Abstract
G protein-coupled receptors that signal bitter taste (T2Rs) are expressed in the mucosal lining of the oral cavity and gastrointestinal (GI) tract. In mice, intragastric infusion of T2R ligands activates Fos expression within the caudal viscerosensory portion of the nucleus of the solitary tract (NTS) through a vagal pathway (Hao S, Sternini C, Raybould HE. Am J Physiol Regul Integr Comp Physiol 294: R33–R38, 2008). The present study was performed in rats to further characterize the distribution and chemical phenotypes of brain stem and forebrain neurons activated to express Fos after intragastric gavage of T2R ligands, and to determine a potential behavioral correlate of this central neural activation. Compared with relatively low brain stem and forebrain Fos expression in control rats gavaged intragastrically with water, rats gavaged intragastrically with T2R ligands displayed significantly increased activation of neurons within the caudal medial (visceral) NTS and caudal ventrolateral medulla, including noradrenergic neurons, and within the lateral parabrachial nucleus, central nucleus of the amygdala, and paraventricular nucleus of the hypothalamus. A behavioral correlate of this Fos activation was evidenced when rats avoided consuming flavors that previously were paired with intragastric gavage of T2R ligands. While unconditioned aversive responses to bitter tastants in the oral cavity are often sufficient to inhibit further consumption, a second line of defense may be provided postingestively by ligand-induced signaling at GI T2Rs that signal the brain via vagal sensory inputs to the caudal medulla.
Keywords: brain stem, denatonium, forebrain, nucleus of the solitary tract
specific sensors within the mucosal lining of the oral cavity and digestive tract play a critical role in the regulation of ingestive and digestive functions, and recent findings suggest that chemosensing in the gut involves at least some of the same signaling molecules that mediate taste in the oral cavity (26). For example, receptors involved in sensing bitter taste (T2Rs) that are expressed in the tongue and palate epithelium (4, 13) are also present in the human and rodent gastrointestinal (GI) tract, along with the functionally linked α-subunit of the taste-specific G protein gustducin (Gαgust) (11, 23, 28, 34, 35). T2Rs comprise a relatively large family of G protein-coupled receptors coded for by 33 and 36 intact genes in humans and rodents, respectively (18, 35). The presence of transcripts corresponding to specific T2Rs that may be activated by the bitter tastants denatonium benzoate (DB) and phenylthiocarbamide (PTC) has been demonstrated in mouse and rat upper GI tract and in a mouse GI enteroendocrine cell line, STC-1 (35). Application of DB, PTC, and other T2R ligands induces rapid Ca2+ signaling in STC-1 cells (5, 35), evidence that the expressed T2Rs are functional. Indeed, DB induces release of cholecystokinin (CCK) from STC-1 cells (5).
The physiological role of putative bitter taste sensors located distal to the oral cavity is not yet clear, but the gastric and duodenal mucosal localization of T2R mRNA (34, 35) makes GI T2Rs particularly well positioned to detect bitter tastants within recently consumed ingesta. We have reported (10) that intragastric gavage of T2R ligands in mice activates neural expression of the immediate-early gene product Fos in the caudal viscerosensory portion of the nucleus of the solitary tract (NTS) and that the Fos response to intragastric T2R ligands is blocked by prior subdiaphragmatic vagotomy. The NTS Fos response to DB gavage also was abolished by prior administration of CCK1 or Y2 receptor antagonists and was absent in CCK1 receptor-knockout mice (10). In rats, gastric infusion of DB increases vagal nerve activity (32) and delays gastric emptying (9). These findings support the view that stimulation of at least a subset of T2Rs in the upper GI tract recruits local receptor signaling pathways, and that the effects are likely transmitted from GI tract to caudal medulla via the vagus nerve.
If natural T2R ligands that are present in swallowed food or fluid can signal the brain via vagal sensory pathways from the gut, how might that sensory information be used, and what additional brain areas might be involved? The perception of bitter taste subsequent to stimulation of T2Rs expressed in the oral cavity is thought to be important for the detection of toxins in food before it is swallowed, because many naturally occurring plant toxins are bitter substances that stimulate T2Rs (9). Similarly, stimulation of T2Rs in the lumen of the GI tract may condition future avoidance of similar ingesta through the process of conditioned flavor avoidance (CFA), thus providing an additional line of defense against potentially toxic compounds. In this regard, Glendinning et al. (9) recently reported that a repeated intragastric infusion of the T2R ligand DB could support a CFA response in rats and mice. In addition, stimulation of gut T2Rs may activate reflexive changes in GI function that contribute to reducing further intake of possibly toxic material and/or limiting its spread from the upper GI tract to more distal absorptive gut regions by decreasing food intake and delaying gastric emptying. The present study was performed in rats to study the effects of intragastric gavage of a mixture of T2Rs ligands, DB alone, or water alone (control treatment) on central patterns of neuronal activation assessed by immunocytochemical localization of the immediate-early gene product Fos. Brain regions targeted for Fos analysis included those that are known to receive and process GI vagal sensory signals, i.e., the caudal medial (visceral) NTS, area postrema (AP), ventrolateral medulla (VLM), lateral parabrachial nucleus (PBN), paraventricular nucleus of the hypothalamus (PVN), and central nucleus of the amygdala (CeA). We further characterized gavage-induced recruitment of identified noradrenergic (NA) neurons within the NTS and VLM, because these neurons are known to convey visceral sensory information to the hypothalamus and limbic forebrain (14, 15, 20, 21). Finally, given the reported ability of intragastric DB to support CFA in rats and mice after repeated exposure (9), and given the known participation of the aforementioned brain regions in CFA learning and expression (20, 21, 25, 30), we also examined the ability of intragastric DB and T2R ligand mixture to support CFA in rats, using a one-trial conditioning and testing paradigm.
MATERIALS AND METHODS
Animals
Experiments were performed with adult male Sprague-Dawley rats (Harlan, San Diego, CA; 180–200 g body wt; n = 15 rats for experiment 1, n = 24 rats for experiment 2). Rats were housed in a controlled environment (20–22°C; 12:12-h light-dark cycle, lights off at 7:00 PM) with ad libitum access to water and pelleted chow (Purina Rat Chow 5012, Ralston, St. Louis, MO), except as noted. All experiments were reviewed and approved by the University of California Davis Institutional Animal Care and Use Committee.
Experiment 1: Central Fos Expression After Intragastric Gavage of T2R Ligands
One cohort of rats (n = 15) was used to examine central Fos expression after intragastric gavage of T2R ligands. Rats were acclimated for 1 wk to daily handling and intragastric water gavage (1.0 ml, once per day for 2 days) before beginning the experiment. After a 15-h overnight fast, awake rats were gavaged at 9:00 AM with either 1.0 ml of double-distilled H2O (ddH2O, control; n = 5), 1.0 ml of DB alone (10 mM; n = 5), or 1.0 ml of a mixture of T2R ligands (in mM: 10 DB, 10 PTC, 5 6-propyl-2-thiouracil, 5 quinine, and 5 d-[−]salicin; n = 5), as in our previous study in mice (10). The mixture was used to stimulate multiple T2R subtypes and maximize the possibility of observing an experimental effect. The individual T2R agonist DB was used to compare its effects on Fos activation (and CFA, see below) with the effects of the T2R mixture. DB and other T2R ligands were purchased from Sigma-Aldrich (St. Louis, MO). Doses were chosen based on prior studies in rodents (3, 10) and the STC-1 enteroendocrine cell line (5).
To ensure that intragastric gavage procedures were effective and uniform, one well-trained experimenter gavaged all animals in this report. Each gavage took 1–2 min. A standard ball-tipped rat intragastric gavage needle (9.5 cm long, Biomedical Research Instruments, Rockville, MD) was passed periorally (PO) to deliver infusate directly to the lumen of the upper GI tract. Care was taken to ensure that there was no fluid on the tip or the outside of the gavage needle before PO passage in order to minimize potential exposure of the oral cavity to the GI infusate.
After intragastric gavage, rats were left undisturbed in their home cages with access to water but not food for 2 h and then deeply anesthetized with pentobarbital sodium (Nembutal, 100 mg/kg body wt ip; Western Medical Supply, Arcadia, CA) and transcardially perfused with 100 ml of heparinized saline (0.1 ml heparin/100 ml 0.9% NaCl) followed by 400 ml of buffer (0.1 M sodium phosphate, pH 7.4) containing 4% paraformaldehyde (Sigma-Aldrich). The 2-h postgavage survival time was based on our previous study in mice (10), and on evidence that stimulus-induced Fos expression typically peaks within 45–60 min after significant neural stimulation and remains elevated at peak levels for an additional 90–120 min before declining back toward baseline.
Histology and immunocytochemistry.
Fixed brains were removed from the skull, postfixed in 4% paraformaldehyde for 24 h at 4°C, and then cryoprotected in 20% sucrose solution. Coronal 35-μm-thick tissue sections were cut from the caudal extent of the medullary dorsal vagal complex through the rostral extent of the corpus callosum with a freezing stage microtome. Sections were collected serially in six adjacent sets and stored at −20°C in cryoprotectant (33). Before immunocytochemical procedures were initiated, sections were removed from cold storage, rinsed for 1 h in buffer (0.1 M sodium phosphate, pH 7.4), immersed for 30 min in buffer containing 1% sodium borohydride, rinsed, immersed for 10 min in buffer containing 0.3% hydrogen peroxide, and rinsed again.
Primary and secondary antisera were diluted in buffer containing 0.3% Triton X-100 (Sigma-Aldrich) and 1% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA). Two adjacent sets of tissue sections from each rat were processed for immunocytochemical localization of nuclear Fos protein with a rabbit polyclonal antiserum (1:50,000; provided by Dr. Philip Larsen, Rheoscience, Ledoeje, Denmark), biotinylated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch), and Vectastain Elite ABC immunoperoxidase reagents (Vector Laboratories, Burlingame, CA). The specificity of the primary antibody for Fos has been reported (22). Sections were reacted with nickel sulfate-intensified diaminobenzidine (DAB) to generate a blue-black nuclear product in Fos-positive cells. One set of Fos-labeled tissue sections was subsequently processed for dual immunoperoxidase localization of the NA synthetic enzyme dopamine β-hydroxylase (DβH) with a monoclonal anti-DβH antibody (1:30,000; Millipore, Billerica, MA) and biotinylated donkey anti-mouse IgG (1:500; Jackson ImmunoResearch). The second set of Fos-reacted sections was processed for dual cytoplasmic localization of calcitonin gene-related peptide (CGRP) with a polyclonal rabbit anti-CGRP antiserum (1:50,000; Peninsula Laboratories, Belmont, CA) and biotinylated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch). DβH or CGRP immunolabeling was visualized by using a nonintensified DAB reaction to generate a brown cytoplasmic reaction product. After dual immunoperoxidase labeling, tissue sections were mounted onto Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA), allowed to dry overnight, dehydrated and defatted in graded ethanols and xylene, and coverslipped with Cytoseal 60 (VWR International, West Chester, PA).
Quantification of neural labeling.
Dual immunoperoxidase-labeled tissue sections were analyzed with a light microscope to determine the number of Fos-positive neurons within brain stem and forebrain regions of interest, including the caudal medial (visceral) NTS, caudal VLM, lateral PBN, central CeA, and PVN. Sections through the brain stem and forebrain (spaced by 240 μm within each set) were viewed with 20–40× microscope objectives to identify labeled neurons. The distribution of DβH or CGRP labeling helped guide the analysis by demarcating neuroanatomic boundaries (15, 20). Cells within each region of interest were counted as Fos-positive if their nucleus displayed visible blue-black immunolabeling, regardless of labeling intensity. Criteria for counting NTS or VLM neurons as DβH-positive (i.e., noradrenergic) included the presence of brown cytoplasmic immunoreactivity surrounding a visible nucleus that was Fos-positive (in double-labeled cells) or was unlabeled. In each rat, cell count data for each region of interest were obtained bilaterally and divided by the number of tissue sections analyzed through that region.
Fos-positive NTS neurons and double-labeled DβH/Fos-positive NTS and VLM neurons were counted according to three rostrocaudal medullary levels defined with respect to the location of the AP: 1) sections caudal to the AP [caudal (c)NTS and cVLM, respectively]; 2) sections through the level of the AP [middle (m)NTS and mVLM, respectively]; and 3) sections rostral to the AP [rostral (r)NTS and rVLM, respectively]. Counts made within the rNTS and rVLM were discontinued at the rostral level at which the NTS moves laterally away from the floor of the fourth ventricle. Within each rat, counts of Fos-positive neurons in the NTS and counts of DβH/Fos-positive neurons in the NTS and VLM were summed at each rostrocaudal level and then divided by the number of sections analyzed to obtain mean counts per section at each level. Fos-positive neurons in the lateral PBN, CeA, and PVN were counted by using both sets of dual immunoperoxidase-labeled tissue sections in order to ensure that counting was performed in similar regions across animals that were defined by the presence of DβH and/or CGRP labeling.
Data analysis.
Cell count data from individual animals were combined within each gavage treatment group to obtain group mean (±SE) counts per section for each brain region of interest. Within each brain region, one- or two-way ANOVAs were used for statistical comparisons of cell count values. Independent variables for each ANOVA included gavage condition (i.e., water, DB, or T2R ligand mixture) and, for NTS and VLM, rostrocaudal level. When ANOVA F-values revealed significant effects of (or interactions among) independent variables, ANOVAs were followed up with post hoc least significant difference (LSD) t-tests. Differences revealed in ANOVA and post hoc tests were considered statistically significant when P < 0.05.
Experiment 2: Conditioned Flavor Avoidance After Intragastric Gavage of T2R Ligands
A sensitive, two-bottle choice paradigm (7, 20) was used to determine whether intragastric gavage of either DB alone or a mixture of T2R ligands induces conditioned avoidance of associated oral flavors. Flavor exposure during CFA training and testing was conducted during the light cycle of the photoperiod, between 1:00 and 3:00 PM. Rats were acclimated to intragastric water gavage (1.0 ml, once per day for 2 days) before beginning the CFA experiment. Rats also were acclimated to one trial of overnight water deprivation followed by morning access to regular drinking water in the same type of graduated drinking cylinder to be used in the CFA experiment. Food was available ad libitum throughout the experiment.
Two cohorts of rats (n = 12/cohort) were used in CFA experiments. The first cohort was used in two separate experiments, with a different pair of novel flavors used in each. The second cohort was used for a third control CFA experiment. Paired flavors included vanilla vs. almond extract (0.5% in water; McCormick, Hunt Valley, MD) for the first CFA experiment in the first cohort, coconut vs. banana extract (0.5% in water; McCormick) for the second CFA experiment in the first cohort, and vanilla vs. almond extract for the control experiment in the second cohort. Preliminary testing in other animals confirmed that flavors within each pair were isopreferred by rats before conditioning; that is, when water-deprived rats were given 30 min access to a two-bottle choice of each pair of novel flavors, cumulative intakes from each bottle did not differ significantly (data not shown).
The first cohort of rats underwent 22-h water deprivation beginning at 3:00 PM. On the following day, half of the group (n = 6) was given 30-min access to a single bottle containing novel almond-flavored water, while the other half (n = 6) was given access to a single bottle containing novel vanilla-flavored water. The left-right position of the drinking bottle on each cage was switched after 15 min, with cumulative intakes recorded after 30 min. Thirty minutes after the end of the novel flavor exposure session, all rats were gavaged intragastrically with 1.0 ml of deionized water. Bottles containing regular drinking water were returned 30 min after gavage, and rats had ad libitum water access for the next 24 h. Rats were then water deprived again for 22 h beginning at 3:00 PM, after which each rat received access to a single bottle of water containing the alternate novel flavor to drink for 30 min (e.g., vanilla for rats that consumed almond in the first exposure session or almond for rats that first consumed vanilla), with bottle positions switched after 15 min and cumulative intakes recorded at 30 min. Thirty minutes after the end of this second flavor exposure, all rats were gavaged intragastrically with 1.0 ml of the same T2R ligand mixture used in the Fos experiment (i.e., in mM: 10 DB, 10 PTC, 5 6-propyl-2-thiouracil, 5 quinine, and 5 d-[−]salicin). Plain drinking water was returned 30 min after gavage, to which rats had ad libitum access for the next 24 h.
Before the two-bottle choice test, rats were water deprived for 22 h beginning at 3:00 PM and then were given 30 min of simultaneous access to two drinking bottles, one bottle containing the flavor previously paired with intragastric water gavage (either almond or vanilla) and the other containing the flavor previously paired with intragastric gavage of T2R ligands. Bottle positions on each cage were switched after 15 min, and cumulative intake from each bottle was recorded at 30 min. Rats were then returned to ad libitum access to regular drinking water for 72 h before the next experiment was initiated.
The general protocol described above for one-bottle novel flavor exposure and subsequent two-bottle choice testing was repeated in a second experiment using the first cohort of rats and in a third control experiment using the second cohort of rats (described further below). In the first cohort of rats, the second experiment evaluated the ability of intragastric gavage of DB alone (10 mM; 1.0 ml in water) to induce CFA, using novel banana vs. coconut flavor extracts paired with gavage of either DB or water. The second cohort of rats was used in a control experiment to reveal whether brief oral exposure to T2R agonists (e.g., as might occur during PO passage of the gavage needle in other rats) is sufficiently aversive to support CFA, with almond vs. vanilla flavor extracts. In this experiment, a Q-tip dipped either in ddH2O (control) or the T2R agonist mixture was swabbed on the back portion of the rat's tongue.
Data analysis.
Flavor preferences displayed by each rat during two-bottle choice tests were determined by dividing the volume consumed from each drinking bottle by the total volume consumed from both bottles during the 30-min test. Individual preference ratios were combined within each experiment to obtain group preference ratios (means ± SE) for each treatment-flavor pairing condition. Outcomes indicating group mean flavor preference ratios that did not differ significantly from each other within an experiment (i.e., close to 50%:50%) were interpreted as an absence of CFA, whereas outcomes indicating significantly shifted preference ratios (e.g., 70%:30%) were interpreted as evidence for conditioned avoidance of the flavor represented by the lower value in the ratio. Paired Student's t-tests were used to determine whether preference ratios were significantly different (P < 0.05).
RESULTS
Experiment 1: Central Fos Expression After Intragastric Gavage of T2R Ligands
Dorsal vagal complex.
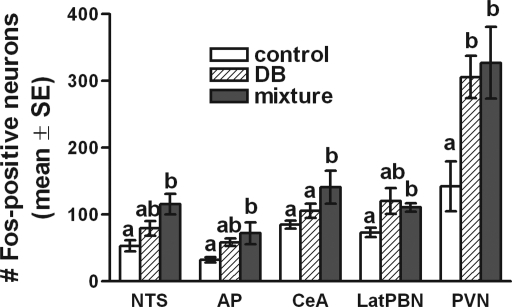
Little or no Fos labeling was observed within the dorsal motor nucleus of the vagus (Fig. 1) or within the rostral (taste) area of the NTS (not shown) in rats from any of the three gavage treatment groups. ANOVA revealed a nearly significant main effect of treatment on Fos labeling within the AP [F(2,12) = 3.82, P = 0.052]. Rats gavaged with DB alone or with the T2R ligand mixture displayed ∼80% and ∼120% more Fos-positive neurons within the AP, respectively, compared with AP Fos counts in water-gavaged controls (Fig. 2). Although the overall effect of treatment group on AP Fos labeling was not quite significant by ANOVA, post hoc LSD comparisons revealed that AP Fos labeling in rats gavaged with the T2R ligand mixture was significantly greater than in water-gavaged control rats (P = 0.019). Other between-group differences in AP Fos labeling were not significant.
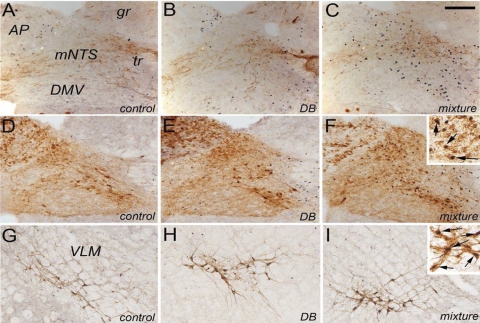
Fig. 1.
Representative color photomicrographs depicting dual immunolabeling for Fos (black nuclear label) and either calcitonin gene-related peptide (CGRP; A–C) or dopamine β-hydroxylase (DβH; D–F) (brown cytoplasmic label) within the medial caudal visceral nucleus of the solitary tract (NTS) and Fos + DβH immunolabeling within the ventrolateral medulla (VLM) (G–I) in rats after gavage of water (control), denatonium benzoate (DB) alone, or a mixture of bitter taste receptor (T2R) ligands (mixture). Neuronal Fos labeling within the area postrema (AP), caudal visceral NTS, and VLM appeared greatest in rats gavaged intragastrically with the T2R agonist mixture. Quantitative data are provided in Figs. 2 and 3 and in Table 1. Insets in F and I provide higher-magnification views of double-labeled (i.e., Fos-positive noradrenergic) neurons (arrows). DMV, dorsal motor nucleus of the vagus; gr, gracile nucleus; mNTS, medial subnucleus of the solitary tract; tr, tractus solitarius. Scale bar = 100 μm, A–I.
Fig. 2.
Effects of intragastric DB and T2R agonist mixture on the number of Fos-positive neurons counted within the caudal visceral NTS (collapsed across rostrocaudal levels), AP, central nucleus of amygdala (CeA), lateral (Lat) parabrachial nucleus (PBN), and paraventricular nucleus of hypothalamus (PVN). For each region, bars represent the average number of Fos-positive neurons per tissue section within each treatment group (means + SE; n = 5/group). Within each region, bars with different letters are significantly different from each other (P < 0.05); bars that share a letter are not different.
ANOVA revealed a significant main effect of intragastric gavage condition on Fos labeling within the caudal medial (visceral) NTS [F(2,12) = 6.78; P = 0.011]. Compared with counts of Fos-positive NTS neurons (averaged across all 3 rostrocaudal levels of the caudal visceral NTS) in control rats, rats gavaged with the T2R ligand mixture displayed significantly more Fos labeling (P = 0.003; Fig. 2). Conversely, NTS Fos labeling in rats gavaged with DB alone did not differ from that in control rats (Fig. 2). Although DB gavage tended to increase the number of Fos-positive neurons in the NTS compared with the control condition (Fig. 2), this increase was not statistically significant (P = 0.148). Conversely, intragastric gavage of the T2R ligand mixture increased the number of Fos-positive NTS neurons by ∼107% compared with water-gavaged control rats, and this increase was significant (P = 0.003; Fig. 2). The difference in NTS Fos labeling after gavage with DB alone compared with the T2R ligand mixture approached but did not quite reach statistical significance (P = 0.056; Fig. 2).
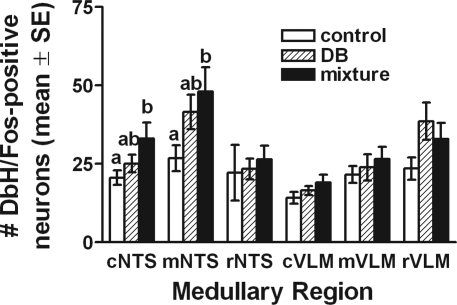
We further explored the effect of intragastric gavage on Fos expression across three rostrocaudal levels of the caudal (visceral) NTS (Table 1). Two-way ANOVA revealed significant main effects of gavage treatment group [F(2,12) = 8.7, P = 0.005] and rostrocaudal level [F(2,24) = 8.3, P = 0.002] on NTS Fos expression but no interaction between treatment group and rostrocaudal level. Post hoc tests revealed that Fos labeling was significantly elevated by >100% at all three rostrocaudal NTS levels in rats gavaged with the T2R agonist mixture compared with Fos labeling in water-gavaged control rats (P < 0.01 at each level; Table 1). Conversely, in rats gavaged with DB alone, Fos levels were significantly greater than in control rats only at the rostrocaudal level of the AP (mNTS, P = 0.037; Table 1). Fos labeling in rats gavaged either with DB alone or with the T2R agonist mixture was statistically similar at caudal and middle levels of the visceral NTS (cNTS, mNTS) but was significantly greater at rostral levels of the visceral NTS (rNTS) in T2R mixture-gavaged rats (P = 0.006; Table 1). However, as mentioned above, little or no Fos expression was observed within the more rostral (taste) region of the NTS (data not shown).
Table 1.
Fos expression and DβH and Fos dual immunoreactivity in rats after intragastric gavage of water (control), DB, or T2R agonist mixture
| Medullary Region |
Fos Immunoreactivity, Fos/section |
DβH and Fos Dual Immunoreactivity, DβH+Fos/section
|
||||
|---|---|---|---|---|---|---|
| Control | DB | Agonist mixture | Control | DB | Agonist mixture | |
| NTS | 22.5±2.2 | 29.1±2.9 | 34.0±5.0* | |||
| cNTS | 41.6±8.1 | 69.6±11.2 | 97.9±13.7* | |||
| mNTS | 54.2±7.9 | 92.6±11.8* | 113.2±14.1* | |||
| rNTS | 70.6±13.3 | 80.6±12.3 | 145.7±20.5*† | |||
| VLM | 19.4±2.3 | 24.8±2.5 | 25.5±3.5 | |||
Values are means ± SE for n = 5 rats/gavage treatment group. Values for Fos immunoreactivity are shown for 3 rostrocaudal levels of caudal visceral nucleus of the solitary tract (NTS): caudal (c)NTS, middle (m)NTS, rostral (r)NTS. Collapsed values for Fos immunoreactivity across rostrocaudal NTS levels are shown in Fig. 2. Values for dual immunoreactivity are collapsed across rostrocaudal levels and summarize the number of activated NTS and ventrolateral medulla (VLM) neurons identified as noradrenergic [i.e., Fos and dopamine β-hydroxylase (DβH)-positive]. See Fig. 3 for a breakdown of noradrenergic NTS and VLM activation by rostrocaudal level. DB, denatonium benzoate; T2R, bitter taste receptor.
P < 0.05 compared with control;
P < 0.05 compared with DB.
Activation of noradrenergic neurons.
Many Fos-positive neurons at each rostrocaudal level of the NTS were noradrenergic, as evidenced by cytoplasmic DβH immunolabeling (see Fig. 1). Statistical comparisons of the number of double-labeled NTS neurons (i.e., Fos + DβH positive) by two-way ANOVA revealed significant main effects of gavage treatment group [F(1,12) = 185.4, P < 0.001] and rostrocaudal NTS level [F(2,24) = 8.7, P = 0.001] on the number of activated neurons but no significant interaction between treatment group and rostrocaudal level. Post hoc tests revealed that within the caudal and middle NTS, the number of activated DβH-positive neurons was significantly greater in rats gavaged with the T2R ligand mixture compared with activation in water-gavaged control rats (P < 0.05 each for cNTS and mNTS; Fig. 3). There were no significant group differences within the rostral portion of the visceral NTS (rNTS) and no significant differences at any rostrocaudal level between DB-gavaged rats and control rats or between DB and T2R agonist mixture-gavaged groups (Fig. 3). When counts of activated NA NTS neurons were collapsed across rostrocaudal levels, rats gavaged with the T2R agonist mixture displayed significantly greater activation compared with water-gavaged control rats (i.e., 51% more; Table 1). NTS NA activation in DB-gavaged rats was 29% higher than in control rats, but this increase was not statistically significant (Table 1).
Fig. 3.
Effects of intragastric DB and T2R agonist mixture on activation of Fos labeling within DβH-immunopositive noradrenergic neurons at each of 3 rostrocaudal levels of the caudal visceral NTS and VLM (c, caudal; m, middle; r, rostral). Bars represent group mean ± SE (n = 5/group) number of double-labeled neurons counted per section. Within each region, bars with different letters are significantly different from each other (P < 0.05); bars that share a letter or have no letter are not different.
The number of activated NA neurons (i.e., Fos + DβH-positive) across three rostrocaudal levels of the VLM was assessed in the same tissue sections analyzed for NTS immunolabeling. Two-way ANOVA revealed significant main effects of gavage treatment group [F(1,12) = 166.21, P < 0.001] and rostrocaudal level [F(2,24) = 31.96, P < 0.001] on the number of activated NA VLM neurons and a nearly significant interaction between treatment group and rostrocaudal level [F(4,24) = 2.64, P = 0.059]. However, post hoc LSD tests failed to reveal statistically significant differences between gavage treatment groups in the number of double-labeled VLM neurons at any of the three rostrocaudal levels (Fig. 3). When counts of activated NA VLM neurons were collapsed across the three rostrocaudal levels, rats gavaged with the T2R agonist mixture displayed 31% more activation compared with water-gavaged control rats (Table 1), and DB-gavaged rats displayed 28% more activation of NA VLM neurons compared with control rats. However, these group differences were not statistically significant (Table 1).
Lateral parabrachial nucleus.
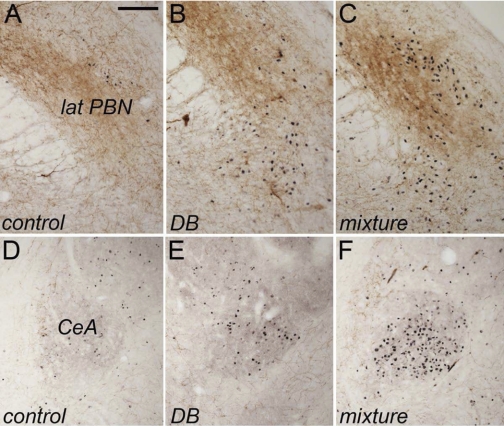
Control rats gavaged intragastrically with water displayed an average of 73.3 ± 6.8 Fos-positive neurons per section bilaterally through the region of the lateral PBN (Figs. 2, 4) that was demarcated by both DβH and CGRP immunolabeling. ANOVA revealed a significant main effect of treatment on the number of Fos-positive neurons within the lateral PBN [F(2,13) = 7.33, P = 0.009]. Rats gavaged with DB alone or with the T2R ligand mixture displayed increases of ∼64% and ∼51%, respectively, in Fos labeling compared with PBN Fos counts in water-gavaged control rats (Figs. 2, 4). Although the average increase in PBN Fos labeling was slightly higher in DB-gavaged rats compared with mixture-gavaged rats (Fig. 2), variability in the data made the increase after DB gavage nonsignificant compared with control rats, although the increase after T2R ligand mixture gavage was significant (P = 0.003; Fig. 2). Fos expression within the lateral PBN did not differ significantly between rats gavaged with DB versus the T2R mixture (Fig. 2). Little or no Fos expression was observed within the medial PBN, which receives oral taste-related signals relayed from more rostral regions of the NTS (data not shown).
Fig. 4.
Representative color photomicrographs depicting dual immunolabeling for Fos (black nuclear label) and DβH (brown axonal and terminal label) within the lateral PBN (A–C) and CeA (D–F) in rats after intragastric gavage of water (control), DB alone, or a mixture of T2R ligands. Neuronal Fos labeling within both regions appeared greatest in rats gavaged intragastrically with the T2R agonist mixture. Quantitative data are provided in Fig. 2. Scale bar = 100 μm, A–F.
Central nucleus of amygdala.
Control rats gavaged intragastrically with water displayed an average of 84.7 ± 6 Fos-positive neurons per section bilaterally through the region of the CeA (Figs. 2, 4) demarcated by DβH (Fig. 4) and CGRP immunolabeling (not shown). ANOVA revealed a significant main effect of treatment on the number of Fos-positive CeA neurons [F(2,14) = 12.7, P = 0.001; Fig. 2]. After intragastric gavage with DB alone, CeA Fos labeling was increased by ∼25% compared with labeling in control rats, but this increase was not significant (Fig. 2). Conversely, after gavage with the T2R ligand mixture, CeA Fos labeling was increased by ∼66% compared with control rats and by ∼34% compared with DB-gavaged rats, and these increases were significant (P = 0.001 for each comparison; Fig. 2).
Paraventricular nucleus of hypothalamus.
Control rats gavaged intragastrically with water displayed an average of 142.6 ± 37.3 Fos-positive PVN neurons per section (Figs. 2, 5). ANOVA revealed a significant main effect of treatment on the number of Fos-positive PVN neurons [F(2, 14) = 5.81, P = 0.017; Fig. 2]. After intragastric gavage with DB alone, PVN Fos labeling was increased by ∼114% compared with labeling in control rats (P = 0.017; Fig. 2). After gavage with the T2R ligand mixture, PVN Fos labeling was increased by ∼129% compared with control rats (P = 0.009; Fig. 2). PVN Fos expression did not differ significantly between the two ligand-gavaged groups (Fig. 2).
Fig. 5.
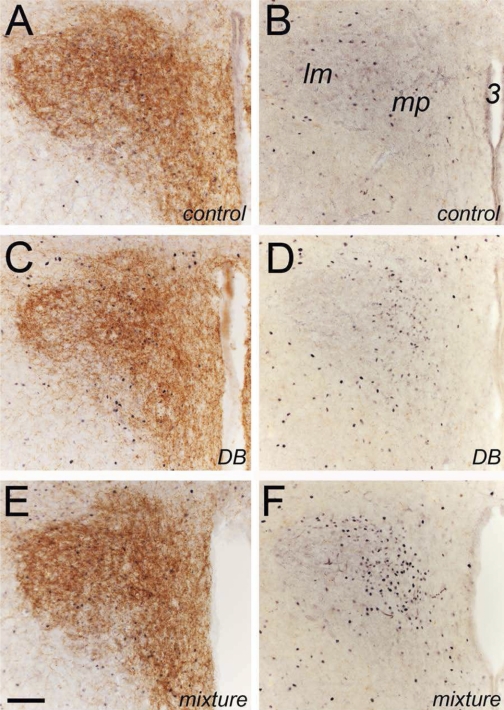
Representative color photomicrographs depicting immunolabeling for Fos (black nuclear label) in sections through the hypothalamic PVN that were double labeled for either DβH (brown axonal and terminal label; A, C, E) or CGRP (B, D, F; note that PVN contains little or no CGRP labeling) after intragastric gavage of water (control), DB alone, or a mixture of T2R ligands. Neural Fos labeling appeared to be increased after intragastric gavage of either DB alone or the T2R ligand mixture. Quantitative data are provided in Fig. 2. Scale bar = 100 μm, A–F. lm, lateral magnocellular; mp, medial parvocellular; 3, third ventricle.
Experiment 2: Conditioned Flavor Avoidance After Intragastric Gavage of T2R Ligands
Test 1: Ability of T2R agonist mixture to induce CFA.
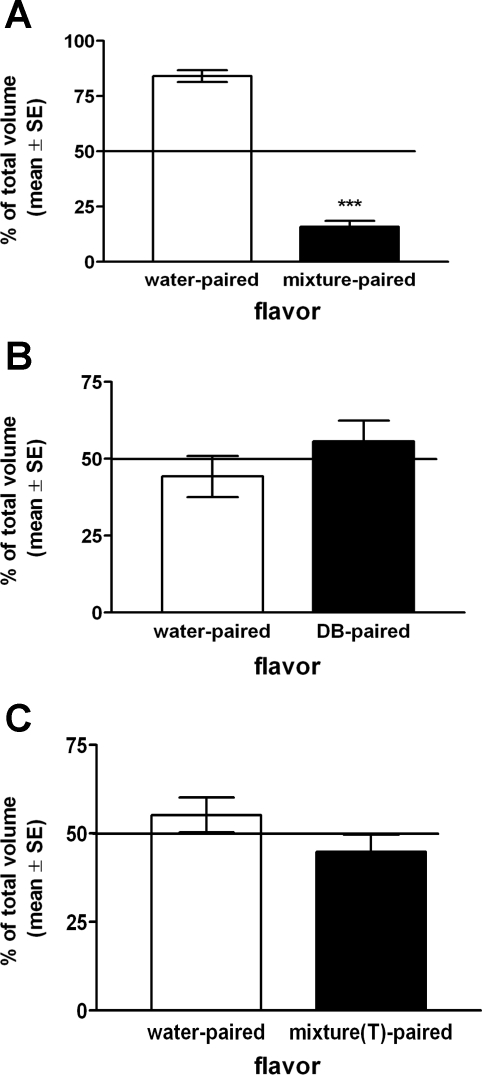
Rats consumed similar volumes of novel vanilla- or almond-flavored water during initial presentation on single-bottle CFA training days (13.5 ± 0.6 ml vanilla, 13.3 ± 0.6 ml almond; range 9–17 ml). In the two-bottle choice test, rats consumed significantly lower volumes of flavors previously paired with intragastric gavage of the T2R agonist mixture (i.e., 2.9 ± 0.9 ml, representing 15.9 ± 2.7% of the total volume consumed from both bottles) compared with intake of flavors paired with intragastric water gavage (i.e., 12.8 ± 1.1 ml, representing 84.1 ± 2.7% of the total volume consumed from both bottles) (Fig. 6A). These preference ratios were significantly different (P < 0.001), evidence for robust conditioned avoidance of flavors previously paired with intragastric gavage of the T2R ligand mixture.
Fig. 6.
Average group preference ratios (% of total volume intake; means ± SE, n = 12/group) for novel flavors in 2-bottle choice tests after flavors were paired with intragastric gavage of water vs. T2R agonist mixture (A), intragastric gavage of water vs. DB (B), or oral exposure to water swabbed on the tongue vs. T2R agonist mixture (C). Horizontal lines indicate expected preference ratios of 50%:50% if there were no effects of flavor-pairing condition. Rats avoided consuming flavors previously paired with intragastric gavage of T2R agonist mixture (A; ***P < 0.05) but did not avoid flavors previously paired with intragastric gavage of DB alone (B) and flavors previously paired with oral exposure to T2R agonist mixture swabbed on the tongue [mixture(T)] (C).
Test 2: Ability of DB alone to support CFA.
Rats consumed similar volumes of novel banana- or coconut-flavored water during initial presentation on single-bottle CFA training days (14.0 ± 0.6 ml banana, 13.1 ± 0.7 ml coconut; range 10–19 ml). In the two-bottle choice test, rats consumed similar volumes of flavors previously paired with intragastric gavage of DB alone (i.e., 8.9 ± 1.1 ml, representing 55.7 ± 6.7% of the total volume consumed from both bottles) compared with intake of flavors paired with intragastric water gavage (i.e., 7.3 ± 1.1 ml, representing 44.3% ± 6.7 of the total volume consumed from both bottles). These preference ratios were not significantly different (P > 0.05), evidence that intragastric gavage of DB alone did not induce CFA (Fig. 6B).
Test 3: Ability of oral exposure to T2R mixture to support CFA.
In the two-bottle choice test, rats consumed similar volumes of flavors previously paired with oral exposure to the T2R ligand mixture swabbed on the tongue (i.e., 6.7 ± 2.1 ml, representing 44.8 ± 4.9% of the total volume consumed from both bottles) compared with intake of flavors paired with oral exposure to water swabbed on the tongue (i.e., 8.8 ± 1.0 ml, representing 55.2 ± 4.92% of the total volume consumed from both bottles). These preference ratios were not significantly different (P > 0.05), evidence that oral exposure to the T2R mixture does not support CFA (Fig. 6C).
DISCUSSION
T2Rs and other taste signaling molecules that are expressed in the mucosal lining of the gut might participate in detecting potentially nutritious or potentially harmful substances after they are swallowed but before they are absorbed, in order to help prepare the organism for subsequent absorptive or protective gut responses (26). For example, many poisonous plants contain bitter alkaloids, and bitter taste sensing in the oral cavity likely evolved as a natural warning system that discourages swallowing of toxic substances (8). Signaling at oral T2Rs by bitter tastants produces inborn (i.e., unconditioned) aversive responses, including immediate rejection of the tastant from the oral cavity, and learning (i.e., conditioning) that supports future avoidance of ingesta with similar sensory properties. Indeed, animals and humans can be protected from accidental poisoning by the addition of one or more T2R ligands to toxic but otherwise palatable commercial products, such as antifreeze. If foods or fluids that contain bitter-tasting substances are swallowed, however, a second line of defense may be provided postingestively by ligand-induced activation of T2Rs expressed within the mucosal lining of the upper GI tract (9). Although several aspects of this hypothesis remain to be tested, results from the present study indicate that intragastric gavage of T2R agonists in rats is sufficient to recruit neural Fos activation in specific brain stem and forebrain regions that have been implicated in CFA, and that CFA is induced by intragastric gavage of T2R ligands.
Glendinning and colleagues (9) recently demonstrated that repeated slow intragastric infusions of DB were able to condition flavor avoidance responses in rats and mice with a multiple-trial conditioning paradigm. Conversely, in the present study, CFA was not evident in rats after intake of novel flavors followed by a single, acute intragastric gavage of 10 mM DB. The differences between these two studies include experimental training and testing protocols, infusion/gavage procedures, DB concentrations and volumes delivered, the number of training sessions before flavor choice tests, and the presence or absence of food restriction. The testing paradigm and doses of DB used are perhaps the most significant differences between our study and theirs that might explain the discrepancy in CFA results after intragastric DB infusion. In the study of Glendinning et al., the effective dose for CFA was sixfold higher than in the present study (i.e., 6 ml of 10 mM self-infused in their study vs. 1 ml of 10 mM infused in the present study). Indeed, the low dose in their study that failed to induce CFA (i.e., 10 ml of 2.5 mM self-infused) is still 2.5-fold higher than the dose used in the present study (1 ml of 10 mM). It is possible that higher DB concentrations might support CFA learning and expression within the training and testing paradigm used in the present study, but this remains to be determined.
In contrast to the absence of a CFA response to flavors previously paired with intragastric DB, our results demonstrated a robust CFA response to flavors paired with intragastric gavage of T2R agonist mixture. These behavioral results are consistent with the significantly greater Fos activation after gavage of agonist mixture compared with Fos activation after DB alone. Although DB gavage produced clear trends toward increased Fos activation in most brain regions, only the mNTS (Table 1) and PVN (Fig. 2) were significantly more activated in rats gavaged intragastrically with DB than in water-gavaged control rats. Conversely, Fos counts in rats gavaged with the T2R agonist mixture were significantly greater than Fos counts in control rats in every brain region examined, except for the VLM. Considered together, these results suggest that activation of GI T2Rs recruits brain stem and forebrain neural pathways that support CFA learning but that differences exist in the potency of different T2R ligands and/or their affinity for T2R transcripts expressed within the gut that engage these central neural mechanisms.
We previously reported (10) that intragastric gavage of T2R ligands in mice activates Fos expression within the caudal visceral portion of the NTS that can be blocked by prior subdiaphragmatic vagotomy, evidence that the NTS activation depends on vagal viscerosensory inputs to the caudal medulla. In the present study, we confirmed in rats that intragastric gavage of T2R ligands activates Fos within the caudal visceral sensory portion of the NTS. We extended those findings by confirming a NA phenotype for many of the NTS neurons activated by T2R ligand gavage and by analyzing Fos expression within other brain regions to which the caudal NTS projects, including the VLM, lateral PBN, CeA, and hypothalamic PVN. These central regions are commonly activated by visceral sensory stimuli, especially those that are transmitted initially to the central nervous system via vagal afferents (14, 21).
We focused our quantification of Fos expression on certain brain stem and forebrain regions that are delineated by DβH and/or CGRP immunolabeling. NA VLM neurons and the CGRP-rich portion of the lateral PBN receive visceral sensory signals relayed from the AP and NTS (21), and the PBN appears to be necessary for CFA learning and expression (1, 6, 12). The CGRP-rich region of the lateral PBN projects heavily to the CeA (24), which also receives inputs from NA NTS and VLM neurons (14). The AP, NTS, VLM, lateral PBN, and CeA are commonly activated by stimuli that support CFA (20, 21, 25, 30). Thus the distribution of Fos activation observed in the present study is consistent with recruitment by a visceral sensory stimulus that can support conditioned avoidance learning and expression (20).
The medial parvocellular subnucleus of the hypothalamic PVN displayed significant and similar Fos activation in rats after intragastric gavage of DB or T2R agonist mixture, consistent with other evidence that GI vagal sensory stimulation can recruit endocrine and nonendocrine components of the PVN (for review, see Ref. 21). Indeed, our results demonstrated that intragastric gavage of DB or the T2R agonist mixture activated similar proportions of DβH-positive NA neurons within the NTS and VLM, although activation trended toward higher levels after gavage of the agonist mixture. Thus it seems likely that PVN Fos activation in both gavage conditions is due to activation of medullary NA neurons that receive vagal sensory input from the GI tract and project to the PVN (21). Other vagal sensory stimuli that activate this pathway include systemic CCK, which recruits GI vagal sensory inputs to the caudal medulla (19) to activate NA NTS and VLM neurons (2, 21) to thereby activate PVN neurons (21). In the present study, PVN Fos activation included the medial parvocellular region, where corticotropin-releasing hormone-positive neurons reside at the apex of the hypothalamic-pituitary-adrenal axis. However, this PVN activation pattern did not correlate with CFA, which was present after gavage of the agonist mixture but not after gavage of DB alone. It is important to point out that CFA learning does not require the unconditioned stimulus (in our study, intragastric T2R ligands) to produce a stressful, nauseogenic, or even aversive response; it is only necessary that the unconditioned stimulus possess signal properties that are registered centrally in association with the conditioned stimulus (in our study, a novel oral flavor) (16, 17). It is possible that PVN activation after T2R ligand gavage includes preautonomic PVN neurons that project to the medullary dorsal vagal complex to modulate vagally mediated control of GI motor and secretory function. For example, oxytocin- and bombesin (gastrin-releasing peptide)-containing projections from the PVN to the dorsal vagal complex provide inhibitory control over GI motility, emptying, and secretion (29), functions that may well be engaged in an adaptive manner by ligand-induced stimulation of gut T2Rs. Further studies are needed to characterize the chemical phenotype and central projections of these activated PVN neurons.
Previous studies have demonstrated that oral application of the bitter tastants quinine, denatonium, and 6-propyl-2-thiouracil (each of which is included in our T2R ligand mixture) activates Fos protein in the medial-dorsal part of the rostral (taste) third of the NTS, which receives synaptic input from oral gustatory sensory neurons, and within the medial PBN, which receives input from the rostral taste region of the NTS (3, 31). Conversely, oral exposure to these bitter tastants fails to activate Fos expression in the caudal medial (visceral) NTS, which primarily receives synaptic input from visceral sensory neurons (3). In the present study, Fos protein after intragastric gavage of T2R ligands was significantly activated within the caudal visceral NTS and lateral PBN, but not within the taste-related rostral NTS or medial PBN, consistent with our previous studies in mice (10) showing that T2R agonist mixture infused directly into the GI tract activates the caudal NTS via subdiaphragmatic vagal nerves. In addition, oral exposure to the T2R agonist mixture was insufficient to support CFA in the present study, providing additional evidence that the neural Fos activation and behavioral results reported here after intragastric infusions are not due to inadvertent exposure of the oral cavity to the T2R ligands during the infusion procedure.
Although our results do not prove that the gavaged T2R ligands exerted their effects via GI T2R receptors, several lines of evidence support this conclusion. Our previous study (10) indicated that vagal inputs to the brain stem are necessary for intragastric T2R ligands to recruit central Fos expression in mice, and that endogenously released CCK and peptide YY (PYY) may play a role in the T2R signaling cascade. Thus T2R ligands can stimulate enteroendocrine cells in the gut wall and induce release of regulatory peptides. Indeed, Gαgust colocalizes with PYY, glucagon-like peptide, and 5-HT in human and rodent GI enteroendocrine cells (23, 28), and T2R ligands activate enteroendocrine STC-1 cells to release CCK (5, 35). However, it is possible that the systemic effects of PTC may, at least in part, contribute to the CFA observed in the present study; it was previously demonstrated that systemic injections of PTC induce conditioned taste aversion in mice (27).
Perspectives and Significance
We have demonstrated that GI gavage of T2R ligands supports a robust conditioned avoidance response in association with significantly increased activation of neurons within the caudal visceral NTS and caudal VLM, including NA neurons, and significant activation within the lateral PBN, CeA, and PVN. We speculate that inadvertent swallowing of a potentially toxic substance that contains one or more T2R ligands can promote activation of one or more types of gut T2Rs, which may protect against future consumption of similar ingesta by engaging central visceral sensory and other neural pathways that support conditioned avoidance behavior.
GRANTS
This work was supported by National Institutes of Health Grants DK-41004 (H. E. Raybould), DK-41301 (C. Sternini), and MH-59911 (L. Rinaman).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agüero A, Arnedo M, Gallo M, Puerto A. Lesions of the lateral parabrachial nuclei disrupt aversion learning induced by electrical stimulation of the area postrema. Brain Res Bull 30: 585–592, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci 27: 13292–13302, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan CY, Yoo JE, Travers SP. Diverse bitter stimuli elicit highly similar patterns of fos-like immunoreactivity in the nucleus of the solitary tract. Chem Senses 29: 573–581, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell 100: 703–711, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Chen MC, Wu SV, Reeve JR Jr, Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol 291: C726–C739, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Cubero I, Puerto A. Lateral parabrachial lesions impair intraperitoneal but not intraventricular methylscopolamine-induced taste aversion learning. Brain Res 871: 113–119, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch JA, Hardy WT. Cholecystokinin produces bait shyness in rats. Nature 10: 266, 1977. [DOI] [PubMed] [Google Scholar]
- 8.Glendinning JI Is the bitter rejection response always adaptive? Physiol Behav 56: 1217–1227, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Glendinning JI, Yiin YM, Ackroff K, Sclafani A. Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiol Behav 93: 757–765, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao S, Sternini C, Raybould HE. Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol 294: R33–R38, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of α-gustducin. Proc Natl Acad Sci USA 93: 6631–6634, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Grancha M, Sánchez-Amate C, Navarro M, Carvajal F, Sánchez-Santed F, Cubero I. Lateral parabrachial lesions disrupt paraoxon-induced conditioned flavor avoidance. Toxicol Sci 91: 210–217, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature 434: 225–229, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Myers EA, Rinaman L. Viscerosensory activation of noradrenergic inputs to the amygdala in rats. Physiol Behav 77: 723–729, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Myers EA, Rinaman L. Trimethylthiazoline supports conditioned flavor avoidance and activates viscerosensory, hypothalamic, and limbic circuits in rats. Am J Physiol Regul Integr Comp Physiol 288: R1716–R1726, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Parker LA Taste avoidance and taste aversion: evidence for two different processes. Learn Behav 31: 165–172, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Parker LA The role of nausea in taste avoidance learning in rats and shrews. Auton Neurosci 125: 34–41, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Parry CM, Erkner A, le Coutre J. Divergence of T2R chemosensory receptor families in humans, bonobos, and chimpanzees. Proc Natl Acad Sci USA 101: 14830–14834, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raybould HE Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol 7: 570–574, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am J Physiol Regul Integr Comp Physiol 293: R1495–R1503, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Rinaman L Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol 28: 50–60, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinaman L, Stricker EM, Hoffman GE, Verbalis JG. Central c-Fos expression in neonatal and adult rats after subcutaneous injection of hypertonic saline. Neuroscience 79: 1165–1175, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol 291: G792–G802, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Schwaber JS, Sternini C, Brecha NC, Rogers WT, Card JP. Neurons containing calcitonin gene-related peptide in the parabrachial nucleus project to the central nucleus of the amygdala. J Comp Neurol 270: 416–426, 1988. [DOI] [PubMed] [Google Scholar]
- 25.St Andre J, Reilly S. Effects of central and basolateral amygdala lesions on conditioned taste aversion and latent inhibition. Behav Neurosci 121: 90–99, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Sternini C Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol 292: G457–G461, 2007. [DOI] [PubMed] [Google Scholar]
- 27.St. John ST, Pour L, Boughter JD Jr. Phenylthiocarbamide produces conditioned taste aversions in mice. Chem Senses 30: 377–382, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol 292: G1420–G1428, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Taché Y, Garrick T, Raybould H. Central nervous system action of peptides to influence gastrointestinal motor function. Gastroenterology 98: 517–528, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Touzani K, Sclafani A. Critical role of amygdala in flavor but not taste preference learning in rats. Eur J Neurosci 22: 1767–1774, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Travers SP Quinine and citric acid elicit distinctive Fos-like immunoreactivity in the rat nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 282: R1798–R1810, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Uneyama H, Tanaka T, Torii K. Gut nutrient sensing by the abdominal vagus. Folia Pharmacol Jpn 124: 210–218, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Watson RE Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7: 155–159, 1986. [DOI] [PubMed] [Google Scholar]
- 34.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 44: 2392–2397, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu SV, Chen MC, Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics 22: 139–149, 2005. [DOI] [PubMed] [Google Scholar]