Abstract
Exercise can activate the hypothalamo-pituitary-adrenocortical (HPA) axis, and regular exercise training can impact how the HPA axis responds to stress. The mechanism by which acute exercise induces HPA activity is unclear. Therefore, the purpose of this study was to test the hypothesis that nitric oxide modulates the neuroendocrine component of the HPA axis during exercise. Female Yucatan miniature swine were treated with N-nitro-l-arginine methyl ester (l-NAME) to test the effect of chronic nitric oxide synthase (NOS) inhibition on the ACTH response to exercise. In addition, we tested the effect of NOS inhibition on blood flow to tissues of the HPA axis and report the effects of handling and treadmill exercise on the plasma concentrations of ACTH and cortisol. Chronic NOS inhibition decreased plasma NOx levels by 44%, increased mean arterial blood pressure by 46%, and increased expression of neuronal NOS in carotid arteries. Vascular conductance was decreased in the frontal cortex, the hypothalamus, and the adrenal gland. Chronic NOS inhibition exaggerated the ACTH response to exercise. In contrast, chronic NOS inhibition decreased the ACTH response to restraint, suggesting that the role of NO in modulating HPA activity is stressor dependent. These results demonstrate that NOS activity modulates the response of the neuroendocrine component of the HPA axis during exercise stress.
Keywords: neuroendocrine, restraint, pigs, N-nitro-l-arginine methyl ester, stress
during the stress response, release of ACTH secretagogues from the hypothalamus initiates the activation of the hypothalamo-pituitary-adrenocortical (HPA) axis. Exercise training can influence HPA function at the level of the hypothalamus and the pituitary, thereby facilitating adaptation to the exercise stimulus (24). Interestingly, adaptation to exercise training affects the HPA axis response to nonexercise stressors (7). To provide further insight into the mechanisms by which regular exercise training can affect HPA function, the manner by which acute exercise bouts stimulate neuroendocrine activity needs to be established. Therefore, this study was designed to test the role of nitric oxide (NO) in the activation of the neuroendocrine component of the HPA axis during exercise.
The primary site of control for the HPA axis resides in a set of neurons within the hypothalamic paraventricular nucleus (PVN) (14). The neurons within the PVN produce corticotropin-releasing hormone and vasopressin (VP) and are capable of integrating extrinsic and intrinsic information so that a glucocorticoid response appropriate for the stressor encountered is achieved (14). Nitric oxide has been shown to affect the activation of these PVN neurons (27, 28, 33), and, at least in rodents, both stimulatory (19, 31) and inhibitory (9, 11, 29) roles for NO in the HPA stress response have been described.
In brain, NO may be derived from any one of three different nitric oxide synthase (NOS) isoforms: neuronal (nNOS), endothelial (eNOS), and inducible (iNOS). In addition to its role in modulating neural activity (33, 34), NO is involved in the control of cerebral vascular tone (13, 15, 21, 23, 39). In fact, NO is the major contributor to cerebral blood flow differences in sleep-wake states (39). Within the hypothalamus, NO can modulate neuroendocrine function and has been suggested to link neuronal-glial-vascular interactions (34).
The purpose of this study was to test the hypothesis that chronic NOS inhibition augments the ACTH response to exercise in adult Yucatan swine. Although NO has previously been suggested to couple neural activity and cerebral blood flow (13, 16), studies examining the role of NO in modulating neuroendocrine activity have not assessed the effects of NO on brain vascular tone. Therefore, this study incorporates blood flow measures and hormonal output data to test the effects of NOS inhibition on the ACTH response to exercise. For interpretation of results, basal HPA activity in female Yucatan swine was established, and the effects of NOS inhibition on the HPA response to another stressor, i.e., restraint, were determined.
METHODS
Animals.
Female Yucatan miniature swine (Sinclair Research Farm, Columbia, MO) were used for this study. All animals were sexually mature, between 22 and 42 kg in weight and between 7 and 13 mo of age. Pigs were housed in a 12:12-h light-dark cycle and maintained in accordance with standards set forth by the University of Missouri Institutional Animal Care and Use Committee. Animals were singly housed in our swine facility and fed Purina minipig chow once daily. Animals were monitored for signs of heat and were not in estrus on test dates. Two groups of animals were used for this study: 1) a group of pigs used to describe HPA axis activity in female Yucatan miniature swine, and 2) a group of pigs used to test the effects of chronic NOS inhibition on HPA activity.
We selected adult Yucatan miniature swine to test the hypothesis that chronic NOS inhibition augments the ACTH response to exercise because there are a number of important similarities between the endocrine, metabolic, and cardiovascular systems of pigs and humans and because these same systems respond similarly to exercise in pigs and humans. Similar physiological characteristics include 1) The maximal oxygen uptake/kg body wt of pigs is similar to that of humans (1, 2, 30); 2) pigs are a good model in which to study lipoprotein metabolism (6) and whole body metabolism (1, 2, 30); 3) pigs redistribute cardiac output, favoring blood flow to active skeletal muscle during exercise in a manner similar to that of humans (1, 2, 30); 4) Miniature pigs exhibit cardiorespiratory adaptations to exercise training that are similar to those reported for humans (1, 2, 30, 35–38). Because of the difference in surface area-to-volume ratio of small rodents vs. large mammals, the relative effects of exercise on metabolism and the cardiorespiratory system are much greater in large mammals like pigs and humans. Finally, the larger body size of pigs allows for sampling of blood during exercise and of tissues in the brain for measurement of regional blood flows. For these reasons, we concluded that pigs are a unique and valuable model of these experiments.
HPA axis activity in female Yucatan swine.
To assess hormonal rhythms and test the effects of blood collection and treadmill activity on ACTH and cortisol levels, animals underwent surgery to have catheters implanted in the jugular vein and connected to a vascular access port on their back. For surgery, animals received xylazine (2 mg/kg) and ketamine (10 mg/kg) administered intramuscularly and then isoflurane in O2. Animals were allowed to recover at least 5 days prior to inclusion in any study.
Hormonal rhythms.
To assess the circadian rhythm of ACTH and cortisol in female pigs, blood samples were collected with automated blood sampling equipment (Accusampler, DiLab, Lund, Sweden). Pigs were individually placed in the sampling pen (1.2 × 1.8 m) for at least 4 days prior to initiation of blood collection. Pigs were connected, and sampling was commenced 2 h posthandling. The blood collection device was programmed to collect 1 ml of blood every hour followed by an infusion of 1 ml of saline. Collected samples were placed in tubes with EDTA and kept at 4°C during the 24-h collection. Importantly, connection of pigs to sampling equipment did not restrict the pig's movement within the pen.
Handling and treadmill exercise.
To assess the hormone response to handling, blood samples were manually collected via vascular access ports while the animal was in its pen and after moving the animal out of its pen. When pigs were moved for blood collection, they were placed on a scale that prevented their movement. For treadmill activity, animals were acclimated to treadmill activity over a 2-wk period (4 days/wk, 5–10 min/day); treadmill speed began at a slow walk and was slowly increased to a jogging pace (8 km/h). After acclimation, animals completed one exercise session per week, for 3 wk, with blood samples being collected prior to and after each exercise session (10 min of treadmill jogging at 8 km/h). Compared with the maximal exercise test described later in this manuscript, no electrical shock was used during treadmill activity in pigs used to assess the hormone response to treadmill jogging. Blood collection to determine the effects of handling and treadmill activity occurred in the afternoon between 1300 and 1700.
Effects of N-nitro-l-arginine methyl ester on blood flow and HPA axis activity.
To determine the effects of N-nitro-l-arginine methyl ester (l-NAME) on tissue blood flow and the hormone response to stress, animals had two catheters surgically placed, one in the left atrium and one in the aorta via the internal thoracic artery. Animals in this study represent a subset of pigs from a larger study examining the effects of l-NAME on exercise responses (22). Pigs recovered from surgery for 8–10 days prior to the exercise test. Restraint blood samples were collected 4 days after surgery. In the treatment group, l-NAME was added to the drinking water (0.01%) for ≥ 4 wk prior to inclusion in studies making l-NAME available to drink whenever they were in their cage.
Exercise and restraint.
Restraint and exercise were used to determine the effect of l-NAME on the HPA response. Both stressors were conducted in the morning between 0800 and 1000. For the restraint stressor, pigs were removed from their pen, picked up by their hind limbs, and placed flat on their back. Several investigators were used to restrain animals by holding down the pig's front legs, rear legs, and head while blood samples were obtained via jugular venipuncture. Blood samples were collected in chilled EDTA Vacutainers within 5 min of placing the animal on its back. The time of collection occurred between 2 and 5 min, and there was not a correlation between time and ACTH or cortisol, nor was there a group difference in mean restraint time.
The exercise stressor involved incremental exercise to exhaustion. Exhaustion was defined as an inability to locomote at the prevailing speed and grade. Every 5 min throughout the protocol, the treadmill speed was changed. Animals began walking at 3.2 km/h, and this walking stage was repeated between 5-min stages of 4.8, 8.0, and 11.3 km/h. If the pig were able to complete the 11.3 km/h stage, a 5% treadmill grade was then added to the speed of 11.3 km/h. Mild electrical shock was used sparingly to encourage running for the maximal exercise test. Between running stages, blood samples were withdrawn from an arterial catheter and placed in chilled EDTA Vacutainers.
Blood flow determination in conscious pigs.
The determination of blood flow was accomplished with the use of radiolabeled microspheres of 15-μm diameter (Perkin Elmer) (1, 5), using methods previously described (22). Approximately 2.2 million microspheres were infused, and only experimental animals with at least 400 microspheres collected in the reference blood sample were used for analysis (3). To obtain mean arterial pressure, the externalized aortic catheter was connected to a blood pressure analyzer (MicroMed). After completion of the exercise protocol, animals were sedated and anesthetized. Following euthanasia, the frontal cortex, hypothalamus, pituitary, and adrenal were dissected and weighed, and radioactivity was determined with a gamma counter (LKB Wallac 1282).
Blood flow was normalized to tissue mass, and vascular conductance was calculated by dividing blood flow by mean arterial pressure. Blood flow to right and left kidney was measured to confirm good microsphere-blood mixing and animals with bilateral difference >15% were excluded from analysis. Anterior and posterior pituitary samples were pooled to ensure a minimum of 200 microspheres per sample (17). Adrenal data are the combined mean values from left and right adrenals, as there was no difference in blood flow between the two sides.
Blood assays.
All blood samples were collected in chilled EDTA containers and centrifuged, and plasma was stored at −80°C until analysis. Both ACTH and cortisol were measured by chemiluminescent assays (Immulite, DPC), as previously described (18). Blood lactate was measured spectrophotometrically by the enzymatic conversion of lactate and NAD to pyruvate and NADH by bovine heart lactate dehydrogenase (LDH) (glycine, 330 mM; hydrazine, 270 mM; NAD 1.3 mM; bovine heart LDH, 200 U/ml; Sigma) (25). NO metabolite (NOx) concentration was determined using a chemiluminescent analyzer (NOA 280i; Sievers, Boulder, CO). Presented mean NOx data are from the larger ongoing study since only a small number of animals in this subset had systemic NOx concentration determined (22).
mRNA analysis of NOS isoforms.
The right common carotid artery was dissected, quickly cleaned of fat and connective tissue using a dissection microscope, and slit longitudinally. Endothelium was scraped from the luminal surface of the vessel into 1.0 ml of TriZOL reagent (MCR, Cincinnati, OH). Total RNA was isolated according to the vendor's recommended protocol. The phenol chloroform extraction of RNA included a separation centrifugation step of 12,000 g at 4°C, and the observation of an undisturbed interphase was used to confirm separation of the RNA from DNA.
First-strand (i.e., cDNA) synthesis was performed on 1.0 μg isolated RNA using reverse transcriptase (200 U/μg) and oligo (dT) primers (Invitrogen, Carlsbad, CA). cDNA was amplified via PCR. Primers for DNA polymerase to amplify eNOS cDNA were 5′-GCC TGA ACA GCA CAG GAG TT-3′ (forward), and 5′-GCT CTT CTA GCC GTG TGT CC-3′ (reverse), designed according to GenBank accession no. GI: 30794678. Primers to amplify nNOS cDNA were 5′-CAA GGG TCA AGA ACT GGG AGA CTG-3′ (forward) and 5′-GTG CTG AGA AGG AAG CAT GAC G-3′ (reverse), developed from published sequence data (32). Primers to amplify iNOS cDNA were 5′-CGT TAT GCC ACC AAC AAT GG-3′ (forward) and 5′-GAG CTG AGC GTT CCA GAC C-3′ (reverse), adapted from published data (12), GenBank accession no. GI: 1777979. GAPDH cDNA was amplified with the primers 5′-TCA AGA AGG TGG TGA AGC AG-3′ (forward) and 5′-TGT CGT ACG AGG AAA TGA GC-3′ (reverse), designed according to GenBank accession no. GI: 2407183. The eNOS, iNOS and nNOS primers were intron spanning and yielded products of 100–200 base pairs, a size requirement for mRNA quantification with SYBR Green real-time PCR technology. Melt curves performed after PCR amplification confirmed the amplification of a single, one-size product.
Real-time PCR cycles (40×; Cepheid SmartCycler, Sunnyvale, CA) consisted of 5 s at 95°C (denaturing), 60 s at 58°C (annealing), and 60 s at 72°C (extension). Annealing and extension times were reduced to 20 s and 30 s, respectively, for nNOS and iNOS. The reaction mixture included Brilliant SYBR Green QPCR Master Mix (dNTPs, DNA polymerase, SYBR Green; Stratagene, La Jolla, CA), MgCl2 (2.5 mM), primers (100 nM), and 5.0 μl cDNA (diluted 5-fold in nuclease-free water). Primer concentrations were increased to 200 nM for nNOS and 300 nM (iNOS). Samples were analyzed in duplicate.
PCR standard curves were generated for the primer set of each NOS isoform, as well as for GAPDH (housekeeping gene). The amplicon of each message of interest was purified, cloned into a plasmid (TOPO TA pCR2.1; Invitrogen, Carlsbad, CA), and propogated in bacteria. Purified constructs were linearized with NcoI and quantified by spectrophotometry. The PCR cycle thresholds for serial dilutions of the constructs were used to generate standard curves for PCR amplification of 5.0 μl of cDNA from each mRNA and recorded as the number of molecules per reaction. This value was normalized to the corresponding value for GAPDH.
Statistical analyses.
Data are presented as means ± SE and analysis was completed using SigmaStat 3.1 software. One-way repeated-measures ANOVA with Holm-Sidak pairwise multiple comparison method was used to determine the effect of time on plasma ACTH and cortisol. A two-way repeated-measures ANOVA was used to compare the effects of treadmill speed and l-NAME on the ACTH and cortisol response to exercise. A Student's t-test was run for other group comparisons.
RESULTS
ACTH and cortisol rhythms.
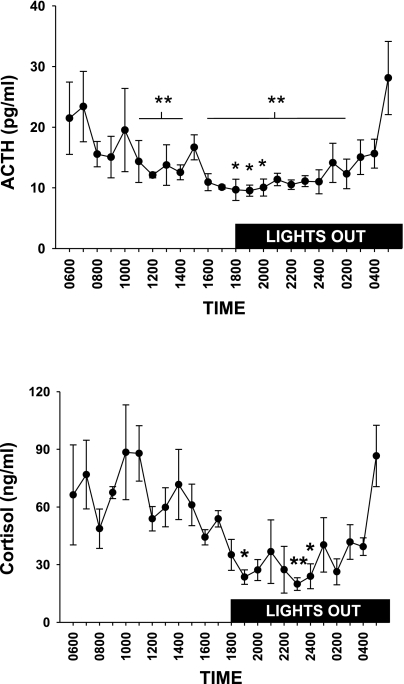
The circadian rhythm for ACTH and cortisol in Yucatan miniature swine is presented in Fig. 1. During this 24-h period, blood sample collection was automated so that there would be no animal handling performed during the experiment. There was a statistically significant main effect of time on ACTH and cortisol. Animals were fed only once per day at ∼1000 h. The increase in morning ACTH and cortisol began prior to the start of the light phase.
Fig. 1.
Circadian rhythm for ACTH (top) and cortisol (bottom) in female pigs (n = 4). There was a significant main effect of time on ACTH and cortisol, and post hoc analysis revealed significant differences between individual points (ACTH: *different from 0700, **different from 0500, P < 0.05; cortisol: *different from 1100 h, **different from 0500 and 1100 h, P < 0.05).
Effects of animal handling and treadmill activity.
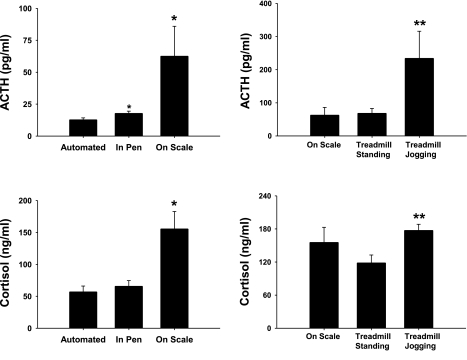
Compared with automated sampling, manual sampling of blood in the pen significantly increased ACTH but did not affect cortisol while removal of the animal from its pen significantly increased both hormones (Fig. 2). There was no difference in ACTH or cortisol between placing animals on the scale or on the treadmill. There was a significant increase in ACTH and cortisol during treadmill exercise.
Fig. 2.
Plasma ACTH (top) and cortisol (bottom) response to manual blood collection (left) and treadmill activity (right). Pen refers to samples collected while animals remained in their pen. Female pigs were removed from pen and placed on scale or treadmill. Treadmill standing is pre-exercise, while treadmill jogging is after the completion of 10 min of treadmill jogging. All samples were collected via vascular access ports. Values are presented as means ± SE: *P < 0.05 vs. automated; **P < 0.05 vs. treadmill standing; n = 10, pen; n = 4, scale; n = 12, treadmill standing and treadmill walking.
Chronic NOS inhibition.
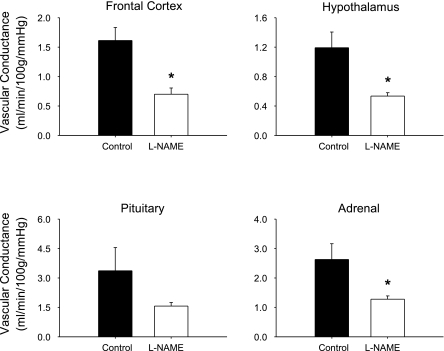
Table 1 presents the effects of chronic NOS inhibition on plasma NOx, mean arterial pressure, and tissue blood flow. Treatment with l-NAME decreased NOx levels by 44%, increased mean arterial blood pressure by 46%, and decreased blood flow to the frontal cortex and the hypothalamus. Blood flow also decreased in the pituitary and adrenal, but these differences were not statistically significant. Because chronic NOS inhibition increased arterial pressure, we calculated the effects of treatment on tissue vascular conductance, and the results are presented in Fig. 3. In response to l-NAME treatment, there was a statistically significant decrease in vascular conductance in the frontal cortex, the hypothalamus, and the adrenal gland. Because l-NAME was administered through the drinking water, water intake for all animals was measured the final week of treatment. There was no difference in water consumption between l-NAME-treated pigs (2,874 ± 248 ml/day) and the control pigs (3,008 ± 969 ml/day).
Table 1.
Effects of chronic NOS inhibition on plasma nitric oxide, mean arterial pressure, and tissue blood flow in pigs
| n | NOx, μM | MAP, mmHg | Frontal cortex, ml·min−1·100 g−1 | Hypothalamus, ml·min−1·100 g−1 | Pituitary, ml·min−1·100 g−1 | Adrenal, ml·min−1·100 g−1 | |
|---|---|---|---|---|---|---|---|
| Control | 4 | 12.2±1.9 | 81±5 | 126±17 | 96±4 | 247±67 | 194±26 |
| l-NAME | 6 | 6.8±0.7* | 119±8* | 83±9* | 63±5* | 192±26 | 149±10 |
MAP, mean arterial pressure.
P < 0.05 vs. control. NOx, nitric oxide metabolite [n = 19, control; n = 14, N-nitro-l-arginine methyl ester (l-NAME)]. Values are presented as means ± SE.
Fig. 3.
The effect of chronic NOS inhibition on tissue vascular conductance in conscious standing pigs (n ≥ 4). N-nitro-l-arginine methyl ester (l-NAME) treatment significantly reduced vascular conductance in the frontal cortex, the hypothalamus and the adrenal but did not significantly change vascular conductance in the pituitary. *P < 0.05 vs. control.
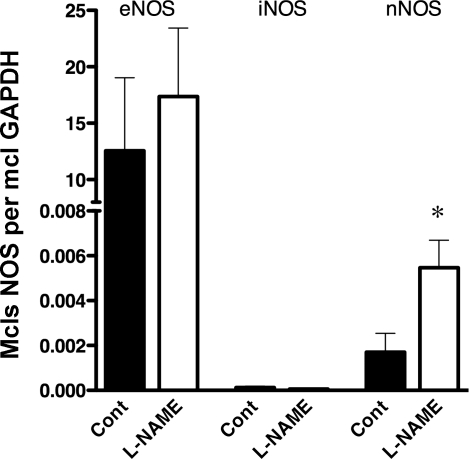
To evaluate the effects of chronic NOS inhibition on vascular NOS expression, we measured expression of message for eNOS, iNOS, and nNOS in carotid artery endothelium. As shown in Fig. 4, real-time PCR analysis reveals that there is substantial expression of eNOS and nNOS, while there is very little iNOS message in carotid endothelium. Also, chronic l-NAME treatment increased expression of nNOS mRNA in carotid artery endothelial cells, but did not significantly alter eNOS or iNOS message (Fig. 4).
Fig. 4.
Effects of chronic nitric oxide synthase (NOS) inhibition on endothelial NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS) mRNA expression in carotid artery endothelial cells. Results are expressed as number of NOS mRNA molecules per molecules of GAPDH mRNA as outlined in text. *P < 0.05 vs. control; n = 7 per group.
Exercise and restraint.
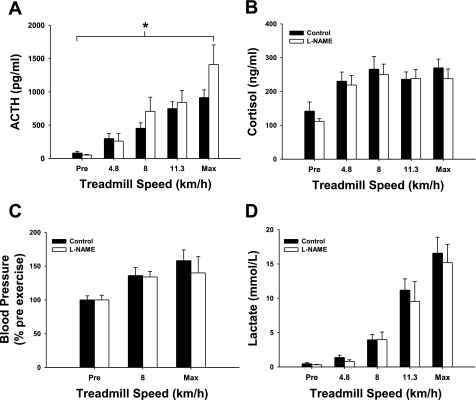
Treadmill walking increased ACTH and cortisol, as shown in Fig. 2. In a separate group of pigs, we determined whether there was a dose-response relationship between treadmill running speed and changes in plasma ACTH and cortisol (Fig. 5). There was a significant main effect of exercise on cortisol, blood pressure, and blood lactate. Statistical analysis of the data set indicates a main effect of l-NAME (P < 0.05) and a main effect of treadmill speed (P < 0.001) on the ACTH response to exercise. The interaction between l-NAME and treadmill speed on the ACTH response to exercise did not reach statistical significance (P = 0.081).
Fig. 5.
The effect of chronic NOS inhibition on the ACTH (A), cortisol (B), blood pressure (C), and blood lactate (D) response to treadmill exercise. Solid bars represent control animals, and open bars represent l-NAME-treated animals. Blood samples were collected prior to initiation of exercise (Pre), during the exercise test upon completion of 5-min stages (4.8, 8, and 11.3 km/h), and once exhaustion was reached (Max). There was a significant main effect of exercise on ACTH, cortisol, blood pressure, and blood lactate. There was a significant main effect of l-NAME on the ACTH response to exercise (*P < 0.05; n ≥ 7 per group). There was not a significant main effect of l-NAME on the cortisol, blood pressure, or blood lactate response to exercise.
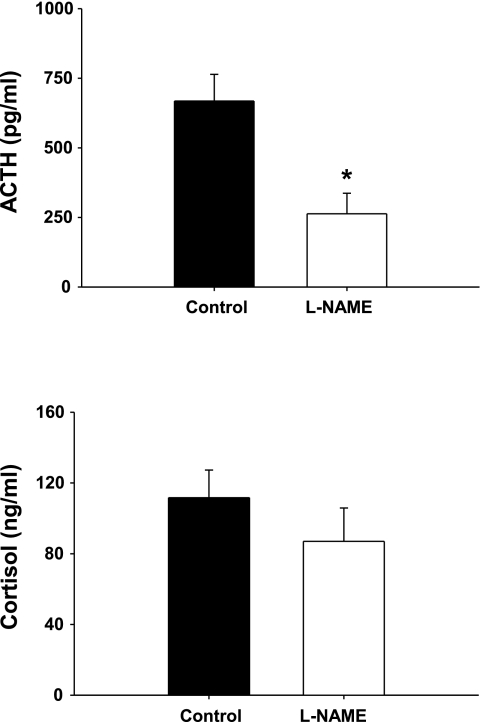
The cortisol response to exercise was not affected by l-NAME treatment (Fig. 5). Chronic NOS inhibition did not alter the percentage change in blood pressure produced by treadmill exercise, nor did it affect the blood lactate response to exercise. Mean pre-exercise blood pressure data are presented in Table 1. Chronic NOS inhibition with l-NAME produced a statistically significant reduction in the ACTH response to restraint (P = 0.01, Fig. 6), but did not affect the cortisol response to restraint.
Fig. 6.
The effect of chronic NOS inhibition on the plasma ACTH (top) and cortisol (bottom) response to restraint. Animals were removed from the pen and placed on their back, and blood samples were collected within 5 min. Data are displayed as means ± SE; *P < 0.01 for l-NAME (n = 11) vs. control (n = 8).
DISCUSSION
The key finding of our study is that chronic NOS inhibition increased the ACTH response to exercise, indicating that nitric oxide modulates the neuroendocrine component of the HPA axis during exercise. The decrease in hypothalamic blood flow in response to chronic l-NAME treatment raises the possibility that manipulations affecting NO bioavailability may affect the neuroendocrine component of the HPA axis through changes in local blood flow.
The establishment of the basal circadian rhythm for ACTH and cortisol in female Yucatan miniature swine demonstrates the benefit of automated sampling and allows for an appreciation of the effects of handling, exercise and restraint on ACTH and cortisol. If the pig is cooperative, one can enter the pen and collect blood samples with no change in cortisol and a small increase in ACTH. Frequent handling, however, would not be ideal for blood sample collection over a 24-h period. Moving the animal out of the pen induces a stress response, and our results indicate that placing pigs on a treadmill induced no greater hormonal change than being placed on the scale. An increase in ACTH and cortisol in response to treadmill walking was still apparent even after a few weeks of acclimation to treadmill walking (data not shown). The large increase in ACTH in response to submaximal treadmill activity is greater in female pigs and did not occur in male pigs (18).
Chronic inhibition of NOS with l-NAME decreased systemic NOx levels and increased blood pressure. The increase in blood pressure would be expected to produce a change in baroreceptor activity (26) but given that l-NAME treatment lasted several weeks, there was enough time for resetting of the baroreceptors to occur (20). The decrease in vascular conductance in the frontal cortex and hypothalamus suggests that NO production helps maintain basal cerebral blood flow, consistent with previous reports (15, 21, 23, 39). Chronic NOS inhibition also appeared to result in compensatory increases in expression of nNOS in carotid artery endothelial cells (Fig. 4). This adaptation may have resulted from chronically decreased NOx levels in the blood.
Our results indicate that chronic NOS inhibition augmented the ACTH response to exercise. Further, our results indicate that this effect may be specific to exercise stress since chronic NOS inhibition decreased the ACTH response to restraint. The observation that NOS inhibition resulted in increased ACTH response to exercise but decreased response to restraint suggests that NO release from NOS can serve as an inhibitor or activator of the neuroendocrine component of the HPA axis in swine. Previous work in rodents has ascribed both stimulatory (19, 31) or inhibitory (9, 11, 29) roles to NO in affecting HPA activity. A stimulatory role for NO is supported by the observation that microinfusion of NO into the PVN increases the release of ACTH (31).
The inhibitory effect of NO during prolonged stressor exposure is consistent with the hypothesis that NO in the hypothalamus plays an inhibitory role during states of increased neuronal activity (34). That is, the time difference in stressor duration between restraint (<5 min) and exercise (45 min) may partly explain the differential effects of l-NAME on ACTH response to exercise vs. restraint. Interestingly, subcutaneous injection of l-NAME in rats initially decreased the ACTH response to electroshocks in rats during the first 5–20 min but increased the ACTH response after 30–60 min compared with controls (28), a similar time pattern to what we observed with pigs during treadmill exercise.
The use of l-NAME, a nonspecific NOS inhibitor, in our study prevents us from ascribing our effects to a specific NOS isoform(s). Results from previous studies using specific NOS inhibitors demonstrate that NOS isoforms differ in their contribution to HPA regulation (8, 9) and that the contribution of different NOS isoforms to HPA regulation can change following repeated stressor exposure (4, 10). In this study, l-NAME was administered to animals for several weeks. Previous work had demonstrated that the effect of l-NAME on the ACTH response can be observed with both acute (2 min prior) and chronic (4 day) administration of l-NAME (29). In a previous study that examined the effects of NO in modulating the HPA response, it was concluded that NO does not affect the ACTH response through its action as a vasoconstrictor (19). This conclusion was based on the fact that use of intracerebroventricular l-NAME did not alter mean arterial blood pressure. It is not surprising that local changes in brain NO bioavailability did not alter systemic blood pressure. However, a lack of change in systemic blood pressure does not necessarily indicate that vasomotor tone in brain arteries was unaffected. Indeed, our results show that l-NAME treatment does increase vasomotor tone in the hypothalamus, resulting in decreased hypothalamic blood flow. Therefore, this may be a mechanism by which NO modulates HPA axis function. This observation suggests that studies altering NO bioavailability in the hypothalamus should consider the fact that blood flow in this region may be altered when NOS synthesis is inhibited.
Perspectives and Significance
Our results identify NOS activity as a modulator of the HPA axis response during acute exercise. The demonstration that chronic NOS inhibition increases the ACTH response to exercise but decreases the ACTH response to restraint suggests that NO release from NOS functions in a context-specific manner to inhibit or activate neuroendocrine activity. Thus, NO is an important modulator of the neuroendocrine component of the HPA axis during exercise in large animals.
GRANTS
This research was supported by National Institutes of Health Grants RR-18276, HL-36088, HL-52490, and AR-048523.
Acknowledgments
The authors would like to thank Dave Harah, Cory Weimer, Miles Tanner, Sean Newcomer, Kevin Eklund, Ann Melloh, Pam Thorne, and Robert Johnson for their assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Distribution of blood flow in muscles of miniature swine during exercise. J Appl Physiol 62: 1285–1298, 1987. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Progressive elevations in muscle blood flow during prolonged exercise in swine. J Appl Physiol 63: 285–291, 1987. [DOI] [PubMed] [Google Scholar]
- 3.Buckberg GD, Luck JC, Payne DB, Hoffman JI, Archie JP, Fixler DE. Some sources of error in measuring regional blood flow with radioactive microspheres. J Appl Physiol 31: 598–604, 1971. [DOI] [PubMed] [Google Scholar]
- 4.Bugajski AJ, Gadek-Michalska A, Bugajski J. The involvement of nitric oxide and prostaglandins in the cholinergic stimulation of hypothalamie-pituitary-adrenal response during crowding stress. J Physiol Pharmacol 57: 463–477, 2006. [PubMed] [Google Scholar]
- 5.Delp MD, Armstrong RB, Godfrey DA, Laughlin MH, Ross CD, Wilkerson MK. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. J Physiol 533: 849–859, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon JL, Stoops JD, Parker JL, Laughlin MH, Weisman GA, Sturek M. Dyslipidemia and vascular dysfunction in diabetic pigs fed an atherogenic diet. Arterioscler Thromb Vasc Biol 19: 2981–2992, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J Appl Physiol 100: 1867–1875, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Gadek-Michalska A, Bugajski J. Nitric oxide mediates the interleukin-1beta- and nicotine-induced hypothalamic-pituitary-adrenocortical response during social stress. J Physiol Pharmacol 56: 491–503, 2005. [PubMed] [Google Scholar]
- 9.Gadek-Michalska A, Bugajski J. Role of nitric oxide in the nicotine-induced pituitary-adrenocortical response. J Physiol Pharmacol 55: 443–455, 2004. [PubMed] [Google Scholar]
- 10.Gadek-Michalska A, Spyrka J, Bugajski J. Psychosocial stress affects the involvement of prostaglandins and nitric oxide in the lipopolysaccharide-induced hypothalamic-pituitary-adrenal response. J Physiol Pharmacol 56: 287–298, 2005. [PubMed] [Google Scholar]
- 11.Givalois L, Li S, Pelletier G. Central nitric oxide regulation of the hypothalamic-pituitary-adrenocortical axis in adult male rats. Brain Res Mol Brain Res 102: 1–8, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Gookin JL, Chiang S, Allen J, Armstrong MU, Stauffer SH, Finnegan C, Murtaugh MP. NF-κB-mediated expression of iNOS promotes epithelial defense against infection by Cryptosporidium parvum in neonatal piglets. Am J Physiol Gastrointest Liver Physiol 290: G164–G174, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Hamel E Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 100: 1059–1064, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci 16: 381–385, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Hudetz AG, Shen H, Kampine JP. Nitric oxide from neuronal NOS plays critical role in cerebral capillary flow response to hypoxia. Am J Physiol Heart Circ Physiol 274: H982–H989, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Iadecola C Regulation of the cerebral microcirculation during neural activity: is nitric oxide the missing link? Trends Neurosci 16: 206–214, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Ishise S, Pegram BL, Yamamoto J, Kitamura Y, Frohlich ED. Reference sample microsphere method: cardiac output and blood flows in conscious rat. Am J Physiol Heart Circ Physiol 239: H443–H449, 1980. [DOI] [PubMed] [Google Scholar]
- 18.Jankord R, Turk JR, Schadt JC, Casati J, Ganjam VK, Price EM, Keisler DH, Laughlin MH. Sex difference in link between interleukin-6 and stress. Endocrinology 148: 3758–3764, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CK, Rivier CL. Nitric oxide and carbon monoxide have a stimulatory role in the hypothalamic-pituitary-adrenal response to physico-emotional stressors in rats. Endocrinology 141: 2244–2253, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Krieger EM Mechanisms of complete baroreceptor resetting in hypertension. Drugs 35 Suppl 6: 98–103, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Lee TJ Nitric oxide and the cerebral vascular function. J Biomed Sci 7: 16–26, 2000. [DOI] [PubMed] [Google Scholar]
- 22.McAllister RM, Newcomer SC, Pope ER, Turk JR, Laughlin MH. Effects of chronic nitric oxide synthase inhibition on responses to acute exercise in swine. J Appl Physiol 104: 186–197, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paravicini TM, Miller AA, Drummond GR, Sobey CG. Flow-induced cerebral vasodilatation in vivo involves activation of phosphatidylinositol-3 kinase, NADPH-oxidase, and nitric oxide synthase. J Cereb Blood Flow Metab 26: 836–845, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Park E, Chan O, Li Q, Kiraly M, Matthews SG, Vranic M, Riddell MC. Changes in basal hypothalamo-pituitary-adrenal activity during exercise training are centrally mediated. Am J Physiol Regul Integr Comp Physiol 289: R1360–R1371, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Passonneau JV, Lowry OH. A Collection of metabolite assays. In: Enzymatic Analysis: A Practical Guide (1st ed.), edited by Passonneau JV and Lowry OH. Totowa, NJ: Humana, 1993, p. 193–194.
- 26.Purinton SC, Wood CE. Oestrogen augments the fetal ovine hypothalamus-pituitary-adrenal axis in response to hypotension. J Physiol 544: 919–929, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riedel W Role of nitric oxide in the control of the hypothalamic-pituitary-adrenocortical axis. Z Rheumatol 59 Suppl 2: II/36–42, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Rivier C Role of gaseous neurotransmitters in the hypothalamic-pituitary-adrenal axis. Ann NY Acad Sci 933: 254–264, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Rivier C, Shen GH. In the rat, endogenous nitric oxide modulates the response of the hypothalamic-pituitary-adrenal axis to interleukin-1 beta, vasopressin, and oxytocin. J Neurosci 14: 1985–1993, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders M, White F, Bloor CM. Cardiovascular responses of dogs and pigs exposed to similar physiological stress. Comp Biochem Physiol 58A: 365–370, 1977. [Google Scholar]
- 31.Seo DO, Rivier C. Microinfusion of a nitric oxide donor in discrete brain regions activates the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol 13: 925–933, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Solhaug MJ, Dong XQ, Adelman RD, Dong KW. Ontogeny of neuronal nitric oxide synthase, NOS I, in the developing porcine kidney. Am J Physiol Regul Integr Comp Physiol 278: R1453–R1459, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Stern JE Nitric oxide and homeostatic control: an intercellular signalling molecule contributing to autonomic and neuroendocrine integration? Prog Biophys Mol Biol 84: 197–215, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Stern JE, Zhang W. Cellular sources, targets and actions of constitutive nitric oxide in the magnocellular neurosecretory system of the rat. J Physiol 562: 725–744, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol 85: 1160–1168, 1998. [DOI] [PubMed] [Google Scholar]
- 36.White FC, Roth DM, Bloor CM. The pig as a model for myocardial ischemia and exercise. Lab Anim Sci 36: 351–356, 1986. [PubMed] [Google Scholar]
- 37.White FC, Sanders M, Bloor CM. Coronary reserve at maximal heart rate in the exercising swine. J Clin Res 1: 31–40, 1981. [Google Scholar]
- 38.White FN Comparative aspects of vertebrate cardiorespiratory physiology. Annu Rev Physiol 40: 471–499, 1978. [DOI] [PubMed] [Google Scholar]
- 39.Zoccoli G, Grant DA, Wild J, Walker AM. Nitric oxide inhibition abolishes sleep-wake differences in cerebral circulation. Am J Physiol Heart Circ Physiol 280: H2598–H2606, 2001. [DOI] [PubMed] [Google Scholar]