Abstract
Many animals communicate in situations that make it difficult to discriminate a species' signals from those of others. Consequently, coexisting species usually have signals that differ by more than the minimum required to prevent overlap in acoustic features. These gaps between signals might facilitate detection and discrimination of degraded signals in noisy natural conditions. If so, perception of signals should have broader scope than production. We investigated this possibility by studying song production and perception of two species of birds in an especially noisy environment, the Amazonian dawn chorus. With software developed for this study, we digitally synthesized songs of two species, as well as intermediate versions of their songs. Experimental playbacks of these synthesized songs to individuals of both species confirmed that perception (as indicated by responses) was broader than production of songs. We propose that broader perception than production of song promotes communication in noisy situations and limits the similarity between signals of coexisting species.
Keywords: animal communication, acoustic partitioning, bird song, neotropical birds, signal detection
1. Introduction
Many animals rely on long-range communication for species recognition, mate selection and territorial defence, but degradation of signals during transmission and irrelevant noise in the background often limit the ability of receivers to detect a signal or to discriminate between signals (Ryan & Brenowitz 1985; Klump 1996; Bradbury & Vehrencamp 1998; Wollerman & Wiley 2002). Multi-species choruses make it especially difficult for animals to detect or to discriminate signals (Wiley 1994; Gerhardt & Huber 2002; Wollerman & Wiley 2002; Brumm & Slabbekoorn 2005).
Features of signals that differ distinctly from those of other species would promote accurate recognition of conspecific signals (Marler 1960; Miller 1982; Nelson 1988; Nelson & Marler 1990). For instance, coexisting species using acoustic signals should partition multidimensional acoustic space, as defined by the acoustic features of their signals. The difference between species' signals, however, is usually more than the minimum required to avoid overlap. Instead, signals occupy regions of acoustic space that are disjunct (separated by gaps; Nelson & Marler 1990), so acoustic space is not continuously occupied by the production of signals. These gaps might serve to reduce errors in detection and discrimination of conspecific signals degraded during propagation through the environment.
It remains unclear whether the regions of acoustic space in which different species respond to signals are also disjunct (Richards & Wiley 1980; Wiley & Richards 1982; Naguib & Wiley 2001). Characteristic responses indicate the processing, or perception, of sensations. Although in many cases it is clear that the sense organs of different species respond to each other's vocalizations, individuals only perceive signals with certain features as conspecific and thus respond to them. Does this perception of signals by coexisting species leave gaps in acoustic space as does the production of signals or does perception fully occupy acoustic space? Greater scope for perception of signals than for their production would accommodate variation in signals introduced by degradation during transmission. Previous reports have indicated that birds respond to signals at least one standard deviation from the population mean (e.g. Nelson & Marler 1990), but none has investigated the entire acoustic space between two species' songs.
Avian dawn choruses in tropical forests provide an example of communication in high levels of background noise from other species. The combination of many species and a brief time in which most of them broadcast their signals limits possibilities for signal divergence. Although it is clear that each species has a distinct species-specific song, it is not clear how the production and perception of these signals are distributed in acoustic space.
To examine this issue, we focused on two species that are distantly related but acoustically similar. Both are suboscines (Passeriformes: Tyranni), a group that includes most of the species of birds in the understorey of Amazonian forests. In comparison with oscines (Passerini), this group has relatively little variation among individuals' songs (Wiley 2005). To test their recognition of songs, we measured the responses of each species to playbacks of its own song, the other species' song and three intermediates. The intermediate versions, spaced along a continuum between the two species' songs, allowed us to determine whether these species partition acoustic space for perception of conspecific songs either disjunctly, as they do for production of songs, or continuously.
2. Material and methods
Fieldwork took place at the Rio Cristalino Private Natural Heritage Preserve, 40 km northeast of Alta Floresta, Mato Grosso, Brazil (9°41′ S, 55°54′ W). In 2004, standardized replicated recordings between 05.30 and 08.30 in mature upland (terra firma) forest detected 51 suboscine species that sang during more than 1 per cent of the total time. To compare their songs in multidimensional acoustic space, we measured 15 acoustic features of songs from three individuals of each species (table 1) and used principal components analysis (PCA) to derive independent variables that are linear combinations of these features. The first four principal components (PCs) had eigenvalues >1 and explained 73 per cent of the variation. To identify nearest neighbours in acoustic space, we calculated the Euclidean distance between species' mean songs in four-dimensional space defined by the first four PCs (see Luther 2008). Twenty-one species had nearest neighbours that were reciprocal. Of these species, we selected the plain-winged antshrike, Thamnophilus schistaceus (PWA), and wing-barred piprites, Piprites chloris (WBP), which had one of the smallest nearest-neighbour distances and populations sufficient for playback experiments. Both are year-round territorial residents. Territories were mapped by following the movements of singing birds and by marking the locations of singing across mutual boundaries.
Table 1.
Parameters of synthesized songs (frequencies in Hz and times in ms).
| length of note | low frequency of note | high frequency of note | distance between notes | no. of elements per note | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| song type | no. of notes | first | average | last | first | average | last | first | average | last | first and second | average | last P. chloris | last T. schistaceus | first | average | last |
| Thamnophilus | |||||||||||||||||
| schistaceus 100% | 9.0 | 180.0 | 125.9 | 121.0 | 1808.0 | 1550.0 | 947.0 | 1808.0 | 1722.0 | 1550.0 | 287.0 | 188.4 | 128.0 | 37.0 | 1.0 | 1.0 | 2.0 |
| T. schistaceus 67%, | |||||||||||||||||
| P. chloris 33% | 8.7 | 159.7 | 112.8 | 109.7 | 1664.7 | 1521.3 | 1119.3 | 1693.3 | 1664.7 | 1550.0 | 236.7 | 189.5 | 120.0 | n.a. | 1.0 | 1.3 | 2.0 |
| T. schistaceus 50%, | |||||||||||||||||
| P. chloris 50% | 8.5 | 149.5 | 106.2 | 104.0 | 1593.0 | 1507.0 | 1205.5 | 1636.0 | 1636.0 | 1550.0 | 211.5 | 190.1 | 116.0 | n.a. | 1.0 | 1.3 | 2.0 |
| T. schistaceus 33%, | |||||||||||||||||
| P. chloris 67% | 8.3 | 139.3 | 99.7 | 98.3 | 1521.3 | 1492.7 | 1291.7 | 1578.7 | 1607.3 | 1550.0 | 186.3 | 190.7 | 112.0 | n.a. | 1.0 | 1.7 | 2.0 |
| P. chloris 100% | 8.0 | 119.0 | 86.6 | 87.0 | 1378.0 | 1464.0 | 1464.0 | 1464.0 | 1550.0 | 1550.0 | 136.0 | 191.8 | 104.0 | n.a. | 1.0 | 2.6 | 1.0 |
| difference between | |||||||||||||||||
| P. chloris and | |||||||||||||||||
| T. schistaceus | 1.0 | 61.0 | 53.6 | 34.0 | 430.0 | 86.0 | 517.0 | 344.0 | 172.0 | 0.0 | 151.0 | 100.2 | 24.0 | 37.0 | 1.0 | 1.0 | 2.0 |
| confidence intervals, | |||||||||||||||||
| P. chloris (n=7) | 1 | 19.3 | 33.7 | 21.7 | 48.2 | 50.3 | 44.0 | 68.1 | 58.9 | 96.3 | 44.6 | 63.7 | 39.0 | n.a. | 0.0 | 0.4 | 0.0 |
| confidence intervals, P. schistaceus | |||||||||||||||||
| (n=7) | 0 | 36.8 | 29.8 | 20.6 | 79.9 | 82.7 | 71.2 | 70.9 | 73.5 | 68.1 | 15.8 | 15.2 | n.a. | 15.5 | 0.0 | 0.0 | 0.0 |
| overlap between | |||||||||||||||||
| species(n=7) | 2 | 12 | 77 | 64 | 86 | 86 | 345 | 86 | 115 | 258 | −12 | 112 | 371 | n.a. | 0 | 2 | −1 |
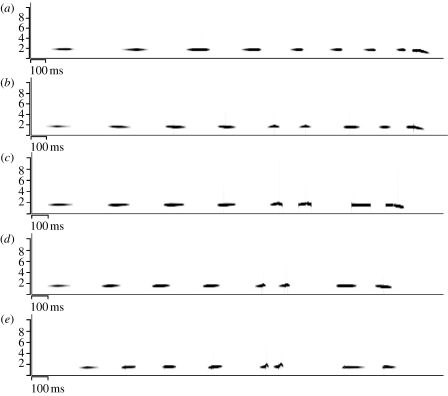
To synthesize songs with intermediate features, we used Soundsynthesis2 (v. 060906, www.unc.edu/∼rwhiley), a program developed for this study, which uses a spreadsheet of frequencies and amplitudes to specify successive smoothed segments of notes with a resolution of 1 ms. We synthesized a mean song for each species as well as songs with features 2/3, 1/2 and 1/3, the distance between the two species' means (figure 1; table 1). We call the synthesized songs the 100, 67, 50, 33 and 0% morphs of a species' song. A 0 per cent morph of one species' song is the same as a 100 per cent morph of the other species' song.
Figure 1.
Sonograms of synthesized songs (see text for explanations of the different morphs). (a) 100% T. schistaceus and 0% P. chloris, (b) 67% T. schistaceus and 33% P. chloris, (c) 50% T. schistaceus and 50% P. chloris, (d) 33% T. schistaceus and 67% P. chloris and (e) 0% T. schistaceus and 100% P. chloris.
Experiments were conducted on eight PWA and eight WBP in September and October 2006. All morphs (100–0%) were presented in randomized order to all individuals of each species. Preliminary trials with both species compared responses to natural and synthetic versions of their own songs in randomized order.
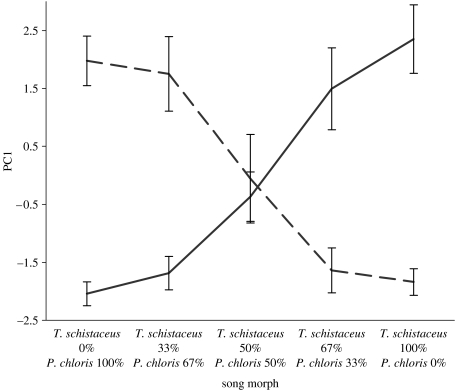
During and after each playback, we recorded nine measures of behavioural response (see the electronic supplementary material). PCA extracted three PCs with eigenvalues >1, which together explained 77 per cent of the variation (see table S1 in the electronic supplementary material). PC1 (51% of the variation) was used as the response variable in all tests. An initial examination showed that the relationship of this variable to the predictor approximated a logistic S-shaped curve (figure 2). Consequently, we treated PC1 as a logistic function of song morph.
Figure 2.
Mean responses (±s.e.) of each species to the different synthesized morphs (high values of PC1 indicate a strong response). Solid line, T. schistaceus; dashed line, P. chloris.
To compare song production by the two species, we measured the same 15 features mentioned above (table 1) in randomly selected songs of seven individuals of each species. PCA extracted four PCs with eigenvalues >1 that together explained 81 per cent of the variation. PC1 and PC2 (61% of the variation) were used to plot 2 standard deviations around the mean signals for each species in two-dimensional signal space. To compare song perception, we analysed individuals' responses to each of the synthesized morphs. PC1 and PC2 (65% of the response variation; see table S1 in the electronic supplementary material) were used to plot 2 standard deviations around the mean responses to each morph in two-dimensional response space.
3. Results
Both species responded similarly to natural and synthetic versions of their own songs. Responses to synthetic songs were slightly stronger, perhaps because the recordings were so clean. For both species, responses were strong to 100 per cent and 67 per cent morphs, moderate to low to 50 per cent morphs and low to absent to 33 per cent and 0 per cent morphs (figure 2). The diminishing responses across the series of morphs indicated continuity in perception along the continuum between the two species' songs. Each species responded to the 50 per cent morph about half as strongly as to the appropriate 100 per cent morph. For these species, there were no gaps in the perception of their songs. By contrast, production of their songs was disjunct. Although most of the features of their natural songs overlapped (table 1), the PCs of all measured features revealed that the two species' songs were separated by a gap in multidimensional acoustic space (see figure S3 in the electronic supplementary material).
4. Discussion
Receivers in natural situations must detect and discriminate conspecific signals in the presence of background noise. Noise in the form of signals from other individuals and species is especially likely to interfere (Gerhardt & Klump 1988; Wollerman & Wiley 2002). Although coexisting species usually evolve distinct signals, little is known about the evolution of perception in coexisting species (Gerhardt 1994; Amezquita et al. 2006). Perception of sounds as conspecific is indicated in our experiments by the characteristic response of a territorial male towards a rival. Our results indicate that two species with similar songs in a noisy environment use signal space disjunctly, with a gap between their signals, but use perceptual space continuously, without a gap.
A greater scope for perception than for production of signals in acoustic space is a plausible adaptation for communication in a noisy environment. During transmission, a signal becomes degraded in complex ways and mixed with background noise (Wiley & Richards 1982; Naguib & Wiley 2001). As a result, signals when received are more variable than when broadcast. In these conditions, receivers cannot afford to be too narrowly focused on the features of a clean signal. Furthermore, signals should differ enough to allow receivers to discriminate between signals despite degradation during transmission. Noisy conditions thus constrain the similarity of coexisting species' signals as a result of the benefits of a broader scope for perception than for production of signals.
Acknowledgments
This research was conducted in accordance with all requirements for the ethical treatment of animals in Brazil and the United States.
Jack Weiss provided statistical advice. Maria Alice dos Santos Silva and Mario Cohn-Haft helped with Brazilian visas, and John Luther, Amy Upgren, Brad Davis, Vitoria da Riva Carvalho and staff of Rio Cristalino RPPN assisted in the field. Amy Upgren and Barbara Ballentine provided comments on the manuscript. Support came from the Mellon Foundation, Explorer's Club, International and Latin American Studies at UNC, the UNC Graduate School and the UNC-CH Biology Department's Behavioral Research Fund. D.A.L. conducted the fieldwork, R.H.W. wrote the program for sound synthesis and both authors participated equally otherwise.
Supplementary Material
Includes supplementary figures and tables
References
- Amezquita A., Hodl W., Lima A.P., Castellanos L., Erdtmann L., Carmozina De Araujo M. Masking interference and the evolution of the acoustic communication system in the Amazonian dendrobatid frog Allobates femoralis. Evolution. 2006;60:1874–1887. doi:10.1554/06-081.1 [PubMed] [Google Scholar]
- Bradbury J.W., Vehrencamp S.L. Sinauer Associates; Sunderland, MA: 1998. Principles of animal communication. [Google Scholar]
- Brumm H., Slabbekoorn H. Acoustic communication in noise. Adv. Study Behav. 2005;35:151–209. doi:10.1016/S0065-3454(05)35004-2 [Google Scholar]
- Gerhardt H.C. The evolution of vocalization in frogs and toads. Annu. Rev. Ecol. Syst. 1994;25:293–324. doi:10.1146/annurev.es.25.110194.001453 [Google Scholar]
- Gerhardt H.C., Huber F. University of Chicago Press; Chicago, IL: 2002. Acoustic communication in insects and anurans: common problems and diverse solutions. [Google Scholar]
- Gerhardt H.C., Klump G.M. Masking of acoustic signals by the chorus background noise in the green tree frog a limitation on mate choice. Anim. Behav. 1988;36:1247–1249. doi:10.1016/S0003-3472(88)80090-3 [Google Scholar]
- Klump G.M. Bird communication in the noisy world. In: Kroodsma D.E., Miller E.H., editors. Ecology and evolution of acoustic communication in birds. Cornell University Press; Ithaca, NY: 1996. pp. 321–338. [Google Scholar]
- Luther, D. A. 2008 The acoustic community and the evolution of acoustic communication. Dissertation, University of North Carolina, Chapel Hill.
- Marler, P. 1960 Bird songs and mate selection. In Animal sounds and communication, vol. 7 (eds W. E. Lanyon & W. N. Tavolga), pp. 348–367. Washington, DC: American Institute of Biological Sciences.
- Miller, E. H. 1982 Character and variance shift in acoustic signals of birds. In Acoustic communication in birds, vol. 1 (eds D. E. Kroodsma & E. H. Miller), pp. 253–295. New York, NY: Academic Press.
- Naguib M., Wiley R.H. Estimating the distance to a source of sound: mechanisms and adaptations for long-range communication. Anim. Behav. 2001;62:825–837. doi:10.1006/anbe.2001.1860 [Google Scholar]
- Nelson D.A. Feature weighting in species song recognition by the field sparrow Spizella pusilla. Behaviour. 1988;106:158–182. doi:10.1163/156853988X00142 [Google Scholar]
- Nelson, D. A. & Marler, P. 1990 The perception of bird song and an ecological concept of signal space. In Comparative perception: complex signals, vol. 2 (eds W. C. Stebbins & M. A. Berkley), pp. 443–478. New York, NY: Wiley.
- Richards D.G., Wiley R.H. Reverberations and amplitude fluctuations in the propagation of sound in a forest—implications for animal communication. Am. Nat. 1980;115:381–399. doi:10.1086/283568 [Google Scholar]
- Ryan M.J., Brenowitz E.A. The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am. Nat. 1985;126:87–100. doi:10.1086/284398 [Google Scholar]
- Wiley R.H. Errors, exaggeration, and deception in animal communication. In: Real L.A., editor. Behavioral mechanisms in evolutionary ecology. University of Chicago Press; Chicago, IL: 1994. pp. 157–189. [Google Scholar]
- Wiley R.H. Individuality in songs of Acadian flycatchers and recognition of neighbours. Anim. Behav. 2005;70:237–247. doi:10.1016/j.anbehav.2004.09.027 [Google Scholar]
- Wiley R.H., Richards D.G. Adaptations for acoustic communication in birds: sound transmission and signal detection. In: Kroodsma D.E., Miller E.H., editors. Acoustic communication in birds. Academic Press; New York, NY; London, UK: 1982. pp. 131–181. [Google Scholar]
- Wollerman L., Wiley R.H. Possibilities for error during communication by neotropical frogs in a complex acoustic environment. Behav. Ecol. Sociobiol. 2002;52:465–473. doi:10.1007/s00265-002-0534-7 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Includes supplementary figures and tables