.39 | 0.20 | 1.12(0.93, 1.35) | 0.496 | |
| 4 | rs1154401 | 100366916 | 0.369 | 0.336 | 2.51 | 0.09 | 1.15(0.97, 1.38) | 0.490 | |
| 4 | rs1154400 | 100367188 | 0.347 | 0.321 | 1.69 | 0.16 | 1.13(0.94, 1.35) | 0.496 | |
| ADH4 | 4 | rs1042364 | 100402752 | 0.314 | 0.285 | 2.11 | 0.12 | 1.15(0.95, 1.38) | 0.490 |
| 4 | rs1126671 | 100405592 | 0.335 | 0.302 | 2.66 | 0.08 | 1.16(0.97, 1.40) | 0.490 | |
| 4 | rs6836440 | 100405684 | 0.976 | 0.976 | 0.00 | 0.80 | 1.01(0.58, 1.75) | 0.976 | |
| 4 | rs2213035 | 100421411 | 0.308 | 0.285 | 1.38 | 0.20 | 1.12(0.93, 1.35) | 0.496 | |
| 4 | rs3762894 | 100423262 | 0.880 | 0.859 | 2.08 | 0.12 | 1.20(0.94, 1.55) | 0.490 | |
| 4 | rs1984364 | 100427961 | 0.313 | 0.287 | 1.66 | 0.16 | 1.13(0.94, 1.36) | 0.496 | |
| ADH6 | 4 | rs3857224 | 100486863 | 0.732 | 0.695 | 3.45 | 0.051 | 1.19(0.99, 1.44) | 0.434 |
| 4 | rs6833176 | 100488341 | 0.508 | 0.491 | 0.60 | 0.36 | 1.07(0.90, 1.27) | 0.678 | |
| 4 | rs4699733 | 100494712 | 0.315 | 0.312 | 0.02 | 0.72 | 1.01(0.84, 1.22) | 0.959 | |
| 4 | rs10008281 | 100499480 | 0.814 | 0.793 | 1.46 | 0.19 | 1.14(0.92, 1.41) | 0.496 | |
| ADH1A | 4 | rs3819197 | 100557687 | 0.802 | 0.774 | 2.61 | 0.09 | 1.19(0.96, 1.46) | 0.490 |
| 4 | rs1229967 | 100564756 | 0.767 | 0.759 | 0.16 | 0.57 | 1.04(0.85, 1.27) | 0.859 | |
| 4 | rs13134764 | 100569351 | 0.430 | 0.385 | 4.54 | 0.027 | 1.21(1.02, 1.43) | 0.434 | |
| 4 | rs904092 | 100571342 | 0.817 | 0.795 | 1.63 | 0.16 | 1.15(0.93, 1.42) | 0.496 | |
| ADH1B | 4 | rs1042026 | 100585644 | 0.754 | 0.733 | 1.30 | 0.21 | 1.12(0.92, 1.36) | 0.510 |
| 4 | rs17033 | 100586123 | 0.927 | 0.909 | 2.36 | 0.10 | 1.27(0.93, 1.73) | 0.490 | |
| 4 | rs1353621 | 100598753 | 0.429 | 0.371 | 7.50 | 0.005 | 1.27(1.07, 1.52) | 0.186 | |
| 4 | rs1159918 | 100600187 | 0.664 | 0.624 | 3.80 | 0.042 | 1.19(1.00, 1.42) | 0.434 | |
| ADH1C | 4 | rs1614972 | 100615333 | 0.753 | 0.751 | 0.01 | 0.74 | 1.01(0.83, 1.23) | 0.969 |
| 4 | rs698 | 100617967 | 0.496 | 0.458 | 2.97 | 0.07 | 1.16(0.98, 1.38) | 0.490 | |
| 4 | rs904096 | 100620762 | 0.498 | 0.456 | 3.93 | 0.038 | 1.19(1.00, 1.41) | 0.434 | |
| 4 | rs1693482 | 100621143 | 0.496 | 0.455 | 3.60 | 0.047 | 1.18(0.99, 1.39) | 0.434 | |
| 4 | rs1693426 | 100623508 | 0.496 | 0.455 | 3.61 | 0.047 | 1.18(0.99, 1.39) | 0.434 | |
| ADH7 | 4 | rs729147 | 100690445 | 0.193 | 0.190 | 0.03 | 0.70 | 1.02(0.82, 1.26) | 0.959 |
| 4 | rs894369 | 100691024 | 0.193 | 0.190 | 0.02 | 0.72 | 1.02(0.82, 1.26) | 0.959 | |
| 4 | rs1154454 | 100695520 | 0.855 | 0.854 | 0.00 | 0.78 | 1.01(0.79, 1.28) | 0.969 | |
| 4 | rs1154456 | 100696774 | 0.347 | 0.335 | 0.37 | 0.44 | 1.06(0.88, 1.26) | 0.722 | |
| 4 | rs1154458 | 100697700 | 0.388 | 0.431 | 3.91 | 0.039 | 1.20(1.00, 1.43) | 0.434 | |
| 4 | rs1154460 | 100698821 | 0.466 | 0.443 | 1.20 | 0.22 | 1.10(0.93, 1.30) | 0.529 | |
| 4 | rs971074 | 100699039 | 0.116 | 0.106 | 0.52 | 0.38 | 1.10(0.84, 1.44) | 0.697 | |
| 4 | rs1573496 | 100706847 | 0.896 | 0.893 | 0.09 | 0.63 | 1.04(0.79, 1.37) | 0.918 | |
| 4 | rs1154469 | 100713357 | 0.651 | 0.646 | 0.06 | 0.66 | 1.02(0.86, 1.22) | 0.943 | |
| 4 | rs1154470 | 100713515 | 0.351 | 0.338 | 0.40 | 0.43 | 1.06(0.89, 1.26) | 0.722 | |
| ALDH1A1 | 9 | rs3764435 | 72746430 | 0.493 | 0.475 | 0.69 | 0.41 | 1.07(0.91, 1.27) | 0.669 |
| 9 | rs1888202 | 72748805 | 0.502 | 0.481 | 0.90 | 0.34 | 1.09(0.92, 1.29) | 0.627 | |
| 9 | rs63319 | 72754338 | 0.520 | 0.489 | 2.09 | 0.15 | 1.13(0.96, 1.34) | 0.490 | |
| 9 | rs8187974 | 72756420 | 0.991 | 0.985 | 1.65 | 0.20 | 1.68(0.75, 3.77) | 0.496 | |
| 9 | rs348457 | 72760108 | 0.454 | 0.421 | 2.32 | 0.13 | 1.14(0.96, 1.35) | 0.490 | |
| 9 | rs2303317 | 72771496 | 0.488 | 0.469 | 0.79 | 0.37 | 1.08(0.91, 1.28) | 0.642 | |
| 9 | rs2773806 | 72780854 | 0.019 | 0.018 | 0.01 | 0.94 | 1.03(0.55, 1.92) | 0.969 | |
| 9 | rs1424482 | 72793111 | 0.340 | 0.324 | 0.67 | 0.41 | 1.08(0.90, 1.29) | 0.669 | |
| 9 | rs8187876 | 72794508 | 0.947 | 0.937 | 0.90 | 0.34 | 1.19(0.83, 1.71) | 0.627 | |
| 9 | rs11143429 | 72837438 | 0.417 | 0.409 | 0.13 | 0.72 | 1.03(0.87, 1.23) | 0.879 | |
| 9 | rs1364451 | 72838314 | 0.891 | 0.880 | 0.58 | 0.45 | 1.11(0.85, 1.45) | 0.678 | |
| 9 | rs6560311 | 72841736 | 0.307 | 0.289 | 0.80 | 0.37 | 1.09(0.90, 1.31) | 0.642 | |
| 9 | rs2249978 | 72856652 | 0.706 | 0.703 | 0.03 | 0.86 | 1.02(0.84, 1.22) | 0.959 | |
| 9 | rs1418187 | 72867214 | 0.764 | 0.757 | 0.17 | 0.68 | 1.04(0.86, 1.27) | 0.859 | |
| 9 | rs4745209 | 72869659 | 0.766 | 0.754 | 0.38 | 0.54 | 1.06(0.87, 1.30) | 0.722 | |
| 9 | rs7862749 | 72873389 | 0.964 | 0.959 | 0.36 | 0.55 | 1.14(0.74, 1.77) | 0.722 | |
| 9 | rs4406477 | 72882012 | 0.367 | 0.365 | 0.01 | 0.93 | 1.01(0.85, 1.20) | 0.969 | |
| ALDH2 | 12 | rs2238151 | 110674553 | 0.327 | 0.302 | 1.58 | 0.21 | 1.12(0.94, 1.35) | 0.496 |
| 12 | rs2238152 | 110677179 | 0.159 | 0.138 | 1.90 | 0.17 | 1.18(0.93, 1.50) | 0.496 | |
| 12 | rs4648328 | 110685508 | 0.157 | 0.138 | 1.51 | 0.22 | 1.16(0.92, 1.47) | 0.496 | |
| 12 | rs4646778 | 110698503 | 0.157 | 0.135 | 2.20 | 0.14 | 1.20(0.94, 1.52) | 0.490 | |
| 12 | rs7296651 | 110709674 | 0.158 | 0.137 | 1.88 | 0.17 | 1.18(0.93, 1.50) | 0.496 | |
SNP, single nucleotide polymorphism.
p-value using weighted FDR approach for ADH genes cluster and unweighted for ALDH genes
q-value was calculated assuming P0 equals 1.
Figure 1 shows the LD of the ADH gene cluster along with significance levels of the 40 SNPs (The two ALDH genes are excluded because of lack of evidence for association). Overall, there was higher LD within each gene and lower LD between genes. Six of the 7 ADH genes formed a region of moderate LD (rs7684986-rs1692126, Fig. 1). All were in very low LD with ADH7, which itself had two separate LD blocks. Among these six ADH genes, ADH5 and ADH4 exhibited high LD between genes (D′ estimates for adjacent SNPs greater than 0.80 for 83% of the comparisons) as well as the region formed by ADH6, ADH1A, and ADH1B (D′ estimates for adjacent SNPs across these 3 genes greater than 0.80 for 73% of the comparisons).
Fig. 1.
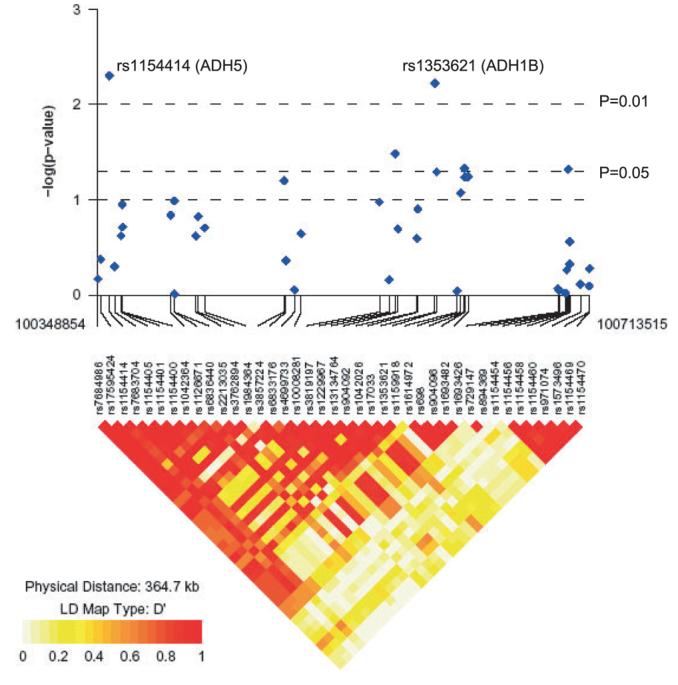
The linkage disequilibrium plot and nonadjusted p-values for markers in the ADH genes cluster. The single nucleotide polymorphisms are listed by chromosome position from pter toward qter.
Haplotype Association
Because of the high LD of SNPs within each ADH gene (with the exception of ADH7), we examined association of haplotypes based on genes instead of algorithm-defined blocks. LD between ADH1A and ADH1B is strong and yields a highly correlated haplotype distribution for the common haplotypes in both genes. The most common haplotype in ADH1A (frequency 42.4%) occurs with the most common haplotype in ADH1B (42.6%); the second and third most common haplotypes at ADH1A are similarly related to the corresponding haplotypes at ADH1B. One to one correspondence of the three common haplotypes results in a high correlation of 0.93 between ADH1A and ADH1B. Therefore, we examined the haplotypic association using all markers in the two genes to form a long haplotype block. For ADH1C, D′ was extremely high among 5 genotyped markers (mean = 0.995). Consequently, two tagging SNPs (rs1614972 and rs1693482) were used for haplotype analysis.
Table 2 displays the results of haplotype analyses. Compatible with the single marker results, haplotypes formed by SNPs in ADH5 and 3 class I ADH genes (ADH1A, ADH1B, ADH1C) showed haplotypic evidence of association with AD. No other ADH and none of the ALDH genes exhibited haplotypic association with AD (data not shown). For ADH5, the strongest effect was seen with a protective haplotype of 7 markers that is overrepresented in controls (8.4% in cases vs. 11.7% in controls, p = 0.01). For ADH1A-ADH1B, two haplotypes of 8 markers are associated with AD, one overrepresented in cases (42.6% vs. 38.0%, p = 0.036), the other more common in controls (0.9% in cases vs. 2.1% in controls, p = 0.02). These results and the strong LD between the two loci suggest that we are observing only a single effect; which one has the functional effect is not clear. The results for ADH1C showed some evidence for a common risk haplotype (cases: 49.4% vs. controls: 45.6%, p = 0.07) and a common protective haplotype (cases: 25.8% vs. controls: 29.5%, p = 0.05).
Table 2.
Haplotypic Association Results for the ADH Genes
| Haplotype frequency |
|||||
|---|---|---|---|---|---|
| Gene | Haplotypes | Case | Control | χ2 | p-value |
| ADH5 | 1111111 | 0.551 | 0.551 | 0.000 | 0.998 |
| 1111222 | 0.180 | 0.163 | 1.161 | 0.281 | |
| 2212222 | 0.119 | 0.109 | 0.499 | 0.480 | |
| 1121111 | 0.084 | 0.117 | 6.382 | 0.012 | |
| 1111122 | 0.046 | 0.042 | 0.180 | 0.672 | |
| 1111121 | 0.021 | 0.019 | 0.084 | 0.772 | |
| ADH1A-ADH1B | 11211121 | 0.426 | 0.380 | 4.411 | 0.036 |
| 21112111 | 0.188 | 0.211 | 1.817 | 0.178 | |
| 12121112 | 0.183 | 0.200 | 0.963 | 0.326 | |
| 11111212 | 0.073 | 0.082 | 0.673 | 0.412 | |
| 11111112 | 0.061 | 0.053 | 0.484 | 0.487 | |
| 12112111 | 0.050 | 0.041 | 1.022 | 0.312 | |
| 11211112 | 0.011 | 0.010 | 0.043 | 0.836 | |
| 21112112 | 0.009 | 0.021 | 5.381 | 0.020 | |
| ADH1C | 12 | 0.495 | 0.456 | 3.233 | 0.072 |
| 11 | 0.258 | 0.295 | 3.784 | 0.052 | |
| 21 | 0.248 | 0.249 | 0.004 | 0.947 | |
Markers in ADH5, ADH1A-ADH1B, and ADH1C gene blocks are as follows:
ADH5: rs7684986-rs17595424-rs1154414-rs7683704-rs1154405-rs1154401-rs1154400; ADH1A-ADH1B: rs3819197-rs1229967-rs13134764-rs904092-rs1042026-rs17033-rs1353621-rs1159918; ADH1C: rs1614972-rs1693482.
Pairwise Interaction
The closely related biological roles of ADH and ALDH gene products in alcohol metabolism suggest strong potential for interaction effects on risk for AD. We explored these interaction effects by analyzing marker-marker interactions. There were 1891 possible pair-wise comparisons of 62 SNPs. Again, the aforementioned FDR approach and threshold were applied to assess the significance of the interaction analysis. We evaluated the probability of a false positive result for each interaction test based on the observed significance level. Because of the moderate sample size and the exploratory nature of this analysis, results of the interaction tests with p-values less than 0.01 are displayed in Table 3; q-values were also provided as an estimate of the confidence of the findings to be true.
Table 3.
Pairwise Interactions Among Markers in the ADH and ALDH Genes (p < 0.01)
| Chromosome 1 | Chromosome 2 | SNP1 | SNP2 | OR_int | χ2 | p-value | q-valuea |
|---|---|---|---|---|---|---|---|
| 4 | 12 | rs3857224 | rs7296651 | 2.320 | 16.500 | 0.00005 | 0.092 |
| 4 | 4 | rs3762894 | rs904092 | 3.797 | 13.770 | 0.00021 | 0.195 |
| 4 | 9 | rs1154460 | rs3764435 | 1.533 | 10.300 | 0.0013 | 0.805 |
| 4 | 9 | rs1154470 | rs3764435 | 1.499 | 9.243 | 0.0024 | 0.805 |
| 4 | 4 | rs904092 | rs1154469 | 1.712 | 9.179 | 0.0024 | 0.805 |
| 4 | 4 | rs1154405 | rs1984364 | 1.596 | 8.875 | 0.0029 | 0.805 |
| 4 | 4 | rs2213035 | rs3762894 | 2.222 | 8.309 | 0.0039 | 0.805 |
| 4 | 4 | rs1984364 | rs6833176 | 1.549 | 8.302 | 0.0040 | 0.805 |
| 4 | 9 | rs1042364 | rs2773806 | 6.532 | 7.898 | 0.0050 | 0.805 |
| 4 | 4 | rs7684986 | rs1159918 | 2.096 | 7.887 | 0.0050 | 0.805 |
| 4 | 4 | rs1154414 | rs1154458 | 1.778 | 7.632 | 0.0057 | 0.805 |
| 4 | 4 | rs1126671 | rs894369 | 1.633 | 7.485 | 0.0062 | 0.805 |
| 4 | 4 | rs10008281 | rs3819197 | 1.749 | 7.467 | 0.0063 | 0.805 |
| 4 | 4 | rs4699733 | rs1159918 | 1.510 | 7.141 | 0.0075 | 0.805 |
| 4 | 9 | rs1984364 | rs4745209 | 1.571 | 7.075 | 0.0078 | 0.805 |
| 4 | 9 | rs1154414 | rs1418187 | 1.939 | 7.033 | 0.0080 | 0.805 |
| 4 | 9 | rs971074 | rs348457 | 1.784 | 7.013 | 0.0081 | 0.805 |
| 4 | 4 | rs7684986 | rs1693482 | 1.856 | 6.969 | 0.0083 | 0.805 |
| 4 | 12 | rs894369 | rs7296651 | 1.813 | 6.927 | 0.0085 | 0.805 |
| 4 | 9 | rs971074 | rs3764435 | 1.685 | 6.921 | 0.0085 | 0.805 |
| 4 | 4 | rs1126671 | rs1159918 | 1.508 | 6.683 | 0.0097 | 0.840 |
| 9 | 9 | rs1888202 | rs4406477 | 1.405 | 6.676 | 0.0098 | 0.840 |
OR_int: odds ratio of interaction term; SNP, single nucleotide polymorphism.
q-value was calculated based on a false discovery rate approach (van den Oord, 2005) using maximum likelihood estimator to first derive P0 (i.e., the number of tests with no effects), and then utilize the P0 to obtain the q-value for each interaction test. The P0 estimate was 0.9994698 (i.e., about one true positive among the 1891 interaction tests based on data distribution).
There were 22 interaction effects with p-values less than 0.01, but only two of them demonstrated a q-value less than 0.2 (i.e., a 20% chance of the positive finding being false). With this more stringent criterion we get only two significant findings. However, as q-values are function of power (lower power means higher q-values) we can also look at these results in a more exploratory fashion by increasing the threshold to avoid missing potential interactions. The remaining interaction tests all had high q-values, indicating that a p-value of approximately 0.0002 is required to give reasonable support for this putative interaction being a true effect in our sample of this size (n = 1088). For comparison, an interaction p-value of 0.001 (the third highest) in this sample and data structure has an 80% chance of being false. Because of the smoothing procedure in FDR calculation, smaller p-values require smaller q-values; the “smoothing” procedure explains why many of the tests all have the same q-values of 0.805.
Markers rs3857224-rs7296651 (intron 6 in ADH6 and intron 12 in ALDH2, respectively) showed significant evidence for interaction with an OR of 2.3 (p = 5 × 10−5). Marker rs3857224 also had a borderline main effect mentioned previously. Marker pair rs3762894/rs904092 (5′ of ADH4 and ADH1A) yielded an OR of 3.8 for the interaction term, but neither showed significant association at the single marker level. One ADH7 coding SNP, rs971074, which did not show significant association with AD in prior analysis was in this list of potential interaction effects with two SNPs in the ALDH1A1 gene. However, the biological function of these possible interactions with rs971074 is unknown.
DISCUSSION
The development of AD is a function of the interplay of genetic and environmental factors. Some proportion of variation in risk for AD may be because of individual differences in metabolism. Interindividual variation in alcohol absorption and metabolism is in part because of allelic variants in the genes coding for alcohol metabolizing enzymes. Previous studies have predominantly reported evidence from a few functional polymorphisms in these genes, but ADH1B*2, ADH1C*1, and ALDH2*2 are rare outside the Asian or native American samples. As a result, these studies gave only limited understanding of the involvement of these genes in AD in Caucasian populations. The present study examined the association of AD with all 7 ADH genes and two genes in the ALDH gene family, as well as possible interactions between these genes in an Irish sample. This comprehensive examination of genes involved in alcohol metabolism is important and may provide insight into AD risk in Caucasians.
Using weighted p-values for ADH gene cluster, we found that our previous reported linkage evidence in this chromosome 4 region tended to increase significance over nonweighted p-values as expected. Marker rs1693482 is the only coding SNP (ADH1C exon6, Ile271Gln) which showed association with AD in our study. Other coding SNPs have previously shown association with alcoholism‡ in different populations (Zintzaras et al., 2006). The SNP that distinguishes ADH1B*1 from ADH1B*2 (rs1229984, Arg47His in exon3) had very low MAF (0.006) in this Irish sample and was excluded from our standard analysis. However, this SNP consistently showed association with AD and several other endopheno-types in previous studies of Caucasians despite its low frequency. When we included rs1229984 in single marker analysis, it exhibited association with AD (weighted p-value = 0.05) in our sample. Another SNP which distinguishes ADH1C*1 from ADH1C*2, (rs698; Val349Phe in exon8) had reasonable MAF and was retained in the standard analysis, and showed weak association with AD (weighted p-value = 0.07). In ALDH2, marker rs671 (Lys504Glu in exon12) had very low MAF (0.001) and was excluded from our analysis.
Edenberg et al. (2006) found significant association of ADH4 with AD in the European-American sample, and a protective effect of ADH1B*3 in African-American sample, which is in prediction as the ADH1B*3 allele has been shown to be restricted to populations of African descent (Han et al., 2005). The significant association of ADH4 with AD was also reported in another European-American sample using a Hardy–Weinberg disequilibrium test (Luo et al., 2005, 2006a). However, the association of ADH4 with AD was not replicated in our Irish sample. Although the reason for this nonreplication is unclear, sample fluctuation and differences in allele frequency estimates and LD structure may all contribute. In our case, the lack of replication is less likely to be because of allele frequency difference: 3 markers (rs3762894, rs1126671, and rs1042364) were genotyped in studies of both Edenberg et al. (2006) and our group, with identical MAF for the latter two markers and a difference of 3% in MAF for rs3762894.
Our study is the first to examine both the ADH and ALDH genes in a European sample. In our data, the ADH genes are in moderate to high LD with each other except for ADH7 (see Fig. 1). The LD structure is compatible with Hapmap CEPH (Centre d'Etude du Polymorphism Humain, the DNA samples provided from U.S. residents with northern and western European ancestry in the International HapMap Project) and Edenberg et al. (2006) data. Noncoding SNPs may have unknown effects on the function of ADH enzymes or may be in LD with other functional or regulatory SNPs. Our main significant findings are in the 3 class I genes (ADH1A, ADH1B, and ADH1C) and the ADH5 gene. The class I enzyme is known to be liver expressed and contributes approximately 70% of the total ethanol oxidizing capacity. Polymorphisms in the class I genes ADH1B and ADH1C previously showed protective effects against AD by causing aversive reactions (Osier et al., 1999; Shen et al., 1997). The protective effects of ADH1B*2, ADH1B*3, and ADH1C*1 are reported across several ethnic groups (African-American, native American, Japanese, Chinese, Taiwanese, Jewish, and Mexican), especially in East Asian populations where the ADH1B*2 and ADH1C*1 predominate and are in strong LD with each other (Goedde et al., 1992; Shen et al., 1997). These allele variants have very low MAF (less than 1%) in other Caucasian and our samples, so their impact on AD should be extremely low in European populations. However, other polymorphisms in these loci may contribute to the risk of AD. Nearly half of our genotyped markers in class I genes are significantly associated with AD singly or in haplotypes. Interestingly, associated markers in ADH1A and ADH1B loci are either upstream of the gene or close to promoter regions, consistent with a possible regulatory effect. Luo et al. (2006b) also reported significant association for ADH1A and ADH1B using diplotype tests in their European-American sample.
The most significant single marker (rs1154414) is in intron 4 of ADH5 (OR = 1.48, p = 0.004). In our data, this marker has high LD with most of the markers in ADH genes except for ADH7. Luo et al. (2006b) also reported significant association for ADH5 for marker rs1154400 using genotypic tests in both European- and African-Americans but the associated genotypes were opposite to those found in our study (they found C/C in European-Americans and T/T in African-Americans) and this may be because of a multilocus effect in ADH5. When only single loci are assessed, the interlocus correlation can contribute to the “flip-flop” association (Lin et al., 2007). Although we did not find an association for marker rs1154400, one of our significant associated marker (rs1154414) is in LD with rs1154400 (D′ = 0.834), and rs1154414 also tags the associated haplotype (1121111 in Table 2) for ADH5. The ADH5 gene codes for a class III ADH and encodes glutathione-dependent formaldehyde dehydrogenase, which has very low affinity for ethanol. This enzyme is an important component of cellular metabolism for the elimination of formaldehyde but has virtually no activity for ethanol oxidation. Unlike most of the ADH genes, which are expressed in specific tissues, ADH5 is expressed ubiquitously. The biological explanation for an ADH5 association with AD is unclear, but may be caused by the extensive LD we observe between rs1154414 and SNPs in other genes in the cluster.
There is one marker each in intron 6 of ADH7 and intron 6 of ADH6 which showed single marker, but not haplotypic, association with AD. The associated marker (rs1154458) in ADH7 is in the second block of this gene, and is in high LD (D′, 0.83 to 0.94) with markers in the associated haplotype of Luo et al. (2006b). ADH7 has been suggested to have a protective effect against AD in Taiwanese Han-Chinese (Osier et al., 2004) despite controversial evidence about whether its effect is independent from the functional polymorphism in ADH1B. The coding SNP (rs971074) does not show association with AD in our sample, but the associated intronic marker (rs1154458) may tag other variants not assessed in the current dataset. In addition, we found that rs971074 has potential interaction effects with two intronic SNPs in the ALDH1A1 gene with OR = 1.7 to 1.8. We observed a weak association for marker rs3857224 (intron 6 in ADH6) with AD, but there is no previous association evidence for ADH6 in the literature. Nevertheless, this marker exhibited the most significant interaction effect (p = 5 × 10−5) with one marker in intron 12 of ALDH2 (rs7296651). This may merit further study to clarify whether it is merely a statistical artifact, or represents a true molecular interaction. In terms of physiologic function, the ADH7 gene is mainly expressed in the upper digestive tract (such as stomach and esophagus) (Farres et al., 1994; Moreno et al., 1994) and the ADH6 gene is expressed mainly in liver (Yasunami et al., 1991; Zhi et al., 2000). ADH7 is most active as a retinol dehydrogenase, and may participate in the synthesis of retinoic acid, a hormone important for cellular differentiation. ADH6 metabolizes a wide variety of substrates, including ethanol, retinol, other aliphatic alcohols, hydroxysteroids, and lipid peroxidation products. Both enzymes are inefficient in ethanol oxidation and are much less important in ethanol metabolism compared with class I and II ADH enzymes. Of potential relevance is that the cases in our sample are mainly severe alcoholics with average AD symptoms of 6 (of 7), who were ascertained in treatment settings. It is possible that the ADH6 and ADH7 genes are related to aspects of the AD phenotype other than clinical diagnosis, such as health effects that result in treatment-seeking or personality traits like extraversion that are associated with substance use (Luo et al., 2007).
To account for multiple testing, we applied several FDR approaches and reported q-values for both single marker and interaction tests. According to the FDR estimate, there is likely to be at least one true positive interaction effect in our data. Although our results showed significant interaction effects in two marker pairs with q-values less than 0.2, it is very hard to say whether the statistical evidence of epistasis can be used to infer biological epistasis at the individual level. If the true genetic model is epistatic without strong main effects of any loci, association tests at the population level have virtually no power to detect these loci (Culverhouse et al., 2002). However, because several of our genotyped ADH genes showed significant to marginal effects in AD, we should have moderate power to detect possible interaction effects among these alcohol metabolism genes, though the underlying biological function of these potential gene-gene or protein-protein interactions will need to be addressed using other sorts of data and study designs.
Other than possible interaction effects, ALDH1A1 and ALDH2 show neither single marker nor haplotypic association with AD in our data. A previously associated variant, ALDH2*2 (rs671) is almost monomorphic in Caucasians (MAF = 0.001 in our sample). In a linkage study we conducted for alcohol-related phenotypes, there was evidence for linkage to age-at-onset of AD in the vicinity of ALDH1A1 gene (9q13-21) and another linkage peak for maximum drinking within 24 hours near the vicinity of ALDH2 (Kuo et al., 2006). Although no significant association was found for AD in the present study for these two ALDH family genes, it would be worthwhile to test for association with those pheno-types producing linkage.
Overall speaking, the current study has several strengths, including unbiased assessment of ADH and two key ALDH genes in a ethnically and culturally homogeneous European sample. Because of the multiple genes and markers involved in the analyses, we adopted methods to control for false discoveries and weighted p-values of single marker associations by prior linkage evidence from the same Irish sample. Nevertheless, this study has some limitations. First, whereas ADH metabolizes the bulk of ethanol within the liver, other enzymes, such as cytochrome P4502E1 and catalase also metabolize ethanol but were not included in this study. Second, there are gender-related differences in hepatic activity of ADH isoenzymes and ALDH as well as differences in body weight and body fluid volume on alcohol metabolism, but these were not controlled for in our analyses. A recent study using the alcohol clamp method to measure alcohol absorption and metabolism found that given the same blood levels of ethanol, there is no gender difference in the rate of alcohol elimination (Ramchandani et al., 2007). Therefore, an alternative way to check this influence maybe to see if the average consumption by genders differs after adjusting for age and body weight. Third, the interaction analyses were exploratory and we applied marker-based tests while it is more ideal to conduct gene sets analysis to study gene-gene interactions. However, the existent statistical method for gene-based interaction tests is not yet well-established. Finally, we genotyped 43 markers in seven ADH genes and 34 markers in ALDH genes. Although our LD patterns are compatible with HapMap data, it remains possible that we do not have a full coverage set of SNPs to detect potential association in these alcohol metabolism genes.
ACKNOWLEDGMENTS
This work was funded by U.S. National Institutes of Health grant R01-AA-11408 (K. Kendler PI) with support from the Irish Health Research Board. Lisa Halberstadt (VCU), Margaret Devitt (HRB) and Victor Robinson (U of Ulster) supervised data collection. Ruth Barrington, Ros Moran and Carol Cronin of the HRB provided administrative support. F.A. O'Neill, the Northern Ireland Blood Transfusion Service of the British National Health Service and the Irish Gardai provided assistance in obtaining control blood samples. At VCU, Helen Wang provided computer assistance, Indrani Ray, John Myers and Cheryl Smith assisted with data management, and Stacey Garnett, Jill Opalesky, and Melissa Hayes provided administrative support. Preparation of this manuscript was supported by a Young Investigator award from the National Alliance for Research on Schizophrenia and Depression to Po-Hsiu Kuo.
Footnotes
We use newer HUGO nomenclature for the ADH genes throughout this paper, including to refer to findings from previous studies (e.g. ADH1B, ADH1C refer to ADH2, ADH3, respectively, in the old nomenclature).
In this meta-analysis, strict criteria for alcoholism was defined by: individuals whose diagnoses using published criteria (DSM-III-R, DSM-IV, CAGE, SAPS, Feighner, ICD-10) and/or alcohol consumption greater than 80 g/d for at least 10 years, and individuals who unequivocally had any type of alcoholic end-organ damage.
REFERENCES
- Borras E, Coutelle C, Rosell A, Fernandez-Muixi F, Broch M, Crosas B, Hjelmqvist L, Lorenzo A, Gutierrez C, Santos M, Szczepanek M, Heilig M, Quattrocchi P, Farres J, Vidal F, Richart C, Mach T, Bogdal J, Jornvall H, Seitz HK, Couzigou P, Pares X. Genetic polymorphism of alcohol dehydrogenase in Europeans: the ADH2*2 allele decreases the risk for alcoholism and is associated with ADH3*1. Hepatology. 2000;31:984–989. doi: 10.1053/he.2000.5978. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JIJ, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Chai YG, Oh DY, Chung EK, Kim GS, Kim L, Lee YS, Choi IG. Alcohol and aldehyde dehydrogenase polymorphisms in men with type I and type II alcoholism. Am J Psychiatry. 2005;162:1003–1005. doi: 10.1176/appi.ajp.162.5.1003. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Loh EW, Hsu YPP, Chen CC, Yu JM, Cheng ATA. Alcohol-metabolising genes and alcoholism among Taiwanese Han men: independent effect of ADH2, ADH3 and ALDH2. Br J Psychiatry. 1996;168:762–767. doi: 10.1192/bjp.168.6.762. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lu RB, Chen YC, Wang MF, Chang YC, Li TK, Yin SJ. Interaction between the functional polymorphisms of the alcohol-metabolism genes in protection against alcoholism. Am J Hum Genet. 1999;65:795–807. doi: 10.1086/302540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ATA, Gau SF, Chen THH, Chang JC, Chang YT. A 4-year longitudinal study on risk factors for alcoholism. Arch Gen Psychiatry. 2004;61:184–191. doi: 10.1001/archpsyc.61.2.184. [DOI] [PubMed] [Google Scholar]
- Crabb DW, Matsumoto M, Chang D, You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc Nutr Soc. 2004;63:49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- Culverhouse R, Suarez BK, Lin J, Reich T. A perspective on epistasis: limits of models displaying no main effect. Am J Hum Genet. 2002;70:461–471. doi: 10.1086/338759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei XL, Tian HJ, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA (A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Xuei XL, Chen HJ, Tian HJ, Wetherill LF, Dick DM, Almasy L, Bierut L, Bucholz KK, Goate A, Hesselbrock V, Kuperman S, Nurnberger J, Porjesz B, Rice J, Schuckit M, Tischfield J, Begleiter H, Foroud T. Association of alcohol dehydrogenase genes with alcohol dependence: a comprehensive analysis. Hum Mol Genet. 2006;15:1539–1549. doi: 10.1093/hmg/ddl073. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Harris L, Carr L. Association of the ADH2*3 allele with a negative family history of alcoholism in African American young adults. Alcohol Clin Exp Res. 2001;25:1773–1777. [PubMed] [Google Scholar]
- Ehlers CL, Spence JP, Wall TL, Gilder DA, Carr LG. Association of ALDH1 promoter polymorphisms with alcohol-related phenotypes in Southwest California Indians. Alcohol Clin Exp Res. 2004;28:1481–1486. doi: 10.1097/01.alc.0000141821.06062.20. [DOI] [PubMed] [Google Scholar]
- Eriksson CJP, Fukunaga T, Sarkola T, Chen WJ, Chen CC, Ju JM, Cheng ATA, Yamamoto H, Kohlenberg-Muller K, Kimura M, Murayama M, Matsushita S, Kashima H, Higuchi S, Carr L, Viljoen D, Brooke L, Stewart T, Foroud T, Su J, Li TK, Whitfield JB. Functional relevance of human ADH polymorphism. Alcohol Clin Exp Res. 2001;25:157S–163S. doi: 10.1097/00000374-200105051-00027. [DOI] [PubMed] [Google Scholar]
- Farres J, Moreno A, Crosas B, Peralba JM, Allalihassani A, Hjelmqvist L, Jornvall H, Pares X. Alcohol dehydrogenase of class IV (sigma sigma-ADH) from human stomach. cDNA sequence and structure/function relationships. Eur J Biochem. 1994;224:549–557. doi: 10.1111/j.1432-1033.1994.00549.x. [DOI] [PubMed] [Google Scholar]
- Goedde HW, Agarwal DP, Fritze G, Meiertackmann D, Singh SM, Beck-mann G, Bhatia K, Chen LZ, Fang B, Lisker R, Paik YK, Rothhammer F, Saha N, Segal B, Srivastava LM, Czeizel A. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum Genet. 1992;88:344–346. doi: 10.1007/BF00197271. [DOI] [PubMed] [Google Scholar]
- Guindalini C, Scivoletto S, Ferreira RGM, Breen G, Zilberman M, Peluso MA, Zatz M. Association of genetic variants in alcohol dehydrogenase 4 with alcohol dependence in Brazilian patients. Am J Psychiatry. 2005;162:1005–1007. doi: 10.1176/appi.ajp.162.5.1005. [DOI] [PubMed] [Google Scholar]
- Han Y, Oota H, Osier MV, Pakstis AJ, Speed WC, Odunsi A, Okonofua F, Kajuna SLB, Karoma NJ, Kungulilo S, Grigorenko E, Zhukova OV, Bonne-Tamir B, Lu RB, Parnas J, Schulz LO, Kidd JR, Kidd KK. Considerable haplotype diversity within the 23 kb encompassing the ADH7 gene. Alcohol Clin Exp Res. 2005;29:2091–2100. doi: 10.1097/01.alc.0000191769.92667.04. [DOI] [PubMed] [Google Scholar]
- Higuchi S. Polymorphisms of ethanol metabolizing enzyme genes and alcoholism. Alcohol Alcohol Suppl. 1994;2:29–34. [PubMed] [Google Scholar]
- Higuchi S, Matsushita S, Masaki T, Yokoyama A, Kimura M, Suzuki G, Mochizuki H. Influence of genetic variations of ethanol-metabolizing enzymes on phenotypes of alcohol-related disorders. Ann N Y Acad Sci. 2004;1025:472–480. doi: 10.1196/annals.1316.058. [DOI] [PubMed] [Google Scholar]
- Kuo PH, Bukszar J, Van den Oord EJCG. Estimating the number and size of the main effects in genome-wide case–control association studies. BMC Proc. 2007;1(Suppl 1):S143. doi: 10.1186/1753-6561-1-s1-s143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo PH, Neale MC, Riley BP, Webb BT, Sullivan PF, Vittum J, Patterson DG, Thiselton DL, van den Oord EJ, Walsh D, Kendler KS, Prescott CA. Identification of susceptibility loci for alcohol-related traits in the Irish Affected Sib Pair Study of alcohol dependence. Alcohol Clin Exp Res. 2006;30:1807–1816. doi: 10.1111/j.1530-0277.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- Lee SL, Hoog JO, Yin SJ. Functionality of allelic variations in human alcohol dehydrogenase gene family: assessment of a functional window for protection against alcoholism. Pharmacogenetics. 2004;14:725–732. doi: 10.1097/00008571-200411000-00003. [DOI] [PubMed] [Google Scholar]
- Lin PI, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: the flip-flop phenomenon. Am J Hum Genet. 2007;80:531–538. doi: 10.1086/512133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XG, Kranzler HR, Zuo LJ, Lappalainen J, Yang BZ, Gelernter J. ADH4 gene variation is associated with alcohol dependence and drug dependence in European Americans: results from HWD tests and case–control association studies. Neuropsychopharmacology. 2006a;31:1085–1095. doi: 10.1038/sj.npp.1300925. [DOI] [PubMed] [Google Scholar]
- Luo XG, Kranzler HR, Zuo LJ, Wang S, Schork NJ, Gelernter J. Diplotype trend regression analysis of the ADH gene cluster and the ALDH2 gene: multiple significant associations with alcohol dependence. Am J Hum Genet. 2006b;78:973–987. doi: 10.1086/504113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XG, Kranzler HR, Zuo LJ, Yang BZ, Lappalainen J, Gelernter J. ADH4 gene variation is associated with alcohol and drug dependence: results from family controlled and population-structured association studies. Pharmacogenet and Genomics. 2005;15:755–768. doi: 10.1097/01.fpc.0000180141.77036.dc. [DOI] [PubMed] [Google Scholar]
- Luo XG, Kranzler HR, Zuo LJ, Zhang HP, Wang S, Gelernter J. ADH7 variation modulates extraversion and conscientiousness in substance-dependent subjects. Am J Med Genet B Neuropsychiatr Genet. 2007 Oct 4; doi: 10.1002/ajmg.b.30589. 2007 (E-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinshausen N, Rice J. Estimating the proportion of false null hypotheses among a large number of independently tested hypotheses. Ann Stat. 2006;34:373–393. [Google Scholar]
- Moreno A, Pares A, Ortiz J, Enriquez J, Pares X. Alcohol dehydrogenase from human stomach: variability in normal mucosa and effect of age, gender, ADH3 phenotype and gastric region. Alcohol Alcohol. 1994;29:663–671. [PubMed] [Google Scholar]
- Mulligan CJ, Robin RW, Osier MV, Sambuughin N, Goldfarb LG, Kittles RA, Hesselbrock D, Goldman D, Long JC. Allelic variation at alcohol metabolism genes (ADH1B, ADH1C, ALDH2) and alcohol dependence in an American Indian population. Hum Genet. 2003;113:325–336. doi: 10.1007/s00439-003-0971-z. [DOI] [PubMed] [Google Scholar]
- van den Oord E. Controlling false discoveries in candidate gene studies. Mol Psychiatry. 2005;10:230–231. doi: 10.1038/sj.mp.4001581. [DOI] [PubMed] [Google Scholar]
- van den Oord E, Sullivan PF. False discoveries and models for gene discovery. Trends Genet. 2003;19:537–542. doi: 10.1016/j.tig.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Oota H, Pakstis AJ, Bonne-Tamir B, Goldman D, Grigorenko E, Kajuna SLB, Karoma NJ, Kungulilo S, Lu RB, Odunsi K, Okonofua F, Zhukova OV, Kidd JR, Kidd KK. The evolution and population genetics of the ALDH2 locus: random genetic drift, selection, and low levels of recombination. Ann Hum Genet. 2004;68:93–109. doi: 10.1046/j.1529-8817.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- Osier MV, Lu RB, Pakstis AJ, Kidd JR, Huang SY, Kidd KK. Possible epistatic role of ADH7 in the protection against alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2004;126B:19–22. doi: 10.1002/ajmg.b.20136. [DOI] [PubMed] [Google Scholar]
- Osier MV, Pakstis AJ, Goldman D, Edenberg HJ, Kidd JR, Kidd KK. A proline-threonine substitution in codon 351 of ADH1C is common in native Americans. Alcohol Clin Exp Res. 2002;26:1759–1763. doi: 10.1097/01.ALC.0000042013.13899.75. [DOI] [PubMed] [Google Scholar]
- Osier M, Pakstis AJ, Kidd JR, Lee JF, Yin SJ, Ko HC, Edenberg HJ, Lu RB, Kidd KK. Linkage disequilibrium at the ADH2 and ADH3 loci and risk of alcoholism. Am J Hum Genet. 1999;64:1147–1157. doi: 10.1086/302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pares X, Farres J, Pares A, Soler X, Panes J, Ferre JL, Caballeria J, Rodes J. Genetic polymorphism of liver alcohol dehydrogenase in Spanish subjects – significance of alcohol consumption and liver disease. Alcohol Alcohol. 1994;29:701–705. [PubMed] [Google Scholar]
- Prescott CA, Madden PAF, Stallings MC. Challenges in genetic studies of the etiology of substance use and substance use disorders: introduction to the special issue. Behav Genet. 2006a;36:473–482. doi: 10.1007/s10519-006-9072-9. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Sullivan PF, Kuo PH, Webb BT, Vittum J, Patterson DG, Thiselton DL, Myers JM, Devitt M, Halberstadt LJ, Robinson VP, Neale MC, van den Oord EJ, Walsh D, Riley BP, Kendler KS. Genome-wide linkage study in the Irish Affected Sib Pair Study of alcohol dependence: evidence for a susceptibility region for symptoms of alcohol dependence on chromosome 4. Mol Psychiatry. 2006b;11:603–611. doi: 10.1038/sj.mp.4001811. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Sullivan PF, Myers JM, Patterson DG, Devitt M, Halberstadt LJ, Walsh D, Kendler KS. The Irish Affected Sib Pair Study of alcohol dependence: study methodology and validation of diagnosis by interview and family history. Alcoholism Clinical And Experimental Research. 2005;29:417–429. doi: 10.1097/01.alc.0000156085.50418.07. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bosron WF, Li TK. Research advances in ethanol metabolism. Pathol Biol. 2001;49:676–682. doi: 10.1016/s0369-8114(01)00232-2. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Kumar SR, Saxena NA, Spero DE, Momenan R, Hommer D. Influence of age and sex on alcohol elimination rates and subjective effects of alcohol. Alcohol Clin Exp Res. 2007;31:51A. [Google Scholar]
- Roeder K, Bacanu SA, Wasserman L, Devlin B. Using linkage genome scans to improve power of association in genome scans. Am J Hum Genet. 2006;78:243–252. doi: 10.1086/500026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YC, Fan JH, Edenberg HJ, Li TK, Cui YH, Wang YF, Tian CH, Zhou CF, Zhou RL, Wang J, Zhao ZL, Xia GY. Polymorphism of ADH and ALDH genes among four ethnic groups in China and effects upon the risk for alcoholism. Alcohol Clin Exp Res. 1997;21:1272–1277. [PubMed] [Google Scholar]
- Spence JP, Liang TB, Eriksson CJP, Taylor RE, Wall TL, Ehlers CL, Carr LG. Evaluation of aldehyde dehydrogenase 1 promoter polymorphisms identified in human populations. Alcohol Clin Exp Res. 2003;27:1389–1394. doi: 10.1097/01.ALC.0000087086.50089.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD. The positive false discovery rate: a Bayesian interpretation and the q-value. Ann Stat. 2003;31:2013–2035. [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genome-wide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliou V, Nebert DW. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genomics. 2005;2:138–143. doi: 10.1186/1479-7364-2-2-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall TL, Carr LG, Ehlers CL. Protective association of genetic variation in alcohol dehydrogenase with alcohol dependence in native American Mission Indians. Am J Psychiatry. 2003;160:41–46. doi: 10.1176/appi.ajp.160.1.41. [DOI] [PubMed] [Google Scholar]
- Wall TL, GarciaAndrade C, Thomasson HR, Carr LG, Ehlers CL. Alcohol dehydrogenase polymorphisms in native Americans: identification of the ADH2*3 allele. Alcohol Alcohol. 1997;32:129–132. doi: 10.1093/oxfordjournals.alcalc.a008246. [DOI] [PubMed] [Google Scholar]
- Whitfield JB. Alcohol dehydrogenase and alcohol dependence: variation in genotype-associated risk between populations. Am J Hum Genet. 2002;71:1247–1250. doi: 10.1086/344287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunami M, Chen CS, Yoshida A. A human alcohol-dehydrogenase gene ADH6 encoding an additional class of isozyme. Proc Natl Acad Sci U S A. 1991;88:7610–7614. doi: 10.1073/pnas.88.17.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Sheng H, Uehara R. A double classification tree search algorithm for index SNP selection. BMC Bioinformatics. 2004;5:89–94. doi: 10.1186/1471-2105-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi X, Chan EM, Edenberg HJ. Tissue-specific regulatory elements in the human alcohol dehydrogenase 6 gene. DNA Cell Biol. 2000;19:487–497. doi: 10.1089/10445490050128412. [DOI] [PubMed] [Google Scholar]
- Zintzaras E, Stefanidis L, Santos M, Vidal F. Do alcohol-metabolizing enzyme gene polymorphisms increase the risk of alcoholism and alcoholic liver disease? Hepatology. 2006;43:352–361. doi: 10.1002/hep.21023. [DOI] [PubMed] [Google Scholar]


