Abstract
Adenosine A1 and A2A receptor subtypes modulate metabolism in adult mammals. This study was designed to determine the role of these receptors in regulating plasma levels of insulin, glucose, and lactate in 20 chronically catheterized fetal sheep (>0.8 term). In normoxic fetuses (PaO2 ∼24 Torr), systemic blockade of A1 receptors with DPCPX (n = 6) increased plasma concentrations of insulin, glucose, and lactate, but antagonism of A2A receptors with ZM-241385 (n = 5) had no significant effects. Intravascular administration of adenosine (n = 9) reduced insulin concentrations and elevated glucose and lactate levels. DPCPX (n = 6) augmented the glycemic and lactatemic responses of adenosine. In contrast, ZM241385 (n = 5) virtually abolished adenosine-induced hyperglycemia and hyperlactatemia. Isocapnic hypoxia (PaO2 ∼13 Torr) suppressed insulinemia and enhanced glycemia and lactatemia, but only the hyperglycemia was blunted by blockade of A1 (n = 6) or A2A (n = 6) receptors. We conclude that 1) endogenous adenosine via A1 receptors depresses plasma concentrations of insulin, glucose, and lactate; 2) exogenous adenosine via A2A receptors increases glucose and lactate levels, but these responses are dampened by stimulation of A1 receptors; and 3) hypoxia, which increases endogenous adenosine concentrations, induces hyperglycemia that is partly mediated by activation of A1 and A2A receptors. We predict that adenosine, via A1 receptors, facilitates at least 12% of glucose uptake and utilization in normoxic fetuses.
Keywords: fetus, hypoxia, metabolism, growth, placenta
adenosine regulates glucose metabolism in mammals through several mechanisms that include inhibiting insulin secretion by islet cells, altering insulin transduction, and facilitating intracellular glucose transport. Interstitial levels of adenosine increase substantially in hypoxia, hypoglycemia, or heightened cellular metabolism that can occur through intense neuronal activity or skeletal muscle contractions. The physiological effects of adenosine are mediated through activation of G protein-liganded cell surface receptors that are subdivided into four adenosine receptor subtypes: A1, A2A, A2B, and A3 (45). These receptors are connected to classical second-messenger pathways that modulate intracellular cAMP production, phospholipase C pathway, and mitogen-activated protein kinases (45, 46). With regard to cAMP, stimulation of A1 receptors, via inhibition of adenylyl cyclase, generally inhibits intracellular cAMP synthesis, while activation of A2A and A2B receptors has the opposite effects.
Glucose promotes A1 and A2A expression in rat cardiac fibroblasts, while insulin suppresses the expression of A1 and A2B receptors (11). Insulin also facilitates the equilibrative transcellular movement of adenosine through stimulation of nucleoside transporters in human umbilical vein endothelial cells (37). Adenosine, in turn, inhibits insulin release via activation of islet A1 receptors (21, 51). Stimulation of A1 receptors also enhances glucose uptake in tissues through mechanisms that are both dependent and independent of insulin (1, 34, 46). Thus, glucose and insulin can either amplify or blunt the metabolic effects of adenosine receptor activity, depending on the direction and extent of changes in their respective concentrations.
The expression of adenosine receptor subtypes (41, 52), and probably their modulation by substrate and insulin, is developmentally regulated, so that the function of adenosine receptors may differ quantitatively and qualitatively in the fetus compared with that in the adult. Moreover, plasma adenosine concentrations in the fetus are four-fold greater than those in the adult (26, 44), which raises the question as to whether adenosine has a more prominent role in fetal glucose metabolism.
Acute hypoxia (PaO2 ∼15 Torr) rapidly increases fetal plasma levels of adenosine (26), which contributes, directly and indirectly, to many hormonal, cardiovascular, and behavioral adaptations that are critical for fetal survival in short periods of acute oxygen deprivation (2, 5, 27, 28, 29, 30, 32, 39). Hypoxia also lowers fetal plasma concentrations of insulin, while it raises levels of glucose and lactate (22, 26). Adenosine, via the A1 receptor, facilitates glycolysis in normoxic adult tissues in vitro (53); thus, adenosine may also be involved in fetal metabolic responses to acute O2 deficiency (25). This study was designed to test the hypothesis that adenosine (ADO) A1 and A2A receptors participate in the regulation of fetal plasma concentrations of insulin, glucose, and lactate under both normoxic and hypoxic conditions.
MATERIALS AND METHODS
The Chancellor's Animal Research Committee approved the surgical procedures and experiments involved in this study. Twenty pregnant ewes (Rambouillet-Columbia cross), anesthetized with halothane, underwent aseptic surgery at about 120 days of gestation (∼0.8 term). Polyvinyl catheters (ID: 1.0 mm, OD: 2.0 mm) were inserted into the right brachiocephalic artery, right external jugular vein, and right carotid artery of the fetus, and another was placed in the amniotic sac (32). Tetracycline (15 mg/kg) was injected intramuscularly in the ewe immediately prior to surgery, and ampicillin (500 mg/l lactated Ringer solution) was infused into the amniotic sac after the completion of surgical procedures and on the first postoperative day. Buprenorthine HCl (0.01 mg/kg body wt) was administrated to the ewe intramuscularly for postoperative analgesia.
The studies were begun at least 4 days after surgery under chronic experimental conditions, when fetal metabolic homeostasis had virtually recovered. The effects of adenosine receptor agonists and antagonists were compared with those of the respective vehicle, which controlled for metabolic changes that might have occurred independently of adenosine. These infusions were performed in varied order and on separate days to minimize the potential for carry-over effects.
Normoxia Endogenous Adenosine
Potent, highly selective, and specific antagonists of adenosine A1 and A2A receptors were administered to determine the metabolic effects of these receptor subtypes. The administration protocol of these agents was the same as that used to block A1 and A2A receptors in fetal sheep that are involved in sleep state and cardio-respiratory regulation (27, 28); moreover, the dosages are consistent with the greater potency of these highly selective receptor antagonists over nonselective adenosine receptor antagonists that block the release of fetal hormones (6, 30, 39).
A1 receptor.
A highly specific and selective A1 receptor antagonist, 9-cyclopentyl-1,3-dipropylxanthine (DPCPX; Tocris Cookson, Ballwin, MO), was dissolved in 0.04 M 2-hydroxypropyl-β-cyclodextrin (Sigma Aldrich, St. Louis, MO) and 0.2 N NaOH (50:50, vol/vol) and diluted with saline to a concentration of 2.5 mg/ml. The antagonist was infused intra-arterially at 1.2 mg·min−1·kg−1 for 10 min, which was followed by a maintenance rate of 0.24 mg·min−1·kg−1 for 50 min.
A2A receptor.
ZM-241385 (Tocris Cookson), a highly selective and specific blocker of A2A receptors, was dissolved in polyethene glycol 400 and 0.1 N NaOH (50:50, vol/vol) and diluted with saline to 0.66 mg/ml. The antagonist was infused intra-arterially at 0.65 mg·min−1·kg−1 for 10 min, and subsequently at 0.056 mg·min−1·kg−1 for 50 min.
Adenosine
A second series of experiments was performed in normoxic fetuses to determine the effects of exogenous adenosine on circulating levels of insulin, glucose, and lactate. Specific antagonists of A1 and A2A receptors were subsequently used to determine whether these receptors were involved in fetal metabolic responses to adenosine.
Adenosine.
Adenosine (Sigma-Aldrich) dissolved in saline was infused (250 μg·min−1·kg−1) for 1 h into the external jugular vein of the fetus. This dose of adenosine increases fetal plasma concentrations of adenosine to levels that have been measured in hypoxic fetal sheep (26, 39).
A1 receptor blockade.
Adenosine was administered intravenously while simultaneously infusing intra-arterially DPCPX.
A2A receptor blockade.
Adenosine was infused intravenously along with intra-arterial administration of ZM-241385.
Hypoxia
In a third series of experiments, fetal hypoxia was induced to establish whether a rise in endogenous adenosine levels contribute to the metabolic perturbations of acute O2 deprivation. Isocapnic hypoxia was induced in the fetus by having the ewe breathe a hypoxic gas mixture (9% O2, 3% CO2, 88% N2) for 1 h. Hypoxia experiments were performed with and without intra-arterial administration of an A1 or A2A receptor antagonist or their respective vehicles, as described previously (27, 28).
Blood Samples
Preductal arterial blood for analysis was withdrawn in the control period, after 10, 30, and 60 min of infusion and after 10 and 30 min of recovery. Arterial blood gases and pH were determined using blood gas electrodes (model 1304; Instrumentation Laboratories, Lexington, MA) with measurements corrected to fetal temperature (39.5°C).
One milliliter of blood was placed in iced tubes (Becton Dickinson, Franklin Lakes, NJ) that contained potassium oxalate, an anticoagulant, and sodium flouride, an inhibitor of glycolysis. The blood samples were centrifuged at 4°C, and the separated plasma was stored at −80°C until analysis. Plasma glucose and lactate concentrations were measured in duplicate by glucose oxidase techniques and lactate oxidase methods, respectively (Yellow Springs Instruments 2700 SELECT Biochemistry Analyzer; YSI, Yellow Springs, OH), with a precision of 2% for both analytes. Plasma insulin concentration was measured in duplicate by radioimmunoassay with reagents from Linco (St. Charles, MO). Intra- and inter-assay coefficients of variance were 6 and 8%, respectively.
The data for each animal were divided by its control value (0 time) to provide standardization for statistical comparisons. The area under the standardized plot for each animal (area under the curve, AUC) was also calculated over the 60 min of infusion. Areas are expressed in units of concentration ratio·min (CRmin). The mean response profiles over time were compared by repeated measures of ANOVA with post hoc comparison of means by Tukey-Fisher least significant difference criterion. Mean AUC comparisons were made by paired t-tests. Differences were significant at P ≤ 0.05. Values reported are expressed as means ± SE.
RESULTS
All experiments were conducted in fetuses with normal arterial blood gases and pH, which were not significantly altered by vehicle administration. The ranges of the mean concentrations for all measurements before the start of infusion were 0.40–0.53 μM for insulin, 0.86 −1.01 mM for glucose, and 1.25–2.11 mM for lactate. With respect to measurements at time 0, the means for control (vehicle) experiments did not differ significantly from those for administration of the respective adenosine receptor antagonists.
Normoxia and Endogenous Adenosine
A1 receptor blockade.
Vehicle infusion (n = 6) did not significantly alter plasma levels of insulin, glucose, or lactate. DPCPX did not significantly alter preductal arterial blood gases or pH. The following summarizes the metabolic effects of DPCPX.
INSULIN.
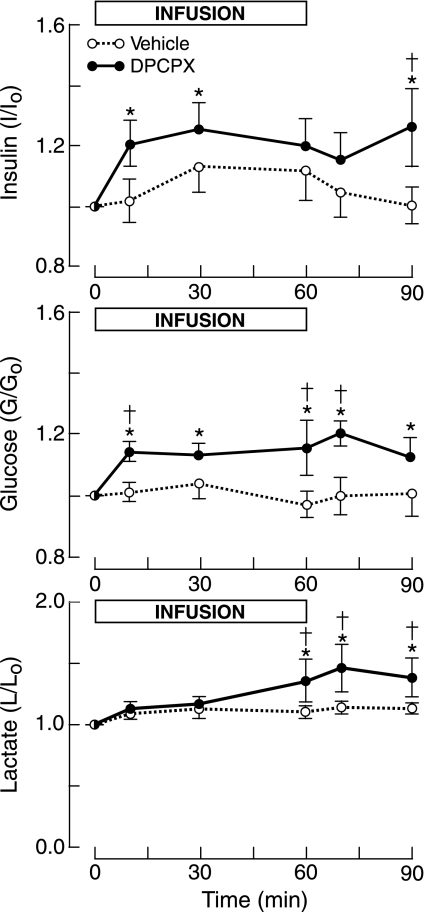
Insulin concentrations rose by at least 20% with DPCPX infusion, although they were not significantly greater than the respective vehicle levels, which increased by ∼10% (Fig. 1). The AUC for the insulin concentration ratio over the 60 min of infusion was greater for DPCPX than for vehicle (Table 1), which was of borderline significance (P = 0.06).
Fig. 1.
Fetal plasma concentration ratios (concentration/concentrationtime 0) of insulin, glucose, lactate relative to levels at time 0 for infusion of the A1-receptor antagonist DPCPX or vehicle. Horizontal bar shows time of infusion. Vertical bars show means ± SE. *P < 0.05 compared with time 0. †P < 0.05 compared with vehicle.
Table 1.
Area under the curve for plasma insulin, glucose, and lactate concentrations over a 1-h infusion of A1 receptor antagonist (DPCPX), A2A receptor blocker (ZM-241385), ADO, or vehicle in normoxic fetal sheep
| Insulin | Glucose | Lactate | |
|---|---|---|---|
| Endogenous ADO | |||
| DPCPX (6) | 72.5±2.8 | 67.8±2.5 | 71.5±4.3 |
| Vehicle (6) | 65.3±4.0 | 60.9±2.3 | 64.9±2.9 |
| P value | 0.06 | 0.03 | 0.21 |
| ZM-241385 (6) | 66.7±5.2 | 62.6±5.9 | 59.7±4.2 |
| Vehicle (6) | 65.8±4.3 | 58.6±1.1 | 58.0±0.7 |
| P value | 0.57 | 0.41 | 0.60 |
| Exogenous ADO | |||
| ADO (9) | 43.8±1.8 | 70.8±2.4 | 89.8±8.6 |
| Vehicle (9) | 63.9±2.8 | 61.5±0.9 | 61.1±0.8 |
| P value | 0.00002 | 0.000001 | 0.0005 |
| ADO + DPCPX (6) | 48.7±1.9 | 72.1±3.0 | 97.1±16.1 |
| ADO + Vehicle (6) | 54.0±4.8 | 63.9±1.5 | 70.0±2.6 |
| P value | 0.31 | 0.05 | 0.04 |
| ADO+ZM-241385 (5) | 67.1±7.4 | 60.6±3.9 | 64.5±5.2 |
| ADO + Vehicle (5) | 50.6±7.0 | 68.8±2.8 | 92.4±13.9 |
| P value | 0.12 | 0.04 | 0.05 |
Number in parentheses represents the number of fetuses. ADO, adenosine. Area under the curve is presented as concentration ratio·min.
GLUCOSE.
Plasma glucose levels, which rose sharply by ∼14% within 10 min of the start of the DPCPX infusion, remained elevated throughout DPCPX infusion and recovery (Fig. 1). This rise in glucose concentrations was also significantly greater than that for vehicle infusion. The AUC for DPCPX was significantly greater than that for vehicle (Table 1).
LACTATE.
DPCPX increased lactate levels by ∼36% by the end of infusion, an elevation that persisted through recovery (Fig. 1). Lactate levels for DPCPX were significantly greater than for vehicle after 60 min of infusion, although the AUC for both infusions did not differ significantly (Table 1).
A2A receptor blockade.
Infusion of ZM-41385 in six fetuses altered neither fetal PaO2, PaCO2, and pH nor plasma levels of insulin, glucose, and lactate.
Normoxia and Exogenous Adenosine
No antagonist.
Adenosine (n = 9) did not significantly change mean PaO2 or PaCO2, but it significantly lowered fetal arterial pH from 7.343 ± 0.006 (time 0) to 7.314 ± 0.009 by the end of infusion (P = 0.01).
INSULIN.
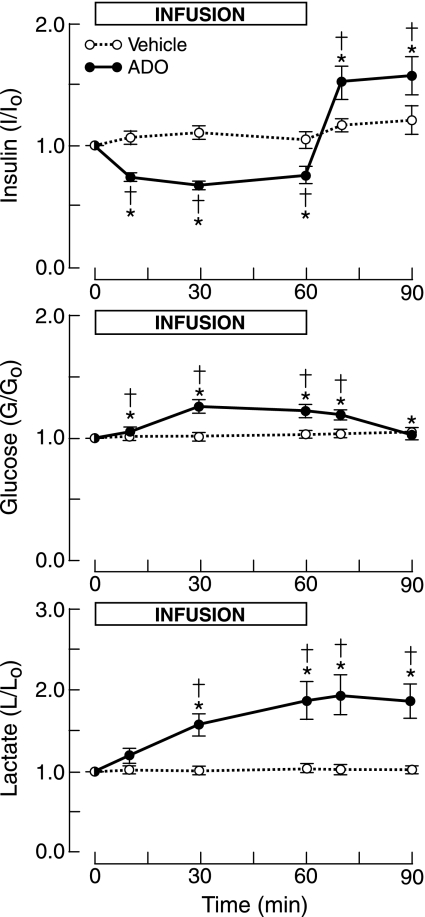
Adenosine reduced plasma insulin concentrations by up to 33%. When the infusion was stopped, insulin levels rapidly rose to levels ∼50% greater than control (Fig. 2).
Fig. 2.
Fetal plasma concentration ratios of insulin, glucose, and lactate in response to infusion of adenosine (ADO) or vehicle. *P < 0.05 compared with time 0. †P <0.05 compared with vehicle.
GLUCOSE.
Adenosine increased mean glucose levels by up to ∼27% (Fig. 2), and these concentrations were significantly greater than for vehicle infusion.
LACTATE.
Mean plasma lactate concentrations rose progressively to a peak increase of ∼90% by the end of the adenosine infusion, which was maintained through the 30-min recovery (Fig. 2).
A1 receptor blockade.
DPCPX or vehicle was infused intra-arterially in six fetuses that received simultaneously an intravenous infusion of adenosine. In vehicle studies, adenosine reduced mean pHa without altering PaCO2 and PaO2, as previously described, and DPCPX did not alter these effects.
INSULIN.
Adenosine with vehicle depressed insulin levels, although the decline did not reach statistical significance (Fig. 3A, Table 1). Insulin concentrations increased significantly after the adenosine infusion had been stopped (Fig. 3A). DPCPX did not significantly alter insulin responses to adenosine.
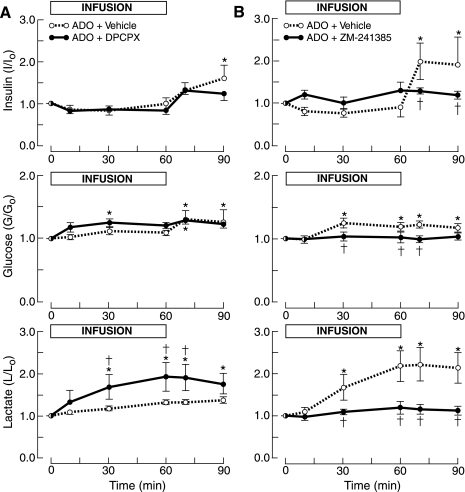
Fig. 3.
Fetal plasma concentration ratios of insulin, glucose, and lactate in response to simultaneous infusion of adenosine and either the A1 receptor (DPCPX) or A2A receptor antagonist (ZM-241385). A: infusion of adenosine with DPCPX or vehicle. B: infusion of adenosine with ZM-241385 or vehicle. *P <0.05 compared with time 0. †P < 0.05 compared with ADO + vehicle.
GLUCOSE.
In these experiments, the adenosine-induced increase in glycemia did not reach statistical significance, although post-infusion levels did so (Fig. 3A). DPCPX enhanced the glycemic response to adenosine, which resulted in a significantly greater proportional increase in glucose levels after 30 min of infusion compared with control, although this concentration did not differ significantly from that for adenosine with vehicle. But the AUC over the hour of infusion of adenosine with DPCPX was significantly greater than for adenosine with vehicle (Table 1).
LACTATE.
The A1 receptor antagonist significantly enhanced the lactatemia induced by adenosine, with lactate concentrations greater than both preinfusion values and time-matched levels for adenosine with vehicle infusion (Fig. 3A).
A2A receptor blockade.
Adenosine was infused to five fetuses that were simultaneously administered ZM-241385 or vehicle. Again, adenosine with vehicle induced a metabolic acidemia without altering Po2 or PaCO2. Mean pHa at 10 [7.330 ± 0.010, ZM-241385 (ZM); 7.302 ± 0.010, vehicle (V), P = 0.01] and 30 min (7.321 ± 0.015, ZM; 7.294 ± 0.008, V, P = 0.003) after the start of ZM-241385 infusion was significantly greater than the respective values for adenosine with vehicle, although the mean value at 60 min was not (7.301 ± 0.005, ZM; 7.278 ± 0.007, V, P = 0.21). PaO2 and PaCO2 were unchanged.
INSULIN.
The experiment-to-control concentration ratio for insulin declined during infusion of adenosine and vehicle, as described previously (Fig. 2), although this fall was not significant in these experiments (Fig. 3B, Table 1). The insulin concentration ratio also increased postinfusion.
A2A receptor antagonism blocked the adenosine-induced decline in the insulin concentration ratio, although this effect did not reach statistical significance (Table 1, Fig. 3B). ZM-241385 prevented the hyperinsulinemia that developed after the adenosine infusion had been terminated.
GLUCOSE.
The administration of adenosine with vehicle was associated with a significant rise in plasma glucose concentrations, as previously observed (Fig. 2), which was abolished by the A2A receptor antagonist (Fig. 3B, Table 1).
LACTATE.
ZM-241385 abolished the increase in lactate levels that were induced by adenosine (Fig. 3B, Table 1).
Hypoxia and Endogenous Adenosine
A1 receptor blockade.
Hypoxia was induced in six fetal sheep that were administered DPCPX or vehicle.
ARTERIAL BLOOD GASES AND PH.
Isocapnic hypoxia was virtually identical for vehicle and DPCPX, with mean PaO2 decreasing from the control of ∼23 Torr to ∼13 Torr. Mean arterial pH fell significantly from 7.331 ± 0.009 at time 0 to 7.118 ± 0.043 (P < 0.0003) after 60 min of O2 deprivation. Mean pH also fell progressively to 7.165 ± 0.026 after 60 min of hypoxia with DPCPX administration, a decline that did not differ significantly from that for vehicle.
INSULIN.
Hypoxia depressed the insulin concentration ratio by up to 40% (to 0.59 ± 0.08, P = 0.23), with a rise in recovery to levels that were twofold greater (P < 0.0002) than unity at time 0. DPCPX did not alter the hypoxia-induced depression of insulin levels (Table 2) or the posthypoxia rise.
Table 2.
Area under the curve for plasma insulin, glucose, and lactate concentrations with 1-h infusion of A1 receptor antagonist (DPCPX), A2A receptor blocker (ZM-241385), or vehicle in six hypoxic fetal sheep
| Insulin | Glucose | Lactate | |
|---|---|---|---|
| DPCPX | 41.7±2.1 | 98.9±9.5 | 251±24 |
| Vehicle | 42.6±3.3 | 123±10 | 253±46 |
| P value | 0.69 | 0.15 | 0.98 |
| ZM-241385 | 40.3±5.9 | 94.8±15.2 | 256±59 |
| Vehicle | 54.6±6.9 | 113±17.5 | 251±38 |
| P value | 0.27 | 0.02 | 0.90 |
Area under the curve is presented as concentration ratio·min.
GLUCOSE.
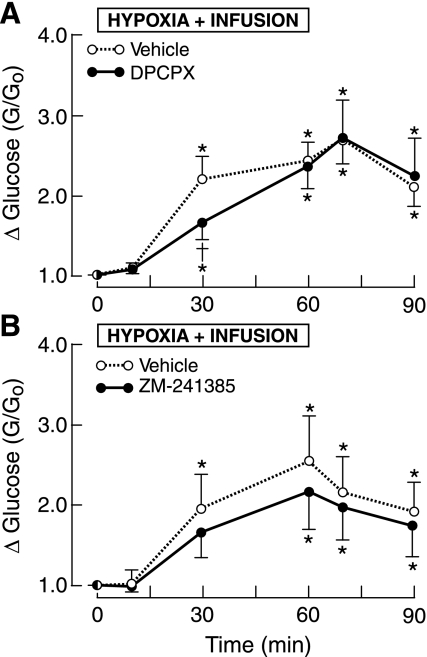
The mean ratio of plasma glucose levels increased two- to threefold in the last 30 min of O2 deficiency (Fig. 4A). DPCPX attenuated the hypoxia-induced rise in glucose at 30 min but not at 60 min. The difference in mean AUC for DPCPX did reach statistical significance (P = 0.15), as shown in Table 2.
Fig. 4.
Fetal plasma concentrations ratios for glucose in 1 h of hypoxia. A: infusion of A1 receptor antagonist or vehicle. B: infusion of A2A receptor antagonist or vehicle. *P < 0.05 compared with time 0. †P <0.05 compared with vehicle.
LACTATE.
Hypoxia increased the mean lactate concentration ratio by nearly eight-fold, and DPCPX did not alter this hyperlactatemia (Table 2).
A2A receptor blockade.
ZM-241385 or vehicle was administered to six fetuses in which isocapnic hypoxia (ΔPaO2, −10 Torr) had been induced for 60 min.
ARTERIAL BLOOD GASES AND PH.
ZM-241385 did not reduce the hypoxia-induced decline in mean arterial pH, which fell to levels similar to those for DPCPX experiments.
INSULIN.
ZM-241385 did not alter the ∼40% reduction in insulin levels that occurred in hypoxia with vehicle infusion.
GLUCOSE.
A2A receptor blockade attenuated the rise in the mean glucose concentration ratio throughout hypoxia, although statistical significance was not achieved for measurements at specific times (Fig. 4B). Analysis of the AUC, which better reflects the total glucose response, showed that ZM-241385 significantly blunted the hyperglycemia elicited by hypoxia (Table 2).
LACTATE.
ZM-241385 did not modify the hyperlactatemia of acute O2 deficiency, which increased ∼8-fold for experiments with the A2A receptor blocker or its vehicle.
DISCUSSION
This study was performed to determine the role of adenosine A1 and A2A receptors in regulating fetal plasma levels of insulin, glucose, and lactate. The results provide new information that 1) endogenous adenosine, via A1 receptors, tonically suppresses plasma concentrations of insulin, glucose, and lactate; 2) exogenous adenosine lowers insulin levels and raises glucose and lactate concentrations; 3) A1 receptors attenuate the hyperglycemia and hyperlactatemia elicited by exogenous adenosine; 4) A2A receptors promote the hypoinsulinemia, hyperglycemia, and hyperlactatemia triggered by exogenous adenosine; and 5) endogenous adenosine, via A1 and A2A receptors, contributes to hypoxia-induced hyperglycemia.
Insulin
Insulin enables glucose to have priority over other substrates for oxidative processes, and it drives oxygen and glucose utilization by fetal skeletal muscle without altering lactate production (18, 35). Insulin sensitivity to glucose in the fetus is the same as in the adult, but the equilibrium set-point is shifted to lower glucose concentrations that favor glucose transfer to the fetus (18).
Normoxia and endogenous ADO.
A1 receptor blockade increased circulating insulin levels, which is consistent with a tonic depression of insulin secretion by islet cells and/or an increase in insulin clearance. ZM-241385 did not alter insulin levels; thus, A2A receptors are not significant modulators of insulin levels under these conditions.
Normoxia and exogenous ADO.
In our previous work, similar rates of adenosine infusion increased fetal plasma epinephrine and norepinephrine concentrations, probably indirectly via stimulation of adenosine A2A receptors associated with activation of the carotid bodies and subsequent stimulation of adrenergic pathways (24, 25, 29). Sympathetic activity inhibits fetal insulin release through activation of α2-adrenergic receptors that modulate islet cell function (19). Thus, the adenosine-induced reduction in insulin levels in the present study is consistent with an A2A receptor-mediated activation of sympathoadrenal pathways, which, in turn, blunt insulin release (13, 22, 29), although other effects of A2A receptor activation cannot be excluded.
Hypoxia and endogenous ADO.
Low O2 tensions also inhibit fetal insulin release via hypoxia-induced rise in adrenergic activity (20, 22). Neither DPCPX nor ZM-241385 changed the depressant effects of hypoxia on plasma levels of insulin; thus, neither A1 nor A2A receptors are critical modulators of autonomic influences on insulin levels under these conditions.
Glucose
In sheep, glucose is almost entirely of endogenous origin, because the rumen converts dietary carbohydrates to short-chain fatty acids. As a result, maternal glucose and insulin levels remain relatively steady throughout the day (38). The placenta, a highly metabolically active exchange organ, facilitates glucose transfer from the maternal circulation to the fetus via glucose transporters (GLUT). The rate of glucose transfer to the fetus, which is 4–6 mg·min−1·kg fetal weight−1, increases about 10-fold over the last half of gestation. About 60% of the rise results from facilitated transfer capacity by the placenta with the balance due to lower fetal glucose concentrations caused by increased fetal insulin levels and sensitivity (16). In sheep, both umbilical glucose uptake and fetal growth diminish toward term in coincidence with the rise in cortisol that precedes parturition.
Fetal glycemia is totally dependent upon placental delivery, because virtually no glucose is released in the unstressed fetus until immediately before term (16). The relatively uniform plasma glucose and insulin concentrations in the fetal sheep reflect the virtual absence of prandial effects on maternal glucose levels. Besides placental glucose transfer, fetal plasma glucose concentrations are regulated by insulin release from pancreatic islets, glucose utilization, and hepatic storage, and glucose production. Overall, glucose accounts for ∼50% of fetal metabolic rate (16, 35), with the central nervous system (an insulin-independent organ) consuming up to 20% of the glucose utilized in sheep and up to 75% in human fetuses.
Normoxia and endogenous adenosine.
In the present study, endogenous adenosine, via A1 receptors, depressed fetal glucose concentrations, as shown by the rise in glycemia that was evident within 10 min after A1 receptor blockade was established. Over all, mean fetal glucose levels in the hour of normoxia with A1 receptor blockade increased by ∼14% over control values, which would have been even greater had insulin concentrations not also risen concomitantly by ∼22%. A1 receptors suppress glycemia by facilitating glucose deposition through enhancement of GLUT4 capacity and promotion of glucose utilization in adult tissues (1, 6, 14, 34, 53); and these mechanisms are likely involved in lowering fetal glucose concentrations.
The involvement of A1 receptors in glucose disposal has relevance to the net transfer of glucose across the placenta through its depressant effects on fetal glycemia. Assuming maternal levels remain constant, the lower fetal glucose levels mediated by A1 receptors increases the glucose concentration gradient across the placenta, which enhances glucose transfer from mother to fetus. The data from the present study suggest that fetal A1 receptors boost the rate of placental transfer and fetal utilization of glucose by ∼12% (17, 36). This is a conservative estimate because the rise in insulin levels with A1 receptor blockade blunted the increase in glycemia (20). These calculations assume a maternal arterial plasma glucose level of 3.89 mM (17, 36) and no other effects of DPCPX on placental glucose transport.
Glucose uptake by skeletal muscle is normally limited by insulin-stimulated glucose transport (9), a process that is enhanced by adenosine via sarcolemmal A1 receptors in active skeletal muscle in vitro (8, 14, 47, 50). Adenosine also promotes glucose transfer in resting skeletal muscle, although not all studies have demonstrated this effect (8, 14, 47, 50). Adenosine augments insulin-stimulated glucose transport in heart muscle and adipocytes (33), with an additional insulin-independent mechanism proposed for myocardium (1). In rodents, the effects of A1 receptor blockade on glucose uptake with a euglycemic hyperinsulinemic clamp can differ qualitatively and quantitatively in the liver, skeletal muscle, and adipose tissue (7). Nonselective blockade of adenosine receptors by caffeine reduces glucose uptake by 24–50% in sedentary and exercised human subjects under a hyperinsulinemic-euglycemic clamp (11, 46).
Normoxia and exogenous adenosine.
DPCPX augmented the hyperglycemic response to adenosine without reducing insulin levels, which is further evidence that A1 receptors are involved in whole-body glucose disposal in the fetus. Contrarily, ZM-241385 abolished adenosine-induced hyperglycemia, indicating involvement of A2A receptors. An indirect effect through stimulation of sympathetic activity and catecholamine release probably is involved in the first 10 min of adenosine infusion when epinephrine levels would be expected to approach threshold levels for raising fetal glucose concentrations (29, 40, 46).
Activation of A2A receptors suppresses macrophage production of tumor necrosis factor-α, a cytokine known to be involved glucose homeostasis (15). Further studies should be performed to explore whether the depressant effects of adenosine on cytokine release modulate fetal plasma glucose responses to hyper-adenosinemic states.
Exogenous adenosine has other noteworthy effects on fetal metabolism. For example, it decreases fetal O2 consumption via stimulation of A1 receptors (2, 23). Activation of central adenosine A2A receptors that inhibit fetal breathing activity also contributes to the reduction in O2 consumption (28).
Hypoxia and endogenous adenosine.
Hypoxia initially increases fetal glucose levels by reducing glucose consumption and by inhibiting insulin release, which results from an elevation in splanchnic nerve activity and plasma catecholamine concentrations. Subsequently, hepatic glycogenolysis contributes to the glycemia (22). Exogenous adenosine also elevates fetal glycemia, which raises the question as to whether high endogenous levels of adenosine associated with O2 deficiency are involved in hypoxia-induced hyperglycemia.
This study indicates that activation of both A1 and A2A receptors contribute to the hyperglycemia of acute O2 deprivation.
Lactate
Intracellular glucose metabolism includes storage as glycogen or fat, oxidation, and production of lactate. The placenta in early gestation (∼0.5 term) produces very little lactate; but, near term, about 30% of glucose uptake by the placenta is converted to lactate, of which 60% enters the fetal circulation (16). The high aerobic production of lactate by the placenta and fetal skeletal muscle results in much higher plasma lactate levels in the fetus than in the adult; and this lactate concentration difference persists across species (4, 16). Driving about 15–20% of total fetal metabolism, lactate is an important carbon source for metabolism in the heart, liver, and adipose tissue of the near-term fetus (16), and, in glucose-deficient states, lactate can substitute for glucose as a substrate for brain metabolism (49).
Normoxia and endogenous adenosine.
A1 receptor blockade significantly increased fetal lactate concentrations in the latter half of DPCPX administration, with maintenance of high levels in the recovery period. The delayed rise in lactate concentrations is consistent with an indirect depressant effect of A1 receptors through modulation of lactate production and/or metabolism.
Normoxia and exogenous adenosine.
Intravascular administration of adenosine greatly elevated lactate concentrations via activation of A2A receptors. The high lactatemia most likely resulted from enhanced glycolysis, although a decline in lactate uptake and metabolism cannot be excluded. The major sources of lactate (e.g., skeletal muscle, heart, liver, placenta) under these conditions have yet to be determined.
Hypoxia and endogenous adenosine.
Hypoxia elicits a dose-dependent rise in fetal lactate concentrations, when PaO2 declines by as little as 5 Torr (48). A major source of fetal lactate in normoxia, the placenta becomes an important site of lactate clearance from the fetal circulation in hypoxia (12, 19). In acute fetal O2 deprivation, hyperlactatemia arises from β-adrenergic stimulation of muscle glycogenolysis by catecholamines, as well as from direct effects of tissue hypoxia (22). Significant gluconeogenesis from lactate does not occur, although it may become a source of glucose upon depletion of hepatic glycogen stores (42).
Nonselective antagonism of adenosine receptors does not alter hypoxia-induced increases in lactate concentrations in fetal plasma (13). The present work with potent, highly selective and specific blockers of A1 and A2A receptors reveals that neither of these adenosine receptor subtypes is significantly involved in modulating fetal plasma lactate concentrations in acute O2 deprivation.
Limitations
We have restricted the study to the most commonly studied A1 and A2A receptor subtypes for which highly selective and specific antagonists are available (27). We have not examined the potential contribution of A2B or A3 receptors or excluded endocrine and metabolic effects that are secondary to perturbations in blood flow. As a regulator of adenylyl cyclase, adenosine could indirectly alter metabolism through effects on catecholamine release or other endocrine perturbations. In hypoxia, the relative contribution of adenosine to glycolysis and lactic acid production may depend on the extent of fetal oxygen availability; thus, further studies should investigate the potential metabolic effects of endogenous adenosine in graded hypoxia. Further studies should also be performed to determine the role of fetal adenosine receptors in the release and/or clearance of insulin, glucose, and lactate.
Although maternal levels of insulin, glucose, and lactate were not measured, the stable fetal concentrations in control experiments indicate that maternal metabolite levels did not significantly change over the experimental hour. Placental transfer of the adenosine receptor antagonists to the maternal circulation potentially could change maternal levels of insulin and metabolites that, in turn, could alter fetal concentrations. Such indirect, maternal effects on fetal measurements are very unlikely. Both the time required for placental passage and the dilution of transferred antagonist by larger maternal blood volume, which is ∼14-fold greater that of the fetus (3, 43), are not consistent with the rapid time course of changes in fetal glucose levels that occurred in these studies. The ovine placenta is effectively impenetrable to insulin.
Adenosine concentrations have been measured in fetal plasma and in brain extracellular fluid (26, 31) but not in the interstium of insulin-sensitive tissues. That adenosine levels are also elevated in the latter tissues is a reasonable assumption based on the low fetal Po2, a major factor regulating adenosine concentrations, and the high levels in plasma and the brain (26, 31, 44).
DPCPX has a high degree of selectivity (>500-fold) for A1 receptors relative to A2A and A3 receptors, with a moderate degree of selectivity relative to A2B receptors. ZM-241385, which has a high degree of affinity for A2A receptors, has moderate selectivity relative to A2B receptors and has little or no interaction with A1 and A3 receptors. The selectivity of adenosine receptor antagonists is species dependent, but our previous work indicates that DPCPX and ZM-241385 can effectively separate the physiological actions of A1 and A2A receptors in sheep but less so relative to A2B receptors (27, 28). As with other pharmacological studies, these experiments do not eliminate the possibility of nonspecific effects of these adenosine receptor antagonists.
In summary, endogenous adenosine via A1 receptors suppresses circulating concentrations of insulin, glucose, and lactate in normoxic fetal sheep. The lower glucose levels appear to reflect at least a 12% enhancement of placental transfer and fetal uptake. Exogenous adenosine increases plasma levels of glucose and lactate through activation of A2A receptors, effects that are apposed by A1 receptor-mediated mechanisms. Hypoxia-induced elevations in endogenous adenosine, via A1 and A2A receptors, help mediate the hyperglycemia of acute O2 deficiency. Thus, adenosine promotes fetal growth via A1 receptors and enhances the hyperglycemia of hypoxia by stimulation of A1 and A2A receptors.
GRANTS
This work was supported in part by National Institute of Child Health and Human Development Grant HD-18478.
Acknowledgments
The authors thank Mohammed Saad for performing the insulin assays and Fanor Bohorquez and Noune Aslanian for technical assistance.
Present address for T. Maeda: Kagoshima Municipal Hospital, Kagoshima, Japan; e-mail: ml.kch.Kagoshima.Kagoshima.jp.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Angello DA, Berne R, Coddington NM. Adenosine and insulin mediate glucose uptake in normoxic rat hearts by different mechanisms. Am J Physiol Heart Circ Physiol 265: H880–H885, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Blood AB, Hunter CJ, Power GG. Adenosine mediates decreased cerebral metabolic rate and increased cerebral blood flow during acute moderate hypoxia in the near-term fetal sheep. J Physiol 553: 935–945, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brace RA Blood volume and its measurement in the chronically catheterized sheep fetus. Am J Physiol Heart Circ Physiol 244: H487–H494, 1983. [DOI] [PubMed] [Google Scholar]
- 4.Carter BS, Moores Russell R Jr, Battaglia FC, Meschia G. Ovine fetal placental lactate exchange and decarboxylation at midgestation. Am J Physiol Endocrinol Metab 264: E221–E225, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Chau A, Rose JC, Koos BJ. Adenosine modulates corticotrophin and cortisol release during hypoxia in fetal sheep. Am J Obstet Gynecol 180: 1272–1277, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Cheng JT, Chi TC, Liu IM. Activation of adenosine A1 receptors by drugs to lower plasma glucose in streptozotocin-induced diabetic rats. Auton Neurosci 83: 127–133, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Crist G, Xu B, LaNoue K, Lang C. Tissue-specific effects of in vivo adenosine receptor blockade on glucose uptake in Zucker rats. FASEB J 12: 1301–1308, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Derave W, Hespel P. Role of adenosine in regulating glucose uptake during contractions and hypoxia in rat skeletal muscle. J Physiol 515: 255–263, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuerger PT, Shearer J, Bracy DP, Posey KA, Pencek RR, McGuinness OP, Wasserman DH. Control of muscle glucose uptake: test of the rate-limiting step paradigm in conscious, unrestrained mice. J Physiol 562: 925–935, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grden M, Podgorska M, Kocbuch K, Szutowicz A, Pawelczyk T. Expression of adenosine receptors in cardiac fibroblasts as a function of insulin and glucose level. Arch Biochem Biophys 455: 10–17, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Greer F, Hudson R, Ross R, Graham T. Caffeine ingestion decreases glucose disposal during a hyperinsulinemic-euglycemic clamp in sedentary humans. Diabetes 50: 2349–2354, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Gu W, Jones CT, Parer JT. Metabolic and cardiovascular effects on fetal sheep of sustained reduction in uterine blood flow. J Physiol 368: 109–129, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guissani DA, Gardner DS, Cox DT, Fletcher AJW. Purinergic contribution to circulatory, metabolic, and adrenergic responses to acute hypoxemia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 280: R678–R685, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Han DH, Hansen PA, Nolte LA, Holloszy JO. Removal of adenosine decreases the responsiveness of muscle glucose transport to insulin and contractions. Diabetes 47: 1671–1675, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Haskó G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Therapeut 113:264–275, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay WW Placental supply of energy and protein substrates to the fetus. Acta Paediatr Suppl 405:13–19, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Hay WW Recent observations on the regulation of fetal metabolism by glucose. J Physiol 572: 17–24, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hay WW, Sparks JW. Placental, fetal, and neonatal carbohydrate metabolism. Clin Obstet Gynecol 28: 473–485, 1985. [DOI] [PubMed] [Google Scholar]
- 19.Hooper SB, Walker DW, Harding R. Oxygen, glucose, and lactate uptake by fetus and placenta during prolonged hypoxemia. Am J Physiol Regul Integr Comp Physiol 268: R303–R309, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Jackson BT, Piasecki GJ, Cohen HE, Cohen WR. Control of fetal insulinsecretion. Am J Physiol Regul Integr Comp Physiol 279: R2179–R2188, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Johansson SM, Salehi A, Sandstrom ME, Westerblad H, Lundquist I, Carlsson PO, Fredholm BB, Katz A. A1 receptor deficiency causes increased insulin and glucagon secretion in mice. Biochem Pharmacol 74: 1628–35, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Jones CT, Roebuck MM, Walker DW, Johnston BM. The role of the adrenal medulla and peripheral sympathetic nerves in the physiological responses of the fetal sheep to hypoxia. J Dev Physiol 10: 17–36, 1988. [PubMed] [Google Scholar]
- 23.Karimi A, Ball KT, Power GG. Exogenous infusion of adenosine depresses whole body O2 use in fetal/neonatal sheep. J Appl Physiol 81: 541–547, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Koos BJ, Chao A, Doany W. Adenosine stimulates breathing in fetal sheep with brainstem section. J Appl Physiol 72: 94–99, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Koos BJ, Chau A, Ogunyemi D. Adenosine mediates metabolic and cardiovascular responses to hypoxia in fetal sheep. J Physiol 488: 761–766, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koos BJ, Doany W. Role of plasma adenosine in breathing responses to hypoxia in fetal sheep. J Dev Physiol 16: 81–85, 1991. [PubMed] [Google Scholar]
- 27.Koos BJ, Maeda T. Adenosine A2A receptors mediate cardiovascular responses to hypoxia in fetal sheep. Am J Physiol Heart Circ Physiol 280: H83–H89, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Koos BJ, Maeda T, Jan C, Lopez G. Adenosine A2A receptors mediate hypoxic inhibition of fetal breathing in sheep. Am J Obstet Gynecol 186: 663–668, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Koos BJ, Mason BA, Ducsay CA. Cardiovascular responses to adenosine in fetal sheep: autonomic blockade. Am J Physiol Heart Circ Physiol 264: H526–H532, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Koos BJ, Mason BA, Ervin MG. Adenosine mediates hypoxic release of arginine vasopressin in fetal sheep. Am J Physiol Regul Integr Comp Physiol 266: R215–R220, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Koos BJ, Mason B, Ogunyemi D, Punla O, Adinolfi A. Hypoxic inhibition of breathing in fetal sheep: relation to brain adenosine concentrations. J Appl Physiol 77: 2734–2739, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Koos BJ, Matsuda K. Fetal breathing, sleep state, and cardiovascular responses to adenosine in sheep. J Appl Physiol 68: 489–495, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Law WR, McLane MP. Adrenergic, insulin, and work interactions with adenosine's effects on in situ myocardial glucose uptake. Cardiovasc Res 31: 691–698, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Liu IM, Lai TY, Tsai CC, Cheng JT. Characterization of adenosine A1 receptors in cultured myoblast C2C12 cells of mice. Auton Neurosci 87: 59–64, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Milley JR Exogenous substrate uptake by fetal lambs during reduced glucose delivery. Am J Physiol Endocrinol Metab 264: E250–E256, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Molina R, Meschia G, Battaglia FA, Hay WW. Gestational maturation of placental glucose transfer capacity in sheep. Am J Physiol Regul Integr Comp Physiol 261: R697–R704, 1991. [DOI] [PubMed] [Google Scholar]
- 37.Munoz G, San Martin R, Farias M, Cea L, Vecchiola A, Casanello P, Sobrevia L. Insulin restores glucose inhibition of adenosine transport by increasing the expression and activity of the equilibrative nucleoside transporter 2 in human umbilical vein endothelium. J Cell Physiol 209: 826–835, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Murata S, Matsuda T, Kiguchi S, Kobayashi M, Cho K, Okuyama K. Effects of long-term administration of KUR-1246, a selective β2-adrenoceptor agonist, on pregnant sheep and their fetuses. BJOG 112: 69–74, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Ogunyemi DA, Koos BJ, Arora CP, Castro LC, Mason BA. Adenosine modulates hypoxia-induced atrial natriuretic peptide release in fetal sheep. Am J Physiol Heart Circ Physiol 269: H282–H287, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Padbury JF, Ludlow JK, Ervin MG, Jacobs HC, Humme JA. Thresholds for physiological effects of plasma catecholamines in fetal sheep. Am J Physiol Endocrinol Metab 252: E530–E537, 1987. [DOI] [PubMed] [Google Scholar]
- 41.Rivkees SA, Zhao Z, Porter G, Turner C. Influences of adenosine on the fetus and newborn. Mol Gen Metab 74: 160–171, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Rudolph CD, Roman C, Rudolph AM. Effect of acute umbilical cord compression on hepatic carbohydrate metabolism in the fetal lamb. Pediatr Res 25: 228–233, 1989. [DOI] [PubMed] [Google Scholar]
- 43.Rumball CWH, Bloomfield FH, Harding JE. Cardiovascular adaptations to pregnancy in sheep and effects of periconceptional undernutrition. Placenta 29: 89–94, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Sawa R, Asakura H, Power GG. Changes in plasma adenosine during simulated birth of fetal sheep. J Appl Physiol 70: 1524–1528, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Schulte G, Fredholm BB. Signaling from adenosine receptors to mitogen-activated protein kinases. Cell Signal 15: 813–827, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Thong FSL, Graham TE. The putative roles of adenosine in insulin- and exercise-mediated regulation of glucose transport and glycogen metabolism in skeletal muscle. J Appl Physiol 27: 152–178, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Thong FSL, Lally JSV, Dyck DJ, Greer F, Bonen A, Graham TE. Activation of the A1 adenosine receptor increases insulin-stimulated glucose transport in isolated rat soleus muscle. Appl Physiol Nutr Metab 32: 701–710, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Towell ME, Figueroa J, Markowitz S, Elias B, Nathanielsz P. The effect of mild hypoxemia maintained for twenty-four hours on maternal and fetal glucose, lactate, cortisol, and arginine vasopressin in pregnant sheep at 122–139 days' gestation. Am J Obstet Gynecol 157: 1550–1557, 1987. [DOI] [PubMed] [Google Scholar]
- 49.Turbow RM, Curran-Everett D, Hay WW Jr, Jones MD Jr. Cerebral lactate metabolism in near-term fetal sheep. Am J Physiol Regul Integr Comp Physiol 269: R938–R942, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Vergauwen L, Hespel P, Richter EA. Adenosine receptors mediate synergistic stimulation of glucose uptake and transport by insulin and by contractions in rat skeletal muscle. J Clin Invest 93: 974–981, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verspohl EJ, Johannwille B, Waheed A, Neye H. Effect of purinergic agonists and antagonists on insulin secretion from INS-1 cells (insulinoma cell line) and rat pancreatic islets. Can J Pharmacol 80: 562–568, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Weaver DR A2a adenosine receptor gene expression in developing rat brain. Brain Res Mol Brain Res 20: 313–327, 1993. [DOI] [PubMed] [Google Scholar]
- 53.Wyatt DA, Edmunds MC, Rubio R, Berne RM, Lasley RD, Mentzer RM Jr. Adenosine stimulates glycolytic flux in isolated perfused rat hearts by A1-adenosine receptors. Am J Physiol Heart Circ Physiol 257: H1952–H1957, 1989. [DOI] [PubMed] [Google Scholar]