Abstract
Purpose
To identify the underlying mechanisms by which lipid mediator lysophosphatidic acid (LPA) acts as a growth factor in stimulating extracellular signal–regulated kinase (ERK) and phosphatidylinositol 3′-kinase (PI3K) during corneal epithelial wound healing.
Methods
Epithelial debridement wounds in cultured porcine corneas and scratch wounds in an epithelial monolayer of SV40-immortalized human corneal epithelial (THCE) cells were allowed to heal in the presence or absence of an epidermal growth factor receptor (EGFR) inhibitor (tyrphostin AG1478), a matrix metalloproteinase inhibitor (GM6001), or a heparin-binding EGF-like growth factor (HB-EGF) antagonist (CRM197) with or without LPA. EGFR activation was analyzed by immunoprecipitation using EGFR antibodies and Western blotting with phosphotyrosine antibodies. Phosphorylation of ERK and AKT (a major substrate of PI3K) was analyzed by Western blotting with antibodies specific to the phosphorylated proteins. Wound- and LPA-induced shedding of HB-EGF was assessed by measuring the release of alkaline phosphatase (AP) in a stable THCE cell line that expressed HB-EGF with AP inserted in the heparin-binding site.
Results
In organ and cell culture models, LPA enhanced corneal epithelial wound healing. LPA-stimulated and spontaneous wound closure was attenuated by AG1478, GM6001, or CRM197. Consistent with the effects on epithelial migration, these inhibitors, as well as the Src kinase inhibitor (PP2), retarded LPA-induced activation of EGFR and its downstream effectors ERK and AKT in THCE cells. Unlike exogenously added HB-EGF, LPA stimulated moderate EGFR phosphorylation; the level of phosphorylated EGFR was similar to that induced by wounding. However, LPA appeared to prolong wound-induced EGFR signaling. The release of HB-EGF assessed by AP activity increased significantly in response to wounding, LPA, or both, and the release of HB-EGF-AP induced by LPA was inhibited by PP2 and GM6001.
Conclusions
LPA accelerates corneal epithelial wound healing through its ability to induce autocrine HB-EGF signaling. Transactivation of EGFR by LPA represents a convergent signaling pathway accessible to stimuli such as growth factors and ligands of G-protein– coupled receptors in response to pathophysiological challenge in human corneal epithelial cells.
The corneal epithelium, like other epithelial barriers in the human body, is continuously subjected to physical, chemical, and biological insults, often resulting in tissue or cell injury and a loss of barrier function. Proper healing of corneal wounds is vital for maintaining a clear, healthy cornea and preserving vision. Corneal epithelium responds rapidly to injury by migrating as a sheet to cover the defect and to reestablish its barrier function.1 Successful wound healing involves a number of processes, including cell migration, proliferation, restratification, matrix deposition, and tissue remodeling.2 Particularly critical are cell migration and proliferation, which are driven by growth factors and other factors released in coordination into the injured area. In a wounded cornea, epithelium plays a central role, not only as a key cell type during repair but also as the source of a number of growth factors. The tear film is potentially another important source of growth factors and cytokines for corneal homeostasis and wound healing.3,4 Prominent among these epithelium-derived factors are ligands for epidermal growth factor receptor (EGFR).1 In addition to peptide growth factors, growth factor–like lipid mediator lysophosphatidic acid (1-acyl-2-hydroxy-sn-glycero-3-phosphate; LPA) is released into the injured cornea. These factors may function synergistically to stimulate cell migration and proliferation to repair a wound.
LPA is an important serum component that affects cell adhesion, migration, proliferation, and survival.5,6 LPA is also released by epithelial cells, platelets, or fibroblasts at sites of injury and inflammation.7,8 LPA has been detected in aqueous humor and lacrimal gland fluid, and corneal injury results in a significant increase in the concentration of LPA.9 Various effects of LPA on the cornea have been reported to date, including the promotion of proliferation in three major cell types of the cornea in a dose-dependent manner, the stimulation of corneal epithelial migration on the stroma,10 and the induction of corneal epithelial and endothelial barrier functions in vitro.11 Thus, LPA appears to play a role in maintaining corneal homeostasis and in preserving its function under pathogenic conditions.
LPA induces cellular responses by binding to specific members of the Edg family of seven transmembrane G-protein–coupled receptors (GPCRs); at least three LPA receptors—LPA1/Edg-2, LPA2/Edg-4, and LPA3/Edg-7—have been identified.12,13 Transcripts for two LPA receptors, LPA1 and LPA3, were detected in rabbit corneal epithelial cells.14 Although LPA signals through classic GPCRs that induce calcium influx and inhibit cAMP generation,15 much interest in postreceptor signaling of LPA has been focused on its growth factor–like effect on the activation of the Ras mitogen-activated protein kinase (MAPK) cascade and phosphatidylinositol 3′-kinase (PI3K).16 Many of the growth-promoting effects of GPCR stimulation are mediated through the activation of receptor tyrosine kinases (RTKs), primarily EGFR, a process called transactivation.17–19 The major mechanism of EGFR transactivation by GPCRs in numerous cell types is mediated by metalloprotease-dependent shedding and subsequent release of growth factor–like substances such as heparin-binding EGF-like growth factor (HB-EGF) and transforming growth factor-α, which bind to and activate EGFR.17,19–21
We recently showed that wounding induces EGFR transactivation through ectodomain shedding of HB-EGF and that this wound-induced activation of EGFR and its coreceptor, erbB2, are required for wound closure in cultured porcine corneas and in human corneal epithelial cells (HCECs).19,22 HB-EGF is synthesized as a type-1 transmembrane protein that can be shed enzymatically to release a soluble 14- to 20-kDa growth factor; the process is called ectodomain shedding.23–25 The released HB-EGF acts through the stimulation of specific cell-surface receptors.26,27 Four related receptor tyrosine kinases have been identified.28–30 They are EGFR/erbB1/HER1, erbB2/HER2/neu, erbB3/HER3, and erbB4/HER4.26 Phosphorylation of EGFR on ligand binding creates docking sites for adaptor proteins and leads to the activation (tyrosine kinase phosphorylation) of effectors such as extracellular signal-regulated kinase (ERK) and PI3K. These two pathways are involved in corneal epithelial wound healing.31–36 Although LPA has effects on corneal epithelial cells that might be attributable to the function of GPCRs,11 the effects of this lipid mediator on processing epithelial migration and proliferation may result from activation of the RTKs and the participation of their downstream signaling pathways. To date, ectodomain shedding of HB-EGF and transactivation of EGFR in transmitting LPA signaling during corneal epithelial wound healing remains elusive.
In this study, we investigated LPA-induced EGFR transactivation in wounded corneal epithelial cells, in porcine corneal organ culture, and in cultured HCECs. Our results showed that EGFR plays a central role in mediating LPA-induced ERK and AKT (a major substrate of PI3K) activation and LPA-enhanced wound closure in vitro.
Materials and Methods
Materials
The following materials were purchased: minimum essential medium (MEM), nonessential amino acid solution, and defined keratinocyte-SFM (Invitrogen, Grand Island, NY); agarose (MP Biomedicals, Irvine, CA); rat tail tendon collagen (Collaborative Biomedical, Medford, MA); keratinocyte basal medium (KBM; BioWhittaker, Walkersville, MD); human recombinant HB-EGF (R&D Systems, Minneapolis, MN); oleoyl-L-α-lysophosphatidic acid (LPA, reconstituted in 10 mg/mL in chloroform/methanol/acetic acid in 95:5:5 ratio), tyrphostin AG1478, and CRM197 (Sigma-Aldrich, St. Louis, MO); GM6001, a hydroxamic acid matrix metalloproteinase (MMP) inhibitor (3-(N-hydroxycarbamoyl)-2-(R)-isobutylpropionyl-L-tryptophan methylamide), and PP2, a Src kinase inhibitor (4-amino-5-(4-chloro-phenyl)-7-(t-butyl) pyrazolo [3,4-d] pyrimidine; Calbiochem, La Jolla, CA); antibodies against human EGFR, phosphorylated ERK1/2 (p42/p44 MAPK), ERK 2 (p42 MAPK), PY20, and PY99 (Santa Cruz Biotechnology, Santa Cruz, CA); antibodies against AKT and phosphorylated AKT (Cell Signaling, Danvers, MA); and other chemicals as necessary (Sigma-Aldrich).
Porcine Corneal Organ Culture
Porcine eyes were obtained from a local abattoir, transported to the laboratory on ice in a moist chamber, and processed for culture within 24 hours. An epithelial wound was made by demarcating an area on the central cornea with a trephine 4 mm in diameter and removing the epithelium within the circle with a surgical scalpel, leaving an intact basement membrane.37 The corneas were then processed for organ culture.38
Corneal-scleral rims, with approximately 4-mm limbal conjunctiva, were excised and rinsed in sterilized phosphate-buffered saline. Excised corneas were placed epithelial side down into a sterile “cup” (a silicon rubber mold). The endothelial corneal cavity was then filled with MEM containing 1% agarose and 1 mg/mL rat tail tendon collagen maintained at 42°C. This mixture was allowed to gel. The cornea and its supporting gel were inverted and transferred to a 35-mm dish. Approximately 2 mL MEM was added dropwise to the surface of the central cornea until the limbal conjunctiva was covered, leaving the epithelium exposed to air. Cornea epithelial wounds were allowed to heal for 48 hours in MEM containing LPA (5 μM), tyrphostin AG1478 (1 μM), CRM197 (10 μg/mL), or GM6001 (50 μM) in 5% CO2 incubator at 37°C.
Determination of Epithelial Wound Healing in Corneal Organ Culture
Corneal epithelial wound repair was monitored daily by fluorescein sodium (0.25%) staining and photographed under a dissecting microscope (Nikon, Tokyo, Japan) with a camera (Kodak MDS290; Eastman-Kodak, Rochester, NY). After 48-hour incubation, the corneas were treated with Richardson staining39 to mark the remaining wound area. The corneas were photographed, and the wound area was quantified by weighing the excised Richardson staining spots from the photograph reprints. Three corneas were used for each treatment; at least two independent experiments were performed (six or more corneas for each treatment). MEM treated alone was used as a control for spontaneous healing, and MEM with LPA was used for enhanced healing. The extent of healing over time was defined as the ratio of the area difference between the original and the remaining wound after 48 hours to the original wound area.
Cell Culture and Migration Studies
SV40-immortalized (THCE) human corneal epithelial cells (HCECs) were generously provided by Kaoru Araki-Sasaki.40 Cells were grown in defined keratinocyte–SFM in a humidified 5% CO2 incubator at 37°C. For wound healing study, cells were seeded onto 12-well plates or 100-mm dishes coated with fibronectin-collagen coating mix (Biological Research Faculty & Facility, Ljamsville, MD) and were grown to 80% confluence in 12-well tissue culture plates. Cells were then starved in KBM overnight and wounded with a sterile 0.1- to 10-μL pipet tip (TipOne; USA Scientific, Ocala, FL) to remove cells by two perpendicular linear scrapes. After washing away suspended cells, the cells were refed with KBM in the presence of LPA (5 μM), AG1478 (1 μM), or CRM197 (10 μg/mL). The wound closure was photographed immediately and 24 hours after wounding near the crossing point to ensure that the same spot was photographed with an inverted microscope (Carl Zeiss, Oberkochen, Germany) equipped with a digital camera (SPOT; Diagnostic Instruments, Sterling Heights, MI). The extent of healing was defined as the ratio of the difference between the original and the remaining wound areas compared with the original wound area. The wound area was determined by the number of pixels in histogram (Adobe Photoshop).
Determination of EGFR, ERK, and AKT Phosphorylation
To assess the effects of wounding, LPA, and HB-EGF on EGFR signaling, growth factor–starved THCE cell monolayers on 100-mm dishes were stimulated with wounding or 5 μM LPA for 15 minutes (see Fig. 3) or for various times (see Fig. 5). To create extensive injury for biochemistry studies, cells were wounded by multiple linear scratches using a cut of 48-well shark’s tooth comb for DNA sequencing gel (Bio-Rad, Hercules, CA) going from one side of the dish to the other. The dish was then rotated, and scrapes were made similarly to the original scrapes at 45°, 90°, and 135°. Cells without any stimulation or inhibitor were used as negative control. For LPA stimulation, the reconstituted LPA was diluted to the final concentrations in KBM, and the media containing LPA were directly added to THCE culture after removal for growth factor starvation or for inhibitor pretreatment. In a preliminary study, 1 to 1000 dilution LPA-reconstituted solution (chloroform/methanol/acetic acid in 95:5:5 ratio) was found to have no effect on THCE cells in terms of wound healing or EGFR activation. For conditions with inhibitors, cells were pretreated with inhibitors for 1 hour and then stimulated (wounding or LPA treatment) in the presence of corresponding inhibitors for 15 minutes. To compare the extents of EGFR phosphorylation, THCE cells were also stimulated with various concentrations of LPA or HB-EGF for 15 minutes (Fig. 4). After each treatment, cells were lysed with RIPA buffer (150 mM NaCl, 100 mM Tris-HCl [pH 7.5], 1% deoxycholate, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 50 mM NaF, 100 mM sodium pyrophosphate, 3.5 mM sodium orthovanadate, proteinase inhibitor cocktail, and 0.1 mM phenylmethylsulfonyl fluoride). Cell lysates of 600-μg proteins were immunoprecipitated with 10-μg antibody against EGFR (agarose conjugate) and immunoblotted with mouse anti–PY99 antibody (1:4000). Experiments were also carried out in reverse with immunoprecipitation of tyrosine-phosphorylated protein PY20 and subsequently with immunoblotting against EGFR. ERK1/2 phosphorylation and ERK2 level were determined using monoclonal antibodies against phospho-ERK1/2 (1:500) and ERK2 (1:4000), respectively. PI3K activation was assessed by Western blotting to detect phospho-AKT with AKT level for equal protein loading.
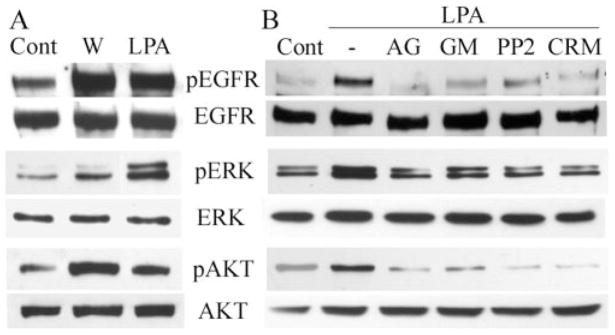
Figure 3.
Wound- and LPA-stimulated phosphorylation of EGFR, ERK, and AKT. (A) Growth factor–starved THCE cells (Cont) were extensively injured or were stimulated with 5 μM LPA for 15 minutes or (B) the cells were pretreated with AG1478 (1 μM), GM6001 (50 μM), PP2 (12.5 μM), or CRM197 (10 μg/mL) for 1 hour and then were stimulated with LPA (5 μM) for 15 minutes. Controls include cells without any stimulation (Cont) or cells stimulated with LPA alone (−). (A, B) Cells were lysed at the end of stimulation, and equal amounts of cell lysates were immunoprecipitated with EGFR antibody and immunoblotted by mouse pY99 antibody against tyrosine-phosphorylated proteins (pEGFR). The same membrane was stripped from the immunoreactivities and reprobed with EGFR antibody (EGFR) to assess the total amount of EGFR precipitated. The same cell lysates were subjected to Western blotting with anti–phospho-ERK1/2 (pERK) and -AKT using ERK2 and AKT levels, respectively, for proper protein loading.
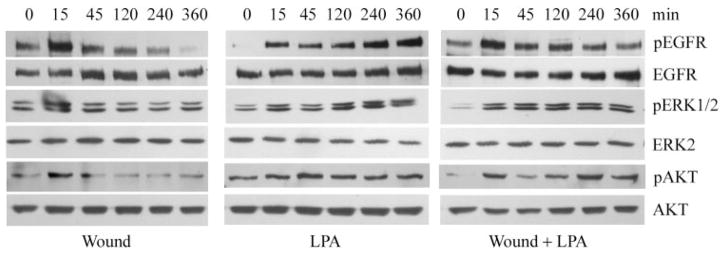
Figure 5.
Comparison of wounding and LPA on EGFR signaling. Cultured THCE cells in 100-mm dishes were starved in KBM overnight. Cells were extensively injured by sequence comb scratching (Wound), LPA (5 μM) treatment, or both (Wound + LPA) and were incubated for the indicated times. Cells were then lysed, and equal amounts of cell lysates were immunoprecipitated with EGFR antibody and immunoblotted by PY99 antibody against tyrosine-phosphorylated proteins (pEGFR). After stripping from the immunoreactivities, the membrane was reprobed with EGFR antibody (EGFR) to assess the amount of protein precipitated. To determine ERK and AKT phosphorylation, the same samples were subjected to Western blot analysis with anti–phospho-ERK1/2 (pERK) or anti–phospho-AKT (pAKT), with ERK2 or AKT levels, respectively, for proper protein loading. As each condition (wounded, LPA treated, and LPA plus wounding) was processed separately for Western blotting, each comparison between the control and treated samples should be made only within each group.
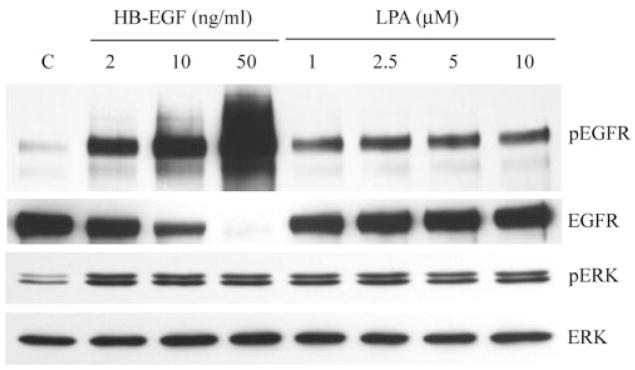
Figure 4.
Comparison of HB-EGF- and LPA-stimulated EGFR and ERK phosphorylation. Growth factor–starved THCE cells were stimulated with different concentrations of HB-EGF or LPA. Fifteen minutes after stimulation, cells were lysed, and equal amounts of cell lysates were immunoprecipitated with EGFR antibody and immunoblotted by pY99 antibody against tyrosine-phosphorylated proteins (pEGFR). The same membrane was stripped from the immunoreactivities and reprobed with EGFR antibody (EGFR) to assess the total amount of EGFR precipitated. The same cell lysates were subjected to Western blotting with anti–phospho-ERK1/2 (pERK) using the ERK2 level for proper protein loading.
Cell Line Expressing HB-EGF-Alkaline Phosphatase Fusion Proteins and Measurement of HB-EGF-AP Shedding
A cell line expressing HB-EGF-alkaline phosphatase (AP) fusion proteins was established by stable transfection of THCE cells with pHB-EGF-AP, as described in an early study.19 Briefly, THCE cells were transfected with the expression plasmid pHB-EGF-AP (Lipofectamine; Life Technologies, Bethesda, MD); in this construct, AP was inserted into the heparin-binding region of HB-EGF.41 Transfected cells were selected with 0.5 μg/mL puromycin. To measure wound- and LPA-induced HB-EGF release, THCE cells expressing HB-EGF-AP41 were cultured in six-well plates and then were growth factor starved overnight. Cells were pretreated with or without PP2 (12.5 μM) or GM6001 (50 μM) for 1 hour and then were extensively injured with a shark’s tooth comb or were stimulated with LPA (5 μM). Cell debris from wounding was rapidly removed, and fresh KBM was added to the cultures. Cells were further cultured in KBM with or without LPA or corresponding inhibitors for 15 minutes. AP activities in the collected media were measured (Great EscAPe SEAP Chemiluminescence Detection Kit; BD Biosciences, Palo Alto, CA) according to the manufacturer’s protocol. Briefly, AP activities were measured, 15-μL conditioned media from each treatment were heated with dilution buffer at 65°C for 30 minutes in a 96-well plate, and assay buffer was added. Chemiluminescence was quantified (GENios Fluorometer; Phenix Research Products, Candler, NC). The readings, after subtracting background luminescence in KBM, were normalized against cellular protein concentration using bovine serum albumin as a standard (BCA kit; Pierce, Rockford, IL) and were shown as relative light units.
Data Analysis
Data are generally expressed as mean (±SD). One-way analysis of variance with Bonferroni correction for pairwise comparisons was used to assess the statistical significance of differences between groups. P < 0.05 was considered statistically significant.
Results
Involvement of EGFR Activation in LPA-Enhanced Corneal Epithelial Wound Closure
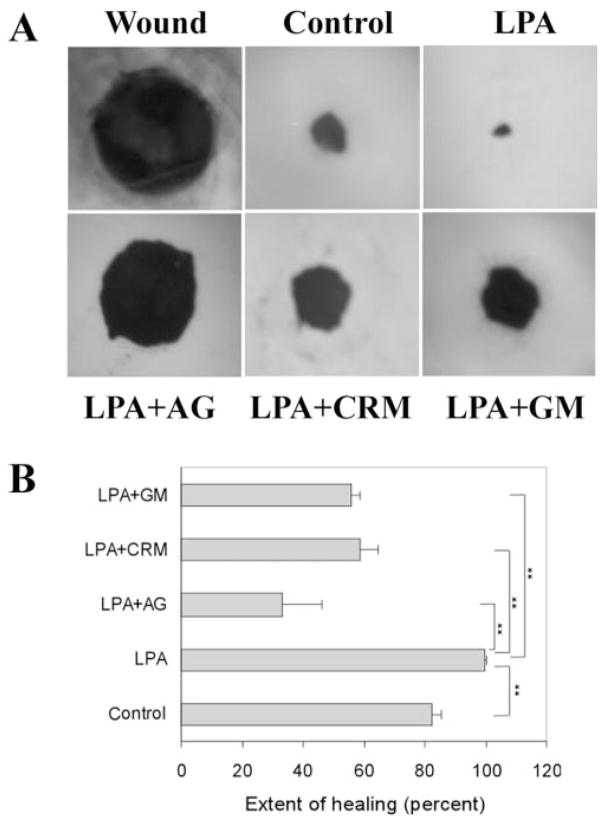
Previous studies have shown that LPA promotes cell migration on the cutting edge of rabbit corneal stoma in organ culture.9,10 To assess the effects of LPA on epithelial wound healing, we used a corneal organ culture model by creating an epithelial debridement wound with a punch 4 mm in diameter in the center of the porcine corneas and tested the effects of LPA on the healing of epithelial wound in an air-lifted culture setting.38,42 In our preliminary study, we tested different concentrations of LPA up to 10 μM and found that 5 μM was sufficient and consistent in accelerating wound closure in cultured porcine corneas. Figure 1 shows the healing of epithelial debridement wound in the presence or absence of 5 μM LPA in cultured porcine corneas. After 48 hours, approximately 82.5% of the untreated wound (control) was covered; in the presence of LPA, the wound was almost completely healed (99.6%, significant increase compared with control; P < 0.01). Tyrphostin AG1478, an EGFR inhibitor, blocked epithelial wound closure in the presence of LPA (33.2% covered; P < 0.01 compared with LPA), suggesting that EGFR activation accounted for spontaneous and LPA-enhanced epithelial wound closure. The release of EGFR ligands is sensitive to MMP inhibitors.20 To determine the effects of MMP activity on LPA-induced corneal wound healing, injured porcine corneas were incubated with GM6001, a hydroxamate metalloproteinase inhibitor. In the presence of GM6001, substantial inhibition of LPA-induced epithelial wound closure occurred (55.9% wound covered, significantly decreased wound healing compared with LPA alone; P < 0.01). To determine whether HB-EGF released from the injured corneas contributes to LPA-accelerated epithelial wound healing, we treated wounded corneas with an HB-EGF antagonist, CRM197,17,43 that attenuated LPA-enhanced epithelial wound closure (58.6% wound covered, significantly reduced wound healing compared with LPA alone; P < 0.01). Among these inhibitors, AG1478 was most effective in blocking spontaneous, HB-EGF-,19 and LPA-stimulated wound closure, suggesting a key role of EGFR signaling in the regulation of corneal epithelial wound healing.
Figure 1.
Corneal epithelial wound healing in cultured porcine corneas. (A) Representative epithelial wound closure induced by LPA in cultured porcine corneas. A 4-mm diameter epithelial wound was made (Wound) and allowed to heal for 48 hours in MEM (Control), MEM containing LPA (5 μM), LPA plus AG1478 (1 μM), LPA plus CRM197 (10 μg/mL) or LPA plus GM6001 (50 μM). Wounded corneas were stained with Richardson staining solution to show the initial (Wound) and the remaining wound. (B) Changes in the extent of healing in cultured porcine corneas treated with different reagents. Coverage of the wound (0 for no migration and 100% for complete covering of the wound bed) was calculated as described in Materials and Methods. LPA accelerated corneal epithelial healing compared with control. However, the abilities of LPA to speed up healing were impaired in the presence of AG1478, CRM197, and GM6001 compared with the control (**P <0.01, one way ANOVA). Data are mean ± SD of at least six corneas from two or more independent experiments.
The involvement of EGFR and its ligand, shed HB-EGF, in epithelial wound healing was also tested in scratch wound on the THCE cell monolayer. Figure 2 shows cell migration toward the center of a scratch wound in cultured THCE cells after 24 hours (day 1). LPA greatly enhanced wound closure (93.44% of wound coverage) compared with the control without LPA (40.17%; n = 3; P < 0.01). Similar to that observed in cultured porcine corneas, AG1478 and CRM197 attenuated spontaneous epithelial wound closure and inhibited epithelial wound closure induced by LPA (33.10% and 43.66% wound coverage, respectively; P < 0.01). With the inhibitors present, the stimulatory effects of LPA on scratch wound closure can still be observed.
Figure 2.
Healing of scratch wound in cultured human corneal epithelial cells. Growth factor–starved THCE cell monolayer cultured in 12-well plates was injured with a sterile 0.1- to 10-μL pipet tip (original wound). Cells were then cultured in KBM (control), KBM containing LPA (5 μM), LPA with or without AG1478 (1 μM), or CRM197 (10 μg/mL) for 24 hours (day 1). (A) Photomicrographs show representative results of three independent experiments. (B) Changes in the extent of wound closure in THCE cells treated with different reagents. Results are presented as extent of healing (total covered as 100%) ± SD of three samples (**P <0.01, one way ANOVA).
Wounding,19 or even introducing gaps in corneal epithelial layers,44 triggers rapid activation of EGFR and its downstream signaling pathways. To examine the response of HCECs to LPA, activation of EGFR signaling was assessed using Western blotting with specific antibodies to tyrosine-phosphorylated EGFR, ERK, and AKT (a major substrate of PI3K) (Fig. 3A). Treatment of THCE cells with 5 μM LPA for 15 minutes resulted in EGFR activation and in elevated phosphorylation of ERK and AKT. As a control, wounding also increased the levels of phosphorylation of these proteins.
To determine whether HB-EGF shedding to release biologically active EGFR ligand is one mechanism by which LPA transactivates EGFR, THCE cells were pretreated with AG1478, GM6001, CRM197, or the Src kinase inhibitor PP245 and then were challenged with LPA. AG1478, as an EGFR-specific inhibitor,46 is shown to block wound-induced EGFR activation in THCE cells.19 As shown in Figure 3B, AG1478 diminished LPA-induced EGFR activation, indicating that LPA-induced EGFR phosphorylation results from autophosphorylation of EGFR. GM6001 (a broad-spectrum MMP inhibitor commonly used to inhibit ectodomain shedding), CRM197 (an HB-EGF antagonist), and PP2 (a Src kinase inhibitor that we recently showed to function as an upstream of EGFR transactivation in wounded HCECs45) also abrogated LPA-induced EGFR activation. Moreover, LPA-induced ERK and AKT phosphorylation was sensitive to these inhibitors, suggesting that LPA elicits MAPK and PI3K pathways, at least in part through HB-EGF shedding-mediated EGFR activation in HCECs.
Taken together, these data suggest that LPA accelerates epithelial wound healing through the metalloproteinase-mediated release of pro-HB-EGF and EGFR transactivation.
Comparison of EGFR Ligand and LPA-Stimulated EGFR and ERK Activation
We previously showed that 50 ng/mL HB-EGF induced maximal stimulatory effects on epithelial wound healing in cultured porcine corneas19 and elicited massive phosphorylation and degradation of EGFR45 that were not observed in cells stimulated by wounding. Given that HB-EGF and LPA significantly stimulate epithelial wound closure in an EGFR-dependent manner, we next compared EGFR activation elicited by these stimuli in THCE cells (Fig. 4). HB-EGF induced dose-dependent increases in EGFR phosphorylation and degradation, as evidenced by a significant decrease in total EGFR precipitated, with maximal effects observed at 50 ng/mL HB-EGF. Although an apparent increase occurred in EGFR phosphorylation from 1 to 2.5 μM LPA, the levels of phosphorylated EGFR remained unchanged between 2.5 and 10 μM LPA, suggesting that 2.5 μM or higher LPA produces maximal stimulation of EGFR activation. Through comparison of the band intensity of phospho-EGFR, it can be seen in Figure 4 that HB-EGF at the concentration tested (2–50 ng/mL) stimulated EGFR phosphorylation to a greater extent than LPA (1–10 μM).
LPA-Stimulated EGFR Activation
We have demonstrated that LPA, at a concentration significantly enhancing epithelial wound closure, stimulated EGFR activation to a level similar to that of wounding but less than that of HB-EGF. We then sought to determine whether LPA influences the duration of EGFR signaling (Fig. 5). As shown previously,19,45 extensive injury made by multiple scratches using a shark’s tooth sequencing comb induced rapid but transient EGFR phosphorylation that peaked at 15 minutes and then declined gradually. By 2 hours, no elevation of EGFR phosphorylation was apparent. Six hours after wounding, almost no EGFR phosphorylation was detectable. Similarly, phospho-ERK and AKT levels at 2 hours or so after wounding were similar to or lower than those of control. LPA also elicited rapid phosphorylation of EGFR, ERK, and AKT in normal and wounded THCE cells. Compared with controls, the activation of EGFR and, more apparently, ERK and PI3K was still detectable 4 and 6 hours after LPA stimulation in normal and wounded THCE cells but not in wounded cells. Thus, by comparing the phosphorylation levels of treated cells and control cells within each set (wounded, LPA treated, or wounded plus LPA), wound-induced EGFR activation was more transient than that induced by LPA in wounded and nonwounded HCECs, suggesting a potential effect of LPA on the duration of EGFR signaling in HCECs.
LPA-Induced Release of HB-EGF-AP
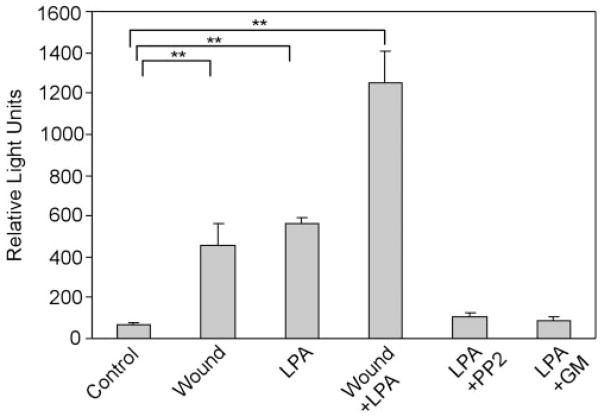
To examine whether LPA induces the proteolytic release of pro-HB-EGF, we used the THCE cell line transfected with plasmids containing the human cDNA with AP inserted into the heparin-binding region of mature human HB-EGF protein (HB-EGF-AP).25,47 HB-EGF released by the transfected cells in response to LPA or wounding was evaluated by measuring AP activities in culture media (Fig. 6). Little AP activity was observed in control cells. However, AP activities increased to 6.6-and 8.1-fold in cells treated with wounding and LPA, respectively (P < 0.01 compared with control); combination wounding and LPA treatment resulted in an 18.1-fold increase in AP activity in the culture medium (P < 0.01 compared with control). Pretreatment with 12.5 μM PP2 or 50 μM GM6001 diminished the LPA-induced increase in AP release. Taken together, these data indicate that wounding or LPA activates EGFR and its downstream signaling by inducing pro-HB-EGF shedding.
Figure 6.
LPA- and wound-induced release of HB-EGF-AP. THCE cells were stably transfected with plasmid pHB-EGF-AP. The HB-EGF-AP expressing cells cultured on six-well plates were pretreated with PP2 (12.5 μM) or GM6001 (50 μM) for 1 hour. Cells were then injured with scratch wound (Wound), stimulated with LPA (5 μM), wound plus LPA, or LPA in the continuous presence of PP2 (LPA + PP2) or GM6001 (LPA + GM). The release of AP in the media collected 15 minutes after stimulation was measured. Results are expressed as relative light units, and each value represents the mean ± SD of three wells. Wounding, LPA, or both significantly increased AP release into the culture medium compared with control (**P <0.01, one way ANOVA).
Discussion
In this study, we used porcine corneal organ culture and cultured HCECs to study the underlying mechanism by which LPA regulates corneal epithelial wound healing. Consistent with previous findings,48,49 we showed that LPA enhanced epithelial wound healing and that wound closure was induced through HB-EGF ectodomain shedding in an MMP- and Src-dependent manner. We further showed that LPA only elicited a moderate increase in EGFR compared with the intensive effects of HB-EGF and that it appeared to prolong EGFR signaling, including activation of the ERK and PI3K/AKT pathways. This ability of LPA to elicit or sustain EGFR transactivation and the activation of its downstream pathways in wounded epithelial cells may be responsible for the accelerated wound healing induced by LPA in cornea. Taken together, our results showed that LPA enhanced corneal wound healing through the induction of EGFR transactivation in HCECs.
LPA is a simple bioactive phospholipid with diverse physiological actions on many cell types.50 It acts through specific GPCRs. Watsky et al.9,51 reported an increase in LPA or LPA-like phospholipids in injured corneas. Nakamura et al.10 used culture blocks of rabbit corneas as a model and showed that the migration of epithelial cells over the cutting edge of corneal stroma was greatly enhanced by LPA. We used epithelial debridement wounds in cultured porcine corneas and scratch wounds in cultured THCE cells and showed that LPA significantly accelerates epithelial wound closure in both models. Given that accelerated corneal wound closure decreases the risk for microbial infection and may prevent vision loss caused by corneal inflammation and scarring, LPA may be therapeutically useful for corneal epithelial wound healing, as shown in skin and intestine.52–54
Other recent studies have shown that not only does LPA signal through classic second-messenger pathways, it also activates Ras and Rho family GTPases to control cell migration, proliferation, and morphogenesis. LPA is well known for its striking effects on cytoskeleton organization and cell shaping; it exerts its effects through the activation of RhoA.8 RhoA activation results in actomyosin-based contractile events such as neurite retraction, cell rounding, and endothelial tight junction opening.55–57 The use of RhoA inhibitors, such as Botulinum C3 exoenzyme, has demonstrated that the effects of LPA on corneal epithelial migration are RhoA dependent.10 Consistent with this observation, our recent study revealed that both wounding and LPA-stimulated Rho activation in migratory THCE cells (our unpublished results, 2006). LPA-mediated activation of Rho GTPases is GPCR dependent, and PI3K has been shown to play a role in switching Rho GTPases to an active state.8,57,58
In addition to Rho GTPase, LPA has been found to elicit ERK and PI3K activation.59,60 ERK1/2 is known to be involved in the regulation of several major cellular events, including survival, growth, secretion, chemotaxis, and motility.61 It has been suggested that PI3K is pivotal for the generation of cell polarity and the regulation of cell migration, particularly in controlling the direction of chemotaxis.62,63 The findings that LPA activates ERK and PI3K revealed a previously unknown mechanism by which LPA mediates an array of physiological processes, mainly cell migration, proliferation, and survival. Recent studies17 have shown that GPCR-mediated ERK1/2 and PI3K activation often occur through the transactivation of RTKs such as EGFR, which leads to sequential activation of the Ras/Raf/MEK/ERK cascade. In this study we showed that LPA induced EGFR transactivation and ERK and PI3K activation in HCECs. LPA-induced ERK and PI3K activation is sensitive to EGFR inhibitor AG1478, suggesting that ERK and PI3K are the downstream signaling effectors of EGFR in response to LPA stimulation in THCE cells. Furthermore, LPA-induced corneal epithelial wound closure, EGFR transactivation, and ERK and PI3K activation were also sensitive to MMP inhibitor GM6001 and to HB-EGF antagonist CRM197. In cultured cells, these inhibitors proportionally inhibited LPA-enhanced and spontaneous wound healing. Although it is not possible for us to precisely determine the percentage inhibited for LPA-stimulated wound healing and for wound healing in general by these inhibitors, our data indicated that the observed effects of LPA on epithelial wound healing may be attributable, at least in part, to the autocrine/paracrine activation of HB-EGF released from the cell surface. The induction of HB-EGF ectodomain shedding by LPA was further confirmed in our study using cell lines expressing HB-EGF with AP inserted into the heparin-binding region.19 Ectodomain shedding and release of ligands to the receptors may initiate crosstalk between GPCRs and EGFR in HCECs, which is consistent with many other cells.64 Thus, we propose that HB-EGF ectodomain shedding is a key event for LPA to enhance corneal epithelial wound healing and that EGFR functions as a central conduit of signaling by different classes of cell surface receptors in corneal epithelial cells.
We observed that HB-EGF (>10 ng/mL) elicited heavy phosphorylation and degradation of EGFR. This is consistent with early studies showing that the rate of EGFR endocytosis can be increased up to 10-fold after ligand activation, leading to acute, ligand-induced receptor degradation.65 Indeed, we showed that HB-EGF stimulated epithelial wound closure in the cornea. The massive EGFR phosphorylation induced by exogenous EGFR ligand may account for the enhanced migration and proliferation during corneal epithelial wound healing.19 However, the intensity of EGFR phosphorylation induced by LPA was not as strong as that by exogenously added HB-EGF and was similar to that induced by wounding (Fig. 3). This should be expected because wounding and LPA both stimulate EGFR activation through the same signal transduction machinery, including, at least in part, ectodomain shedding of HB-EGF. The availability of the ligands or the tight regulation of EGFR ligand sheddase may limit the amount of ligand(s) released and the scale of EGFR activation to a level sufficient to trigger the activation of downstream effectors such as ERK and PI3K/AKT but not EGFR degradation. How might LPA enhance wound closure? Our study showed that the presence of LPA prolonged the activation of EGFR, ERK, and AKT, possibly because wounding and LPA have synergetic effects on the release of endogenous HB-EGF in an MMP-sensitive manner in vitro. Thus, though the action through GPCRs and the effects on Rho GTPases certainly contribute to LPA-stimulated epithelial wound closure, the ability of LPA to transactivate EGFR and to sustain the activation of downstream signaling, such as ERK and PI3K, is also important for the observed physiological function. Interaction or cross-talk of these signaling pathways may coordinate or determine the epithelial cell signaling that regulates wound healing.
In summary, LPA stimulates corneal epithelial wound healing, at least in part, by facilitating HB-EGF release and providing an autocrine/paracrine ligand to EGFR. Phosphorylated EGFR in turn activates downstream signaling pathways, leading to accelerated cell migration and wound closure. However, unlike exogenous ligands of the EGFR, LPA stimulates moderate EGFR activation with undetectable receptor degradation. This moderate activation appears to be sufficient for prolonging ERK and PI3K activation and for facilitating epithelial migration, proliferation, and wound healing. Understanding the mechanisms of LPA on corneal epithelial wound healing may provide clues for therapeutic application of LPA on corneal wound healing–related problems such as persistent corneal epithelial defects and recurrent corneal erosion.
Acknowledgments
The authors thank Stay Erndt and Ying Long for their dedication, Jessica Yu and Stacy Erndt for reading the manuscript, and Judith Abrams for performing statistical analysis of the data.
Supported by National Institutes of Health/National Eye Institute Grants R01EY10869 and EY14080 and by Research to Prevent Blindness.
Footnotes
Disclosure: K.-P. Xu, None; J. Yin, None; F.-S.X. Yu, None
References
- 1.Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med. 2001;226:653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- 2.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Weng J, Mohan R, et al. Hepatocyte growth factor and hepatocyte growth factor receptor in the lacrimal gland, tears, and cornea. Invest Ophthalmol Vis Sci. 1996;37:727–739. [PubMed] [Google Scholar]
- 4.Wilson SE, Chen L, Mohan RR, Liang Q, Liu J. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res. 1999;68:377–397. doi: 10.1006/exer.1998.0603. [DOI] [PubMed] [Google Scholar]
- 5.Jalink K, Hordijk PL, Moolenaar WH. Growth factor-like effects of lysophosphatidic acid, a novel lipid mediator. Biochim Biophys Acta—Rev Cancer. 1994;1198:185–196. doi: 10.1016/0304-419x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 6.Tigyi G. Physiological responses to lysophosphatidic acid and related glycero-phospholipids. Prostaglandins Other Lipid Mediators. 2001;64:47–62. doi: 10.1016/s0090-6980(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 7.Goetzl EJ, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J. 1998;12:1589–1598. [PubMed] [Google Scholar]
- 8.van Leeuwen FN, Giepmans BN, van Meeteren LA, Moolenaar WH. Lysophosphatidic acid: mitogen and motility factor. Biochem Soc Trans. 2003;31:1209–1212. doi: 10.1042/bst0311209. [DOI] [PubMed] [Google Scholar]
- 9.Liliom K, Guan Z, Tseng JL, Desiderio DM, Tigyi G, Watsky MA. Growth factor-like phospholipids generated after corneal injury. Am J Physiol. 1998;274:C1065–C1074. doi: 10.1152/ajpcell.1998.274.4.C1065. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura M, Nagano T, Chikama T-i, Nishida T. Role of the small GTP-binding protein rho in epithelial cell migration in the rabbit cornea. Invest Ophthalmol Vis Sci. 2001;42:941–947. [PubMed] [Google Scholar]
- 11.Yin F, Watsky MA. LPA and S1P increase corneal epithelial and endothelial cell transcellular resistance. Invest Ophthalmol Vis Sci. 2005;46:1927–1933. doi: 10.1167/iovs.04-1256. [DOI] [PubMed] [Google Scholar]
- 12.An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem. 1998;273:7906–7910. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- 13.Bandoh K, Aoki J, Hosono H, et al. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274:27776–27785. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- 14.Wang DA, Du H, Jaggar JH, Brindley DN, Tigyi GJ, Watsky MA. Injury-elicited differential transcriptional regulation of phospholipid growth factor receptors in the cornea. Am J Physiol Cell Physiol. 2002;283:C1646–C1654. doi: 10.1152/ajpcell.00323.2002. [DOI] [PubMed] [Google Scholar]
- 15.van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Cunnick JM. Trans-regulation of epidermal growth factor receptor by lysophosphatidic acid and G protein-coupled receptors. Biochim Biophys Acta Mol Cell Biol Lipids. 2002;1582:100–106. doi: 10.1016/s1388-1981(02)00143-9. [DOI] [PubMed] [Google Scholar]
- 17.Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 18.Jorissen RN, Walker F, Pouliot N, Garrett TPJ, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 19.Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:813–820. doi: 10.1167/iovs.03-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 21.Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001;8:11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- 22.Xu KP, Riggs A, Ding Y, Yu FS. Role of ErbB2 in corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2004;45:4277–4283. doi: 10.1167/iovs.04-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raab G, Higashiyama S, Hetelekidis S, et al. Biosynthesis and processing by phorbol ester of the cells surface-associated precursor form of heparin-binding EGF-like growth factor. Biochem Biophys Res Commun. 1994;204:592–597. doi: 10.1006/bbrc.1994.2500. [DOI] [PubMed] [Google Scholar]
- 24.Goishi K, Higashiyama S, Klagsbrun M, et al. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell. 1995;6:967–980. doi: 10.1091/mbc.6.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gechtman Z, Alonso JL, Raab G, Ingber DE, Klagsbrun M. The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the Raf/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J Biol Chem. 1999;274:28828–28835. doi: 10.1074/jbc.274.40.28828. [DOI] [PubMed] [Google Scholar]
- 26.Hynes NE, Horsch K, Olayioye MA, Badache A. The ErbB receptor tyrosine family as signal integrators. Endocr Relat Cancer. 2001;8:151–159. doi: 10.1677/erc.0.0080151. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter G, Wahl M. The epidermal growth factor family. In: Sporn M, Ab R, editors. Peptides, Growth Factors and Their Receptors. I. New York: Springer-Verlag; 1991. pp. 69–171. [Google Scholar]
- 28.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 29.Riese DJ, 2nd, Komurasaki T, Plowman GD, Stern DF. Activation of ErbB4 by the bifunctional epidermal growth factor family hormone epiregulin is regulated by ErbB2. J Biol Chem. 1998;273:11288–11294. doi: 10.1074/jbc.273.18.11288. [DOI] [PubMed] [Google Scholar]
- 30.Olayioye M, Neve R, Lane Ha, Hynes N. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandrasekher G, Kakazu AH, Bazan HE. HGF- and KGF-induced activation of PI-3K/p70 s6 kinase pathway in corneal epithelial cells: its relevance in wound healing. Exp Eye Res. 2001;73:191–202. doi: 10.1006/exer.2001.1026. [DOI] [PubMed] [Google Scholar]
- 32.Chandrasekher G, Bazan HE. Corneal epithelial wound healing increases the expression but not long lasting activation of the p85alpha subunit of phosphatidylinositol-3 kinase. Curr Eye Res. 1999;18:168–176. doi: 10.1076/ceyr.18.3.168.5372. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Akhtar RA. Epidermal growth factor stimulation of phosphatidylinositol 3-kinase during wound closure in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1997;38:1139–1148. [PubMed] [Google Scholar]
- 34.Zhang Y, Akhtar RA. Effect of epidermal growth factor on phosphatidylinositol 3-kinase activity in rabbit corneal epithelial cells. Exp Eye Res. 1996;63:265–275. doi: 10.1006/exer.1996.0115. [DOI] [PubMed] [Google Scholar]
- 35.Glading A, Chang P, Lauffenburger D, Wells A. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- 36.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zieske J, Gipson I. Protein synthesis during corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1986;27:1–7. [PubMed] [Google Scholar]
- 38.Foreman DM, Pancholi S, Jarvis EJ, McLeod D, Boulton ME. A simple organ culture model for assessing the effects of growth factors on corneal re-epithelialization. Exp Eye Res. 1996;62:555–564. doi: 10.1006/exer.1996.0065. [DOI] [PubMed] [Google Scholar]
- 39.Richardson K, Jarett L, Finke E. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- 40.Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 41.Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Mouse preimplantation blastocysts adhere to cells expressing the trans-membrane form of heparin-binding EGF-like growth factor. Development. 1996;122:637–645. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- 42.Xu KP, Li XF, Yu FS. Corneal organ culture model for assessing epithelial responses to surfactants. Toxicol Sci. 2000;58:306–314. doi: 10.1093/toxsci/58.2.306. [DOI] [PubMed] [Google Scholar]
- 43.Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem. 1995;270:1015–1019. doi: 10.1074/jbc.270.3.1015. [DOI] [PubMed] [Google Scholar]
- 44.Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- 45.Xu K, Yin J, Yu F. Src-family tyrosine kinases in wound- and ligand-induced epidermal growth factor receptor activation in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:2832–2839. doi: 10.1167/iovs.05-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 47.Uchiyama-Tanaka Y, Matsubara H, Mori Y, et al. Involvement of HB-EGF and EGF receptor transactivation in TGF-beta-mediated fibronectin expression in mesangial cells. Kidney Int. 2002;62:799–808. doi: 10.1046/j.1523-1755.2002.00537.x. [DOI] [PubMed] [Google Scholar]
- 48.Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci. 2000;41:1346–1355. [PubMed] [Google Scholar]
- 49.Nakamura Y, Sotozono C, Kinoshita S. The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp Eye Res. 2001;72:511–517. doi: 10.1006/exer.2000.0979. [DOI] [PubMed] [Google Scholar]
- 50.Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- 51.Watsky MA, Griffith M, Wang DA, Tigyi GJ. Phospholipid growth factors and corneal wound healing. Ann N Y Acad Sci. 2000;905:142–158. doi: 10.1111/j.1749-6632.2000.tb06546.x. [DOI] [PubMed] [Google Scholar]
- 52.Balazs L, Okolicany J, Ferrebee M, Tolley B, Tigyi G. Topical application of the phospholipid growth factor lysophosphatidic acid promotes wound healing in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;280:R466–R472. doi: 10.1152/ajpregu.2001.280.2.R466. [DOI] [PubMed] [Google Scholar]
- 53.Sturm A, Dignass AU. Modulation of gastrointestinal wound repair and inflammation by phospholipids. Biochim Biophys Acta Mol Cell Biol Lipids. 2002;1582:282–288. doi: 10.1016/s1388-1981(02)00182-8. [DOI] [PubMed] [Google Scholar]
- 54.Deng W, Balazs L, Wang DA, Van Middlesworth L, Tigyi G, Johnson LR. Lysophosphatidic acid protects and rescues intestinal epithelial cells from radiation- and chemotherapy-induced apoptosis. Gastroenterology. 2002;123:206–216. doi: 10.1053/gast.2002.34209. [DOI] [PubMed] [Google Scholar]
- 55.Kranenburg O, Poland M, van Horck FPG, Drechsel D, Hall A, Moolenaar WH. Activation of RhoA by lysophosphatidic acid and Galpha 12/13 subunits in neuronal cells: induction of neurite retraction. Mol Biol Cell. 1999;10:1851–1857. doi: 10.1091/mbc.10.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amerongen GPvN, Vermeer MA, van Hinsbergh VWM. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 2000;20:127e–133e. doi: 10.1161/01.atv.20.12.e127. [DOI] [PubMed] [Google Scholar]
- 57.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 58.Han J, Luby-Phelps K, Das B, et al. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Z, Liu Z, Meier KE. Lysophosphatidic acid as a mediator for pro-inflammatory agonists in a human corneal epithelial cell line. Am J Physiol Cell Physiol. 2006;291:C1089–C1098. doi: 10.1152/ajpcell.00523.2005. [DOI] [PubMed] [Google Scholar]
- 60.Hong JH, Oh SO, Lee M, et al. Enhancement of lysophosphatidic acid-induced ERK phosphorylation by phospholipase D1 via the formation of phosphatidic acid. Biochem Biophys Res Commun. 2001;281:1337–1342. doi: 10.1006/bbrc.2001.4517. [DOI] [PubMed] [Google Scholar]
- 61.Luttrell LM. Composition and function of G protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity. J Mol Neurosci. 2005;26:253–264. doi: 10.1385/JMN:26:2-3:253. [DOI] [PubMed] [Google Scholar]
- 62.Haugh JM, Codazzi F, Teruel M, Meyer T. Spatial sensing in fibro-blasts mediated by 3′ phosphoinositides. J Cell Biol. 2000;151:1269–1280. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 64.Higashiyama S, Nanba D. ADAM-mediated ectodomain shedding of HB-EGF in receptor cross-talk. Biochim Biophys Acta. 2005;1751:110–117. doi: 10.1016/j.bbapap.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Wiley HS. Trafficking of the ErbB receptors and its influence on signaling. Exp Cell Res. 2003;284:78–88. doi: 10.1016/s0014-4827(03)00002-8. [DOI] [PubMed] [Google Scholar]