Abstract
Diabetes is a growing public health concern, and animal models of this disease are necessary for a full understanding of disease pathogenesis, progression, clinical sequelae, and treatment options. In particular, nonhuman primate models of diabetes are important because of their close genetic relationship to humans. Although numerous Old World primate models have been described, few studies have examined the possibility of using New World monkeys as an animal model for this disease. Streptozotocin (STZ) is a common diabetogenic drug that selectively destroys beta cells after uptake via the GLUT2 glucose transporter. Induction of diabetes using STZ was attempted in common marmosets (Callithrix jacchus). These animals showed increases in blood glucose consistent with diabetes only at STZ doses markedly greater than those used in other primate species. Additionally, all animals showed pathological evidence of acute renal and liver toxicity secondary to the treatment. In a subsequent comparative study of various nonhuman primates, GLUT2 immunostaining in pancreatic islets was used as a marker for sensitivity to STZ. Immunostaining of islets from a variety of nonhuman primate species indicated a reduced expression of pancreatic GLUT2 in New compared with Old World monkeys; this finding explains their resistance to diabetic induction with STZ. Furthermore, there were age-dependent differences in GLUT2 expression, with aged and infant macaques showing reduced expression. We conclude that New World monkeys are an inappropriate model for diabetes induction with STZ and that, with all primate species, it is important to consider the animals’ age before diabetic induction with STZ is attempted.
Keywords: diabetes mellitus, streptozotocin, common marmoset
diabetes is a disease that affects millions of people worldwide, and its prevalence is growing. The significant increase in the annual incidence of diabetes mellitus in the past 30 years indicates that this chronic disease is a growing public health concern (13, 18). Animal models of diabetes provide an important means by which to study disease pathogenesis, progression, sequelae, and various treatment options. Nonhuman primate models are particularly relevant because of their close genetic and physiological similarity to humans.
One of the common models of diabetic induction is selective destruction of beta cells with the chemotherapeutic agent streptozotocin (STZ). STZ is a well-known diabetogenic drug consisting of a methyl-nitrosurea group linked to d-glucose. It selectively destroys pancreatic beta cells by DNA alkylation and depletion of NAD (15, 22). It is commonly used to induce a diabetes-like condition in rodent models, as well as in several species of Old World nonhuman primates (OWP), including cynomolgus macaques, rhesus macaques, vervet monkeys, pig-tailed macaques, and baboons (2, 11, 23).
Here we describe a pilot study designed to investigate the potential to develop a New World primate (NWP) model of diabetes by chemical induction with STZ. An NWP model would have several advantages over OWP models: ease of handling, reduced space requirements, and decreased risk of zoonotic disease exposure. The diabetogenic potential of STZ in the common marmoset, an NWP routinely utilized in biomedical research, was examined. STZ proved to be ineffective at inducing sustained elevations in blood glucose in the marmoset, resulting in our hypothesis that there exists a natural species-specific variation in the susceptibility of pancreatic islet cells to STZ-induced toxicity.
Numerous studies have shown that the action of STZ is dependent on the action of the GLUT2 glucose transporter (2–5, 9, 17). GLUT2 is 1 of 11 identified glucose transporters (GLUT). The class I facilitative transporters, GLUT1, GLUT2, GLUT3, and GLUT4, differ in their kinetics, tissue expression, and glucose specificity. GLUT2 is a low-affinity transporter with a high Km, so it is expressed predominantly in kidney, pancreas, and liver to allow fast equilibration of glucose (and subsequent release of insulin) with a constant number of receptors (20, 24). The glucose moiety in the structure of STZ interacts with the GLUT2 transporter, facilitating transport into the cell. GLUT2-knockout mice are not susceptible to STZ (9). Similarly, species with low GLUT2 expression in their pancreas, such as humans, are highly resistant to the diabetogenic effect of STZ (5). Various studies have shown that the degree of GLUT2 expression in pancreatic islet cells correlates to the dose of STZ needed to induce diabetes, with higher GLUT2 expression corresponding to lower necessary doses (2, 5, 9).
We suggest that the inability to induce diabetes in common marmosets may be due to species-specific variation in GLUT2 expression in the pancreatic islets. In an effort to investigate this hypothesis, we used immunohistochemical methods to study GLUT2 expression in various species of primates, including common marmosets, cotton-top tamarins, squirrel monkeys, owl monkeys, vervet monkeys, cynomolgus macaques, and rhesus macaques.
MATERIALS AND METHODS
STZ treatment in common marmosets.
The diabetogenic potential of STZ was assayed in a group of four common marmosets. All animals were housed at the New England Primate Research Center and maintained in accordance with the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. The facility is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International, and all work was approved by Harvard Medical School's Standing Committee on Animals.
Animals were dosed with STZ (Sigma-Aldrich, St. Louis, MO) delivered via an intravenous catheter placed in the saphenous vein under ketamine sedation (15 mg/kg im; Ketaset, Fort Dodge, Overland Park, KS). STZ was reconstituted in 20 mM sodium citrate buffer at pH 4.5 and administered immediately after preparation via intravenous bolus. Target dose was based on a reported effective cumulative dose range of 1,465–1,800 mg/m2 in cynomolgus macaques (1, 16). Marmosets A and B were dosed with STZ at 120 mg/kg on days 0 and 21. This treatment course was designated low-dose STZ, with a cumulative dose of 1,440 mg/m2. Moderate-dose treatment was subsequently attempted in marmosets C and D, with a dose of 90 mg/kg administered on days 0 and 14 followed by 100 mg/kg on days 28 and 63, for a cumulative dose of 2,280 mg/m2. An additional dose of 120 mg/kg was administered to marmosets C and D on day 105, which brought the cumulative dose for marmosets C and D to 3,000 mg/m2 and was designated high-dose STZ. After each STZ dosing, 0.9% NaCl was administered intravenously at a rate of 5 ml/h for a total of 10 ml. Hand-caught marmosets were lightly sedated with ketamine, and blood samples were collected by routine phlebotomy techniques. All samples were collected in the early morning before feeding but without an overnight fast. Blood glucose levels were assayed using an i-STAT hand-held blood chemistry analyzer (Abbott Point of Care, East Windsor, NJ). Baseline blood glucose ranges were established on the basis of samples collected from study animals at four to six time points: 147.03–241.85 [194.44 (SD 47.41)] mg/dl. Animals were euthanized by intravenous pentobarbital sodium overdose for evaluation of histopathological changes in pancreas, liver, and kidneys.
Case selection and tissue samples.
Archival, formalin-fixed, paraffin-embedded tissues from 55 animals were included in this study. All cases had been previously submitted for necropsy at the New England Primate Research Center. Samples were chosen on the basis of species, age, and histological identification of pancreas, kidney, and brain without evidence of significant pathology or autolysis. Sections of pancreas stained with hematoxylin-eosin were additionally screened and excluded on the basis of the presence of pancreatic islet amyloid deposition. Species examined included common marmosets (Callithrix jacchus), cotton-top tamarins (Saguinus oedipus), owl monkeys (Aotus trivurgatus), squirrel monkeys (Saimiri sciureus), rhesus macaques (Macaca mulatta), cynomolgus macaques (Macaca fascicularis), and vervet monkeys (Cercopithecus aethiops). Three to four animals per species were selected, and the pancreas from each animal was evaluated for GLUT2 expression. Kidneys were examined in a subset of animals. For evaluation of variation in GLUT2 expression by age, 21 marmosets and 13 rhesus monkeys of various ages were examined. Samples were chosen to include the following age classifications: neonates, infants, juveniles, adults, and aged adults.
GLUT2 and insulin immunohistochemistry.
For GLUT2 and insulin immunohistochemistry, 5-μm sections were deparaffinized in xylene, rehydrated in graded alcohol, and rinsed in distilled water. Sections were incubated with 3% hydrogen peroxide for 5 min to block endogenous peroxidases and rinsed in Tris-buffered saline for 5 min. For GLUT2 immunostaining, heat-induced epitope retrieval was accomplished by 20 min of heating in a microwave oven; then the samples were cooled for 20 min and rinsed. Nonspecific antigen binding was blocked by incubation of slides for 10 min with a commercially available protein blocking solution (Dako Cytomation, Carpinteria, CA). Slides were incubated for 30 min with rabbit anti-GLUT2 polyclonal antibody (Chemicon International, Temecula, CA) at a dilution of 1:5,000 or rabbit anti-insulin polyclonal antibody (Dako Cytomation) at a dilution of 1:150. Rabbit immunoglobulin fraction at a dilution of 1:15,000 for GLUT2 or 1:288 for insulin was used to stain irrelevant control slides. Sections were washed for 5 min and incubated for 30 min with biotinylated goat anti-rabbit immunoglobulins at a dilution of 1:200. Color was developed for 30 min using freshly prepared ABC Elite solution (Vectastain Elite ABC Kit, Vector Laboratories), and diaminobenzidine tetrahydrochloride dihydrate was applied as a chromogen, as previously described (8). Sections were counterstained with Mayer's hematoxylin, dehydrated through graded ethanol, and cleared in xylene, and coverslips were applied.
Immunohistochemical evaluation.
Sections were visualized for determination of positivity and scored using an ordinal scale. Within pancreatic islets, the scoring system was based on the number of GLUT2-positive cells within pancreatic islets. A negative score indicated a lack of staining in the vast majority of islets examined. A score of + corresponded to few or scattered positive cells in occasional islets, ++ to samples with moderate staining in most islets, and +++ to samples with strong staining in all islets.
A similar system was used for kidney sections, with a negative score indicating no observed staining, + corresponding to weak or scattered staining, ++ corresponding to moderate staining in localized areas, and +++ corresponding to strong staining in most areas. The scorer was blinded to the species of animal at the time of scoring.
Staining of cerebral neurons and/or myenteric plexus neurons was used for positive control tissue (21).
RESULTS
High-dose STZ treatment required to induce sustained hyperglycemia.
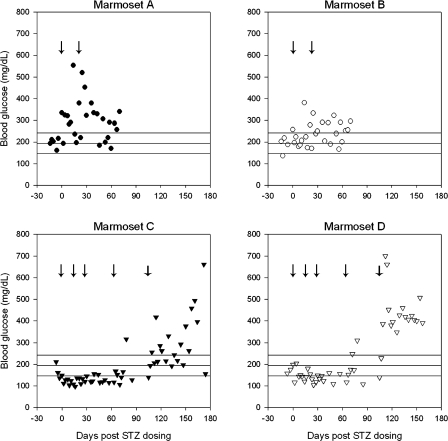
Although marmosets treated with low-dose STZ (cumulative dose 1,440 mg/m2) demonstrated higher mean blood glucose levels over the course of treatment, increases in blood glucose were not sustained. Low-dose STZ-treated animals demonstrated a mean blood glucose of 272.10 (SD 86.24) mg/dl compared with the mean baseline value of 194.44 (SD 47.41) mg/dl (P < 0.0001 by 2-tailed Student's t-test). Animals developed measured increases in blood glucose that ranged as high as a maximum of 554 mg/dl for marmoset A and 381 mg/dl for marmoset B each on day 14 after dosing. Despite these increases, only 56.0% of measures were considered above the baseline blood glucose range for samples collected over the course of the low-dose STZ treatment.
Marmosets C and D did not develop sustained elevations in blood glucose following the moderate-dose STZ regimen, consisting of a cumulative STZ dose of 2,280 mg/m2. In fact, mean blood glucose levels were lower than baseline over the course of treatment until day 105, with treated animals demonstrating a mean blood glucose of 143.00 (SD 42.08) mg/dl (P < 0.00001 by 2-tailed Student's t-test). For the high-dose STZ regimen, a fifth dose of STZ was administered on day 105, resulting in a cumulative dose of 3,000 mg/m2. After this final dose, marmosets C and D demonstrated significant elevations in blood glucose levels over baseline, with a mean of 360.63 (SD 135.06) mg/dl (P < 0.00001 by 2-tailed Student's t-test). This regimen was more effective for marmoset D, with 95.4% of values measuring above the baseline blood glucose range, than for marmoset C, with 63.6% of values measuring above the baseline blood glucose range. Blood glucose measures and STZ doses are summarized in Table 1. The longitudinal trends in blood glucose following STZ administration are depicted in Fig. 1.
Table 1.
Mean blood glucose and percent blood glucose above baseline range for marmosets treated with high, moderate, and low doses of STZ
| Cumulative Dose, mg/m2 | Treatment Course | Blood Glucose, mg/dl | Values Above Normal Blood Glucose Range, n (%) | |
|---|---|---|---|---|
| Baseline | 0 | 194.44 (47.41) | 4 of 20 (20.0%) | |
| Low-dose STZ | 1,440 | 90 mg/kg on days 0 and 21 | 272.10 (86.24) | 28 of 50 (56.0%) |
| Marmoset A | 303.28 (99.41) | 17 of 25 (68.0%) | ||
| Marmoset B | 240.92 (57.26) | 11 of 25 (44.0%) | ||
| Moderate-dose STZ | 2,280 | 90 mg/kg on days 0 and 14; 100 mg/kg on days 28 and 63 | 143.00 (42.08) | 8 of 62 (12.9%) |
| Marmoset C | 134.73 (38.66) | 2 of 31 (6.4%) | ||
| Marmoset D | 152.18 (44.50) | 6 of 31 (19.4%) | ||
| High-dose STZ | 3,000 | Above doses+120 mg/kg on day 105 | 360.63 (135.06) | 35 of 44 (79.5%) |
| Marmoset C | 300.81 (125.82) | 14 of 22 (63.6%) | ||
| Marmoset D | 434.53 (109.08) | 21 of 22 (95.4%) |
Values were calculated over the course of treatment as follows: for low-dose streptozotocin (STZ) beginning on day 2, for moderate-dose STZ from day 2 to day 105, and for high-dose STZ beginning on day 107. Blood glucose values are means (SD). Baseline blood glucose range =147.03-241.85 mg/dl.
Fig. 1.
Longitudinal changes in blood glucose after streptozotocin (STZ) administration in the common marmoset. Horizontal lines indicate mean blood glucose for healthy control marmosets and upper and lower limits of baseline blood glucose based on 1 SD from the mean (n = 8 surveyed at 2 time points). Arrow indicates STZ dosing points for each animal. Thicker arrows for marmosets C and D correspond to high-dose treatment at day 105.
Histopathology.
For further investigation of the effect of STZ on renal and pancreatic pathology, marmosets A and B were humanely euthanized at 70 days after STZ administration. Marmoset C was euthanized at day 174 and marmoset D at day 157 after STZ administration. Complete necropsies and histopathological examinations were performed.
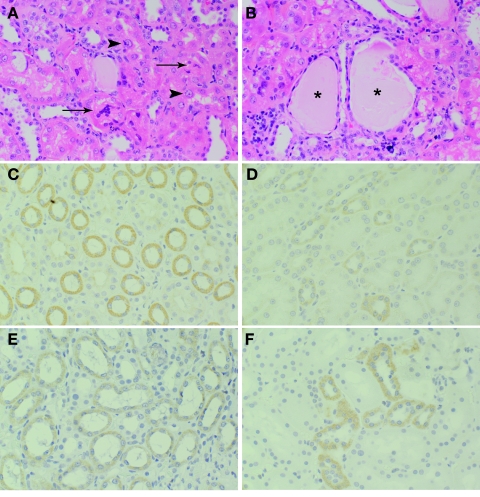
All animals showed evidence of renal tubular degeneration and necrosis characterized by marked anisocytosis and anisokaryosis of tubular epithelial cells, cytoplasmic vacuolization and cellular swelling, and desquamation of epithelial cells into tubular lumina (Fig. 2). There was multifocal dilation of tubules and collecting tubules by cellular and proteinaceous casts. In two cases, mild lymphoplasmacytic interstitial infiltrate and multifocal random mineralization of renal tubules were observed. In all cases, there was evidence of a regenerative response, including flattened tubular epithelial cells with plump nuclei and prominent nucleoli. The changes were more severe in the two animals that received high STZ doses at day 105 (marmosets C and D). The renal histopathological observations are most consistent with a nephrotoxic insult.
Fig. 2.
A and B: hematoxylin-eosin-stained sections of kidneys from STZ-treated marmosets. Note tubular degeneration and necrosis with sloughing of necrotic epithelial cells into tubular lumina (arrow), cellular swelling and vacuolation (arrowhead), and dilation of tubules by proteinaceous fluid (*). C–F: GLUT2 staining in kidney of cynomolgus macaques (C and D) and common marmosets (E and F). Note diffuse positive staining in epithelial cells of proximal convoluted tubules (D and F) and collecting tubule epithelial cells (C and E).
Animals also showed evidence of hepatocellular degeneration and necrosis characterized by severe multifocal hepatocyte vacuolation, anisocytosis and anisokaryosis with marked cytomegaly, individual hepatocytic necrosis, and focal areas of hemorrhage and neutrophilic inflammation. These changes were most prominent in the animals treated with high doses of STZ (marmosets C and D).
Pancreases from the treated animals were morphologically unremarkable. There was no evidence of necrosis, degeneration, beta cell proliferation, or islet inflammation.
Insulin staining.
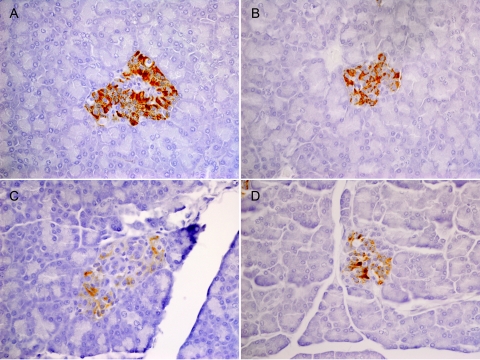
Pancreatic samples collected from all marmosets were immunostained with insulin antibody. Marmosets A and B showed diffuse, positive reactivity in islets, despite treatment with STZ, indicating that the insulin-producing beta cells were not destroyed by the low-dose STZ treatment. In the two animals that received higher STZ doses at day 105, marmosets C and D, insulin staining was retained but was less intense than that observed in the other animals (Fig. 3).
Fig. 3.
Insulin staining of marmoset islets after low-dose (A and B) and high-dose (C and D) STZ treatment. Note slight reduction in staining intensity after high-dose STZ but overall retention of insulin production after low- and high-dose STZ.
Pancreatic GLUT2 expression between species.
We examined 22 archived samples from a variety of nonhuman primate species, including common marmosets (n = 3), rhesus macaques (n = 3), cynomolgus macaques (n = 4), squirrel monkeys (n = 2), owl monkeys (n = 3), cotton-top tamarins (n = 3), and vervet monkeys (n = 4), to determine species differences in GLUT2 expression.
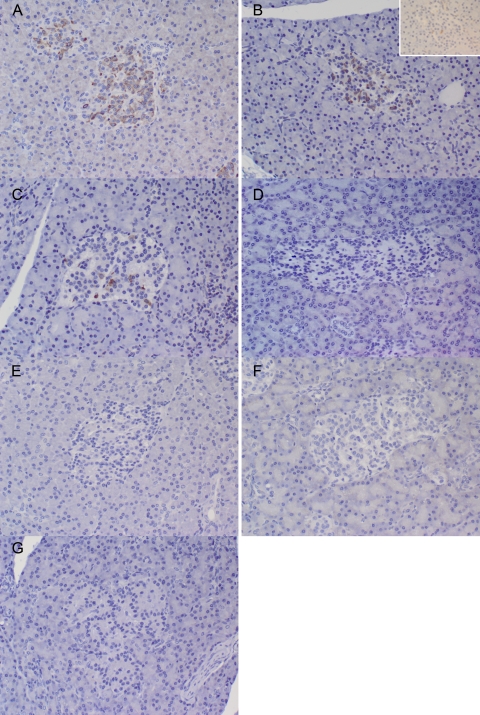
Immunohistochemistry results comparing species differences in GLUT2 expression are summarized in Table 2. All species showed staining in neurons of the cerebral cortex, which acted as a positive control. In New World monkeys, staining was nearly uniformly negative for GLUT2 in the pancreatic islet cells (Fig. 4). In one tamarin and one owl monkey, there was occasional weak GLUT2-positive staining in scattered islets. In contrast, in most Old World species, staining was diffusely and strongly positive in islet tissue (Fig. 4). The lone exception is vervet monkeys, in which staining was mostly weak in the islet tissue.
Table 2.
Staining of islet and kidney tissues in New and Old World monkeys
| Animal No. |
Staining Intensity |
|
|---|---|---|
| Islets | Kidneys | |
| Macaca fascicularis | ||
| 1 | ++ | ++ |
| 2 | +++ | +++ |
| 3 | +++ | NE |
| 4 | ++ | NE |
| Macaca mulatta | ||
| 5 | ++ | ++ |
| 6 | ++ | ++ |
| 7 | +++ | NE |
| Cercopithecus aethiops | ||
| 8 | + | ++ |
| 9 | + | ++ |
| Saguinus oedipus | ||
| 10 | Weak + | ++ |
| 11 | − | + |
| 12 | − | NE |
| Callithrix jacchus | ||
| 13 | − | ++ |
| 14 | − | ++ |
| 15 | − | + |
| Saimiri sciureus | ||
| 16 | − | NE |
| 17 | − | ++ |
| Aotus trivurgatus | ||
| 18 | − | ++ |
| 19 | Weak + | NE |
| 20 | − | ++ |
Staining intensity was scored as follows: −, no staining; +, ++, and +++, mild, moderate, and intense staining, respectively. NE, not examined.
Fig. 4.
Differential staining with GLUT2 immunostain of pancreatic islet cells from New and Old World primates. A–C: positive staining in cynomolgus macaques, rhesus macaques, and vervet monkeys, respectively. Inset in B: staining in aged rhesus macaque, with less staining intensity than in young adult macaque in main image. D, E, F, and G: negative staining results in common marmosets, owl monkeys, squirrel monkeys, and cotton-top tamarins, respectively. Note less intense staining in vervet than in rhesus or cynomolgus islet cells.
Kidney GLUT2 staining.
All animals’ kidneys (14 of 14) stained positively for GLUT2 expression, with the majority revealing moderate to strong signal. In most animals, the most intense staining was observed in collecting tubule epithelial cells, although staining was observed in proximal convoluted tubule epithelium as well in all animals (Fig. 2).
Age group GLUT2 expression.
Several studies in humans and rodents suggested that age is a factor in susceptibility to STZ, possibly related to changes in glucose transport with age (2, 19, 25). Tissues from 21 additional marmosets of various ages were evaluated for pancreatic islet GLUT2 expression. Marmosets are generally divided into five main age groups: neonates (<1 mo of age), infants (1–6 mo of age), juveniles (<2 yr old), adults (2–7.5 yr old), and aged adults (>7.5 yr old). No GLUT2 staining was observed in pancreatic islet tissue of any of the animals.
In agreement with previous studies utilizing human and rodent tissues, GLUT2 expression varied with age in islet tissue from rhesus macaques. In general, GLUT2 expression was diminished in very young and very old animals compared with juvenile and adult rhesus monkeys, which showed moderate to strong GLUT2 expression (Fig. 4). The results are summarized in Table 3.
Table 3.
Rhesus macaque islet cell GLUT2 expression by age group
| Age Group | GLUT2 Expression |
|---|---|
| Neonate | |
| 0.03 yr | − |
| 0.03 yr | + |
| Infant | |
| 0.2 yr | + |
| 0.4 yr | + |
| Juvenile | |
| 1.6 yr | +++ |
| 1.7 yr | ++ |
| 2.8 yr | ++ |
| 2.8 yr | +++ |
| 4 yr | +++ |
| Adult | |
| 5.3 yr | ++ |
| 9 yr | ++ |
| Older adult | |
| 16 yr | + |
| 17 yr | + |
| Aged adult | |
| 19 yr | + |
| 19 yr | + |
| 20 yr | ++ |
Staining intensity was scored as described in Table 2 footnote.
DISCUSSION
Our results suggest that pancreatic islet GLUT2 expression varies markedly between New and Old World species of monkeys and correlates well with observed species susceptibility to the diabetogenic effects of STZ. Low levels of pancreatic GLUT2 expression coupled with high levels of expression in renal tubules explains not only the apparent resistance to STZ demonstrated by common marmosets but also the renal toxicity. In these animals, blood glucose elevations were only seen in marmosets treated with STZ at nearly twice the effective dose used in cynomolgus macaques, while all animals showed histopathological evidence of acute renal tubular necrosis and hepatic necrosis, regardless of STZ dose. Additionally, staining of pancreatic islets with insulin antibody was only somewhat affected by STZ treatment, indicating that STZ was minimally effective at destroying insulin-producing pancreatic beta cells. The toxic side effects of STZ in this species outweigh the diabetogenic effects.
In contrast to the observations in marmosets, pancreatic GLUT2 expression was much greater in OWP and expression was greater in the pancreas than in renal tubules. There are a number of reports showing that OWP are susceptible to STZ-induced diabetes, with minimal to no side effects at appropriate doses (2, 11, 23, 25). At higher STZ doses, cynomolgus macaques are reported to exhibit severe renal tubular necrosis (2).
It is likely that animals are able to compensate for subclinical renal damage at low STZ doses and regenerate damaged epithelium, whereas higher STZ doses result in irreversible damage. This theory is supported by the finding in humans that mild renal defects are reversible but more serious deficits due to continued drug treatment result in irreversible renal toxicity (22). Although limited dose regimens were examined in the marmoset, because of the profound lack of GLUT2 expression in NWP islets relative to renal expression, we submit that the threshold for renal toxicity would be exceeded before that of the pancreatic beta cells. Subsequently, higher doses of STZ would result in renal tubular necrosis before any measurable and long-lasting increases in blood glucose concentrations. This lack of beta cell selectivity limits the usefulness of STZ in the species of NWP examined.
It is surprising that mean blood glucose over the course of treatment was higher in the low- than in the moderate-dose STZ-treated marmosets. The moderate-dose STZ group also demonstrated a lower mean blood glucose over the course of treatment than at baseline. Possible explanations for this variability include alterations in blood glucose metabolism, alterations in food intake secondary to STZ-associated renal or hepatic toxicity, or increased loss of glucose into the urine through damaged renal tubular epithelium, resulting in glucosuria and decreased blood glucose. Because it is difficult to exclude confounding due to associated STZ toxicity, the usefulness of STZ for induction of a diabetes-like state in the common marmoset is limited.
Alloxan is another diabetogenic drug commonly utilized in animal models of diabetes mellitus. In contrast to STZ, alloxan affects the glucokinase transporter and GLUT2 transporter and causes beta cell death via the generation of toxic free radicals. However, prior transport into the cell via GLUT2 for subsequent redox cycling is necessary for this toxic mechanism. As with STZ, mouse GLUT2-knockout cell lines are highly resistant to the toxic effects of alloxan (3, 4, 6, 9, 10). This similar mechanism of transport suggests that alloxan may also demonstrate a lack of effectiveness in NWP.
NWP and OWP diverged from each other ∼35–40 million years ago and have undergone numerous taxonomic divergences within their respective groups. Humans and the great apes deviated from Old World monkeys much later, ∼27 million years ago (14). Surprisingly, responses of humans to STZ and GLUT2 expression profiles are similar to those of NWP, although they are more closely related to those of OWP (5, 25). This implies that 1) there were two separate evolutionary events leading to a loss of pancreatic GLUT2 expression in NWP and humans or 2) OWP gained pancreatic GLUT2 expression after diverging from the human/great ape lineage.
Previous reports have also noted differences in STZ susceptibility depending on animals’ age, with younger animals having decreased sensitivity to STZ's toxic effects (2, 19, 25). Here, we show low expression of GLUT2 in the islets of very young and very old rhesus macaques compared with juvenile and adult macaques. This age discrepancy also explains the relatively low level of GLUT2 staining in islets from vervet monkeys. The only available vervet tissue was from aged vervet monkeys, and it is likely that younger animals would have shown stronger immunostaining for GLUT2. It is well reported that vervets are susceptible to the diabetogenic and toxic effects of STZ (12, 23). Age should, therefore, play an important factor when animals are selected for diabetes induction using STZ or alloxan.
Spontaneous type 2 diabetes in the aged rhesus macaque is a well-described model and recapitulates many of the symptoms of diabetes in humans. Affected macaques begin to demonstrate evidence of impaired glucose tolerance, insulin resistance, and hyperinsulinemia at ≥10 yr of age, with a subset of animals developing reduced fasting insulin levels and progressing to overt diabetes (7). Although GLUT2 expression is reduced in aged macaques, it is not known whether the metabolic changes associated with this naturally occurring type 2 diabetes would be exacerbated by the administration of STZ.
On the basis of these results, we propose that NWP are not an appropriate choice of species for STZ induction of diabetes. The toxic side effects of the drug and inability to induce sustained hyperglycemia preclude the use of STZ in this species. Although there would be many benefits to this model, the inability to induce diabetes in marmosets without undue toxicity and lack of transporter expression in squirrel monkeys, owl monkeys, and tamarins suggest that different agents, surgical pancreatectomy, or dietary models be should be attempted in these species.
Perspectives and Significance
Although nonhuman primate animal models of disease are highly beneficial because of their genetic and physiological similarity to humans, the phylogenetic divergences among groups of nonhuman primates contribute to significant species-specific differences. Here we have shown a wide variation in pancreatic islet expression of GLUT2 among OWP and NWP and suggest that this variation limits the usefulness of the STZ-induced diabetes model in the NWP. Although the NWP continues to hold promise as a model for the study of diabetes, further work should focus on mechanisms of glucose transport at the level of the beta cell in these species and on development of dietary models.
GRANTS
This work was supported by National Center for Research Resources Grant P51 RR000168-47.
Acknowledgments
Present address of E. L. Moeller: Massachusetts General Hospital, Center for Comparative Medicine, Charlestown, MA 02129.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Campanile N, Rood PP, Yeh P, Casu A, Bottino R, Cooper DK. Acute gastric dilatation after porcine islet transplantation in a cynomolgus monkey—case history and review of the literature. Xenotransplantation 14: 265–270, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Dufrane D, van Steenberghe M, Guiot Y, Goebbels RM, Saliez A, Gianello P. Streptozotocin-induced diabetes in large animals (pigs/primates): role of GLUT2 transporter and beta-cell plasticity. Transplantation 81: 36–45, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Elsner M, Guldbakke B, Tiedge M, Munday R, Lenzen S. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia 43: 1528–1533, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Elsner M, Tiedge M, Guldbakke B, Munday R, Lenzen S. Importance of the GLUT2 glucose transporter for pancreatic beta cell toxicity of alloxan. Diabetologia 45: 1542–1549, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Elsner M, Tiedge M, Lenzen S. Mechanism underlying resistance of human pancreatic beta cells against toxicity of streptozotocin and alloxan. Diabetologia 46: 1713–1714, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Gai W, Schott-Ohly P, Schulte IM, Walde S, Gleichmann H. Differential target molecules for toxicity induced by streptozotocin and alloxan in pancreatic islets of mice in vitro. Exp Clin Endocrinol Diabetes 112: 29–37, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Hansen B, Tigno X. The rhesus monkey (Macaca mulatta) manifests all features of human type 2 diabetes. In: Animal Models of Diabetes: Frontiers in Research, edited by Shafrir E. Boca Raton, FL: CRC, 2007, p. 251–270.
- 8.Horvath CJ, Hunt RD, Simon MA, Sehgal PK, Ringler DJ. An immunohistologic study of granulomatous inflammation in SIV-infected rhesus monkeys. J Leukoc Biol 53: 532–540, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Hosokawa M, Dolci W, Thorens B. Differential sensitivity of GLUT1- and GLUT2-expressing beta cells to streptozotocin. Biochem Biophys Res Commun 289: 1114–1117, 2001. [DOI] [PubMed] [Google Scholar]
- 10.im Walde SS, Dohle C, Schott-Ohly P, Gleichmann H. Molecular target structures in alloxan-induced diabetes in mice. Life Sci 71: 1681–1694, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Jonasson O, Jones CW, Bauman A, John E, Manaligod J, Tso MO. The pathophysiology of experimental insulin-deficient diabetes in the monkey. Implications for pancreatic transplantation. Ann Surg 201: 27–39, 1985. [PMC free article] [PubMed] [Google Scholar]
- 12.Kavanaugh K, Flynn M, Zhang L, Collins J, Rankin S, Sajuthi A, Butler PC, Wagner JD. Development of a nonhuman primate model of type 1 diabetes in African green monkeys (Abstract). JAALAS 46: 126, 2007. [Google Scholar]
- 13.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of type 1 diabetes—the analysis of the data on published incidence trends. Diabetologia 42: 1395–1403, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Purvis A A composite estimate of primate phylogeny. Philos Trans R Soc Lond B Biol Sci 348: 405–421, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Rerup CC Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev 22: 485–518, 1970. [PubMed] [Google Scholar]
- 16.Rood PP, Bottino R, Balamurugan AN, Smetanka C, Ezzelarab M, Busch J, Hara H, Trucco M, Cooper DK. Induction of diabetes in cynomolgus monkeys with high-dose streptozotocin: adverse effects and early responses. Pancreas 33: 287–292, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes 43: 1326–1333, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Sloan FA, Bethel MA, Ruiz D Jr, Shea AH, Feinglos MN. The growing burden of diabetes mellitus in the US elderly population. Arch Intern Med 168: 192–199, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Theriault BR, Thistlethwaite JR Jr, Levisetti MG, Wardrip CL, Szot G, Bruce DS, Rilo H, Li X, Gray GS, Bluestone JA, Padrid PA. Induction, maintenance, and reversal of streptozotocin-induced insulin-dependent diabetes mellitus in the juvenile cynomolgus monkey (Macaca fascilularis). Transplantation 68: 331–337, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Thorens B Glucose transporters in the regulation of intestinal, renal, and liver glucose fluxes. Am J Physiol Gastrointest Liver Physiol 270: G541–G553, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Thorens B GLUT2 in pancreatic and extra-pancreatic gluco-detection. Mol Membr Biol 18: 265–273, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Weiss RB Streptozocin: a review of its pharmacology, efficacy, and toxicity. Cancer Treat Rep 66: 427–438, 1982. [PubMed] [Google Scholar]
- 23.White JA, Bolstridge MC, Downing HJ, Helm EH, Klomfass HJ. Diabetogenic drugs in the vervet monkey. S Afr Med J 48: 273–276, 1974. [PubMed] [Google Scholar]
- 24.Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr 89: 3–9, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Wright JR Jr. Human beta cells are exceedingly resistant to streptozotocin in vivo. Endocrinology 143: 2491–2495, 2002. [DOI] [PubMed] [Google Scholar]