Abstract
Background
Previously, we presented evidence that at physiologic concentrations the green tea catechin, epigallocatechin gallate (EGCG), inhibited attachment of gp120 to the CD4 molecule on T cells, but the downstream effects of EGCG upon HIV-1 infectivity were not determined.
Objective
To evaluate the inhibition of HIV-1 infectivity by EGCG and begin preclinical development of EGCG as a possible therapy.
Methods
Peripheral blood mononuclear cells, CD4+ T cells, and macrophages were isolated from blood of HIV-1 uninfected donors. HIV-1 infectivity was assessed by an HIV-1 p24 enzyme linked immunoassay. Cell survival was assessed by cell viability by trypan blue exclusion assay; cell growth by thymidine incorporation; and apoptosis by flow cytometric analysis of Annexin-V binding.
Results
EGCG inhibited HIV-1 infectivity on human CD4+ T cells and macrophages in a dose-dependent manner. At a physiologic concentration of 6μM, EGCG significantly inhibited HIV-1 p24 antigen production across a broad spectrum of both HIV-1 clinical isolates and laboratory-adapted subtypes [B (p<0.001), C, D, and G (p<0.01)]. The specificity of the EGCG-induced inhibition was substantiated by the failure of EGCG derivatives lacking galloyl and/or pyrogallol side groups to alter HIV-1 p24 levels. EGCG-induced inhibition of HV-1 infectivity was not due to cytotoxicity, cell growth inhibition, nor apoptosis.
Conclusion
We conclude that by preventing the attachment of HIV-1-gp120 to the CD4 molecule, EGCG inhibits HIV-1 infectivity. As this inhibition can be achieved at physiologic concentrations, the natural anti-HIV agent, EGCG, is a candidate as an alternative therapy in HIV-1 therapy.
Keywords: EGCG, HIV, p24, subtypes, lymphocytes, alternative therapy, green tea, apoptosis, cytotoxicity, proliferation
INTRODUCTION
Human immunodeficiency virus type-1 (HIV-1) infection ultimately results in impaired specific immune function by virtue of the initial binding of the HIV-1 virion envelope glycoprotein 120 (gp120) to the CD4 receptor in complex with a chemokine receptor on the T-cell surface. HIV infection ensues from the viral entry and the subsequent destruction of T cells eventually leads to acquired immunodeficiency syndrome (AIDS).1 HIV-1 isolates are distinguishable by the main co-receptor used for cell entry, chemokine receptors CXCR4 (X4) or CCR5 (R5).1, 2 Three groups of HIV-1 have been described, M, N, and O, based on genome differences. Most HIV-1 infections are caused by group M viruses, and these are divided into 9 subtypes (A–D, F–H, J, and K) with B and C as the most common.3
At present, due to continuous emergence of drug resistance and side-effects of current drugs, more and more efforts have been spent on searching for more effective anti-HIV drugs.4 A variety of natural products, such as ribosome inactivating proteins, alkaloids, flavonoids, and polyphenols possess promising anti-HIV activity. 5–7 Among these are flavonoids that inhibit reverse transcriptase (RT), induce interferons which inactivate viral protease8 and down-regulate the expression of HIV co-receptors such as CCR2b, CCR3 and CCR5.9
Catechins and theaflavins are two groups of natural polyphenols found in green tea and black tea, respectively.10 The four main catechin derivatives include (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC), and (−)-epigallocatechin gallate (EGCG). EGCG is found to be the most prevalent and active catechin of green tea due to the pyrogallol and galloyl moieties with physiologic concentrations ranging from 0.1 to 10 μM.11, 12 Seven cups of green tea (containing 118mg EGCG, each, would result in a mean peak plasma level, within the physiologically relevant range, of 1.0 μM. 13, 14 Among the biomodulative properties of EGCG are the anti-inflammatory15, 16 and anti-allergic effects such as inhibition of type IV allergic responses17 and histamine release,18, 19 as well as anti-oxidative, anti-tumor, and antiviral activities.20–22
EGCG, has been reported to inhibit HIV-1 replication by targeting several steps in the HIV-1 life cycle, such as interfering with the RT and protease activity, blocking gp120–CD4 interaction by binding to CD4, and inactivating virions, but (almost) all of these observations were at high non-physiologic concentrations (i.e. >10μM) EGCG.12, 23–25
Our previous study demonstrated evidence of high affinity binding of EGCG to the CD4 molecule with a Kd of 10 nM with subsequent inhibition of gp120 binding to human CD4+ T cells. EGCG binds in the same molecular pocket on CD4 as does HIV-1-gp120. EGCG, at 0.170μM, (a concentration equivalent to that obtained by the consumption of 2 cups of green tea) is able to reduce the attachment of gp120 to CD4 by a factor of between 10-fold and 20-fold.7 In the present study we demonstrate that the inhibition of HIV-1-gp120 binding to the CD4 receptor by the green tea catechin, EGCG, is specifically responsible for the inhibition of HIV-1 infectivity at physiologic concentrations. Moreover, the EGCG-induced inhibition is effective across a broad spectrum of HIV-1 subtypes and without compromising the survival of lymphocytes.
METHODS
Reagents
The following reagents (and their sources) were used: Catechin derivatives including EGCG, (−) catechin, ECG and EGC and theaflavin (Sigma, St. Louis, MO); phytohemmaglutinin (PHA) (Remel, Lenexa, KS) antiretoviral drugs, azidothymidine (AZT) and ritonavir (gifts of Dr. Jason Kimata, Dept. Molecular Virology and Microbiology, Baylor College of Medicine, Houston, TX).
Human primary cell isolations
Human studies issues
Informed consent for HIV-1–negative donor blood was obtained and donor selection was made according to the Guidelines of the Gulf Coast Regional Blood Bank (Houston, TX) in a manner approved by the Institutional Review Board at Baylor College of Medicine (H16902).
Peripheral blood mononuclear cells (PBMC) isolation
Fresh PBMC were prepared from blood of HIV-1 uninfected donors (Gulf Coast Regional Blood Bank, (Houston, TX) as previously described.7
Isolation of CD4+ human T cells
CD4+ T cells were positively selected from platelet-depleted human leukopaks to obtain a highly purified CD4+ Tcell population as previously described.7
Isolation of human macrophages
Following PBMC isolation, cells were resuspended in RPMI growth media 1640 (Invitrogen, Carlsbad, CA) supplemented with 1% heat-inactivated human blood type AB serum (Sigma-Aldrich, St. Louis, MO). PBMC were cultured in plates, for 1 h at 37°C, 5% CO2. The cells were washed with Dulbecco’s phosphate buffered saline (DPBS) Invitrogen, Carlsbad, CA) to remove non-adherent cells and resuspended in RPMI 1640. Cells were incubated at 37°C, 5% CO2 for seven days with feeding (100% volume exchange) every 3–4 days.
Lymphocyte Survival Assessment
Cell viability
Cell viability was measured by the ability of living cells to exclude trypan blue vital dye. Living cells that did not take up the stain were counted and expressed as the percentage of the total count of the untreated control.
Measurement of lymphocyte proliferation
PBMC were cultured in 96-well plates with RPMI 1640 medium with 10% fetal bovine serum (FBS) in the presence or absence of various stimulators for 2 days at 37°C in 5% CO2. The cells were pulsed with 1 mCi per well of tritiated thymidine (New England Nuclear, Wilmington, Del) during the last 18 hours of culture and harvested.26
Detection of apoptosis by flow cytometry
Following treatment for 24 hours with different concentrations of EGCG, (−)-catechin or medium, PBMC were double-stained with fluorescein isothiocyanate (FITC)–conjugated Annexin-V and propidium iodide (PI) (BioVision, Mountain View, CA). The cells were analyzed by flow cytometry. Annexin-V(−)PI(−) cells were considered viable cells; Annexin-V(+)PI(−) cells, early apoptotic cells; Annexin-V(+)PI(+) cells, late apoptotic cells; and Annexin-V(−)PI(+) cells, necrotic cells. Data was collected in a FACS Calibur (Becton Dickinson, San Jose, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR).
HIV-1 p24 antigen production experimental protocol
HIV-1 Group M isolates
The following reagents were utilized and obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS (DAIDS), (NIAID, NIH, Bethesda, MD.): clinical isolates; HIV-192UG038, (subtype D, X4) (#1744); HIV-1JV1083, (subtype G, R5) (#3191 from A. Abimiku); HIV-196USNG31, (subtype C, X4/R5) (#4110 from D. Ellenberger); HIV-194UG114, (subtype D, R5) and (#386 from DAIDS). Also utilized were clinical isolate, HIV-1UC5 (subtype B, R5),(a gift of Dr. Janet Lathey of the University of California, San Diego, CA) and laboratory-adapted HIV-1SF162: (subtype B, R5), (gift of Dr. Dorothy Lewis, Dept. Molecular Virology & Microbiology, Baylor College of Medicine, Houston, Texas), -HIV-189.6 (subtype B, R5/X4), and HIV-1Ba-L (subtype B, R5), (#510 and contributed by S. Gartner).
HIV infection assay
PBMC were cultured in RPMI 1640 medium supplemented with 20% FBS 3% IL2, 5 μg/mL, PHA-P (Sigma-Aldrich, St. Louis, MO), and allowed to stimulate for 1–3 days (37°C, 5% CO2). Following stimulation, cells were cultured in RPMI 1640 with 20% FBS, and 5% IL2. Following pre-incubation with the test agent, cells were plated in 24-well plates at a density of 1×106 PBMC/mL. Each well, except for the negative virus control, received sufficient virus to achieve a concentration of 1,000 50 % tissue culture infectious dose (TCID50) units of HIV-1 stock per 1×106 PBMC/mL and incubated overnight. Following overnight incubation, wells were washed with PBS to remove virus. Following washing, cultures were transferred to a 48-well cell culture plate (0.5×106 PBMC/0.5 mL per well) in triplicate.
HIV-1 p24 Enzyme-Linked ImmunoSorbent Assay (ELISA)
ELISA determinations were performed using the RetroTek HIV-1 p24 antigen ELISA kit (Zeptometrix, Buffalo, NY) on culture supernatants. In each experiment, the positive inhibition control was treatment with AZT (10 μM), or ritonavir (1.4μM) if used, and the negative inhibition control was no treatment. Briefly, supernatant from the positive virus control was tested at day 5–6 to determine growth of virus. On day 7, supernatant was harvested and transferred to 96-well microplates where p24 antigen was released by adding 20 mL of the ELISA lysis buffer. The procedure is included in the p24 ELISA kit and was used without modification. The lower limit of the assay is 4.0 pg/ml. Calculations of the p24 content were calculated in an Excel spreadsheet using a Forecast formula contained within the program menu.
Statistical Analysis
Flow cytometry
Data generated from the flow cytometer was recorded and statistically analyzed using Coulter software. Calculation of fluorescence (expressed as median value of fluorescence emission curve) was conducted after conversion of logarithmically amplified signals into values on a linear scale. The statistical significance was calculated by using Student t test.
Other Data
Statistical significance (based on the mean of each experimental replicate group) was determined by using the Student t test with confirmation by analysis of variance (ANOVA), utilizing SigmaStat software (Systat, Point Richmond, CA). In all tests, p <0.05 was considered statistically significant.
RESULTS
Effect of EGCG on HIV-1 infectivity: p24 antigen production
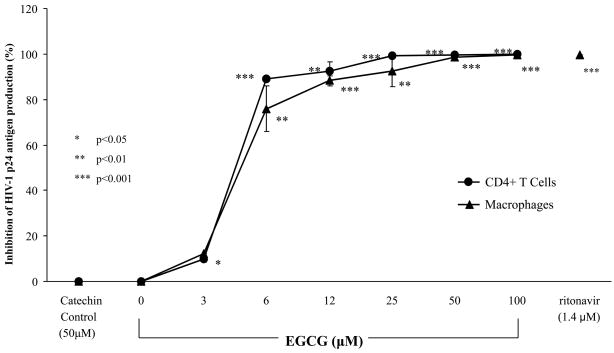
EGCG inhibited p24 antigen production in a dose-dependent manner on freshly isolated human primary CD4 receptor positive cells, CD4+ T cells and macrophages (Fig. 1). EGCG inhibited the subtype B, R5-HIV-1Ba-L in both CD4+ T cells and macrophages cells with significant inhibition observed at the physiologic concentration of 6μM (p<0.001, p<0.01, respectively) and at higher concentrations (12–100μM) (p< 0.001) with a 50% inhibitory concentration (IC50) of 4.5μM. Control catechin, (−)-catechin, was utilized as it does not contain the pyrogallol and galloyl moieties attributed to the specificity of EGCG resulting in no significant effect on HIV-1 p24 levels. Comparison of (−)-catechin to that of EGCG on HIV-1 antigen levels, at two time points (4 and 7 days), resulted in no significant changes in pattern of inhibition (Table I). The positive control, antiretroviral drug, ritonavir (1.4μM), induced 100% (p<0.001) inhibition of HIV-1 p24 antigen in our system, thereby substantiating the validity of our assay system.
FIG 1.
EGCG-induced inhibition of HIV-1 infectivity. HIV-1 p24 antigen production in freshly isolated human CD4+ T cells (●) and macrophages (▲) was measured by ELISA with subtype B, R5-HIV-1Ba-L for 7 days in the presence of EGCG, medium, control catechin, (−)-catechin, or ritonavir. The data are expressed as means ±SD of three separate experiments.
Table I.
EGCG-Induced HIV-1 p24 Antigen Production Time Course
| Inhibition of HIV-1 p24 antigen production (%) ‡ |
||
|---|---|---|
| Day 4 | Day 7 | |
| Agent | ||
| EGCG | ||
| 6 | 83.0±0.57* | 75.9±5.5* |
| 12 | 94.9±2.1** | 86.3±5.1* |
| 25 | 99.9±2.4** | 97.4±2.3** |
| 50 | 99.6±1.0** | 99.6±0.6** |
| 100 | 100±0.1** | 99.9±0.05** |
| (−)-catechin | ||
| 10 | 9.09 ±1.7 | 7.15±2.6 |
| 25 | 11.9±2.2 | 10.0±3.4 |
| 50 | 18.2±2.0 | 25.7±2.1 |
| ritonavir | ||
| 1.4 μM | 99.9 ± 0.01** | 99.9±0.02** |
Clinical isolate, subtype B, R5 HIV-1UC5
Data expressed as means ±SD of percent inhibition over control of three experiments.
p< 0.01;
p< 0.001
Specificity of EGCG-induced HIV-1 infectivity
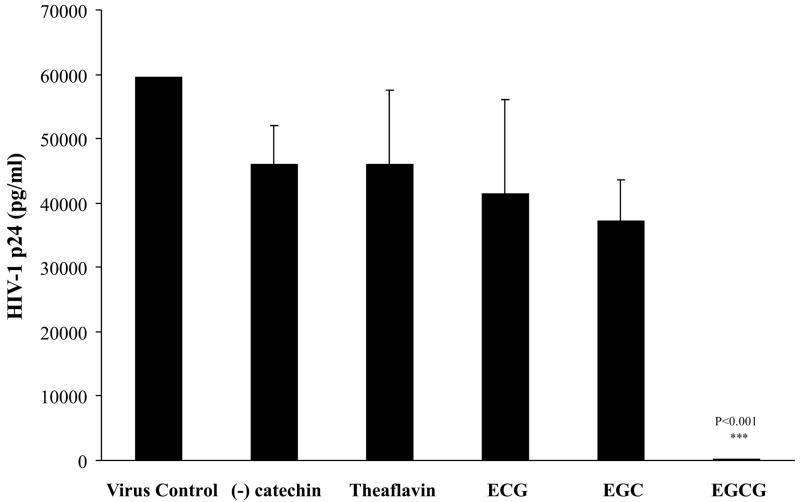
Demonstration of the specificity of the specific green tea catechin, EGCG, on HIV-1 infectivity inhibition was made by the incubation of various EGCG derivatives (50 μM), well beyond physiologic concentrations, in our assay system. As both the pyrogallol and galloyl moieties are required for the attributes of EGCG, we evaluated p24 antigen production of ECG which possesses only the galloyl group, while EGC possesses only the pyrogallol group, (−)-catechin possesses neither the pyrogallol and galloyl groups, and theaflavin which is a black tea polyphenol (Fig. 2). Only EGCG significantly induced inhibition of HIV-1 p24 antigen production (p<0.001). Therefore, the effect of EGCG to induce HIV-1 infectivity is specific and most likely due to the molecular makeup of possessing both the pyrogallol and galloyl moieties.
FIG 2.
Effect of catechins on HIV-1 p24 antigen production in human lymphocytes. HIV-1 p24 antigen production was measured in the presence and absence of the green tea catechins, (−)-catechin, ECG, EGC, and EGCG at 50μM and the black tea catechin, theaflavin at 20μM. The data are expressed as means ±SD of three separate experiments.
Antiviral activity of EGCG across HIV global subtypes, strains, and clinical isolates
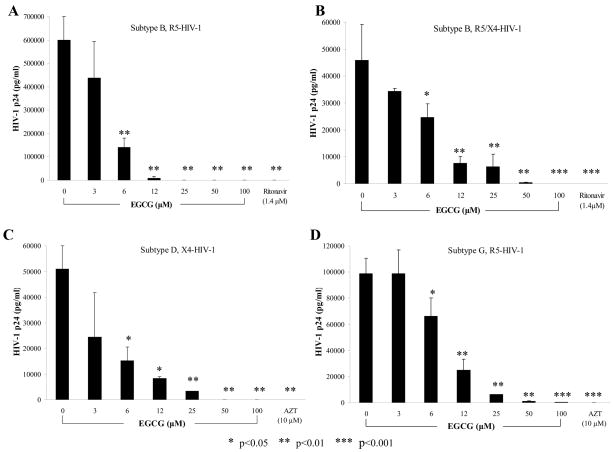
We investigated the robustness of the EGCG-induced HIV-1 infectivity inhibitory response across a spectrum of various HIV subtypes, tropisms, and clinical isolates (Fig. 3). EGCG, in a dose-dependent manner inhibited HIV-1p24 antigen levels in laboratory-adapted subtype B, R5-HIV-1SF162 (IC50 of 4.5μM) (Fig. 3A), laboratory-adapted subtype B, R5/X4-HIV-189.6 (IC50 of 8.0 μM) (Fig. 3B), clinical isolate subtype D, X4-HIV-192UG038 (IC50 of 9.0 μM) (Fig. 3C) and clinical isolate subtype G, R5-HIV-1JV1083 (IC50 of 9.0μM) (Fig. 3D). p24 antigen production of HIV-1 isolates of subtype B and G was significantly inhibited at 6 μM (p<0.01) and subtype D at (p<0.05). These isolates exhibited significant inhibition of HIV-1 p24 antigen production due to EGCG at concentrations 12–100μM (p<0.05 – p<0.001) over cultures grown in the absence of EGCG (0μM). All of these viral isolates showed the expected sensitivity to the antiretroviral drugs, AZT or ritonavir, under these experimental conditions.
FIG 3.
EGCG inhibition of HIV-1 p24 antigen production in human macrophages using different HIV-1 subtypes; A) subtype B, R5-HIV-1SF162, B) subtype B, R5/X4-HIV-189.6, C) subtype D, X4-HIV-192UG038 and D) subtype G, R5-HIV-1JV1083. The data are expressed as means ±SD of three experiments for A) and B); of two experiments for C) and D)..
The results of antiviral experiments using a more expanded range of laboratory-adopted and clinical isolates of varying subtypes and strains of HIV-1 are shown in Table II. The IC50 values from the HIV-1 p24 assay for EGCG are provided. EGCG inhibited a wide range of HIV-1 isolates from diverse sources. EGCG potently inhibited both laboratory-adapted subtypes of HIV-1 as well as clinical isolates from HIV-1 infected patients. The IC50 ranged from the most potent for both clinical and laboratory-adapted subtype B, R5-HIV-1UC5, or SF162, at the physiological concentration of 4.5μM, to that for the clinical isolates of subtypes C, X4/R5-HIV-196USNG31, and subtype D, R5-HIV-194UG114, of 12μM. All of the viral isolates showed the expected sensitivity to the antiretroviral drugs, AZT or ritonavir, under these experimental conditions (data not shown).
Table II.
Inhibition of HIV-1 Infection by EGCG Across Subtypes
| HIV-1 | Inhibition of p24 antigen production | ||
|---|---|---|---|
| Subtype | Tropism | Isolate | IC50 (μM) |
| B | R5 | SF162 | 4.5 ±0.4 |
| B | R5 | UC5* | 4.5 ±1.0 |
| B | X4/R5 | 89.6 | 8.0 ±0.8 |
| C | X4/R5 | 96USNG31* | 12.0 ±1.6 |
| D | R5 | 94UG114* | 12.0 ±0.7 |
| D | X4 | 92UG038* | 9.0 ±1.0 |
| G | R5 | JV1083* | 9.0 ±0.5 |
Clinical isolate
Data are depicted as means ±SD of four experiments (subtype B, R5 isolates) or two experiments (all other isolates).
EGCG does not affect the survival of human lymphocytes
No significant cytotoxicity, determined by the trypan blue exclusion assay, was observed in PBMC at physiologic levels of EGCG and no significant cytotoxicity was induced by EGCG until a concentration of 100μM (73%) (p<0.05) (data not shown). Similar results were attained in HIV-1-infected cell cultures (data not shown) with a 50% cytotoxicity concentration (CC50) of >100 μM. Cell viability was not altered due to increased exposure over time to EGCG (data not shown).
Furthermore, EGCG, at micromolar concentrations likely to be achieved in humans, 1–10 μM, exerted no significant change in normal proliferative responses on human lymphocytes and 50μM, hardly achievable at physiological conditions, was necessary for the effect to be significant (44%) (p<0.05) (data not shown).
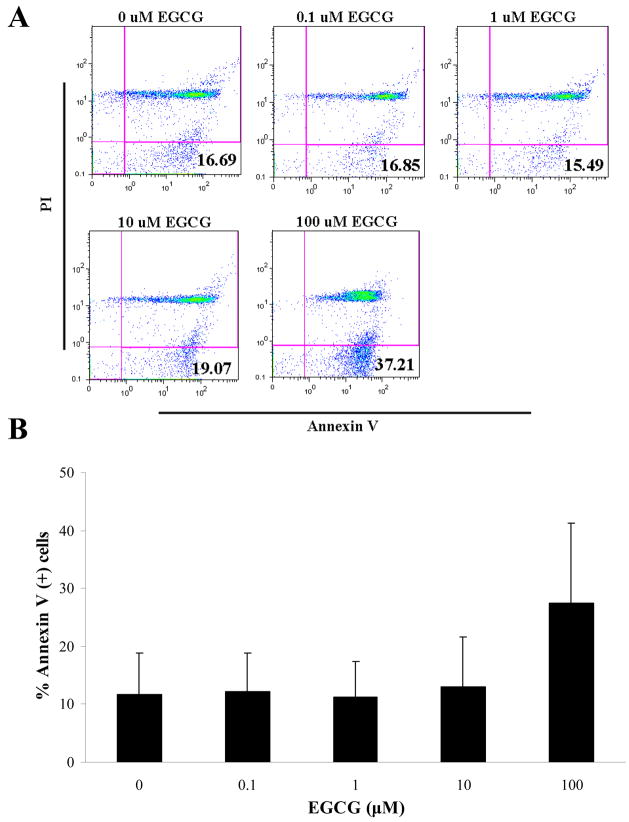
EGCG-induced HIV-1 p24 inhibition is not linked to induction of apoptosis
Determination of whether the mechanism of EGCG induction of HIV-1 p24 antigen production inhibition is via apoptosis induction was made by examination of the translocation of phosphatidlyserine from the internal to the external leaflet of the cell membrane by dual staining with fluorescene-conjugated Annexin-V and PI. After treatment of human PBMC with EGCG (1–10μM), the percentage of Annexin-V-positive and PI-negative cells was almost identical to that in a population of non-treated cells (0μM EGCG), while treatment with a non-physiological concentration of EGCG (100μM) resulted in a non-significant trend toward an increase of cells undergoing apoptosis (Fig 4).
FIG 4.
Effect of EGCG on apoptosis of human lymphocytes. Flow cytometric determination of apoptosis on PBMC after 24 hours in the presence or absence of EGCG. A) Representative histogram of Annexin-V (x-axis) and PI (y-axis) staining. Percent Annexin +/PI− are indicated in quadrant 4. B) Graphic representation of histogram data in A compared to baseline. A) representative of two experiments and B) expressed as means ±SD.
DISCUSSION
A number of natural products mainly derived from plants have proven effective in suppressing HIV replication and progress. Calanolide derivatives, pokeweed antiviral proteins and sulphated polysaccharides are only but a few of the natural compounds with antiviral activities.27, 28 In the present study, we have demonstrated the downstream outcome of the EGCG-induced inhibition of HIV-1-gp120 attachment to the CD4 molecule, that of the inhibition of HIV-1 infectivity.
As our previous study had shown that the CD4 molecule on human T cells was the target in the EGCG-induced inhibition of HIV-1-gp120 attachment (6306), we investigated the effect EGCG had on HIV-1 p24 antigen production in these cells as well as in another CD4+ population, macrophages. EGCG exerted a dose-dependent inhibition of HIV-1 p24 antigen production on both CD4+ T cells and macrophages at physiologic concentrations of EGCG (<10μM) a finding not previously demonstrated.12, 23, 25 Moreover, the specificity of the EGCG-induced HIV-1 infectivity was substantiated by the inability of various other catechins to significantly inhibit HIV-1 p24 antigen production. Most notably, the absence of effect by EGC (lacks the galloyl moiety), ECG (lacks the pyrogallol moiety), and (−)-catechin, (lacks both the pyrogallol and galloyl moieties), demonstrates the specificity of the EGCG-induced effect which requires both the pyrogallol and galloyl moieties. The specificity of these moieties for EGCG-induced effects have been observed in cancer studies.29
Next we investigated the efficacy of EGCG over a broad spectrum of HIV-1 subtypes. Different subtypes might be transmitted with different levels of efficiency and might differ in their pathogenic potential.3 Differences exist in the biologic properties in the various group M HIV-1 subtypes. Subtype D HIV-1 leads to faster disease progression than the other subtypes. 30 Subtype C HIV-1 seems to be spreading more rapidly worldwide than other subtypes.31 R5 viruses predominate primary HIV-1 infection for subtypes A, B, C and D, whereas X4 or R5X4 viruses emerge in about 50% of late HIV disease 32 HIV-1 p24 antigen levels were significantly inhibited by EGCG in a broad range of HIV-1 subtypes and receptor usage. Furthermore, these results were achieved on samples utilizing HIV-1 clinical isolates as well as laboratory isolates.
This broad specificity of EGCG for HIV-1 subtype suggests that EGCG may be an effective alternative therapy against HIV-1 infection. Like vaccines, current HIV treatments such as nucleoside and non-nucleoside reverse transcriptase inhibitors as well as protease inhibitors were developed in Western countries based on an HIV-1 subtype B model. As these and other medications become available in the developing world, the efficacy of these drugs for non-B subtypes becomes a significant issue. Both the natural resistance of the different viruses in drug naïve individuals as well as the propensity of the virus for developing drug resistance after treatment are major concerns.33
Evaluation of cytotoxicity in the HIV-1-infected cultures, revealed that EGCG did not decrease cell viability. The cell viability of human lymphocytes and macrophages was not significantly inhibited by EGCG over a range of 1–100μM. Studies by other investigators using human cell lines found the CC50 of EGCG to be well above the concentrations investigated in this study. In T cell lines, H9 and MT-2, the CC50 were 175 μM and 191 μM, respectively;12, 25, 27 in the monocytic cell line, THP-1, 440 μM;25 and in the human fibroblast cell line, MRC-5, 146 μM.12 The CC50 in human fibroblasts was shown to be 256μM34 and in human neutrophils and keratinocytes, EGCG (100 μM) exhibited 90% viability.16, 35 In our studies, proliferative responses on human lymphocytes were not significantly affected by EGCG at physiologic concentrations. Significant inhibition was not observed until 50μM EGCG had been reached and similar responses have been demonstrated in mouse splenic T cells and thymocytes.36 Finally, analysis of apoptotic events by Annexin-V-binding flow cytometry demonstrated that EGCG did not induce apoptosis at physiologic concentrations. EGCG has been shown to selectively inhibit cell growth and induce apoptosis in cancer cells without adversely affecting normal cells.37–40 In normal human cells, studies have shown that EGCG does not induce apoptosis. In human monocytes,41 human T cells,42 and the B cell line, Ramos43 showed that EGCG (50 μM) did not induce apoptosis. In human fibroblasts, EGCG (250μM) did not induce apoptosis.34 All of these studies reinforce our findings that physiologic concentrations of EGCG are not harmful to cells nor is inhibition of HIV-1 infectivity caused by cytotoxicity or apoptosis.
No studies to date have evaluated the safety, efficacy, and pharmacokinetic characteristics of EGCG in HIV-1-infected individuals. There have been several in-depth studies of the safety and pharmacokinetics of EGCG in healthy and cancer subjects.22, 44–49 On the basis of the reported adverse events and clinical laboratory data in the trials, EGCG has been found to be safe and well tolerated by the study subjects and the reported adverse events were rated as mild events.22, 44, 45 Other studies suggest that EGCG administration may be less likely to affect the pharmacokinetics of commonly used pharmaceutical drugs, in particular many of the available antiretroviral agents.47, 50, A Phase I study of EGCG in HIV-infected patients is a logical next step in the development as a possible complementary and alternative medicine for HIV infection.
We conclude that a crucial aspect of translating the observed effects of EGCG to clinically relevant strategies is the necessity of proving that physiological relevant concentrations inhibit HIV-1 replication. The findings of our study provide the basis of the mechanism and downstream effects on HIV-1 infectivity. Thus, EGCG may represent a potential low-cost inhibitor of global HIV-1 infection that could be utilized at least as adjunctive anti-HIV therapy.
Acknowledgments
Supported by National Institutes of Health grant AT003084
We thank Melinda D’Souza and Ashley McMullen for their technical assistance.
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- AZT
Azidothymidine
- CC50
50% cytotoxicity concentration
- DAIDS
Division of AIDS
- DPBS
Dulbecco’s phosphate buffered saline
- EC
Epicatechin
- ECG
Epicatechin gallate
- EGC
Epigallocatechin
- EGCG
Epigallocatechin gallate
- ELISA
Enzyme linked immunoassay
- FBS
Fetal bovine serum
- FITC
Fluorescein isothiocyanate
- gp120
Glycoprotein 120
- HIV-1
Human immunodeficiency virus type-1
- IC50
50% inhibitory concentration
- PBMC
Peripheral blood mononuclear cells
- PHA
Phytohemmaglutinin
- PI
Propidium iodide
- RT
Reverse transcriptase
- TCID50
50% Tissue culture infectious dose
Footnotes
Disclosure: None to declare
CLINICAL IMPLICATIONS
Development of the green tea catechin, EGCG, as an alternative therapy in HIV-1 infection may result in a potentially non-toxic effective treatment across a broad spectrum of HIV-1 isolates.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kwong P, Wyatt R, Robinson J, Sweet R, Sodroski J, Hendrickson W. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–59. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell G, Spector S. CCL2 increases X4-tropic HIV-1 entry into resting CD4+ T cells. J Biol Chem. 2008:18. doi: 10.1074/jbc.M804112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen M, Hellmann N, Levy J, DeCock K, Lange J. The spread, treatment, and prevention of HIV-1: evolution of a global pandemic. The Journal of Clinical Investigation. 2008;118(4):1244–54. doi: 10.1172/JCI34706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R, Gu Q, Wang Y, Zhang X, Yang L, zhou J, et al. Anti-HIV-1 activities of compounds isolated from the medicinal plant Rhus chinensis. Journal of Ethnopharmacology. 2008;117(2):249–56. doi: 10.1016/j.jep.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Wang X, Yi Y, Lee K. Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorg Med Chem Lett. 2003a;13:1813–5. doi: 10.1016/s0960-894x(03)00197-5. [DOI] [PubMed] [Google Scholar]
- 6.Cos P, Maes L, Vanden Berghe D, Hermans N, Pieters L, Vlietinck A. Plant substances as anti-HIV agents selected according to their putative mechanism of action. Journal of Natural Products. 2004;67:284–93. doi: 10.1021/np034016p. [DOI] [PubMed] [Google Scholar]
- 7.Williamson MP, McCormick TG, Nance CL, Shearer WT. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: Potential for HIV-1 therapy. J Allergy Clin Immunol. 2006;118:1369–74. doi: 10.1016/j.jaci.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Havsteen B. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 9.Nair M, Kandaswami C, Mahajan S, Nair H, Chawda R, Shanahan T, et al. Grape seed extract proanthocyanidins downregulate HIV-1 entry coreceptors, CCR2b, CCR3 and CCR5 gene expression by normal peripheral blood mononuclear cells. Biological Research. 2002;35:421–31. doi: 10.4067/s0716-97602002000300016. [DOI] [PubMed] [Google Scholar]
- 10.Leung L, Su Y, Chen R, Zhang Z, Huang Y, Chen Z. Theaflavins in black tera and catechins in green tea are equally effective antioxidants. J Nutr. 2001;131:2248–51. doi: 10.1093/jn/131.9.2248. [DOI] [PubMed] [Google Scholar]
- 11.Kanadzu M, Lu Y, Morimoto K. Dual function of (−)-epigallocatechin gallate (EGCG) in healthy human lymphocytes. Cancer Letters. 2006;241:250–5. doi: 10.1016/j.canlet.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Lu H, Zhao Q, He Y, Niu J, Debnath AK, et al. Theaflavin derivatives in black tea and catechin derivaties in green tea inhibit HIV-1 entry by targeting gp41. Biochimica et Biophysica Acta. 2005;1723:270–81. doi: 10.1016/j.bbagen.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Wang Z, Li H, Chen L, Sun Y, Gobbo S, et al. Analysis of plasma and urinary tea Polyphenols in human subjects. Cancer Epidemiology, Biomarkers and Prevention. 1995;4:393–9. [PubMed] [Google Scholar]
- 14.Lambert J, Yang C. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutation Research. 2003;523–524:201–8. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- 15.Dona M, Dell’Aica I, Calabrese F, Benelli R, Morini M, Albini A, et al. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003;170:4335–41. doi: 10.4049/jimmunol.170.8.4335. [DOI] [PubMed] [Google Scholar]
- 16.Takano K, Nakaima K, Nitta M, Shibata F, Nakagawa H. Inhibitory effect of (−)-epigallocatechin 3-gallate, a polyphenol of green tea, on neutrophil chemotaxis in vitro and in vivo. Journal of Agricultural and Food Chemistry. 2004;52:4571–6. doi: 10.1021/jf0355194. [DOI] [PubMed] [Google Scholar]
- 17.Hofbauer R, Frass M, Gmeiner B, Handler S, Speiser W, Kapiotis S. The green tea extract epigallocatechin gallate is able to reduce neutrophil transmigration through monolayers of endothelial cells. Wien Klin Wochenschr. 1999;111:278–82. [PubMed] [Google Scholar]
- 18.Yamashita K, Suzuki Y, Matsui T, Yoshimaru T, Yamaki M, Suzuki-Karasaki M, et al. Epigallocatechin gallate inhibits histamine relase from rat basophilic leukemia (RBL-2H3) cells: role of tyrosine phosphorylation pathway. Biochem Biophys Res Commun. 2000;274:603–8. doi: 10.1006/bbrc.2000.3200. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki M, Yoshino K, Maeda-Yamamoto M, Miyase T, Sano M. Inhibitory effects of tea catechins and O-methylated derivatives of (−)-epigallocatechin-3-O-gallate on mouse type IV allergy. J Agric Food Chem. 2000;48:5649–53. doi: 10.1021/jf000313d. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Wang J, Deng F, Hu Z, Wang H. Green tea extract and its major component epigallocatechin gallate inhibits hepatitis B virus in vitro. Antiviral Research. 2008;78:242–9. doi: 10.1016/j.antiviral.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Song JM, Lee KH, Seong BL. Antiviral effect of catechins in green tea on influenza virus. Antiviral Research. 2005;68:66–74. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003 Oct;133(10):3262S–7S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- 23.Fassina G, Buffa A, Benelli R, Varnier OE, Noonan DM, Albini A. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea as a candidate anti-HIV agent. AIDS. 2002 Apr 12;16(6):939–41. doi: 10.1097/00002030-200204120-00020. [DOI] [PubMed] [Google Scholar]
- 24.Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, et al. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J Allergy Clin Immunol. 2003 Nov;112(5):951–7. doi: 10.1016/s0091-6749(03)02007-4. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi K, Honda M, Ikigai H, Hara Y, Shimamura T. Inhibitory effects of (−)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1) Antiviral Res. 2002 Jan;53(1):19–34. doi: 10.1016/s0166-3542(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 26.Patke C, Shearer W. gp120- and TNF-alpha-induced modulation of human B cell function: proliferation, cyclic AMP generation, Ig production, and B-cell receptor expression. J Allergy Clin Immunol. 2000 May;105(5):975–82. doi: 10.1067/mai.2000.105315. [DOI] [PubMed] [Google Scholar]
- 27.Asres K, Seyoum A, Veersham C, Bucar F, Gibbons S. Naturally derived anti-HIV agents. Phytother Res. 2005;19:557–81. doi: 10.1002/ptr.1629. [DOI] [PubMed] [Google Scholar]
- 28.De Clercq E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med Res Rev. 2000 Sep;20(5):323–49. doi: 10.1002/1098-1128(200009)20:5<323::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Zaveri NT. Synthesis of a 3,4,5-trimethoxybenzoyl ester analogue of epigallocatechin-3-gallate (EGCG): a potential route to the natural product green tea catechin, EGCG. Org Lett. 2001 Mar 22;3(6):843–6. doi: 10.1021/ol007000o. [DOI] [PubMed] [Google Scholar]
- 30.Baeten J, Chohan B, Lavreys L, Chohan V, McClelland R, Certain L, et al. HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J Inf Dis. 2007;195:1177–80. doi: 10.1086/512682. [DOI] [PubMed] [Google Scholar]
- 31.McCutchan F. Understanding the genetic diversity of HIV-1. AIDS. 2000;14(Suppl 3):S31–S44. [PubMed] [Google Scholar]
- 32.UNAIDS and WHO. UNAIDS/WHO epidemiological fact sheets on HIV/AIDS and sexually transmitted diseases, 2006 update. 2006 WHO http://wwwwhoint/hiv/pub/epidemiology/pubfacts/en/
- 33.Butler I, Pandrea I, Marx P, Apetrei C. HIV genetic diversity: Biological and public health consequences. Current HIV Research. 2007;5(1):23–45. doi: 10.2174/157016207779316297. [DOI] [PubMed] [Google Scholar]
- 34.Babich H, Krupka M, Nissim H, Zuckerbraun H. Differential in vitro cytotoxicity of (−)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. Toxicology in Vitro. 2005;19:231–42. doi: 10.1016/j.tiv.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor κB in cancer cells versus normal cells. Archives of Biochemistry and biophysics. 2000;376(2):338–46. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- 36.Hu Z, Toda M, Okubo S, Hara Y, Shimamura T. Mitogenic activity of (−) epigallocatechin gallate on B-cells and investigation of its structure-function relationship. International Journal Immunopharmac. 1992;14(8):1399–407. doi: 10.1016/0192-0561(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 37.Shammas M, Neri P, Koley H, Batchu R, Bertheau R, Munshi V, et al. Specific killing of multiple myeloma cells by (−)-epigallocatechin-3-gallate extracted from green tea: biological activity and therapeutic implications. Blood. 2006;108:2804–10. doi: 10.1182/blood-2006-05-022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landis-Piwowar K, Kuhn D, Wan S, Chen D, Chan T, Dou Q. Evaluation of proteasome-inhibitory and apoptosis-inducing potencies of novel (−)-EGCG analogs and their prodrugs. International Journal of Molecular Medicine. 2005;15:735–42. [PubMed] [Google Scholar]
- 39.Roy A, Baliga M, Katiyar S. Epigallocatechin-3-gallate induces apoptosis in estrogen receptor-negative human breast carcinoma cells via modulation in protein expression of p53 and Bax and caspase-3 activation. Molecular Cancer Therapeutics. 2005;4(1):81–90. [PubMed] [Google Scholar]
- 40.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66(5):2500–5. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 41.Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, et al. Epigallocatechin gallate induces apoptosis of monocytes. J Allergy Clin Immunol. 2005 Jan;115(1):186–91. doi: 10.1016/j.jaci.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Aktas O, Prozorovski T, Smorodchenko A, Savaskan N, Lauster R, Kloetzel P, et al. Green tea Epigallocatechin-3-gallate mediates T cellular NF-kB inhibition and exerts neuroprotection in autoimmune encephalomyelitis. The Journal of Immunology. 2004;173:5794–800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- 43.Noda C, He J, Takano T, Tanaka C, Kondo T, Tohyama K, et al. Induction of apoptosis by epigallocatechin-3-gallate in human lymphoblastoid B cells. Biochemical and Biophysical Research Communications. 2007;362:951–7. doi: 10.1016/j.bbrc.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 44.Chow H, Cai Y, Alberts D, Hakim I, Dorrl R, Shahi F, et al. Phase I Pharmacokinetic Study of Tea Polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–8. [PubMed] [Google Scholar]
- 45.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003 Aug 15;9(9):3312–9. [PubMed] [Google Scholar]
- 46.Sleasman JW, Goodenow MM. HIV-1 infection. J Allergy Clin Immunol. 2003;111(2):S582–S92. doi: 10.1067/mai.2003.91. [DOI] [PubMed] [Google Scholar]
- 47.Chow HH, Hakim I, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin Cancer Res. 2005;11(12):4627–33. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 48.Ullman U, Haller J, Decourt J, Girault N, Girault J, Richard-Caudron A, et al. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J of International Medical Research. 2003;31:88–101. doi: 10.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]
- 49.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66(2):1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 50.Donovan J, Chavin K, DeVane C, Taylor R, Wang J, Ruan Y, et al. Green tea (Camellia Sinensis) extract does not alter cytochrome P450 3A4 or 2D6 activity in healthy volunteers. DMD. 2004;32(9):906–8. doi: 10.1124/dmd.104.000083. [DOI] [PubMed] [Google Scholar]