Abstract
Mate choice is mediated by a range of sensory cues, and assortative mating based on these cues can drive reproductive isolation among diverging populations. A specific feature of mormyrid fish, the electric organ discharge (EOD), is used for electrolocation and intraspecific communication. We hypothesized that the EOD also facilitates assortative mating and ultimately promotes prezygotic reproductive isolation in African weakly electric fishes. Our behavioural experiments using live males as well as EOD playback demonstrated that female mate recognition is influenced by EOD signals and that females are attracted to EOD characteristics of conspecific males. The dual function of the EOD for both foraging and social communication (including mate recognition leading to assortative mating) underlines the importance of electric signal differentiation for the divergence of African weakly electric fishes. Thus, the EOD provides an intriguing mechanism promoting trophic divergence and reproductive isolation between two closely related Campylomormyrus species occurring in sympatry in the lower Congo rapids.
Keywords: assortative mating, Campylomormyrus, electric organ discharge, ecological speciation, weakly electric fish (mormyridae)
1. Introduction
A variety of different sensory cues are known to play a key role in mate choice (Jiggins et al. 2001; Kingston & Rossiter 2004; Bridle et al. 2006; Boul et al. 2007). These cues can drive reproductive isolation between diverging populations by facilitating assortative mating. Here, we report a case of female mate recognition (assortment) based on species-specific electrical signals in weakly electric fishes (genus Campylomormyrus) from the Congo River. Using their electric organ discharge (EOD), mormyrid fishes sense objects by active electrolocation (Lissmann & Machin 1958; von der Emde 1999). Beside this, the EOD signal plays an essential role in social communication (for reviews, see Moller 1995; Bullock et al. 2005; Ladich et al. 2006). Based on multilocus genetic analyses, we have demonstrated that EOD types are indicative for evolutionary divergence between Campylomormyrus species of the lower Congo (Feulner et al. 2006). The high diversity of sympatrically occurring species is reflected in the vast variety of EOD signals in this genus.
We hypothesized that the EOD is used in mate recognition and that female preference for EOD signals of conspecific males facilitates assortative mating, thereby ultimately providing a prezygotic isolation mechanism. This study focused on Campylomormyrus compressirostris and related species. We predicted a preference of female C. compressirostris for conspecific males and their EODs over closely related, sympatric Campylomormyrus rhynchophorus males, which exhibit a much longer EOD (figure 1a,b), but no discrimination between conspecifics and the distantly related Campylomormyrus tamandua, whose EOD is more similar to conspecific males. We tested this hypothesis by assessing female preferences for conspecific males in comparison to the two different male types. To examine the specific role of the EOD for female discrimination, we replaced males by playback signals.
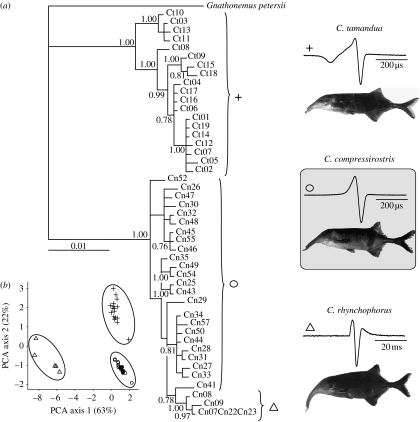
Figure 1.
(a) Bayesian phylogeny based on 1999 bp combined of the mitochondrial cytochrome b and the nuclear ribosomal S7 gene. Branch lengths are proportional to number of character changes. Bayesian support is indicated if >0.75. Representative photos and examples of EOD wave types for the three species examined are shown to the right. (b) EOD divergence among species demonstrated in a PCA using 10 EOD characteristics (§2). Different symbols refer to different species (triangle, C. rhynchophorus; circle, C. compressirostris; plus, C. tamandua).
2. Material and methods
(a) Phylogenetic analysis
Bayesian phylogenetic inference was performed on a combined dataset of the mitochondrial cytochrome b and the nuclear ribosomal S7 gene (1999 bp) (Feulner et al. 2006). A tree of previously examined species (Feulner et al. 2007) was expanded by several new specimens used in the behavioural experiment (GenBank accession numbers EU664340–EU664374). In total, 49 individuals were examined.
(b) EOD analysis
EOD divergence among species was examined by principal component analysis (PCA) of 10 EOD characteristics. The following EOD characteristics were measured for a total number of 46 specimens (C. compressirostris: 6 males, 16 females, 2 unsexed specimens; C. tamandua: 6 males, 11 females; C. rhynchophorus: five males) using a custom-made programme: duration and relative amplitude of the two main phases (maximum and minimum) and the optional pre and post phase (before or after the main positive and negative phase, not exhibited by all species), and amplitude and frequency of the peak of the Fast Fourier Transformation (FFT). The amplitude of the phases was evaluated relative to the peak-to-peak amplitude. The first and last 2 per cent deviation from the peak-to-peak amplitude was taken as the starting and endpoint of each EOD.
(c) Behavioural experiments
All specimens used in the experiments were imported from Kinshasa and occur in sympatry in the lower Congo. They were raised separately, so that all fishes considerably exceeded 40 per cent of the maximal species size, thus ensuring that they have reached sexual maturity (total length: C. compressirostris 13.0–18.5 cm, C. rhynchophorus 23.5–30.0 cm and C. tamandua 19.0–21.0 cm). All males showed a kink in their anal fin base indicating sexual maturity. Prior to the experiments, females were isolated and breeding conditions were achieved by simulating low conductivity, rainy season conditions (Kirschbaum & Schugardt 2002; Schugardt & Kirschbaum 2004, see also for maintenance conditions). As a result of the careful application of our established procedures to stimulate breeding, all the females in our experiments were ready to spawn. This was evidenced by an enlarged body cavity. Two females released eggs shortly after the experiment. Ripe C. compressirostris females (n=6) could choose between a conspecific and a heterospecific ripe male located behind a grid on either side of a large test tank (160×50×50 cm). The central zone of this tank containing the female had a width of 96 cm. Two zones of 32 cm each (adjacent to each male) were considered the ‘preference zones’. A plastic tube was provided in the centre of each zone for shelter. Females were videotaped overnight (i.e. in darkness) under infrared illumination and the fish's location was scored every 2 min. On the second day, the experiment was repeated with males switched between sides. The total number of observations was 660 per female. To test if the EOD alone was sufficient to trigger female mate recognition, male-specific EODs were played back into the tank. Each female (n=7) was simultaneously presented with two digitally synthesized signals consisting of independently controlled EOD waveforms. One sequence of pulsed intervals was chosen at random from a single C. compressirostris male recorded (see the electronic supplementary material for a detailed description of the setup). The time the focal female spent in the preference zone near the active EOD playback was recorded for 1040 s (eight intervals of 130 s with signals interchanged between sides). Female preference was calculated as the proportion of observations or time spent associating with the conspecific male or playback. Data were analysed with a generalized linear mixed model of the binomial family with ‘female identity’ included as a random factor using R (http://www.r-project.org/).
3. Results
Phylogenetic relationships and species assignment were revealed by genotyping and morphological inspection (figure 1a). In our behavioural experiment, we contrasted closely related sympatric species, C. compressirostris and C. rhynchophorus, with a distant relative C. tamandua. The sister species C. compressirostris and C. rhynchophorus produced very distinct EODs that differ largely in their duration. The EOD of C. tamandua was more similar to that of C. compressirostris than to C. rhynchophorus (figure1b; table 1). In agreement with previous studies, our data did not suggest any difference between female and male EOD in C. compressirostris (C. numenius morph C (Feulner et al. 2006) revised as C. compressirostris (Feulner et al. 2007)). This was evidenced by a single species-specific cluster of data points in the PCA analysis of 10 EOD characteristics. This analysis clearly separated the three different species, but did not indicate differences between sexes within species, as both sexes were represented in our sampling.
Table 1.
Mean and standard errors (s.e.) of 10 EOD measurements used in PCA (duration and amplitude of the EODs pre phase, main positive, main negative, and post phase and the peak amplitude and frequency of the FFT).
| mean | s.e. | |
|---|---|---|
| duration pre phase [ms] | ||
| C. compressirostris | 0.00 | 0.00 |
| C. rhynchophorus | 0.00 | 0.00 |
| C. tamandua | 0.06 | 0.00 |
| amplitude pre phase [%] | ||
| C. compressirostris | 0.00 | 0.00 |
| C. rhynchophorus | 0.00 | 0.00 |
| C. tamandua | 5.02 | 0.31 |
| duration main positive [ms] | ||
| C. compressirostris | 0.10 | 0.00 |
| C. rhynchophorus | 3.23 | 0.22 |
| C. tamandua | 0.09 | 0.00 |
| amplitude main positive [%] | ||
| C. compressirostris | 39.83 | 0.33 |
| C. rhynchophorus | 31.72 | 2.07 |
| C. tamandua | 37.36 | 0.32 |
| duration main negative [ms] | ||
| C. compressirostris | 0.05 | 0.00 |
| C. rhynchophorus | 4.00 | 0.33 |
| C. tamandua | 0.04 | 0.00 |
| amplitude main negative [%] | ||
| C. compressirostris | 60.17 | 0.33 |
| C. rhynchophorus | 68.28 | 2.07 |
| C. tamandua | 62.64 | 0.32 |
| duration post phase [ms] | ||
| C. compressirostris | 0.00 | 0.00 |
| C. rhynchophorus | 12.95 | 0.28 |
| C. tamandua | 0.02 | 0.00 |
| amplitude post phase [%] | ||
| C. compressirostris | 0.00 | 0.00 |
| C. rhynchophorus | 18.81 | 1.71 |
| C. tamandua | 1.74 | 0.24 |
| amplitude peak FFT [V2/Hz] | ||
| C. compressirostris | 8643.75 | 1606.85 |
| C. rhynchophorus | 255.15 | 100.98 |
| C. tamandua | 7700.65 | 1747.30 |
| frequency peak FFT [Hz] | ||
| C. compressirostris | 7578.53 | 126.82 |
| C. rhynchophorus | 107.42 | 5.98 |
| C. tamandua | 6318.93 | 116.34 |
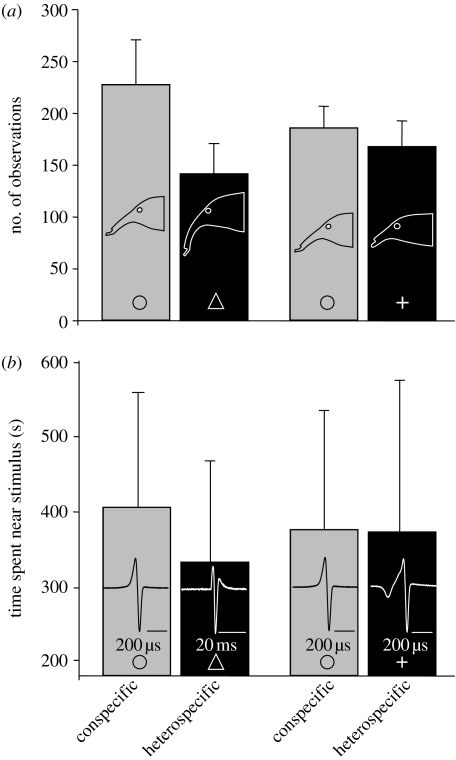
In both experiments, C. compressirostris females preferred conspecific males over C. rhynchophorus males. By contrast, C. compressirostris females neither discriminated between conspecific and C. tamandua males nor their playback signals (figure 2). Association with the conspecific male/EOD playback signal was greater when the female was given a choice between conspecific and C. rhynchophorus males than when the female could choose between conspecific and C. tamandua males (generalized linear mixed model, male stimulus: t=−3.33, p=0.007; EOD playback: z=−3.81, p<0.001).
Figure 2.
(a) Fish–fish interactions (n=6) and (b) fish-playback interactions (n=7). Association behaviour of C. compressirostris females with conspecific and heterospecific males (a) and EODs presented alone (b; mean±s.e.). Different symbols refer to different species (triangle, C. rhynchophorus; circle, C. compressirostris; plus, C. tamandua).
4. Discussion
Our results demonstrated that female mate recognition was influenced by EOD signals and that females associated with EODs characteristic of conspecific males. Our playback experiment was designed to evaluate the importance of the EOD waveform for species discrimination. Other possible discrimination mechanisms such as amplitude or sequence of pulse intervals were kept constant. In this way, we provided strong evidence for mate recognition between sympatrically occurring sister species based on EOD waveform characteristics.
Hence the EOD has the potential to generate assortative mating and function as a prezygotic isolation mechanism in these weakly electric fishes. Although this mechanism allowed discrimination of the closely related C. rhynchophorus, which showed a divergent EOD (figure 1a,b), discrimination apparently failed between EODs that are more similar to those of conspecific males (as in C. tamandua). Male mate recognition (Arnegard et al. 2006) and species recognition (Markowski et al. 2008) in other mormyrid genera showed an asymmetric response as well. Note here that our experiments were carried out with untrained ‘naive’ females, as trained fish do have the ability to distinguish differences smaller than intraspecific waveform variability (Paintner & Kramer 2003).
The evolution of a divergent longer EOD in C. rhynchophorus in conjunction with assortative mating might have promoted reproductive isolation and divergence between the closely related and sympatrically occurring C. rhynchophorus and C. compressirostris. This EOD divergence coincides with differences in the shape of the rostrum (figure 1a), which suggests a possible role for trophic niche segregation (Feulner et al. 2007). The EOD might be a trait allowing direct transmission of ecologically caused divergent selection to a form of reproductive isolation (Kirkpatrick & Ravigne 2002; Rundle & Nosil 2005). Indeed, there is evidence that the EOD is involved in foraging for insect larvae in electric fish (von der Emde & Bleckmann 1998) and EOD duration might be adaptive for the detection of different preys (Meyer 1982; von der Emde & Ringer 1992). The dual function; (i) electrolocation used in foraging and (ii) social communication, including assortative preferences (as evidenced in this study), points towards ecological divergence pleiotropically effecting assortative mating in this system.
We argue that species-specific changes in EOD linked to female preference for that signal, provide an intriguing mechanism for divergence through assortative mating. The electric sense can be considered a key innovation not only regarding its primary function, i.e. electrolocation, but also as an efficient trait for ‘electric’ mate recognition.
Acknowledgements
Experiments were approved by the Deputy for Animal Welfare at the University of Potsdam to comply with legal requirements.
K. Manteuffel and A. Schneider provided technical assistance. Financial support is acknowledged from the German Science Foundation (DFG; Priority Program SPP-1127 ‘Adaptive Radiation—Origin of Biological Diversity’; TI 349/1). We appreciated the discussion with Matthew E. Arnegard, Jessica Stapley, and Carole Smadja.
Supplementary Material
Details on EOD playback setup.
A. SPI of the sequences used for constructing the playback signals. B. Sequence shown in A together with the simultaneously presented second sequence. C. Cumulative histogram (binwidth 1 ms) showing the count and distribution of EOD intervals for a full stimulus sequence of 8 individual trials.
References
- Arnegard M.E., Jackson B.S., Hopkins C.D. Time-domain signal divergence and discrimination without receptor modification in sympatric morphs of electric fishes. J. Exp. Biol. 2006;209:2182–2198. doi: 10.1242/jeb.02239. doi:10.1242/jeb.02239 [DOI] [PubMed] [Google Scholar]
- Boul K.E., Funk W.C., Darst C.R., Cannatella D.C., Ryan M.J. Sexual selection drives speciation in an Amazonian frog. Proc. R. Soc. B. 2007;274:399–406. doi: 10.1098/rspb.2006.3736. doi:10.1098/rspb.2006.3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle J.R., Saldamando C.I., Koning W., Butlin R.K. Assortative preferences and discrimination by females against hybrid male song in the grasshoppers Chorthippus brunneus and Chorthippus jacobsi (Orthoptera: Acrididae) J. Evol. Biol. 2006;19:1248–1256. doi: 10.1111/j.1420-9101.2006.01080.x. doi:10.1111/j.1420-9101.2006.01080.x [DOI] [PubMed] [Google Scholar]
- Bullock T.H., Hopkins C.D., Popper A.N., Fay R.R. Springer Handbook of Auditory Research. Springer; New York, NY: 2005. Electroreception. [Google Scholar]
- Feulner P.G.D., Kirschbaum F., Schugardt C., Ketmaier V., Tiedemann R. Electrophysiological and molecular genetic evidence for sympatrically occuring cryptic species in African weakly electric fishes (Teleostei: Mormyridae: Campylomormyrus) Mol. Phylogenet. Evol. 2006;39:198–208. doi: 10.1016/j.ympev.2005.09.008. doi:10.1016/j.ympev.2005.09.008 [DOI] [PubMed] [Google Scholar]
- Feulner P.G.D., Kirschbaum F., Mamonekene V., Ketmaier V., Tiedemann R. Adaptive radiation in African weakly electric fish (Teleostei: Mormyridae: Campylomormyrus): a combined molecular and morphological approach. J. Evol. Biol. 2007;20:403–414. doi: 10.1111/j.1420-9101.2006.01181.x. doi:10.1111/j.1420-9101.2006.01181.x [DOI] [PubMed] [Google Scholar]
- Jiggins C.D., Naisbit R.E., Coe R.L., Mallet J. Reproductive isolation caused by colour pattern mimicry. Nature. 2001;411:302–305. doi: 10.1038/35077075. doi:10.1038/35077075 [DOI] [PubMed] [Google Scholar]
- Kingston T., Rossiter S.J. Harmonic-hopping in Wallacea's bats. Nature. 2004;429:654–657. doi: 10.1038/nature02487. doi:10.1038/nature02487 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M., Ravigne V. Speciation by natural and sexual selection: models and experiments. Am. Nat. 2002;159:S22–S35. doi: 10.1086/338370. doi:10.1086/338370 [DOI] [PubMed] [Google Scholar]
- Kirschbaum F., Schugardt C. Reproductive strategies and developmental aspects in mormyrid and gymnotiform fishes. J. Physiol. Paris. 2002;96:557–566. doi: 10.1016/S0928-4257(03)00011-1. doi:10.1016/S0928-4257(03)00011-1 [DOI] [PubMed] [Google Scholar]
- Ladich F., Collin S.P., Moller P., Kapoor B.G. Science Publisher; Enfield, NH: 2006. Communication in fishes. [Google Scholar]
- Lissmann H.W., Machin K.E. The mechanism of object location in Gymnarchus niloticus and similar fish. J. Exp. Biol. 1958;35:451–486. [Google Scholar]
- Markowski B., Baier B., Kramer B. Differentiation in electrical pulse waveforms in a pair of sibling Dwarf Stonebashers Pollimyrus castelnaui and P. marianne: possible mechanisms and functions (Mormyridae, Teleostei) Behaviour. 2008;145:115–135. doi:10.1163/156853908782687223 [Google Scholar]
- Meyer J.H. Behavioral-responses of weakly electric fish to complex impedances. J. Comp. Physiol. 1982;145:459–470. doi:10.1007/BF00612811 [Google Scholar]
- Moller P. Chapman & Hall; London, UK: 1995. Electric fishes history and behavior. [Google Scholar]
- Paintner S., Kramer B. Electrosensory basis for individual recognition in a weakly electric, mormyrid fish, Pollimyrus adspersus (Gunther, 1866) Behav. Ecol. Sociobiol. 2003;55:197–208. doi:10.1007/s00265-003-0690-4 [Google Scholar]
- Rundle H.D., Nosil P. Ecological speciation. Ecol. Lett. 2005;8:336–352. doi:10.1111/j.1461-0248.2004.00715.x [Google Scholar]
- Schugardt C., Kirschbaum F. Control of gonadal maturation and regression by experimental variation of environmental factors in the mormyrid fish, Mormyrus rume proboscirostris. Environ. Biol. Fishes. 2004;70:227–233. doi:10.1023/B:EBFI.0000033340.49266.f3 [Google Scholar]
- von der Emde G. Active electrolocation of objects in weakly electric fish. J. Exp. Biol. 1999;202:1205–1215. doi: 10.1242/jeb.202.10.1205. [DOI] [PubMed] [Google Scholar]
- von der Emde G., Bleckmann H. Finding food: senses involved in foraging for insect larvae in the electric fish Gnathonemus petersii. J. Exp. Biol. 1998;201:969–980. doi: 10.1242/jeb.201.7.969. [DOI] [PubMed] [Google Scholar]
- von der Emde G., Ringer T. Electrolocation of capacitive objects in 4 species of pulse-type weakly electric fish. 1. Discrimination performance. Ethology. 1992;91:326–338. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details on EOD playback setup.
A. SPI of the sequences used for constructing the playback signals. B. Sequence shown in A together with the simultaneously presented second sequence. C. Cumulative histogram (binwidth 1 ms) showing the count and distribution of EOD intervals for a full stimulus sequence of 8 individual trials.