Abstract
The generality of asymmetric reproductive isolation between reciprocal crosses suggests that the evolution of isolation mechanisms often proceeds in reciprocal asymmetry. In hermaphroditic snails that copulate simultaneously and reciprocally, asymmetry in premating isolation may not be readily detectable because the failure of the symmetric performance of courtship would prevent copulation from occurring. On the other hand, through their prolonged copulation, snails discriminate among mates when exchanging spermatophores for their benefit and thus may exhibit asymmetric reproductive isolation during interspecific mating. However, no clear case of reciprocal asymmetry has been found in reproductive isolation between snail species. Here we show a discrete difference in hybridization success between simultaneous reciprocal copulations between two species of pulmonate snails. Premating isolation of Bradybaena pellucida (BP) and Bradybaena similaris (BS) is incomplete in captivity. In interspecific copulation, BP removes its penis without transferring a spermatophore, while BS sires hybrids by inseminating BP. Thus, ‘male’ BP or ‘female’ BS rejects the other individual, while female BP and male BS accept each other, so that the two sexes of either BP or BS oppose each other in mate discrimination. Our results are a clear example of asymmetry in reproductive isolation during simultaneous reciprocal mating between hermaphroditic animals.
Keywords: hermaphroditism, cryptic reproductive isolation, mating behaviour, copulation duration, Gastropoda
1. Introduction
Incomplete reproductive isolation between related species provides useful opportunities to elucidate the evolutionary processes of isolation mechanisms. Between incompletely isolated species, reproductive barriers are often differentially effective in reciprocal crosses (Tiffin et al. 2001; Turelli & Moyle 2007). In dioecious animals and hermaphroditic animals that perform only one of the two sexual roles in a particular mating event, the reciprocal asymmetry of reproductive isolation can be tested by simple comparison between the two species/sex combinations. This approach is not applicable to hermaphrodites with simultaneous reciprocal copulation, because each partner acts as a male and a female at the same time, unless they mate unilaterally between species. Pulmonate gastropods that mate simultaneously and reciprocally perform courtship behaviours symmetrically between partners, prior to simultaneous and reciprocal eversions and insertions of the penes (e.g. Asami et al. 1998). Thus, in reciprocally asymmetric premating isolation, one species would reject the other species in the ‘male’ or ‘female’ role despite accepting that species in the opposite sexual role. This discordance in mate discrimination between sexes within individuals may entirely prevent their courtship and penial insertion, because these behavioural steps must proceed simultaneously and symmetrically between partners. Consequently, asymmetry in premating isolation may be more difficult to detect in simultaneously and reciprocally mating hermaphrodites than in other animals.
Without strong premating isolation, however, courtships could proceed symmetrically and lead the partners to the stage of simultaneous reciprocal copulation (figure 1a). While copulation often lasts several hours in pulmonates (Tompa 1984), the partners examine each other before transferring a spermatophore (Leonard 2006). Thus, they may exhibit reciprocal asymmetry in prezygotic isolation during mating between species. Cryptic reproductive isolation through these processes could drive rapid speciation (Coyne & Orr 2004). However, few studies have explored the possible mode or evolutionary implications of asymmetric or cryptic reproductive isolation between species of hermaphroditic animals, despite increasing interest in their mating systems and sexual selection (Michiels 1998; Chase & Vaga 2006; Leonard 2006; Baur 2007).
Figure 1.
(a) Simultaneous reciprocal copulation of Bradybaena similaris (BS) and (b) penial removal by Bradybaena pellucida (BP) from BS in the middle of copulation. Arrows indicate the everted penes. In (b), the BP penis is no longer connected to the BS snail.
Here we show asymmetry in reproductive isolation during simultaneous reciprocal mating between the sibling species of land snails Bradybaena pellucida Kuroda & Habe (hereafter, BP) and Bradybaena similaris (Rang; BS). We discovered that only interspecific mating allows BP to produce hybrids, because BP removes its penis from the partner without transferring a spermatophore whereas BS transfers a spermatophore normally.
2. Material and methods
We collected juveniles of BP from Tateyama, Chiba (34° 55′ 22″ N, 139° 52′ 8″ E) and juveniles of BS from Shimo-shingashi, Saitama, Japan (35° 53′ 21″ N, 139° 31′ 3″ E), where each species occurs allopatrically. To obtain virgin adults, we raised them individually to maturity. We followed the breeding protocol of Asami & Ohbayashi (1999) except as indicated below.
We conducted mate choice experiments using virgin adults in two designs, 2+1 and 2+2. In the 2+1 design, we kept three snails in a Petri dish (150 mm diameter/20 mm depth) with moist paper towel, either two BP and one BS or two BS and one BP, and recorded which copulated first. Under random mating, conspecific and interspecific copulations would occur with the probabilities of one out of three and two out of three, respectively. In the 2+2 design, we kept two individuals of each species in the container and recorded the first copulating pair. Under random mating, the probability of conspecific and interspecific copulations would be one out of six and two out of three, respectively.
To examine the relative reproductive success of conspecific and interspecific pairs, we first allowed additional virgin adult pairs to copulate in containers (109×79×32 mm) for three weeks. For oviposition, we then separated each individual in a flowerpot (120 mm upper diameter, 100 mm height) with sterilized sand. We fed them with food composed of ground cat food, chicken egg shell, oat meal and chlorella pills in a dry weight ratio of 3 : 4 : 2 : 1. We collected eggs once a week for two months.
Using different pairs, we examined spermatophore transfer by dissecting partners as soon as copulation ended. We judged successful transfer as the presence of a spermatophore in the female tract between the vagina and the bursa copulatrix. To examine copulation behaviours, we kept additional pairs in containers (87×57×18 mm) with moist paper towel and the standard food at 25°C under a daily cycle of 16 L : 8 D with infrared light for 24 hours. We recorded their behaviour using a video recorder. After a pair started copulation, we moved the pair out of the recording room to examine their behaviour directly under ceiling lights until either one of the partners removes its penis from the other. This procedure caused no detectable change in copulation behaviour. We recorded the elapsed time from penial exposure to removal from the partner as copulation duration.
Each interspecific pair provides one individual output for each of the two species, while each conspecific pair provides two individual outputs of one species. Thus, for every statistical comparison of performance between interspecific and conspecific pairs, we randomly selected either one of the two individual outputs of each conspecific pair.
3. Results
(a) Symmetric premating isolation
In the 2+1 mate choice experiment, both BP and BS mated with conspecifics more frequently than with each other (table 1). The proportions of conspecific mating did not differ between the two modes, 2BP+1BS and 1BP+2BS (Fisher's exact probability test, p>0.9), showing no difference between the two species in the strengths of assortative mating. In the 2+2 design, the strengths of assortative mating were also similar for BP and BS (Fisher's exact probability test, p=0.353).
Table 1.
Assortative mating between B. pellucida (BP) and B. similaris (BS). (Parentheses indicate the expected number of pairs under random mating.)
| 2+2 desgin | 2+1 desgin | |||||
|---|---|---|---|---|---|---|
| 2BP+2BS | 2BP+1BS | 1BP+2BS | ||||
| obs. | exp. | obs. | exp. | obs. | exp. | |
| BP×BP | 12 | (5) | 15 | (7.7) | — | — |
| BP×BS | 12 | (20) | 8 | (15.3) | 9 | (16) |
| BS×BS | 6 | (5) | — | — | 15 | (8) |
| total no. pairs | 30 | 23 | 24 | |||
| X2 probability | 0.001 | 0.001 | 0.002 | |||
(b) Asymmetric hybridization
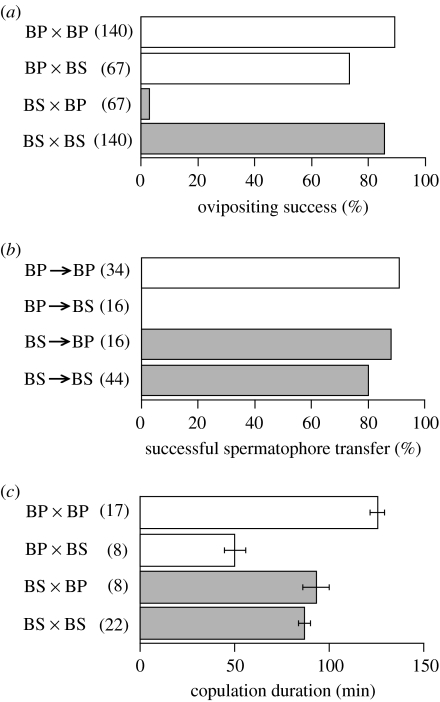
Most individuals of conspecific pairs reproduced successfully (table 2). However, out of 67 interspecific pairs, both species reproduced as a female in only two, and only BP reproduced in 47 (figure 2a). We used the BS outputs in the two pairs and the BP outputs in the 47 pairs to compare with the performance in conspecific pairs. Ovipositing individuals reproduced similarly regardless of the partner species in terms of number of eggs (Mann–Whitney test, BP, p=0.957; BS, p=0.213), number of clutches (BP, p=0.708; BS, p=0.168), clutch sizes (BS, p=0.755) and hatchability (BP, p=0.694; BS, p=0.907), although BP produced smaller clutches when mated with BS (p=0.039).
Table 2.
Reproductive success of B. pellucida (BP) and B. similaris (BS).
| cross type | sp. | no. paired individuals | no. ovipositing individuals | no. eggs/day/ovipositing individual±s.e. | no. clutches±s.e. | clutch size±s.e. | hatching rate±s.e. |
|---|---|---|---|---|---|---|---|
| BP×BP | BP | 140 | 125 | 0.91±0.09 | 3.39±0.45 | 25.20±1.63 | 0.91±0.02 |
| BP×BS | BP | 67 | 49 | 0.91±0.14 | 3.32±0.40 | 19.98±1.66 | 0.88±0.04 |
| BS | 67 | 2 | 0.76±0.18 | 2.00±1.00 | 26.83±8.17 | 0.79±0.21 | |
| BS×BS | BS | 140 | 120 | 1.33±0.12 | 4.50±0.54 | 25.55±1.08 | 0.92±0.01 |
Figure 2.
Asymmetry of reproductive isolation during simultaneous reciprocal mating between B. pellucida (BP) and B. similaris (BS). (a) Proportions of ovipositing individuals. (b) Proportions of individuals that received a spermatophore. (c) Copulation duration. Open and filled bars indicate the means (±s.e.) for BP and BS, respectively. Values in parentheses indicate the numbers of individuals.
(c) Asymmetric isolation during mating
In interspecific copulations, no BS received a spermatophore, but 87.5 per cent of the BP did (figure 2b). In conspecific copulations, BS received a spermatophore in 79.5 per cent of copulations and BP in 91.2 per cent. Thus, only spermatophore transfer from BP to BS was blocked, while BS transferred a spermatophore to BP and BS similarly (Fisher's exact probability test, p=0.176). To compare with the performance in conspecific pairs, we used the outputs of BP in randomly sampled eight interspecific pairs and the outputs of BS in the remaining eight interspecific pairs. Intraspecific copulation lasted longer in BP than BS (Mann–Whitney test, p<0.001; figure 2c). However, BP removed its penis from the partner in interspecific crosses earlier than that in intraspecific crosses (p<0.001; figure 1b), whereas BS did not change copulation duration significantly (p=0.372; figure 2c).
4. Discussion
Despite mutually symmetric performance of courtship and penial eversion, BS seldom received a spermatophore from BP while BS inseminated BP normally. Terrestrial pulmonates form a spermatophore during copulation and transfer it rapidly near the end of copulation (Tompa 1984). Thus, most probably, the early termination of copulation between female BS and male BP mechanically prevented the insemination of BS. The rare hybrids that BS did produce hatched normally, indicating no gametic incompatibility. Therefore, our results represent an example of asymmetry in reproductive isolation during simultaneous reciprocal copulation, instead of postzygotic or postmating prezygotic isolation (Howard 1999).
The early penial removal by BP must have resulted from interspecific rejection by either male BP or female BS. In the former case, male BP rejects female BS while female BP accepts male BS. In the latter, female BS rejects male BP while male BS accepts female BP. This type of behavioural disparity between sexes within individuals is logically inevitable in asymmetric isolation between simultaneous hermaphrodites. Our study demonstrates that it occurs in reality and causes costs to BP and BS in terms of paternal and maternal reproductive successes, respectively. Their F1 and F2 hybrids exhibit heterosis and only weak breakdown in reproductive success, respectively (Wiwegweaw et al. in press). Furthermore, indigenous BP and unindigenous BS in Japan probably began to be partly sympatric only a few 100 yr ago (Asami et al. 1997). Thus, their asymmetric isolation is not ascribable to reinforcement of a premating barrier such as genital mismatch (Eberhard 1985) and prompts further interest to examine other closely related species.
Either BP or BS must have sensed their differences chemically or physically during copulation. Their assortative mating suggests that they discriminate based on the differences in sexual pheromones. The inner wall of the penis, which directly contacts the partner's genitalia after eversion for copulation, exhibits discrete differences in morphology between the two species (Seki et al. 2008). The high variability of penial microsculpture in pulmonates is useful in species level taxonomy (e.g. Sutcharit & Panha 2006) and suggests rapid diversification through sexual selection and a function in physical mate recognition during copulation (Seki et al. 2008). Whether BP inserts the penis into the genital orifice of BS initially remains to be examined by the anatomy of copulating pairs.
Oviposition by BP or BS in interspecific pairs cannot be ascribed to selfing because the clutch sizes and hatchabilities were normal, unlike those values known for selfing in BS (Ueshima & Asami 2003; Asami & Asami 2008). Their hybrid production suggests that in sympatry, the introgression of mtDNA may be biased in the direction from BP to BS in contrast to reciprocally symmetric flow of nuclear markers. However, mate choice experiments in closed containers may underestimate the strength of premating isolation in natural conditions, where they may be separated more effectively by different microhabitat preferences (Seki et al. 2002, 2008) and sex pheromones. Examining the pattern of possible introgression in the wild between these indigenous and alien species of snails will be of great interest.
Acknowledgments
We thank J. Murray and R. Cowie for their comments and N. Asami and M. Asami for their field collections. This study was supported by JSPS Grants-in-Aid for Scientific Research to T.A. and a Hitachi Scholarship to A.W.
References
- Asami T., Asami N. Maintenance mechanism of a supergene in the terrestrial pulmonate Bradybaena similaris. Basteria. 2008;72:119–127. [Google Scholar]
- Asami T., Ohbayashi K. Effects of oviposition substrate on lifetime fecundity of the terrestrial pulmonate Bradybaena similaris. J. Conchol. 1999;36:1–9. [Google Scholar]
- Asami T., Yamashita H., Park J., Ishikawa H. Geographical distribution of the land snail Bradybaena pellucida (Pulmonata: Bradybaenidae) Yuriyagai. 1997;5:31–42. [Google Scholar]
- Asami T., Cowie R.H., Ohbayashi K. Evolution of mirror images by sexually asymmetric mating behavior in hermaphroditic snails. Am. Nat. 1998;152:225–236. doi: 10.1086/286163. doi:10.1086/286163 [DOI] [PubMed] [Google Scholar]
- Baur B. Reproductive biology and mating conflict in the simultaneously hermaphroditic land snail Arianta arbustorum. Am. Malacol. Bull. 2007;23:157–172. doi:10.4003/0740-2783-23.1.157 [Google Scholar]
- Chase R., Vaga K. Independence, not conflict, characterizes dart-shooting and sperm exchange in a hermaphroditic snail. Behav. Ecol. Sociobiol. 2006;59:732–739. doi:10.1007/s00265-005-0103-y [Google Scholar]
- Coyne J.A., Orr H.A. Sinauer; Sunderland, MA: 2004. Speciation. [Google Scholar]
- Eberhard W.G. Harvard University Press; Cambridge, MA: 1985. Sexual selection and animal genitalia. [Google Scholar]
- Howard D.J. Conspecific sperm and pollen precedence and speciation. Ann. Rev. Ecol. Sys. 1999;30:109–132. doi:10.1146/annurev.ecolsys.30.1.109 [Google Scholar]
- Leonard J.L. Sexual selection: lessons from hermaphrodite mating systems. Integr. Comp. Biol. 2006;46:349–367. doi: 10.1093/icb/icj041. doi:10.1093/icb/icj041 [DOI] [PubMed] [Google Scholar]
- Michiels N.K. Mating conflicts and sperm competition in simultaneous hermaphrodites. In: Birkhead T.R., Møller A.P., editors. Sperm competition and sexual selection. Academic Press; London, UK: 1998. pp. 219–254. [Google Scholar]
- Seki K., Inoue S., Asami T. Geographical distributions of sibling species of land snails Bradybaena pellucida and B. similaris in the Boso peninsula. Venus. 2002;61:41–48. [Google Scholar]
- Seki K., Wiwegweaw A., Asami T. Fluorescent pigment distinguishes between sibling snail species. Zool. Sci. 2008;25:1212–1219. doi: 10.2108/zsj.25.1212. doi:10.2108/zsj.25.1212 [DOI] [PubMed] [Google Scholar]
- Sutcharit C., Panha S. Taxonomic review of the tree snail Amphidromus Albers, 1850 (Pulmonata: Camaenidae) in Thailand and adjacent areas: subgenus Amphidromus. J. Molluscan. Stud. 2006;72:1–30. doi:10.1093/mollus/eyi044 [Google Scholar]
- Tiffin P., Olson M.S., Moyle L.C. Asymmetrical crossing barriers in angiosperms. Proc. R. Soc. B. 2001;268:861–867. doi: 10.1098/rspb.2000.1578. doi:10.1098/rspb.2000.1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompa A.S. Land snails (Stylommatophora) In: Tompa A.S., Verdonk N.H., Van Den Biggelaar J.A.M., editors. The Mollusca. vol. 7. Academic Press; Orlando, FL: 1984. pp. 47–140. [Google Scholar]
- Turelli M., Moyle L.C. Asymmetric postmating isolation: Darwin's corollary to Haldane's rule. Genetics. 2007;176:1059–1088. doi: 10.1534/genetics.106.065979. doi:10.1534/genetics.106.065979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueshima R., Asami T. Single-gene speciation by left-right reversal. Nature. 2003;425:679. doi: 10.1038/425679a. doi:10.1038/425679a [DOI] [PubMed] [Google Scholar]
- Wiwegweaw, A., Seki, K., Utsuno, H. & Asami, T. In press. Fitness consequences of reciprocally asymmetric hybridization between simultaneous hermaphrodites. Zool. Sci. [DOI] [PubMed]