Abstract
Given the high costs of avian obligate brood parasitism, host individuals are selected to reject parasitic eggs they recognize as foreign. We show that rejection may not necessarily follow egg discrimination when selective removal of the parasitic egg is difficult. We studied egg rejection behaviour in a small host of the common cuckoo Cuculus canorus, the eastern olivaceous warbler Hippolais pallida, by experimental parasitism with model and real non-mimetic cuckoo eggs and video recordings of host behaviour. Hosts pecked 87 per cent (20 out of 23) of the model eggs but eventually accepted 43.5 per cent (10 out of 23) of them. A similar pattern was found for real cuckoo eggs, which were all pecked, but as many as 47 per cent (7 out of 15) of them were accepted. To our knowledge, this is the first demonstration of a cuckoo host discriminating against real parasitic eggs but often accepting them. Our results also show that in host species experiencing difficulties in performing puncture ejection, non-mimetic cuckoo eggs may avoid rejection by means of their unusually high structural strength.
Keywords: brood parasitism, egg rejection, cuckoo, olivaceous warbler
1. Introduction
Avian obligate brood parasites generally impose high fitness costs on their hosts, resulting in sophisticated coevolutionary arms races (Davies & Brooke 1989; Moksnes et al. 1990). The most ubiquitous host antiparasite defence is egg discrimination and rejection, to which some parasites have responded by evolving mimetic eggs (Rothstein & Robinson 1998). However, despite the apparent benefits of rejection, there is substantial variation both among and within host species used by cuckoos in their responses to foreign eggs (Davies & Brooke 1989; Moksnes et al. 1990; Martín-Gálvez et al. 2007; Stokke et al. 2008). Possible explanations invoke a lack of necessary genetic variation underpinning egg discrimination, or discrimination developing with age/experience or having only evolved in some host populations sympatric with the brood parasite (Rothstein & Robinson 1998; Stokke et al. 2005; Røskaft et al. 2006). Furthermore, because rejection may be costly, some hosts also modify their rejection decisions according to the perceived risk of parasitism (Moksnes et al. 1993; Lindholm 2000; Soler et al. 2000). Rejection costs involve recognition errors, accidental destruction of own eggs and/or simply energetic expenditure (Spaw & Rohwer 1987; Davies et al. 1996; Røskaft et al. 2002). Besides mimicry, the unusually strong shells of parasitic eggs (Spaw & Rohwer 1987; Picman & Pribil 1997) may render rejection especially costly for small-billed hosts that have to puncture the parasitic egg in order to remove it (puncture ejection) or desert the clutch (Moksnes et al. 1991; Røskaft et al. 1993; Antonov et al. 2006). Selection should thus favour flexible host responses and hosts may recognize the foreign egg but choose to tolerate it under some circumstances (Rothstein & Robinson 1998). Nevertheless, the underlying mechanisms of rejection decisions within and across the different host species are still poorly understood (Davies et al. 1996; Stokke et al. 2005). Despite some indirect demonstrations of conditional host responses (Davies & Brooke 1989; Moksnes et al. 1993), there is very little direct evidence for acceptance of cuckoo eggs once host individuals have discriminated against them (Lindholm 2000; Soler et al. 2000). To our knowledge, this phenomenon has never been shown in a situation of hosts confronted with real cuckoo eggs. Here, we demonstrate that discrimination without rejection occurs in the eastern olivaceous warbler Hippolais pallida (hereafter olivaceous warbler), a small puncture-ejecting host of the common cuckoo Cuculus canorus (hereafter cuckoo).
2. Material and methods
This study was performed in northwestern Bulgaria where olivaceous warblers have been frequently parasitized by cuckoos (Antonov et al. 2007). Host nests were experimentally parasitized on the day the last egg was laid, up to 2 days after clutch completion. None of the experimental nests was parasitized by the cuckoo. For a better understanding of host rejection modes, we used both artificial and real cuckoo eggs. Foreign eggs were painted non-mimetic to minimize the confounding influence of recognition problems. Each nest was visited daily for 6 days following experimental parasitism to ascertain acceptance or rejection. The foreign egg was considered rejected if it was ejected or the parasitized clutch deserted; otherwise acceptance was assumed.
Experiments with artificial cuckoo eggs (n=23) were conducted in 2003 and 2006. Model eggs were made of polymer clay and painted with acrylic paint to resemble the immaculate blue eggs of the redstart Phoenicurus phoenicurus cuckoo gens. Model and real cuckoo eggs used in this study did not differ significantly in either egg length (22.6±0.45 mm versus 22.0±1.00 mm, Welch t=1.91, d.f.=17.8, p=0.07) or width (16.5±0.40 mm versus 16.4±0.46 mm, t=0.61, d.f.=27.5, p=0.55). Furthermore, model egg weights (3.2–4.1 g) were within the range of natural cuckoo egg weights from the same general area (2.5–5.0 g; A. Antonov, B. G. Stokke, A. Moksnes & E. Røskaft 2008, unpublished data). The paint coating allowed detection of host pecking in terms of clearly recognizable bill prints. Here, we designated pecked but non-deserted model eggs as accepted. A set of another 15 nests was set as controls to account for desertions unrelated to brood parasitism.
Experiments with real cuckoo eggs (n=15) were carried out in 2007–2008. Unincubated cuckoo eggs were collected from abandoned or multiply parasitized great reed warbler Acrocephalus arundinaceus or marsh warbler Acrocephalus palustris nests from the same general area. Cuckoo eggs were painted with dense black spots by using a black indelible ink pen so that little of the background was visible.
Host behaviour was video recorded in 14 nests experimentally parasitized with real cuckoo eggs as well as another seven nests which were not manipulated (controls). The nests were videotaped daily for a period of 2 hours for up to 4 days if the foreign egg was not rejected within this interval. Timing of the video recordings was randomized among nests and time of day. The control nests were filmed for only 2 hours and were compared with the first video recording session of experimental nests. We recorded the time hosts were incubating, looking in the nest cup and pecking the foreign egg, expressed as a percentage of the time there was a bird at the nest. Pecking effort was classified as weak or strong following Antonov et al. (2008a). Statistical analyses were performed in R v. 2.5.1 (http://www.R-project.org).
3. Results
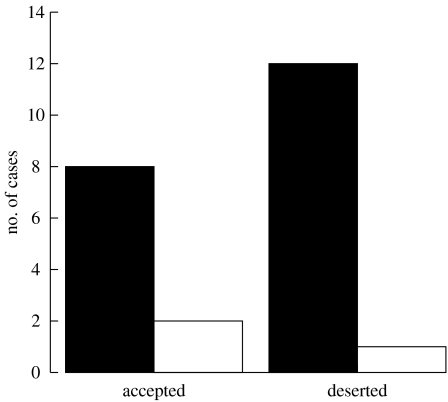
The majority of model eggs were pecked, but 43.5 per cent (10 out of 23) of them were accepted, leading to no significant association between the presence of pecking and rejection decision (Fisher's exact probability test, p=0.56; figure 1). As revealed by video recordings, all the real cuckoo eggs were also pecked but as many as 46.7 per cent (7 out of 15) of them were accepted. Rejection rates of non-mimetic model and real cuckoo eggs did not differ significantly (Χ12=0.02, p=0.89). Model eggs were only rejected by desertion. Five out of eight real cuckoo eggs were rejected by desertion and the other three were ejected. No control nests were deserted.
Figure 1.
The presence of pecking marks on non-mimetic model cuckoo eggs experimentally introduced in olivaceous warbler nests in relation to host rejection decision. Black bars, peck marks; white bars, no peck marks.
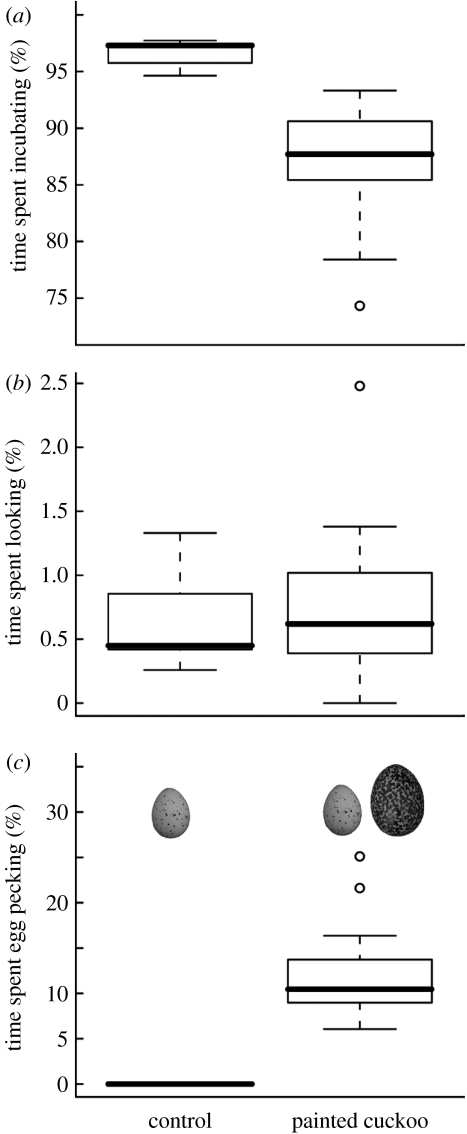
Hosts at experimental and control video-recorded nests did not differ significantly in the amount of time they were present at the nest (Mann–Whitney test, W=40, p=0.54, n1=14, n2=7) or spent looking in the nest cup (W=42, p=0.63; figure 2). However, experimentally parasitized hosts spent approximately 11 per cent less time incubating than the control ones (W=98, p<0.0001; figure 2). This was attributable to pecking, because no hosts at the control nests pecked their own eggs (figure 2). Regardless of the video recording day, strong pecking was detected at 36 per cent (5 out of 14) of the nests. Two birds that pecked strongly subsequently deserted, the other two ejected the cuckoo egg and the remaining one accepted it. The host at the latter nest also damaged and ejected one of its own eggs but failed to puncture the cuckoo egg and finally accepted it. The remaining 64 per cent (9 out of 14) of the hosts only showed weak pecking. Of these, six accepted and three deserted.
Figure 2.
Time budgets of olivaceous warblers experimentally parasitized with non-mimetic cuckoo eggs and control nests.
4. Discussion
Our results show that olivaceous warblers are not able to grasp cuckoo eggs and can only remove them selectively by puncture ejection. The latter, however, seems to be difficult for this host species as evidenced by the low number of observed ejections and the high incidence of desertions. Ejection also accounted for only a minority (12%, 2 out of 17) of rejections of naturally laid cuckoo eggs (Antonov et al. 2007). Video recordings showing strong pecking followed by desertion demonstrated that at least some of the desertions represent a failure at puncture ejection rather than nest abandonment in the first place (cf. Moksnes et al. 1993).
To our knowledge, this is the first study to show discrimination without rejection of real parasitic eggs in a cuckoo host (cf. Lindholm 2000). Discrimination was evidenced by the clear pecking marks left on the model eggs as well as by the egg-pecking behaviour documented by the video recordings. Pecking also led to a reduction in the time experimentally parasitized hosts were incubating in relation to the unmanipulated individuals. It may be argued that acceptance rates may be overestimated for the model eggs, owing to their artificiality (e.g. Rothstein 1977; Underwood & Sealy 2006). However, the fact that real cuckoo eggs were rejected at a very similar rate suggests no appreciable bias in inferences from artificial eggs in this particular host species. All but one of the hosts that accepted real cuckoo eggs exhibited only weak pecking on the video recordings. We have shown in another host species, the marsh warbler, that weak pecking is a reliable indicator of egg discrimination, but it is insufficient to result in successful puncture of the cuckoo egg (Antonov et al. 2008a). Because we did not film olivaceous warbler nests continuously, we cannot be sure whether and to what extent acceptors were also pecking strongly, i.e. tried their best to puncture the cuckoo egg. Nevertheless, at least one individual was indeed recorded as pecking at the maximal strength, and even damaged one of its own eggs, but failed to puncture the cuckoo egg and eventually accepted it. This shows that acceptances ‘forced’ by the impossibility to puncture the hard-shelled cuckoo egg do occur sometimes (cf. Antonov et al. 2008b). Thus, the puncture resistance hypothesis, which was originally proposed to explain the adaptive significance of increased structural strength of cowbird eggs (Spaw & Rohwer 1987), may also be applicable to some hosts of the cuckoo in which puncture ejection is difficult or impossible. As hosts rearing a cuckoo chick suffer a complete reproductive loss, it seems puzzling why desertion does not always follow unsuccessful puncture ejection attempts. However, even among cuckoo hosts, a higher level of phenotypic plasticity in rejection decisions is expected if ejection is costly and the risk of parasitism varies considerably (Rothstein & Robinson 1998; Lindholm 2000).
Acknowledgements
Our study complied with the legal regulations of Bulgaria.
Special thanks to Steve Rothstein, Fiona Pring and one anonymous referee for their constructive comments.
References
- Antonov A., Stokke B.G., Moksnes A., Kleven O., Honza M., Røskaft E. Eggshell strength of an obligate brood parasite: a test of the puncture resistance hypothesis. Behav. Ecol. Sociobiol. 2006;60:11–18. doi:10.1007/s00265-005-0132-6 [Google Scholar]
- Antonov A., Stokke B.G., Moksnes A., Røskaft E. First evidence of regular common cuckoo, Cuculus canorus, parasitism on eastern olivaceous warblers, Hippolais pallida elaeica. Naturwissenschaften. 2007;94:307–312. doi: 10.1007/s00114-006-0189-8. doi:10.1007/s00114-006-0189-8 [DOI] [PubMed] [Google Scholar]
- Antonov A., Stokke B.G., Moksnes A., Røskaft E. Getting rid of the cuckoo Cuculus canorus egg: why do hosts delay rejection? Behav. Ecol. 2008;19:100–107. doi:10.1093/beheco/arm102 [Google Scholar]
- Antonov A., Stokke B.G., Moksnes A., Røskaft E. Does the cuckoo benefit from laying unusually strong eggs? Anim. Behav. 2008;76:1893–1900. doi:10.1016/j.anbehav.2008.08.016 [Google Scholar]
- Davies N.B., Brooke M.D. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J. Anim. Ecol. 1989;58:225–236. doi:10.2307/4996 [Google Scholar]
- Davies N.B., Brooke M.D.L., Kacelnik A. Recognition errors and probability of parasitism determine whether reed warblers should accept or reject mimetic cuckoo eggs. Proc. R. Soc. B. 1996;263:925–931. doi:10.1098/rspb.1996.0137 [Google Scholar]
- Lindholm A.K. Tests of phenotypic plasticity in reed warbler defences against cuckoo parasitism. Behaviour. 2000;137:43–60. doi:10.1163/156853900501863 [Google Scholar]
- Martín-Gálvez D., Soler J.J., Martínez J.G., Krupa A.P., Soler M., Burke T. Cuckoo parasitism and productivity in different magpie subpopulations predict frequencies of the 457bp allele: a mosaic of coevolution at a small geographic scale. Evolution. 2007;61:2340–2348. doi: 10.1111/j.1558-5646.2007.00194.x. doi:10.1111/j.1558-5646.2007.00194.x [DOI] [PubMed] [Google Scholar]
- Moksnes A., Røskaft E., Braa A.T., Korsnes L., Lampe H.M., Pedersen H.C. Behavioral responses of potential hosts towards artificial cuckoo eggs and dummies. Behaviour. 1990;116:64–89. doi:10.1163/156853990X00365 [Google Scholar]
- Moksnes A., Røskaft E., Braa A.T. Rejection behavior by common cuckoo hosts towards artificial brood parasite eggs. Auk. 1991;108:348–354. [Google Scholar]
- Moksnes A., Røskaft E., Korsnes L. Rejection of cuckoo (Cuculus canorus) eggs by meadow pipits (Anthus pratensis) Behav. Ecol. 1993;4:120–127. doi:10.1093/beheco/4.2.120 [Google Scholar]
- Picman J., Pribil S. Is greater eggshell density an alternative mechanism by which parasitic cuckoos increase the strength of their eggs? J. Ornithol. 1997;138:531–541. doi:10.1007/BF01651384 [Google Scholar]
- Røskaft E., Rohwer S., Spaw C.D. Cost of puncture ejection compared with costs of rearing cowbird chicks for northern orioles. Ornis Scand. 1993;24:28–32. doi:10.2307/3676406 [Google Scholar]
- Røskaft E., Moksnes A., Meilvang D., Bicik V., Jemelikova J., Honza M. No evidence for recognition errors in Acrocephalus warblers. J. Avian Biol. 2002;33:31–38. doi:10.1034/j.1600-048X.2002.330106.x [Google Scholar]
- Røskaft E., Takasu F., Moksnes A., Stokke B.G. Importance of spatial habitat structure on establishment of host defenses against brood parasitism. Behav. Ecol. 2006;17:700–708. doi:10.1093/beheco/ark019 [Google Scholar]
- Rothstein S.I. Cowbird parasitism and egg recognition of the northern oriole. Wilson Bull. 1977;89:21–32. [Google Scholar]
- Rothstein S.I., Robinson S.K. Oxford University Press; New York, NY: 1998. Parasitic birds and their hosts. Studies in coevolution. [Google Scholar]
- Soler M., Palomino J.J., Martín-Vivaldi M., Soler J.J. Lack of consistency in the response of Rufous tailed Scrub Robins Cercotrichas galactotes towards parasitic common cuckoo eggs. Ibis. 2000;142:151–154. doi:10.1111/j.1474-919X.2000.tb07699.x [Google Scholar]
- Spaw C.D., Rohwer S. A comparative study of eggshell thickness in cowbirds and other passerines. Condor. 1987;89:307–318. doi:10.2307/1368483 [Google Scholar]
- Stokke B.G., Moksnes A., Røskaft E. The enigma of imperfect adaptations in hosts of avian brood parasites. Ornithol. Sci. 2005;4:17–29. doi:10.2326/osj.4.17 [Google Scholar]
- Stokke B.G., Hafstad I., Rudolfsen G., Moksnes A., Møller A.P., Røskaft E., Soler M. Predictors of resistance to brood parasitism within and among reed warbler populations. Behav. Ecol. 2008;19:612–620. doi:10.1093/beheco/arn007 [Google Scholar]
- Underwood T.J., Sealy S.G. Grasp-ejection in two small ejecters of cowbird eggs: a test of bill-size constraints and the evolutionary equilibrium hypothesis. Anim. Behav. 2006;71:409–416. doi:10.1016/j.anbehav.2005.06.004 [Google Scholar]