Abstract
Obesity is a resilient and complex chronic disease. One potential causative factor in the obesity syndrome is leptin resistance. Leptin behaves as a potent anorexic and energy-enhancing hormone in most young or lean animals, but its effects are diminished or lacking in the obese state associated with a normal genetic background. Emerging evidence suggests that leptin resistance predisposes the animal to exacerbated diet-induced obesity (DIO). Elevation of central leptin in young, lean rats induces a leptin resistance that precludes obesity on a chow diet but accelerates high-fat (HF)-induced obesity. Similarly, chronic dietary fructose consumption evokes a leptin resistance that causes obesity only upon HF exposure. Inherent central leptin insensitivity also contributes to dietary weight gain in certain obesity-prone rats. Conversely, aged, leptin-resistant animals are obese with continuous chow feeding and demonstrate aggravated obesity when challenged with an HF diet. Additionally, a submaximal central blockade with a leptin antagonist leads to obesity on both chow and HF diets, as is the case in rodents with leptin receptor deficiency of genetic origin. Despite the differences in the incidence of obesity on a chow diet, all of these forms of leptin resistance predispose rodents to aggravated HF-mediated obesity. Moreover, once leptin resistance takes hold, it aggravates DIO, and the leptin resistance and obesity compound one another, promoting a vicious cycle of escalating weight gain.
Keywords: age, STAT3, leptin antagonist, fructose
leptin, a product of the obesity gene (or ob gene) (68), is a key regulator of feeding and energy expenditure (14). This adipocyte-derived hormone was once heralded to be an antiobesity agent. While leptin is effective in certain individuals bearing congenital leptin deficiencies (17) or lipodystrophies (45, 46), as a monotherapy, leptin has been disappointing in humans and rodents with common obesity, that is, obesity associated with elevated serum leptin under a normal genetic background (23, 30, 65). Leptin production increases proportionally with adiposity, and leptin levels are high in rodent and human models of diet-induced or adult-onset obesity. Yet, the increased leptin fails to curtail the progression of obesity (22, 23, 30, 34, 65). This apparent leptin ineffectiveness is identified as leptin resistance.
The notion of leptin resistance conjures different interpretations, and its complex nature gives way to several definitions. In the most general terms, leptin resistance is described as the failure of elevated circulating leptin to reduce common obesity. This resistance may be due to an inability of leptin reaching target sites within the brain (resistance to peripherally administered leptin) (4) and/or impaired cellular responses within selected neurons in defined brain regions (central leptin resistance) (15, 42, 43). In rodents, leptin resistance is often noted as the reduced sensitivity with respect to the anorectic response to exogenous leptin introduced either peripherally or centrally.
Extensive research efforts have been made to examine the cause(s), characteristics, and metabolic consequences of the leptin resistance. This review will summarize some recent data and evidence arguing that leptin monotherapy, in and of itself, is destined to be a failed strategy to treat obesity and that leptin resistance is a predisposing factor for diet-induced obesity (DIO).
Animal Models of Leptin Resistance
There are several models of naturally occurring, programmed leptin resistance, including that displayed in seasonal animals (27, 28, 63) or during pregnancy/lactation (2, 21), where increased food consumption and weight gain are necessary to meet the particular biological demands. Additionally, several genetic models of obesity associated with defective or absent leptin receptors reflect a tachyphylaxis to leptin responses (17). In reality, in most models of leptin resistance such as DIO, the resistance is relative, characterized by significant reductions in either leptin sensitivity or efficacy, but some leptin receptor activity remains. The underlying mechanisms are not clearly delineated. One form of leptin resistance is associated with a defective transport of serum leptin across the blood-brain barrier (BBB) (4) and thus, the ratio of cerebral spinal fluid leptin to serum leptin level is diminished (10). This resistance to peripherally administered leptin is characteristic of high-fat (HF)-induced obesity (47, 64) and occurs relatively soon after initiating HF feeding and at a time when rodents remain responsive to centrally administered leptin (47). The mechanism may involve the interference of elevated triglycerides with leptin BBB transport (3), presumably resulting in insufficient leptin levels within the brain. The second form of leptin resistance is characterized by blunted responses to centrally administered leptin (central leptin resistance) and is often referred to as cellular leptin resistance (41, 43). It is associated with impaired leptin signaling events within specific brain regions. Molecular mechanisms contributing to this cellular leptin resistance and impaired signaling may include increased activity of phosphotyrosine phosphatase 1B and elevated levels of suppressor of cytokine signaling 3 (SOCS3). The latter mediates its inhibitory action through binding to leptin-stimulated phosphorylation of tyrosine 985 on the long-form functional leptin receptor (43). On the other hand, manipulations that enhance leptin signaling confer a lean or dietary-resistant phenotype (7, 8, 25, 40), suggesting that diminished leptin receptor signaling may indeed be responsible for cellular leptin resistance. The reader is referred to recent reviews for an in-depth analysis of leptin signaling events associated with leptin resistance (42, 43).
Leptin Resistance in DIO Animals
Leptin resistance is a hallmark of DIO. Rodents usually become obese when fed an HF diet, and the temporal development of diet-induced leptin resistance is dependent on the species and strain of the rodent (22, 35, 64). Obese rodents have elevated leptin levels but blunted leptin sensitivity. They initially develop a resistance to peripheral leptin administration, but continued HF feeding will eventually induce central leptin resistance (13, 35, 64). The leptin-resistant rats display reduced leptin transport across BBB (4), decreased signal transducer and activator of transcription protein 3 (STAT3) phosphorylation and P-STAT3 transcription binding capacity (13), and marred melanocortin release in certain areas in the hypothalamus (15). This leptin resistance is presumably reversible if the HF diet is withdrawn (65).
The degree of DIO is not uniform. For example, most Sprague-Dawley (SD) rats after weaning spontaneously divide into two groups when exposed to HF diet, those resistant to the diet-induced weight gain (referred to as Dietary Resistant), and those particularly susceptible to the weight gain (referred to as Dietary Prone) (30). The susceptibility to DIO may be due to inherent differences in leptin sensitivity (31, 32). When SD rats selectively bred to develop or resist dietary obesity were subject to an acute leptin challenge, the Dietary Prone rats demonstrated diminished hypothalamic leptin-induced STAT3 phosphorylation compared with the Dietary Resistant rats prior to the development of obesity (31). Although no cause and effect was established, this evidence supports the notion that leptin resistance promotes obesity. Despite advancing knowledge, the exact cause for the diet-induced leptin resistance is still unclear, and many questions remain: is it due to the energy-rich diet, the elevated leptin, increases in compensatory mechanisms, and/or a gradual neural network rewiring following chronic HF feeding? Because the HF food consumption has more to do with the reward properties of the palatable food rather than the caloric value, the endocannabinoid system and/or mesolimbic dopamine circuits may also have a role in HF feeding behavior and the resultant obesity (6, 12, 24, 37).
Leptin Resistance in Aging Animals
Besides DIO, another common form of obesity is age-related or adult-onset obesity. Humans demonstrate a steady increase in body weight and adiposity through early senescence followed by a decline in latter life (60). In our aging rat model, the Fisher 344 x Brown Norway (F344xBN) rat, body weight and adiposity follow a steady increase into early senescence (from 3 to 24 mo) and then a decline from 24 to 30 mo (34). For the purpose of this review, we will consider only the portion of the adult life span in which body weight and adiposity are increasing and refer to this as age-related obesity. At 24 mo, aged F344xBN rats have 3–4 times higher serum leptin relative to that at 3 mo (34). Despite this hyperleptinemia, obesity persists, and the adiposity level at 24 mo amounts to ∼ 400% of that at 3-mo. These aged obese rats exhibited little or no anorectic or weight loss responses to peripherally infused leptin (26) as opposed to a dose-dependent food suppression and weight reduction in young rats in response to similarly infused leptin, demonstrating a leptin resistance associated with age-related obesity. Similar leptin resistance is also indicated in aged Wistar rats (18), and caloric restriction was shown to be effective in these animals to partially restore leptin responsiveness (19).
The age-related leptin resistance consists of both a peripheral and central component (69). Young rats respond more robustly to either peripheral or central leptin administration than the aged (26), but the responses to central leptin infusion in the aged are greater compared with the minimal responses observed following peripheral infusion (26, 51, 52).
Leptin-Induced Leptin Resistance in Young Lean Animals
There is clear evidence that prolonged leptin treatment in young, lean rodents can induce leptin resistance. Male Long-Evans rats receiving a 21-day chronic subcutaneous leptin infusion lost the anorectic response to leptin half way into the treatment. A subsequent peripheral high-dose leptin challenge failed to alter 24-h food intake in the leptin-treated rats (36). Significantly diminished hypothalamic leptin receptor message and protein levels appeared to provide one explanation for this leptin resistance (36). In another case in which leptin was infused chronically into the lateral cerebroventricle in adult male SD rats for 28 days, the animals had an initial decrease in food intake, but then developed resistance to the satiety action of leptin after 3 wk (49). In a study from Friedman laboratory, a supraphysiological dose of leptin was used first to deplete fat mass and reduce body weight (39). Upon an abrupt withdrawal of the leptin supplement, a sudden peripheral leptin deficiency was created that generated diminished immune and reproductive functions. When half of the mice were subsequently allowed to eat ad libitum, they displayed hyperphagia and regained the lost weight. The other half of the mice though, with their food intake restricted to the normal preexperimental consumption, continued to display low levels of leptin and blunted responses to an exogenous leptin challenge both in terms of prevention of the regain of lost weight and restoration of deficient immune and reproductive function (39). It was concluded that high-dosage leptin treatment induced a state of acquired leptin resistance. Using transgenic mice overexpressing human leptin within adipose tissue, Qiu et al. (48) found that the mice had lower body weight and less adiposity at a young age, but regained the lost adiposity and body weight at an older age. Such data again demonstrate that leptin insensitivity develops over time.
We have established our own rat model of central leptin elevation-induced leptin resistance. This was achieved using an recombinant adeno-associated virus (rAAV)-based gene transfer system to deliver the gene coding for rat leptin (rAAV-leptin) directly into the brain and was intended to gain insights into the relationship between elevated central leptin and leptin resistance. Leptin overexpression in the hypothalamus of the lean F344xBN rats continued up to 300 days without evidence of abatement and resulted in a 75% elevation in cerebrospinal fluid (CSF) leptin levels (57). The animals initially responded to rAAV-leptin with reduced food intake, elevated oxygen consumption, and a substantial decrease in body weight, and the body fat reduced to near zero by day 10 (57). However, food intake slowly returned to the control level over time and the enhancement in oxygen consumption was also lost (58). Despite lower body mass in the rAAV-leptin-treated vs. control rats at day 300, the rAAV-leptin rats maintained their regular food intake, whereas control rats displayed the expected food suppression in response to a 7-day leptin intracerebroventricular infusion, confirming a leptin-induced leptin-resistant state (58). The fact that leptin overexpression in the central nervous system could evoke hypothalamic leptin resistance in rats devoid of obesity argues that elevated central leptin is one independent factor causal to acquired leptin resistance.
In humans, elevated serum leptin predicts the subsequent development of metabolic syndrome at 5 and 10 years (20), and CSF levels of leptin are augmented with obesity in humans, even though the ratio of CSF to peripheral leptin is diminished (10). However, the physiological significance of increased central leptin remains unclear. Conceivably, the CSF leptin levels in common obesity may be abnormally high due to peripheral hyperleptinemia and could contribute to diet-induced central leptin resistance, or these levels are, in fact, insufficient owing to the defect in leptin transport across the BBB associated with DIO.
The multiple examples of leptin treatment described, whether transgenic, pharmacological leptin administration, or leptin central gene therapy, may not have a naturally occurring physiological and/or pathological counterpart; nevertheless, they provide valuable research tools to study the role of leptin or hyperleptinemia per se in homeostasis regulation. Moreover, the results from these various studies demonstrate a common phenomenon of leptin-induced leptin resistance and underscore the destined failure of using leptin monotherapy to combat obesity.
Dietary Fructose and Leptin Resistance
We discovered a unique model of leptin resistance associated with chronic high-fructose feeding in which neither obesity nor leptin treatment was involved (62). Epidemiological studies suggest a relationship between the consumption of fructose-enriched products and increased rates of obesity (9). We hypothesized that leptin resistance may provide the link between dietary fructose and obesity. We fed a group of SD rats a fructose-free control or a high-fructose diet (60% fructose) for 6 mo and tested for leptin sensitivity both before and after the diet. Initially, all rats displayed an anorectic response to intraperitonial leptin injection, but after 6 mo, only the control rats responded (62). At this point, both groups had similar body weights, total body adiposity, or serum leptin, insulin, or glucose except for elevated serum triglycerides with high fructose consumption. The fructose-fed rats nonetheless showed blunted food response and decreased hypothalamic STAT3 phosphorylation compared with controls (62), demonstrating a chronic fructose consumption-induced leptin resistance. Similar findings indicating a fructose-induced leptin resistance were reported in an abstract from Chotiwat et al. (11). Elevated serum triglycerides mar leptin transport across the BBB (3), which implicates a potential insufficiency in leptin reaching target sites within the brain resulting from the fructose-induced leptin resistance.
Leptin Resistance Predisposes Rodents to DIO
It is clear in animals with normal endogenous levels of circulating leptin that leptin therapy, be it by administration or overexpression, initially induces a weight and adipose loss, but eventually those animals regain the lost weight and adiposity and achieve body weight parity with control counterparts. Despite the presence of acquired leptin resistance, body weight of the leptin-induced, leptin-resistant animals never exceeds that of control animals. The amount of chow diet consumed in these animals without leptin supplement likely represents homeostatic caloric requirement for natural growth (with little reward incentive). The growth-related body weight appears to be rigorously defended by animals despite the transient period of negative energy balance due to exogenous leptin treatment. These observations are consistent with the so-called “adiposity (weight) set-point” theory (29, 33) in which animals fiercely protect a predetermined level of adiposity (or body weight), presumably to ensure critical immune and reproductive functions for survival. By and large, the acquired leptin-induced leptin resistance appears to be “quiescent” in chow-fed animals as long as additional metabolic challenges are not superimposed.
However, upon exposure to an HF diet, a different picture emerges: leptin-resistant animals display exacerbated weight and adiposity gain compared with corresponding HF-fed, non-leptin-resistant controls. In fact, all leptin-induced, aged-related, and fructose-fed leptin-resistant animals faired worse in face of the HF exposure.
In our leptin-induced, leptin-resistant model, leptin resistance was produced following 94 days of central rAAV-leptin gene therapy in the young lean rats (54). When switched to an HF diet, the rAAV-leptin-treated, HF-fed rats consumed a 36% greater amount of calories, grew considerably heavier (Fig. 1A), and accumulated 26% more visceral fat relative to HF-fed leptin-sensitive controls. Hence, the acquired leptin resistance predisposes these rats to DIO. A similar outcome was discovered with the transgenic mice overexpressing human leptin in adipose tissue. The leaner transgenic mice gained considerably more body weight and adiposity on an HF diet compared with the wild-type control mice (44).
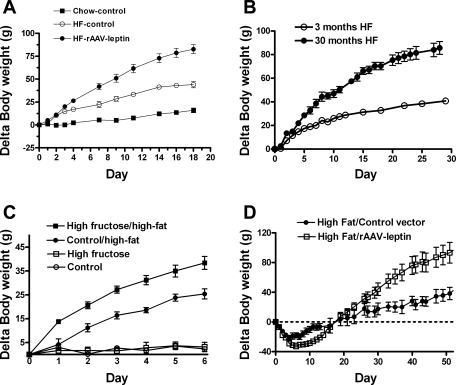
Fig. 1.
A: body weight gain following a high-fat (HF) diet in rats pretreated for 94 days with control vector or rAAV-leptin compared with chow-fed rats pretreated with control vector. Values are means ± SE of 8 rats/group. In some cases, SE bars are less than the size of the data point (P < 0.001 for difference in body weight gain between all pairs by one-way ANOVA). (A is modified from Ref. 70). B: body weight gain following HF feeding in 3-mo-old and 30-mo-old rats. Data are means ± SE of 21 3-mo-old and 5 30-mo-old rats. Body weight gain in the 30-mo-old rats was greater than the 3-mo-old rats beginning at day 3 (P < 0.01, one-way ANOVA). (B is adapted from Ref. 26). C: body weight gain in rats pretreated with a fructose free or high fructose diet for 6 mo and then either maintained on the fructose-free control or high-fructose diets or switched to a 60% high-fat diet. Values are means ± SE of 5–6 animals/group (P < 0.01 for difference between high fructose/high fat and control/high fat weight gain beginning at day 1. (C is adapted from Ref. 62). D: body weight gain following administration of control vector or rAAV-leptin in DIO rats. The rAAV-leptin or control vectors were administered in rats raised on an HF diet for 5 mo and continued on the HF diet throughout the experiment. Values are means ± SE of 6 rats/group (P < 0.05 for difference in slopes beginning at day 20 after vector administration). (D is adapted from Ref. 61).
A similar scenario occurred in aged leptin-resistant rats. For instance, the 30-mo-old F344xBN rats consumed more calories and exhibited a larger weight gain relative to young lean rats on an HF diet (Fig. 1B) (26), implicating a heightened susceptibility to dietary obesity associated with the age-related leptin resistance.
In the unique case of chronic fructose consumption, rats developed leptin resistance despite the normalcy of all other metabolic markers except for the increased serum triglycerides (62). There was no evidence of obesity until a subsequent 2-wk-long HF challenge was applied. The rats preexposed to fructose consumed more calories, gained more body weight, and had increased adiposity compared with correspondingly fed rats preexposed to the fructose-free diet (Fig. 1C). This leptin resistance resembles that of leptin-induced leptin resistance, in that weight gain was avoided unless an HF diet was provided.
Presence of a continuous high-energy diet complicates the DIO leptin resistance model. We describe here two aspects of leptin responsiveness: prior to HF feeding and after consumption of an HF diet. With respect to the former is the case of Dietary Prone rats that exhibit rapid weight gain with HF feeding. These rats have diminished response to leptin prior to HF feeding, and presumably, this leptin resistance predisposes the rats to dietary obesity (31). Conversely, those rats that are diet resistant have corresponding greater responses to leptin prior to HF feeding, suggesting that leptin sensitivity protects against DIO. These data support the idea that leptin resistance predisposes to DIO.
Feeding an HF or combination of HF, sugar, and salt (Western diet) during pregnancy was reported to subject offspring to development of later obesity (5, 38). Although leptin resistance was not examined in these studies, consumption of the HF diet during gestation, lactation, or postweaning resulted in hyperleptinemia, indicative of a presumed leptin-resistant state. These facts seem to imply that maternal/parental leptin resistance may be an important contributing factor predisposing the offspring to obesity.
While the issue about whether the leptin resistance associated with DIO is secondary or causal to obesity is unsettled, a new question arises: does this leptin resistance sustain and/or worsen obesity? Little information is available to address this concern at the present time. One of our recent studies may give some clues. We subjected diet-induced obese rats (5-mo HF fed) to central rAAV-leptin gene therapy; they responded with a transient, mild anorexia and weight reduction, reflecting a partial but not complete leptin-resistant state (61). The lost body weight was regained in 14 days. However, as the leptin therapy continued, the trajectory of HF-mediated increase in weight gain became twofold greater in the rAAV-leptin-treated compared with HF-control animals (Fig. 1D). Thus, central leptin therapy in rats with existing dietary obesity evidently accelerated diet-induced weight gain. We interpret these findings to indicate that the partially leptin-resistant DIO rats developed a full leptin-induced leptin resistance rather quickly, which triggered the heightened weight gain in response to the continued HF feeding. A further contention would be that the leptin-induced resistance worsens dietary obesity.
Physiological relevance of leptin resistance evoked by pharmacological leptin therapy or central leptin overexpression remains to be tested, but animals possessing this kind of acquired leptin resistance, as well as those with diet-induced or age-related leptin resistance, all lack the ability to properly regulate energy balance when challenged with an HF diet. Collectively, leptin resistance is a predisposing factor for DIO. On the contrary, increasing leptin sensitivity (thus dampening leptin resistance) through genetic disruption of inhibitory factors of leptin signaling events reduces body weight and protects against DIO (7, 25, 40). These findings seem to lay some ground for claiming a physiological significance of leptin resistance in obesity, especially in the context of HF feeding.
Insights from a Leptin Antagonist
Leptin resistance is associated with reduced leptin receptor activity.
Whether central leptin resistance is partially due to reduced leptin receptors is controversial, but mounting evidence demonstrates diminished leptin receptor-mediated signaling, including both STAT3 and phosphatidylinositol-3-OH kinase pathways, is associated with leptin resistance (50, 53). In addition, leptin upregulation of the downstream anorectic neuropeptide proopiomelanocortin and the release of its cleaved product α-melanocyte-stimulating hormone is impaired in the hypothalamus with leptin resistance (15, 57). Alternatively, disruption of the inhibitory components of the leptin signaling pathway, including phosphotyrosine phosphatase 1B (7, 66) or SOCS3 (25, 40) enhances leptin sensitivity and/or confirms resistance to dietary obesity. Thus, diminished leptin receptor activity appears to be integral to leptin resistance. Leptin receptor activity, for the purpose of this discussion, refers to both the stimulation of the immediate leptin-receptor signaling events and all subsequent signaling and downstream physiological events that are dependent on the initial ligand-receptor interaction. The obesity phenotype resultant from either lack of leptin or leptin receptors due to genetic mutations highlights the importance of leptin receptor pathway in long-term body weight maintenance. Conceivably, even a minor impairment in the leptin receptor pathway could result in obesity over time. The recent availability of a leptin receptor antagonist has afforded us a special tool to simulate various degrees of leptin blockade and examine the long-term physiological consequences of this form of leptin resistance. We predict minimal metabolic effects from leptin antagonist treatment in leptin-resistant vs. leptin-responsive rats if leptin resistance is the result of downregulated leptin receptors, diminished signaling, or receptors functionally uncoupled from downstream physiological responses. In this case, we consider the extent of the metabolic responses to the leptin antagonist to be a measure of functional leptin receptor activity.
Leptin receptor blockade.
The in vivo action of the leptin antagonist was first characterized in our young, lean F344xBN rats maintained on a chow diet (67). Simultaneous central administration of leptin and increasing doses of the leptin antagonist revealed a dose-dependent inhibition of leptin-induced hypothalamic STAT3 phosphorylation. A 7-day infusion of the leptin antagonist alone produced an increase in food intake and weight gain (67). When administered in the presence of exogenous leptin during the infusion, the leptin antagonist blocked the leptin-mediated anorexic effects and body weight reduction as well as the increase in brown adipose tissue UCP1 protein and hypothalamic STAT3 phosphorylation. Although long-term antagonist infusion is impractical, if this feat was accomplished, we would anticipate a gradual build-up of fat mass and body weight and eventual obesity. Another group also found the same leptin antagonist protein useful to investigate the effects of early postnatal leptin disruption on long-term leptin sensitivity and metabolic phenotype (1).
The leptin antagonist was next employed to gain insights into the relationship between leptin resistance, leptin receptor activity, and metabolic outcomes. Central leptin resistance was induced in lean rats by chronic leptin overexpression mediated by rAAV-leptin vector delivery (55). At day 153, rAAV-leptin and rAAV-control rats were given centrally either vehicle or a rat leptin antagonist for 14 days (55). Food intake, body weight, adiposity, and brown adipose tissue UCP1 mRNA expression increased with the antagonist infusion in controls but elevated only marginally in rAAV-leptin rats (55). The lack of significant physiological responses to the antagonist treatment is indicative of diminished leptin receptor activity accompanying the central leptin resistance and resonates with the notion that defective leptin receptor function underlies leptin resistance.
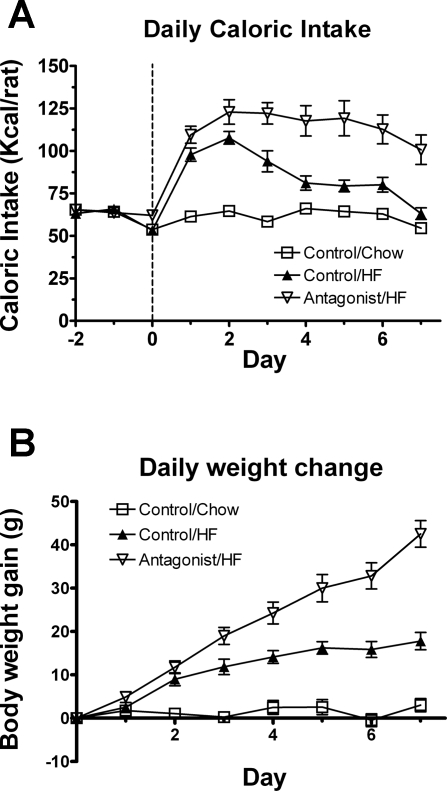
The leptin antagonist also revealed the role of leptin receptor activity in hedonic HF consumption (67). When rats were provided with an HF diet (60% kcal as fat) in the presence of the antagonist (25 μg/day, lateral ventricle infusion) or vehicle for 7 days and were compared with vehicle-infused chow-fed control rats, the daily caloric intake of both HF groups peaked on day 2. However, the HF-induced caloric hyperphagia was normalized to a level isocaloric with regular chow intake by day 7 in the vehicle-infused HF group, whereas the hyperphagia remained in the HF antagonist-infused group (Fig. 2A). Moreover, the HF-mediated weight gain was exaggerated by the leptin antagonist treatment (Fig. 2B). These results indicate an important inhibitory function of endogenous leptin or leptin receptor activity in the counterregulation of hedonic feeding (reward aspect of eating behavior).
Fig. 2.
Daily caloric intake (A) and body weight gain (B) in HF-fed rats following a 7-day infusion of antagonist (25 μg/day) or vehicle and in chow-fed rats following vehicle infusion. The antagonist or vehicle infusion and HF feeding started at day 0. Values are means ± SE of 9 antagonist/HF, 8 control/HF, and 8 control/chow rats. Food intake data are expressed as caloric intake per day, based on 3.10 kcal/g of chow and 5.24 kcal/g of HF diet. Caloric intake and body weight significantly differed between antagonist/HF and control/HF beginning at day 1 (P < 0.01). (Figure 2 is adapted from Ref. 67).
Submaximal chronic leptin receptor blockade.
Generally, the development of leptin resistance upon HF feeding, with age or prolonged leptin therapy, is gradual, involving a process complicated by interactions between the diet, aging, and various leptin-dependent and independent pathways controlling food consumption and energy expenditure. We introduced a chronic submaximal leptin receptor blockade via central gene delivery of a mutated rat leptin gene coding the leptin antagonist protein (59), attempting to directly address the role of daily central leptin receptor activity in the control of energy homeostasis in naturally growing, young, lean animals in a chronic manner. Young, lean, chow-raised F344xBN rats received rAAV-control or rAAV-leptin antagonist for 190 days. During this time, the rats were maintained on a chow diet for 80 days and then switched to a 60% HF diet until day 140, after which they were returned to the chow diet until the end of the experiment (59). The normal growth-related and diet-induced body weight gain was exacerbated in the antagonist group. Feed efficiency, expressed as the body weight gain per unit of food consumed, was increased during both the chow and HF feeding periods, respectively. Because full leptin receptor blockade achieved via central infusion of the leptin antagonist protein in a previous study abolished the normalization of HF-induced hyperphagia (Fig. 2A) (67) as opposed to only a small delay in the normalization process upon HF exposure with antagonist overexpression, we consider that the rAAV-leptin antagonist produced just a submaximal central receptor blockade. In addition to its impact on energy intake, the antagonist also affected physical activity. The rAAV-leptin antagonist rats displayed substantially less wheel-running activity when provided free access to running wheels for 6 days during both the chow and HF feeding periods. On the contrary, wheel-running activity was increased by more than twofold in response to leptin overexpressing in the hypothalamus (59). At death, adiposity and serum leptin levels were greater in the antagonist group. In aggregates, submaximal central leptin receptor blockade leads to greater adiposity, accelerates dietary weight gain, and diminishes wheel-running activity. These observations validate the critical role of fully responsive leptin receptors in long-term regulation of energy homeostasis and hedonic feeding.
Perspective and Significance
This review examined several models of leptin resistance and the role of leptin resistance in the susceptibility to dietary obesity. Only some of the leptin-resistance models (leptin antagonist blockade and aged obese rats) exhibit heightened weight and adiposity gain on a chow diet, while all models discussed demonstrate obesity in the presence of an HF diet. Thus, the leptin resistance appears to be reinforcing “reward eating” beyond caloric energy requirements. Overconsumption of palatable food could be mediated by activation of reward circuitry involving opioids and dopamine or an impairment in a pathway (or pathways) mediating satiation of the palatable diet (16).
Whereas the hypothalamus, in particular, the arcuate nucleus, has been identified as important in regulating the caloric requirements, other regions in the hypothalamus, such as lateral hypothalamus and extrahypothalamus areas including the amygdale, prefrontal cortex, nuclear accumbens, and ventral tegmental area (VTA), are implicated in the reward properties of food (12, 43). Leptin receptors are identified on dopamine-containing neurons within the VTA and were found to suppress dopaminergic neuron firing rate (24). They act through the JAK-STAT signaling pathway and decrease food consumption upon leptin action. The fact that a chronic reduction in leptin receptor activity in the VTA by siRNA knockdown enhances sensitivity to highly palatable food underscores an important role of leptin receptor function in the regulation of reward feeding behavior (24). Our own data also support a counterregulatory mechanism by which leptin modulates HF feeding: leptin receptor blockade prolonged the caloric hyperphagia induced by an HF diet (67). Although, the inputs from multiple brain regions are integrated to determine food ingestion, hedonic feeding driven by the VTA has been suggested to be able to overcome the caloric requirements of homeostatic regulatory properties of the hypothalamus (12).
Two of the leptin resistance models described herein, leptin-induced leptin resistance (54) and fructose-induced leptin resistance (62), accelerated the onset and exacerbated the extent of HF-induced obesity. However, in neither case, did leptin resistance result in obesity on chow diet. For example, rats with central overexpression of leptin initially respond to leptin with a reduction in body weight, and then as leptin resistance develops, the lost body weight is regained until it reaches parity with control animal (54, 56, 58). Despite this leptin resistance, the body weight of the leptin-induced leptin-resistant animals never exceeded that of control animals unless an HF diet was provided. A similar outcome is manifested in fructose-induced leptin-resistant rats, i.e., obesity only develops with HF feeding (62). Conversely, aged, leptin-resistant animals with a deficit in leptin signaling and blunted leptin responses display a steady gain in adiposity on standard chow diet and demonstrate aggravated obesity when challenged with an HF diet (26, 34, 51). Likewise, treatment with a leptin antagonist at a submaximal blockade level leads to obesity on chow diet and increased susceptibility to HF-induced weight gain (59). This resonates with genetic models of leptin receptor deficiency that also result in obesity on both a chow and HF diet. It appears that acquired leptin resistance, including leptin induced and fructose induced, promote obesity only in association with overconsumption of palatable diet, whereas the other forms of leptin resistance evoke obesity regardless of diets. This suggests that the former forms of acquired leptin resistance are fundamentally different than partial blockade or full disruption of leptin receptor activity and possibly involve preferential dysregulation of hedonic eating rather than long-term homeostatic regulatory mechanisms.
In a presumed scenario when leptin receptor activity within the hypothalamic homeostatic brain circuitry falls below a threshold level, obesity may occur regardless of diet compositions, whereas specific defects within the brain reward circuitry may increase the susceptibility to HF-induced obesity. By such a speculation, if leptin-induced leptin resistance differentially impairs the leptin receptors involved in the reward properties of food ingestion as opposed to those involved in the homeostatic regulation of the caloric requirement for animals, then the consequences of the former aspect of the resistance would only be revealed with the introduction of an HF diet. The HF-induced hyperphagia is usually transient, indicating that diminished energy expenditure and/or increased food efficiency are other likely mechanisms underlying long-term leptin-resistance-mediated obesity. These ideas remain to be tested.
The DIO model is complex, and the relationship between leptin resistance and the development of this type of obesity is unclear. Data from Dietary Prone animals point to a potential causal role of preexisting deficient leptin receptor signaling (i.e., some degree of leptin resistance) in predisposing the animals to HF-induced obesity (31). Additionally, chronic leptin treatment in DIO rats exacerbates HF-induced obesity [Fig. 1D; (61)], an observation implicating a preferential disruption in counterregulation of motivational eating rather than homeostatic feeding. Lacking definitive evidence, our current discussion of the potential impact of leptin resistance on dietary obesity remains speculative and likely too simplistic, and further investigations in this regard are warranted. The quest ahead is to elucidate the exact factors initiating leptin resistance, the role of this resistance in hedonic feeding and adaptation to positive energy balance in relationship to obesity development, and more importantly, whether restoration of leptin responses in selective brain regions reverses DIO.
In today's society with an overabundance of readily available high-caloric food, leptin resistance is likely to be prevalent. As leptin resistance takes hold, each subsequent exposure to high-density food may escalate dietary weight gain, causing a vicious spiral of increasing obesity. The predictable devastating metabolic consequences associated with ever-perpetuating obesity underpin the importance in unraveling the mystery of leptin resistance for both the prevention and intervention of obesity.
GRANTS
This work was supported by the National Institutes of Health and the Medical Research Service of the Department of Veterans Affairs.
Acknowledgments
Major contributors to these studies were Michael Matheny, Jiejing Zhang, Melanie Judge, Alexandra Shapiro, Jared Wilsey, Gang Li, and Nihal Tümer.
REFERENCES
- 1.Attig L, Solomon G, Ferezou J, Abdennebi-Najar L, Taouis M, Gertler A, Djiane J. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int J Obes (Lond) 32: 1153–1160, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Augustine RA, Grattan DR. Induction of central leptin resistance in hyperphagic pseudopregnant rats by chronic prolactin infusion. Endocrinology 149: 1049–1055, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53: 1253–1260, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am J Physiol Endocrinol Metab 285: E10–E15, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bayol SA, Farrington SJ, Stickland NC. A maternal “junk food” diet in pregnancy and lactation promotes an exacerbated taste for “junk food” and a greater propensity for obesity in rat offspring. Br J Nutr 98: 843–851, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bellocchio L, Cervino C, Pasquali R, Pagotto U. The endocannabinoid system and energy metabolism. J Neuroendocrinol 20: 850–857, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12: 917–924, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjorbaek C, Myers MG Jr. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest 117: 1354–1360, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79: 537–543, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348: 159–161, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Chotiwat CSC, Teff K, Harris RBS. Feeding a high-fructose diet induces leptin resistance in rats (Abstract). Appetite 49: 284, 2007. [Google Scholar]
- 12.Cota D, Barrera JG, Seeley RJ. Leptin in energy balance and reward: two faces of the same coin? Neuron 51: 678–680, 2006. [DOI] [PubMed] [Google Scholar]
- 13.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105: 1827–1832, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci 1: 445–450, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Erlanson-Albertsson C How palatable food disrupts appetite regulation. Basic Clin Pharmacol Toxicol 97: 61–73, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Farooqi IS, O'Rahilly S. Monogenic obesity in humans. Annu Rev Med 56: 443–458, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Galaz C, Fernandez-Agullo T, Campoy F, Arribas C, Gallardo N, Andres A, Ros M, Carrascosa JM. Decreased leptin uptake in hypothalamic nuclei with ageing in Wistar rats. J Endocrinol 171: 23–32, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Galaz C, Fernandez-Agullo T, Perez C, Peralta S, Arribas C, Andres A, Carrascosa JM, Ros M. Long-term food restriction prevents ageing-associated central leptin resistance in Wistar rats. Diabetologia 45: 997–1003, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Franks PW, Brage S, Luan J, Ekelund U, Rahman M, Farooqi IS, Halsall I, O'Rahilly S, Wareham NJ. Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes Res 13: 1476–1484, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Grattan DR, Ladyman SR, Augustine RA. Hormonal induction of leptin resistance during pregnancy. Physiol Behav 91: 366–374, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94: 8878–8883, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282: 1568–1575, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51: 801–810, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10: 734–738, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Judge MK, Zhang J, Tumer N, Carter C, Daniels MJ, Scarpace PJ. Prolonged hyperphagia with high-fat feeding contributes to exacerbated weight gain in rats with adult-onset obesity. Am J Physiol Regul Integr Comp Physiol 295: R773–R780, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krol E, Tups A, Archer ZA, Ross AW, Moar KM, Bell LM, Duncan JS, Mayer C, Morgan PJ, Mercer JG, Speakman JR. Altered expression of SOCS3 in the hypothalamic arcuate nucleus during seasonal body mass changes in the field vole, Microtus agrestis. J Neuroendocrinol 19: 83–94, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Kronfeld-Schor N, Richardson C, Silvia BA, Kunz TH, Widmaier EP. Dissociation of leptin secretion and adiposity during prehibernatory fattening in little brown bats. Am J Physiol Regul Integr Comp Physiol 279: R1277–R1281, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Levin BE Factors promoting and ameliorating the development of obesity. Physiol Behav 86: 633–639, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283: R941–R948, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 286: R143–R150, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Levin BE, Dunn-Meynell AA, Ricci MR, Cummings DE. Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am J Physiol Endocrinol Metab 285: E949–E957, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Levin BE, Patterson CM. Exercising the obese brain: resetting the defended body weight. Endocrinology 146: 1674–1675, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Matheny M, Nicolson M, Tumer N, Scarpace PJ. Leptin gene expression increases with age independent of increasing adiposity in rats. Diabetes 46: 2035–2039, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord 24: 639–646, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Martin RL, Perez E, He YJ, Dawson R Jr, Millard WJ. Leptin resistance is associated with hypothalamic leptin receptor mRNA and protein downregulation. Metabolism 49: 1479–1484, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Matias I, Cristino L, Di Marzo V. Endocannabinoids: some like it fat (and sweet too). J Neuroendocrinol 20, Suppl 1:100–109, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Mitra A, Alvers KM, Crump EM, Rowland NE. Effect of high-fat diet during gestation, lactation, or postweaning on physiological and behavioral indexes in borderline hypertensive rats. Am J Physiol Regul Integr Comp Physiol 296: R20–R28, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montez JM, Soukas A, Asilmaz E, Fayzikhodjaeva G, Fantuzzi G, Friedman JM. Acute leptin deficiency, leptin resistance, and the physiologic response to leptin withdrawal. Proc Natl Acad Sci USA 102: 2537–2542, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10: 739–743, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Morrison CD Leptin resistance and the response to positive energy balance. Physiol Behav 94: 660–663, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munzberg H, Myers MG Jr. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci 8: 566–570, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70: 537–556. 2008. [DOI] [PubMed] [Google Scholar]
- 44.Ogus S, Ke Y, Qiu J, Wang B, Chehab FF. Hyperleptinemia precipitates diet-induced obesity in transgenic mice overexpressing leptin. Endocrinology 144: 2865–2869, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med 346: 570–578, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Park JY, Chong AY, Cochran EK, Kleiner DE, Haller MJ, Schatz DA, Gorden P. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: the effect of long-term leptin therapy. J Clin Endocrinol Metab 93: 26–31, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prpic V, Watson PM, Frampton IC, Sabol MA, Jezek GE, Gettys TW. Differential mechanisms and development of leptin resistance in A/J versus C57BL/6J mice during diet-induced obesity. Endocrinology 144: 1155–1163, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Qiu J, Ogus S, Lu R, Chehab FF. Transgenic mice overexpressing leptin accumulate adipose mass at an older, but not younger, age. Endocrinology 142: 348–358, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Sahu A Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J Neuroendocrinol 14: 796–804, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Sahu A, Metlakunta AS. Hypothalamic phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP pathway of leptin signalling is impaired following chronic central leptin infusion. J Neuroendocrinol 17: 720–726, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Scarpace PJ, Matheny M, Moore RL, Tumer N. Impaired leptin responsiveness in aged rats. Diabetes 49: 431–435, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Scarpace PJ, Matheny M, Shek EW. Impaired leptin signal transduction with age-related obesity. Neuropharmacology 39: 1872–1879, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Scarpace PJ, Matheny M, Tumer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience 104: 1111–1117, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Scarpace PJ, Matheny M, Tumer N, Cheng KY, Zhang Y. Leptin resistance exacerbates diet-induced obesity and is associated with diminished maximal leptin signalling capacity in rats. Diabetologia 48: 1075–1083, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Scarpace PJ, Matheny M, Zhang Y, Cheng KY, Tumer N. Leptin antagonist reveals an uncoupling between leptin receptor signal transducer and activator of transcription 3 signaling and metabolic responses with central leptin resistance. J Pharmacol Exp Ther 320: 706–712, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Scarpace PJ, Matheny M, Zhang Y, Shek EW, Prima V, Zolotukhin S, Tumer N. Leptin-induced leptin resistance reveals separate roles for the anorexic and thermogenic responses in weight maintenance. Endocrinology 143: 3026–3035, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Scarpace PJ, Matheny M, Zhang Y, Tumer N, Frase CD, Shek EW, Hong B, Prima V, Zolotukhin S. Central leptin gene delivery evokes persistent leptin signal transduction in young and aged-obese rats but physiological responses become attenuated over time in aged-obese rats. Neuropharmacology 42: 548–561, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Scarpace PJ, Matheny M, Zolotukhin S, Tumer N, Zhang Y. Leptin-induced leptin resistant rats exhibit enhanced responses to the melanocortin agonist MT II. Neuropharmacology 45: 211–219, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Scarpace PJ, Matheny M, Shapiro A, Zhang Y. Leptin antagonist overexpression reduces voluntary wheel running and demonstrates the importance of fully responsive leptin receptors in energy homeostasis. In: Proceedings of the Obesity Society's 2008 Annual Scientific Meeting, Phoenix, AZ, 2008, P0146.
- 60.Schwartz R Obesity in the Elderly. New York: Dekker, 1998.
- 61.Shapiro A, Matheny M, Zhang Y, Tumer N, Cheng KY, Rogrigues E, Zolotukhin S, Scarpace PJ. Synergy between leptin therapy and a seemingly negligible amount of voluntary wheel running prevents progression of dietary obesity in leptin-resistant rats. Diabetes 57: 614–622, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol 295: R1370–R1375, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tups A, Ellis C, Moar KM, Logie TJ, Adam CL, Mercer JG, Klingenspor M. Photoperiodic regulation of leptin sensitivity in the Siberian hamster, Phodopus sungorus, is reflected in arcuate nucleus SOCS-3 (suppressor of cytokine signaling) gene expression. Endocrinology 145: 1185–1193, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR Jr. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 99: 385–390, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Widdowson PS, Upton R, Buckingham R, Arch J, Williams G. Inhibition of food response to intracerebroventricular injection of leptin is attenuated in rats with diet-induced obesity. Diabetes 46: 1782–1785, 1997. [DOI] [PubMed] [Google Scholar]
- 66.Wilsey J, Matheny MK, Scarpace PJ. Oral vanadium enhances the catabolic effects of central leptin in young adult rats. Endocrinology 147: 493–501, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Matheny MK, Tumer N, Mitchell MK, Scarpace PJ. Leptin antagonist reveals that the normalization of caloric intake and the thermic effect of food after high-fat feeding are leptin dependent. Am J Physiol Regul Integr Comp Physiol 292: R868–R874, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Scarpace PJ. Circumventing central leptin resistance: lessons from central leptin and gene delivery. Peptides 27: 350–364, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav 88: 249–256, 2006. [DOI] [PubMed] [Google Scholar]