Abstract
The physiological properties of vertebrate skeletal muscle typically show a scaling pattern of slower contractile properties with size. In fishes, the myotomal or swimming muscle reportedly follows this pattern, showing slower muscle activation, relaxation and maximum shortening velocity (Vmax) with an increase in body size. We asked if the muscles involved in suction feeding by fishes would follow the same pattern. We hypothesized that feeding muscles in fishes that feed on evasive prey are under selection to maintain high power output and therefore would not show slower contractile properties with size. To test this, we compared contractile properties in feeding muscles (epaxial and sternohyoideus) and swimming muscle (myotomal) for two members of the family Centrarchidae (sunfish): the bluegill (Lepomis macrochirus) and the largemouth bass (Micropterus salmoides). Consistent with our predictions, the Vmax of myotomal muscle in both species slowed with size, while the epaxials showed no significant change in Vmax with size. In the sternohyoideus, Vmax slowed with size in the bluegill but increased with size in the bass. The results indicate that scaling patterns of contractile properties appear to be more closely tied to muscle function (i.e. locomotion versus feeding) than overall patterns of size.
Keywords: sunfish, feeding, swimming, scaling
1. Introduction
Muscle contraction speed determines the absolute speed of kinematic movement, and thus organismal performance. Therefore, understanding the physiological and physical bases of muscle contraction speed is critical to analysing and evaluating the effects of selection on animal performance (Marden 1995) and comparing relative performance among animals (Wakeling & Johnston 1998). One factor known to strongly affect muscle contraction speed is body size: smaller animals tend to move more quickly in relative terms than larger animals (Hill 1950), and it has been shown that intrinsic muscle contractile velocity decreases with size (Altringham & Johnston 1990; Seow & Ford 1991; Rome 1992).
Body size might affect muscle contraction in two ways. First, increased body size might change the mechanical requirements of muscles, requiring slower contraction speed to maintain important mechanical efficiency or effectiveness (e.g. Hill 1950). Second, muscles might slow because of limitations imposed by metabolic scaling (Kleiber 1961), for instance, to accommodate reduced mass-specific ATP delivery to myosin (Dobson & Headrick 1995). One way to address this problem is to look at the muscle that is not associated with locomotion, because the functional demands of locomotor muscle may be integrally tied to body size, while those associated with feeding may only be tied to the relative size of feeding structures. In the present study, we compared feeding (SH, sternohyoideus; EP, epaxial) and locomotor muscle (MY, white myotomal) within two species of centrarchid fish: the largemouth bass (Micropterus salmoides, Lacepede 1820) and the bluegill sunfish (Lepomis macrochirus, Rafinesque 1819). Both the SH and the EP are thought to contribute to feeding in both species (Lauder et al. 1986; Carroll 2004). The parallel comparison among muscle fibre types within these species offered an opportunity to address the physiological basis underlying the scaling of muscle contraction kinetics. While many aspects of muscle kinetics were measured and are reported, we focus our discussion on length-specific Vmax, because this variable is an accurate measure of the rate at which an active muscle fibre can convert ATP into mechanical work (Lieber 2002), and permits comparisons with other studies (e.g. Rome 1992). We hypothesized that Vmax of feeding muscle would scale differently with size from that of locomotor muscle, reflecting differences in functional requirements placed on each muscle.
2. Material and methods
(a) Animals
The largemouth bass (M. salmoides) and the bluegill sunfish (L. macrochirus) were obtained from Kurtz Fish Farm, Chester County, PA. The fishes were maintained at 25°C and fed live fishes. Comparisons were made primarily between two size classes of each species (table 1), because these were most readily commercially available at the time of the research. All handling of experimental animals was reviewed by the Widener University Institutional Animal Care and Use Committee.
Table 1.
Sizes of fishes used in physiological analysis of the scaling of swimming versus feeding muscle in the largemouth bass and the bluegill sunfish (SL, standard length).
| species | size group | mass (±s.d.) grams | SL (±s.d.) cm | n |
|---|---|---|---|---|
| largemouth bass | small | 4.0±1.5 | 6.0±0.6 | 18 |
| mediuma | 81.7±13.9 | 16.5±1.0 | 5 | |
| large | 327.9±155.0 | 24.9±4.3 | 15 | |
| bluegill | small | 2.9±1.8 | 4.9±1.9 | 15 |
| large | 105.4±22.9 | 14.8±1.1 | 13 |
Sternohyoideus only.
(b) Physiology experiments
Epaxial, sternohyoideus and white myotomal muscle bundles were used to examine contractile properties (see Thys (1997) for anatomical and functional distinction between EP and MY muscles). Live muscle bundles were extracted and prepared as described previously and muscle mechanics experiments were carried out at 25°C using a customized muscle mechanics apparatus (Coughlin & Carroll 2006). Activation conditions for each bundle were optimized to generate the maximal tetanic force. For tetanic contractions, time of activation (TA) was defined as the time from 10 to 90 per cent of maximum isometric stress. Time of relaxation (TR) was the time from 90 to 10 per cent of peak isometric stress. Maximum shortening velocity (Vmax), maximum steady-state power (Wmax) and optimal shortening velocity (Vopt, the velocity at which the highest power is attained, expressed as the ratio of Vopt/Vmax) were determined using an isovelocity ramp method (Coughlin & Carroll 2006). At the end of each experiment, the fibre area of the live muscle bundles was determined histologically, and live muscle fibre area was used to calculate isometric tension (Coughlin & Carroll 2006). Tension ranged from approximately 30 kN m−2 for EP in the small bass (range=12–45 kN m−2) and the bluegill (range=15–44 kN m−2) and for MY in the small bluegill (range=17–42 kN m−2) to 100–150 kN m−2 for all other muscle samples. This variability is similar to that found in Van Wassenbergh et al. (2007).
(c) Statistical analysis
Regressions were determined for the relationship of each contractile property (TA, TR, Vopt/Vmax, Wmax and Vmax) with fish size (using total length). Plots of log(contractile property) as a function of log(total length) are given for EP, SH and MY muscle of each species. For significant regressions, the r2 value (on the graph) and the scaling exponent (in the figure legend) are given. All traits were treated as continuous variables based on the assumption that size classes used represented ends of a continuum rather than discrete life-history stages. This is consistent with the relatively continuous growth observed in these species (e.g. Wainwright & Shaw 1999).
3. Results
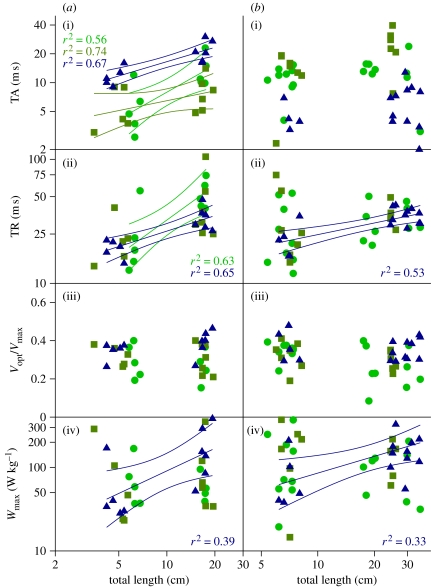
Times to activation and deactivation slowed with size in the swimming and feeding muscles in the bluegill (figure 1). For instance, fish size was significantly correlated with increased TA for all three muscle types and for TR for SH and EP muscles. In the bass, the only significant relationship was a slowing of TR with increasing fish size for EP (figure 1).
Figure 1.
Scaling of muscle contractile properties from isometric and isovelocity ramp contractions in (a(i)–(iv)) the bluegill and (b(i)–(iv)) the bass swimming and feeding muscles. TA, TR, optimal shortening velocity (Vopt/Vmax) and maximum power output (Wmax) are defined in the text. Linear regressions (with 95% confidence intervals) are plotted for statistically significant relationships of log(contraction property) versus log(total length). For TA in the bluegill, the scaling exponents are +0.50 for myotomal muscle (squares),+0.86 for epaxial (triangles) and +0.94 for sternohyoideus (circles). For TR in the bluegill, the scaling exponents are +0.41 for epaxial and +0.93 for sternohyoideus. Finally, the bluegill Wmax has a scaling exponent of +1.59. In the bass, only two relationships are significant—the epaxial muscle has a scaling exponent of +0.31 for TR and +0.64 for Wmax.
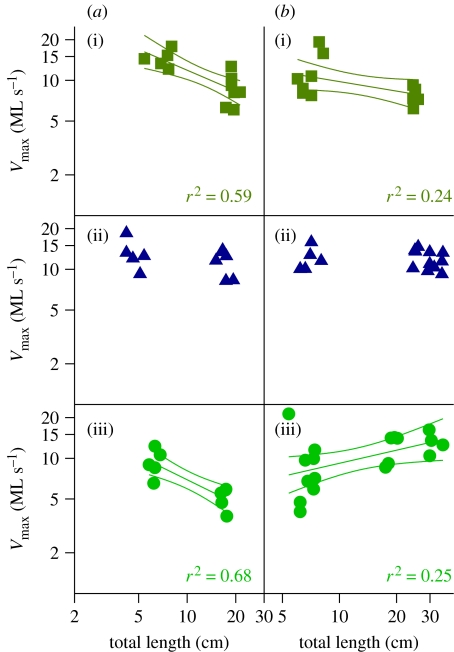
Muscle shortening velocity displayed scaling patterns that differed between fish species. In the bluegill, the Vmax of MY and SH muscles slowed with size, (figure 2), but the EP muscle was not significantly affected by size. In the bass, the Vmax of MY muscle significantly slows with size, the EP muscle shows no scaling effect with regard to Vmax and the SH muscle displays an increase in Vmax with size (figure 2). The Wmax of the EP muscle increased with size in both the bass and the bluegill, while Vopt of all muscles (expressed as the ratio Vopt/Vmax) remained close to 0.3 for all muscle types in both species (figure 1).
Figure 2.
Scaling of Vmax in (a) the bluegill and (b) the bass swimming and feeding muscles. Linear regressions (with 95% confidence intervals) are plotted for statistically significant relationships of log(Vmax) versus log(total length) ((i) myotomal, (ii) epaxial and (iii) sternohyoideus). The scaling exponents are −0.41 for the bluegill myotomal muscle, −0.23 for the bass myotomal, −0.59 for the bluegill sternohyoideus and +0.32 for the bass sternohyoideus.
4. Discussion
We compared feeding (EP and SH) and locomotor muscles (MY) in both the largemouth bass and the bluegill. Feeding structures in the largemouth bass tend to grow isometrically with respect to the body size, while those in the bluegill tend to grow allometrically (Wainwright & Shaw 1999). In both species, Vmax of myotomal muscles decreased with size with exponent values similar to those found in other intraspecies studies (e.g. approx. −0.34, James et al. 1998). However, Vmax in the anterior EP muscle mass, the primary feeding muscle in both species (Lauder et al. 1986; Carroll & Wainwright 2006), did not slow with size. While regional differences in function and protein expression have been described before for fish muscle (James et al. 1998; Thys et al. 2001), to our knowledge, this is the first time that such profound differences in the scaling of muscle patterns have been shown within individuals. In comparison, only slight differences have been found in interspecific comparisons of fast and slow fibres in mammals (Seow & Ford 1991; Rome 1992).
That EP shortening velocity did not slow with size in both species, in contrast to MY muscle, strongly suggests that scaling patterns in muscles are tied to functions and not to general patterns of metabolic scaling. Exactly why these differences in scaling exist is not apparent from this study. White axial muscle is used primarily in burst swimming and C-starts, and reduced undulatory speed with size may be required to maintain hydrodynamic efficiency (e.g. efficiency of vortex shedding, Triantafyllou et al. 2005) during these behaviours. Indeed, muscle shortening velocity in vivo does in fact slow in absolute terms, to maintain a constant V/Vmax during the power stroke of C-starts (James & Johnston 1998). Thus, this reduction of Vmax with size in MY may be a physiological mechanism to maintain locomotor performance throughout ontogeny.
Feeding muscle velocity did not decrease with size. Size-related decreases in Vmax of feeding muscles would be unfavourable because they would reduce the speed of skeletal kinematics and the power output during suction feeding. Interestingly, feeding kinematics do decrease in absolute speed with size in both the bluegill and the largemouth bass (Richard & Wainwright 1995; Wainwright & Shaw 1999). In the largemouth bass, time to activation is invariant with size for feeding muscles (figure 1), there is no reduction in the velocity of contraction in any suction feeding muscle (figure 2) and scaling of feeding skeletal structures is nearly isometric in the largemouth bass (Richard & Wainwright 1995). If absolute shortening velocity does not change with size (figure 2), then these observed reductions in kinematic speed indicate a reduction in relative shortening velocity and, thus, skeletal loading must be increasing faster, with size, than recruited muscle stress. Based on this analysis, therefore, a reduction in in vivo V/Vmax is predicted in the bass as size increases. It can be assumed that kinematics would slow more than they do if EP Vmax decreased with size.
In contrast to our results, Van Wassenbergh et al. (2007) found a decrease in the optimal in vitro cycle frequency for power production in the hypaxial and protractor hyoideus of a clarid catfish, Clarias gariepinus, which indicates a reduction in Vmax with fish length. Although the relative quantitative contribution of these muscles to overall suction feeding power production is not established, their results indicate that reduced kinematic speed in these fish may have a physiological rather than mechanical basis.
Acknowledgements
All handling of experimental animals was reviewed by the Widener University Institutional Animal Care and Use Committee.
This work was supported by grants from Widener University. The comments, criticism and suggestions of three anonymous reviewers greatly improved this manuscript.
References
- Altringham J.D., Johnston I.A. Scaling effects on muscle function: power output of isolated fish muscle fibers performing oscillatory work. J. Exp. Biol. 1990;151:453–468. doi: 10.1242/jeb.158.1.261. [DOI] [PubMed] [Google Scholar]
- Carroll A.M. Muscle activation and strain during suction feeding in the largemouth bass Micropterus salmoides. J. Exp. Biol. 2004;207:983–991. doi: 10.1242/jeb.00862. doi:10.1242/jeb.00862 [DOI] [PubMed] [Google Scholar]
- Carroll A.M., Wainwright P.C. Muscle function and power output during suction feeding in largemouth bass, Micropterus salmoides. Comp. Biochem. Phys. A. 2006;143:389–399. doi: 10.1016/j.cbpa.2005.12.022. doi:10.1016/j.cbpa.2005.12.022 [DOI] [PubMed] [Google Scholar]
- Coughlin D.J., Carroll A.M. In vitro estimates of power output by epaxial muscle during feeding in largemouth bass. Comp. Biochem. Phys. A. 2006;145:533–539. doi: 10.1016/j.cbpa.2006.08.026. doi:10.1016/j.cbpa.2006.08.026 [DOI] [PubMed] [Google Scholar]
- Dobson G.P., Hedrick J.P. Bioenergetic scaling: metabolic design and body size constraints in mammals. Proc. Natl Acad. Sci. USA. 1995;92:7317–7321. doi: 10.1073/pnas.92.16.7317. doi:10.1073/pnas.92.16.7317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.V. The dimensions of animals and their muscular dynamics. Sci. Prog. Lond. 1950;38:209–230. [Google Scholar]
- James R.S., Cole N.J., Davies M.L.F., Johnston I.A. Scaling of intrinsic contractile properties and myofibrillar protein composition of fast muscle in the fish Myoxocephalus scorpius L. J. Exp. Biol. 1998;201:901–912. doi: 10.1242/jeb.201.7.901. [DOI] [PubMed] [Google Scholar]
- James R.S., Johnston A.I. Scaling of muscle performance during escape responses in the first Myoxocephalus scorpius L. J. Exp. Biol. 1998;201:913–923. doi: 10.1242/jeb.201.7.913. [DOI] [PubMed] [Google Scholar]
- Kleiber M. Wiley; New York, NY: 1961. The fire of life: an introduction to animal energetics. [Google Scholar]
- Lauder G.V., Wainwright P.C., Findeis E. Physiological mechanisms of aquatic prey capture in sunfishes: functional determinants of buccal pressure changes. Comp. Biochem. Physiol. A. 1986;84:729–734. doi:10.1016/0300-9629(86)90396-8 [Google Scholar]
- Lieber R.L. Lippincott Williams & Wilkins; Philadelphia, PA: 2002. Skeletal muscle structure, function & plasticity: the physiological basis of rehabilitation. [Google Scholar]
- Marden J.H. Evolutionary adaptation of contractile performance in muscle of ectothermic winter-flying moths. J. Exp. Biol. 1995;198:2087–2094. doi: 10.1242/jeb.198.10.2087. [DOI] [PubMed] [Google Scholar]
- Richard B.A., Wainwright P.C. Scaling the feeding mechanism of largemouth bass (Micropterus salmoides): kinematics of prey capture. J. Exp. Biol. 1995;198:419–433. doi: 10.1242/jeb.198.2.419. [DOI] [PubMed] [Google Scholar]
- Rome L.C. Scaling of muscle fibres and locomotion. J. Exp. Biol. 1992;168:243–252. doi: 10.1242/jeb.168.1.243. [DOI] [PubMed] [Google Scholar]
- Seow C.Y., Ford L.E. Shortening velocity and power output of skinned muscle fibers from mammals having a 25 000-fold range of body mass. J. Gen. Physiol. 1991;97:541–560. doi: 10.1085/jgp.97.3.541. doi:10.1085/jgp.97.3.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thys T. Spatial variation in epaxial muscle activity during prey strike in largemouth bass (Micropterus salmoides) J. Exp. Biol. 1997;200:3021–3031. doi: 10.1242/jeb.200.23.3021. [DOI] [PubMed] [Google Scholar]
- Thys T.M., Blank J.M., Coughlin D.J., Schachat F. Longitudinal variation in muscle protein expression and contraction kinetics of largemouth bass axial muscle. J. Exp. Biol. 2001;204:4249–4257. doi: 10.1242/jeb.204.24.4249. [DOI] [PubMed] [Google Scholar]
- Triantafyllou M.S., Hover F.S., Techet A.H., Yue D.K.P. Review of hydrodynamic scaling laws in aquatic locomotion and fishlike swimming. Appl. Mech. Rev. 2005;58:226–237. doi:10.1115/1.1943433 [Google Scholar]
- Van Wassenbergh S., Herrel A., James R.S., Aerts P. Scaling of contractile properties of catfish feeding muscles. J. Exp. Biol. 2007;210:1183–1193. doi: 10.1242/jeb.000109. doi:10.1242/jeb.000109 [DOI] [PubMed] [Google Scholar]
- Wainwright P.C., Shaw S.S. Morphological basis of kinematic diversity in feeding sunfishes. J. Exp. Biol. 1999;202:3101–3110. doi: 10.1242/jeb.202.22.3101. [DOI] [PubMed] [Google Scholar]
- Wakeling J.M., Johnston I.A. Muscle power output limits fast-start performance in fish. J. Exp. Biol. 1998;201:1505–1526. doi: 10.1242/jeb.201.10.1505. [DOI] [PubMed] [Google Scholar]