Abstract
The origins of the sympathetic nervous system (SNS) innervation of white adipose tissue (WAT) have been defined using the transneuronal viral retrograde tract tracer, pseudorabies virus. Activation of this SNS innervation is acknowledged as the principal initiator of WAT lipolysis. The central control of WAT lipolysis may require neural feedback to a brain-SNS-WAT circuit via WAT afferents. Indeed, conventional tract tracing studies have demonstrated that peripheral pseudounipolar dorsal root ganglion (DRG) sensory cells innervate WAT. The central nervous system projections of WAT afferents remain uncharted, however, and form the focus of the present study. We used the H129 strain of the herpes simplex virus-1 (HSV-1), an anterograde transneuronal viral tract tracer, to define the afferent circuits projecting from WAT to the central nervous system. Siberian hamster inguinal (IWAT) or epididymal WAT was injected with H129 and the neuraxis processed for HSV-1 immunoreactivity. We found substantial overlap in the pattern of WAT sensory afferent projections with multiple SNS outflow sites along the neuraxis, suggesting the possibility of WAT sensory-SNS circuits that could regulate WAT SNS drive and thereby lipolysis. Previously, we demonstrated that systemic 2-deoxy-d-glucose (2DG) elicited increases in the SNS drive to IWAT. Here, we show that systemic 2DG administration also significantly increases multiunit spike activity arising from decentralized IWAT afferents. Collectively, these data provide structural and functional support for the existence of a sensory WAT pathway to the brain, important in the negative feedback control of lipid mobilization.
Keywords: 2-deoxy-d-glucose, lipolysis, sympathetic nervous system, electrophysiology
feedback from lipid stores to the central nervous system (CNS) has been suggested to contribute to the relative stability of total body fat in some individuals, but not in others (for a review, see Ref. 1). Historically, the idea of a lipid feedback signal became prominent with Kennedy's “lipostatic theory” (24), and this notion apparently was affirmed with the discovery of leptin, the largely adipocyte-derived cytokine thought by some to reflect total body fat stores (for a review, see Ref. 21). As with any feedback system, one controlling lipid stores would require not only afferent signals from white adipose tissue (WAT), but also effectors to increase or decrease the lipid stores appropriately. As with any feedback system, one controlling lipid stores would require not only afferent signals from WAT, but also effectors to increase or decrease the lipid stores appropriately. Whether such a system exists or needs to exist for total body fat to be stable is debatable (46).
Efferent neural control of WAT is well recognized. We and others have established that the central control of WAT lipolysis is via activation of the sympathetic nervous system (SNS) innervation of WAT (for a review, see Refs. 7 and 8). Specifically, we provided the first direct neuroanatomical evidence of SNS innervation of WAT by labeling the postganglionic sympathetic innervation of WAT using conventional tract tracers (48) and then by defining the origins of the sympathetic outflow circuits from brain to WAT using pseudorabies virus (PRV), a viral retrograde transneuronal tract tracer (e.g., Refs. 3, 20, 39). Moreover, we find that lipid mobilization is closely correlated with increases in the sympathetic drive to WAT as measured by norepinephrine turnover (NETO; Ref. 48, a neurochemical of SNS drive). Finally, local destruction of WAT SNS innervation, but not adrenal demedullation, blocks WAT lipid mobilization normally triggered by lipolytic stimuli (for a review, see Refs. 7 and 8) unequivocally demonstrating that the activation of SNS innervation to WAT is the principal initiator of lipolysis.
By contrast to the sympathetic neural efferent circuitry innervating WAT, the afferent pathways mediating feedback from WAT to the CNS remain poorly characterized. Although leptin has been suggested to provide a humoral feedback signal from WAT to the brain, the imprecise correlation of circulating leptin concentrations with body fat levels, as well as the saturability of the transport system for this cytokine into the brain (5) argues that it may be insufficient for the negative feedback control of lipolysis.
Alternatively, or perhaps in addition to circulating factors, such as leptin or insulin (9), adiposity levels could be conveyed to the brain via neural afferents. Sensory innervation of WAT was shown directly when a conventional retrograde tract tracer was applied to laboratory rat WAT, resulting in labeling of the pseudounipolar (a.k.a. bipolar) neurons of dorsal root ganglia (DRG) (18). In addition, while the present study was in progress, it was reported that injections of the cholera toxin b subunit (CTb) injections into rat retroperitoneal WAT suggest sensory nerve projections to the nucleus gracilis (Gr) of the brainstem (26). Additional anatomical evidence comes from the immunohistological identification of peptides classically associated with sensory nerves such as calcitonin gene-related peptide and substance P in WAT nerves (37).
Although the neuroanatomical evidence to date shows DRG-labeled sensory neurons [and scant Gr labeling (26)] using traditional tract tracers, the central pattern of sensory projections from WAT remains incompletely defined. To this end, we used the H129 strain of the herpes simplex virus-1 (HSV-1), a transneuronal viral tracer that spreads anterogradely (6, 17, 33, 43, 50), in contrast to the retrograde of PRV, to trace the multi-synaptic ascending afferent circuit from inguinal WAT (IWAT) or epididymal WAT (EWAT) through the CNS of Siberian hamsters. Immunohistochemical detection of H129 has been previously used in this manner to successfully identify CNS regions that receive viscerosensory inputs from the rat stomach wall (33). In the present study, Siberian hamsters were chosen because the SNS and sensory innervation of WAT have been most extensively studied in this species both histologically (e.g., 3, 20, 36, 39, 41, 48) and functionally (e.g., 10, 10, 49). In addition, as a first step toward understanding the function of WAT sensory nerves, we measured the effects of intraperitoneal (ip) administration of 2-deoxy-d-glucose (2DG), the glucoprivic agent that triggers marked increases in sympathetic drive to the periphery, including WAT (i.e., NETO) (11) on multiunit neurophysiological action potentials from decentralized sensory afferent fiber bundles arising from inguinal WAT.
METHODS
Animals.
Adult male Siberian hamsters (n = 25) derived from our colony were singly housed in a long-day photoperiod (16:8-h light-dark cycle, lights on at 0200) at 22°C and received water and Purina rodent chow (#5001; 3.4 kcal/g) ad libitum. Animals were acclimated to single housing 1 wk before H129 injections. All procedures were approved by the Georgia State University and Albert Einstein College of Medicine Institutional Animal Care and Use Committees and were in accordance with the Public Health Service and United States Department of Agriculture guidelines.
H129 injections.
H129 injections and subsequent housing were conducted using Biosafety Level 2 standards. The animals were anesthetized with isoflurane during the injection procedure. The target incision area was shaved and then wiped with 50% ethanol. An incision was made around the right hindquarter to reveal the surface of the right IWAT, or in the lower ventral region to reveal the right EWAT. For each WAT pad, a series of injections were made of H129 (gift from Dr. Richard Dix, Georgia State University) directly into five loci within the target fat pad (1.0 × 108 pfu/ml; 150 nl/loci) to evenly distribute the virus using a 1.0-μl microsyringe. After each 150-nl injection, the syringe was left in place for 60 s to prevent efflux of virus. The syringe needle entry site was then wiped with sterile saline-soaked gauze. Finally, the incision was closed with sterile sutures, and wound clips and nitrofurozone powder (nfz Puffer; Hess & Clark, Lexington, KY) were applied to minimize the risk of bacterial infection.
It is noteworthy that the above precautionary procedures, as well as the small injection volumes used here, helped assure against leakage of virus to label nonrelevant neural circuits. We also conducted additional control pilot experiments. Specifically, in some animals, the same virus titer and volume was placed on the surface of exposed WAT; this resulted in no infection of cells in the DRG, spinal cord or brain (data not shown), whereas direct localized injections of H129 into fat depots resulted in infection of these structures. Moreover, in pilot studies, surgical isolation of WAT pads from the surrounding tissues before H129 injection resulted in a pattern of infection indistinguishable from that of pads injected in their natural in situ position/condition, indicating that the observed infections after direct WAT microinjections derived from the sensory nerves innervating the tissue and not surrounding tissues (data not shown).
A time-course analysis of viral progression through the sensory afferents was performed for hamsters injected with H129 into IWAT to track the progression of the virus through peripheral and central sensory relays. After injection they were transferred to clean cages equipped with ventilated filter-tops and killed at 24 h (n = 4), 48 h (n = 4), 72 h (n = 4), 96 h (n = 4), or 114 h (n = 5) postinjection. Animals injected with H129 into EWAT (n = 4) were killed at 114 h to compare infection pattern with animals that were injected with H129 into IWAT killed at the same postinjection survival time. The parameters for this procedure, including the optimal survival time postinoculation for infection into the rostral forebrain and the virus titer/load, were determined in pilot studies.
Histology.
At termination, the animals were overdosed with pentobarbital sodium (300 mg/kg) and perfused transcardially first with heparinized (0.02%) saline followed by phosphate buffered (0.1 M; pH 7.4) paraformaldehyde (4% w/v). Bilateral nodose ganglia, sympathetic thoracic 13 (T13) ganglia, and DRG along the cervical, thoracic, and lumbar regions of the spinal cord were carefully removed from IWAT-injected hamsters. Only brains and spinal cords were collected from hamsters injected into EWAT. All samples were postfixed in the same fixative (see directly above) overnight at 4°C and cryoprotected in sucrose (30% wt/vol; with 0.1% sodium azide). The brains were sliced at 30 μm on a freezing stage sliding microtome. The bilateral nodose ganglia, DRG, T13 sympathetic ganglia and spinal cords were sliced on a cryostat at 20 μm and thaw-mounted onto gelatin-coated slides.
Immunohistochemistry for HSV-1.
Every sixth brain section was incubated sequentially in the primary HSV-1 antibody (1:750,000; DakoCytomation, Carpinteria, CA) overnight, biotinylated secondary antibody (goat anti-rabbit; 1:750; Vector Laboratories, Burlingame, CA) for 2 h, and in avidin-biotin complex (ABC; 1.75 μl/ml of each avidin and of biotin, Vector Laboratories) for 1 h. Diaminobenzidine (0.1 mg/ml; Sigma Chemicals, St. Louis, MO) was used as a peroxidase substrate in the presence of 0.0025% hydrogen peroxide to produce a chromogen for visualization. All steps in the immunohistochemistry procedure were performed at 22°C. The sections were mounted onto gelatin-coated slides, counterstained with cresyl violet, dehydrated in ethanol, delipidated in xylenes, and then coverslipped. Spinal cords, nodose ganglia, DRG, and T13 sympathetic ganglia sections were thaw-mounted onto gelatin coated-slides and processed for immunohistochemistry for HSV-1 identically to that described above for free-floating brain sections, except that incubation times were increased to 3 days for 1° Ab, 1 day for 2° Ab, and 1 day for ABC.
Data analysis and imaging.
All brain sections processed were observed for HSV-1 immunoreactivity microscopically from the level of the hypothalamic preoptic area (POA) rostrally through the brainstem caudally using an Olympus BX41 microscope. Images were captured digitally with an Olympus DP70 and acquired using Adobe Photoshop (v7.0, San Jose, CA). A mouse brain atlas (32) was used in identifying brain regions, as there is no Siberian hamster brain atlas commercially available, and the size and shape of most mouse brain areas are similar to that of Siberian hamsters. Infected cell numbers were counted from the brains of animals that survived 114 h postinoculation along the rostro-caudal extent of the individual brain regions on both sides and then averaged across the animals for each injection group (i.e., IWAT: n = 4, or EWAT: n = 4).
Peripheral neurophysiological recordings.
All electrophysiological studies were performed in accordance with animal protocols approved by the Albert Einstein College of Medicine Animal Care and Use Committee and in accordance with the Public Health Service and U.S. Department of Agriculture guidelines. Hamsters (n = 4) were anesthetized with ketamine/xylazine via an intraperitoneal injection. A femoral vein catheter was placed in the left femoral vein using silicone tubing (0.12′′ ID × 0.25′′ OD; SF Medical, Hudson, NY). Core temperature was measured via a flexible rectal probe (YSI instruments, Yellow Springs OH) and maintained at 36–37°C with a water-driven heating pad (Gamma Medical Systems, Frederick, MD) and an infrared heating lamp when necessary. A surgical plane of anesthesia was maintained by 0.1-ml intravenous injections of ketamine/xylazine as necessary to maintain areflexia to toe pinch. Heart rate and blood oxygenation was monitored throughout all experiments by a tail pulse oximeter (Nonin Medical, Plymouth, MI). A 2-cm incision was made along the ventrum to expose the IWAT pad. A 1-cm segment of the inguinal nerve was exposed by isolating the IWAT pad from the surrounding tissue. The lateral edges of the exposed pad were packed with gelfoam to minimize bleeding and to form a well that held a small pool of warmed (37°C) mineral oil over the recording sites along the nerve penetrating the IWAT pad.
After obtaining baseline neurophysiological recordings, hamsters were given an intraperitoneal injection of 2DG (500 mg/kg body mass). Although this would be a large 2DG dose for laboratory rats [e.g., 100 mg/kg (30)], this dose is necessary to produce physiological responses in Siberian hamsters (e.g., increased plasma glucose, circulating catecholamines; T. J. Bartness, unpublished observations) but are below those that induce torpor in this species (e.g., 16).
Neurophysiological recording and data analysis.
A pair of Teflon-coated tungsten microelectrodes (5–12 MOhm impedance; AM Systems, Carlsburg, WA) with 2- to 4 mm exposed tips were used for all differential peripheral WAT nerve recordings. A hydraulic micromanipulator (Narishige, Tokyo, Japan) was used to advance the recording electrode pair. Afferent discharges were fed to a Grass P511 AC preamplifier and monitored on line with a Haer audio monitor and Tektronix digital and analog oscilloscopes. In addition, amplified signals were fed to a dual time-amplitude window discriminator (BAK Electronics Inc., AD Converter Model DDIS-1) to determine the number of spikes occurring above basal voltage. Isolated units were triggered to appear on a second digital oscilloscope (Tektronix 2211, Beaverton, OR) following an interposed delay (BAK AD-1, 0.1–3-ms delay range, Gaithersburg, MD) to confirm that each isolated unit produced a reliable and unique time-amplitude signature. All isolated units above baseline were also recorded in real time using PowerLab Chart (ver. 5) (AD instruments, Colorado Springs, CO) and subsequently analyzed using the PowerLab spike histogram module (AD Instruments; ver. 5.3). A digital oscilloscope record was simultaneously written to disk for 50 consecutive spontaneously occurring spikes to confirm the consistency of the isolated units above baseline. A significant multiunit response to a 2DG stimulus was defined as a change in the number of total discharges in the 30–60 s postinjection interval of at least 1.5 standard deviations from the average number occurring in 30–60 bins of spontaneous preinjection activity. These criteria are consistent with previous studies that have successfully recorded and analyzed peripheral vagal afferent and the solitary tract nucleus (Sol) neurophysiological responses to alimentary tract stimulation (34).
Statistical analyses.
The electrophysiological recordings were analyzed statistically using an ANOVA for repeated measures (GraphPad Prism Software, San Diego, CA; GPM ver. 4.0). Differences between means were considered significant if P < 0.05. Exact probabilities and test values were omitted for simplicity and clarity of presentation.
RESULTS
Animals killed between 24 h and 114 h postinjection did not show obvious signs of illness; however, they were less mobile and appeared to spend less time in their nests (a potential sign of discomfort) closer to 114 h postinjection.
Twenty-four-hour postinjection with H129.
HSV-1-immunoreactive (ir) cells were not found at any level of the neuroaxis, including in the spinal cord or sensory (DRG, nodose ganglia) or sympathetic (T13) ganglia.
Forty-eight-hours postinjection with H129.
HSV-1-ir cells were found in the ipsilateral DRG and the ipsilateral T13 (4 of 4 animals). There were no HSV-1-ir cells found in the nodose ganglia, spinal cord, or in the brain.
Seventy-two hours postinjection with H129.
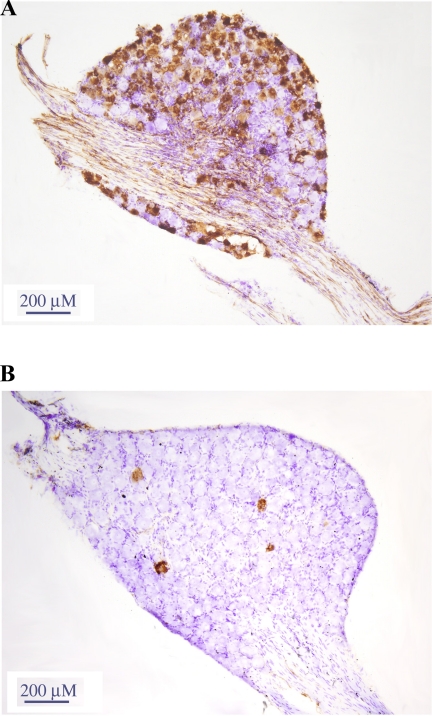
HSV-1-ir cells were found bilaterally in the DRG (4 of 4 animals; Fig. 1; more ipsilaterally [Fig 1A] than contralaterally, however [Fig 1B]). HSV-1-ir cells also appeared bilaterally in the T13 ganglia (1 of 4 animals with more ipsilateral, than contralateral infection) and in the dorsal horn of the lower thoracic and upper lumbar spinal cord (Fig. 2A), including Clarke's nucleus. There were no infected cells found in either nodose ganglion.
Fig. 1.
H129 strain of the herpes simplex virus-1 (HSV-1) injected into inguinal white adipose tissue (IWAT) caused HSV-1 immunoreactivity (-ir) in the ipsilateral dorsal root ganglion (and dorsal horn in the lower thoracic and lumbar spinal cord segments) at 48 h. H129 infection found in the ipsilateral (A) and contralateral (B) dorsal root ganglion (DRGs) at 72 h postinjection was detected using standard immunohistochemistry against HSV-1 with diaminobenzidine peroxidase-generated (brown) chromogen. Sections were then counterstained using cresyl violet (blue).
Fig. 2.
HSV-ir in the thoracolumbar spinal cord dorsal horn 72 h after H129 injections into the ipsilateral inguinal white adipose tissue (A). Ninety-six hours postinoculation, spinal cord H129 infection progressed into the ipsilateral intermediolateral horn (IML) and ventral horn (B). H129 infection was detected using standard immunohistochemistry against HSV-1 (DAB peroxidase-generated chromogen; brown). Sections were counterstained (cresyl violet; blue). Arrows indicate HSV-1-ir fiber from the dorsal horn to the intermedial lateral horn. CC: central canal.
Ninety-six hours postinjection with H129.
HSV-1-ir cells were observed bilaterally in the nodose ganglia (1 of 4 animals, although predominantly ipsilateral to the injection with scant infection contralaterally), as well as in the intermediolateral (IML) horn and region of the thoracic spinal cord (Fig. 2B). In the brainstem, HSV-1-ir cells were found in the Gr and cuneate (Cu) nuclei, raphe nuclei (magnus, RMg; pallidus, RPa), lateral paragigantocellular (LPGi), dorsal paragigantocellular (DPGi), gigantocellular alpha (GiA), reticular regions (interstitial, Irt; lateral LRt; rostroventrolateral, RVL), prepositus hypoglossal nucleus (Pr), ventral spinocerebellar tract (vsc), A5 cell group, subcoeruleus nucleus (dorsal/-ventral; SubCD/-V), Sol, and dorsal motor nucleus of the vagus nerve (DMV). In the midbrain, HSV-1-ir cells were in the periaqueductal gray (PAG; particularly the ventrolateral subdivision). Forebrain infections were found most abundantly in the paraventricular (PVH) and periventricular (Pe) hypothalamic nuclei. Scattered HSV-1-ir cells also were found in the lateral (LH), dorsomedial (DM) and posterior (PH) hypothalamic nuclei.
One-hundred-and-fourteen hours postinjection with H129.
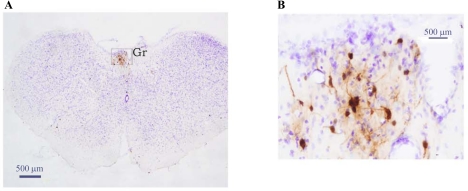
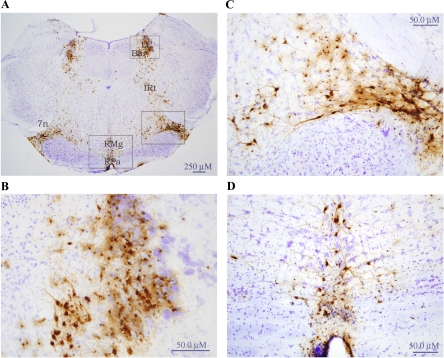
By 114 h, the infection was more intense in the regions identified at 72 h and 96 h, including the nodose ganglia (Fig. 3), as well as in the brainstem. In terms of the latter, HSV-1-ir cells were found in the Gr (Fig. 4), Irt (Fig. 5), LRt, LPGi, RVL, vsc, DMV, Sol, facial nuclei regions (7N), A5 (Fig. 5, A and C), A1/C1, locus coeruleus (LC; Fig. 5, A and B), Barrington's nucleus (Bar; Fig. 5, A and B), RMg (Fig. 5, A and D), Rpa (Fig. 5, A and D), Raphe cap (RC; Fig. 6, A and B), lateral parabrachial (LPB; Fig. 6, A and B) and SubCD/-V (Fig. 6, A and C). In addition, HSV-1-ir cells were found in several midbrain sites such as the olivary regions and rubral areas, including the retrorubral field. In the forebrain, infection was most impressive in the PVH (Fig. 7) and Pe, and less impressive in the zona incerta and thalamic reuniens (Re) and its immediate surrounding regions (i.e., ventral reuniens and xiphoid; Re/Vre/Xi; see Fig 7). HSV-ir cells also were found scattered across various hypothalamic regions including the LH, DM, PH, POA (see Table 1 for a more complete list).
Fig. 3.
H129 injected into inguinal white adipose tissue caused infection in the nodose ganglion, predominantly ipsilateral to the site of injection (A). B: scant H129 infection in the contralateral nodose ganglion is shown for comparison. HSV-ir cells:brown.
Fig. 4.
Low-magnification photomicrograph illustrating HSV-1-ir cells in the gracile nucleus (Gr; A) 114 h after injection into IWAT. B: Gr outlined in A is magnified for better visibility. HSV-ir cells:brown; cresyl violet:blue.
Fig. 5.
H129 injected unilaterally into hamster inguinal white adipose tissue resulted in HSV-ir cells in the brain at 114 h postinjection. A: low-magnification photomicrograph illustrating HSV infection in the brainstem. B: locus coeruleus (LC) and Barrington's nuclei (Bar) outlined in Fig. 5A is magnified for better visibility. C: A5 area outlined in A is magnified. D: raphe (magnus: RMg, pallidus: RPa) regions outlined in A under higher magnification. HSV-ir cells:brown; cresyl violet:blue. 7n, facial nerve, or root; A5, noradrenaline cells; IRt, intermediate reticular nucleus.
Fig. 6.
A: photomicrograph illustrating H129 infection in the caudal midbrain ∼ rostral brainstem 114 h after injection into IWAT. B: Raphe cap (RC)/lateral parabrachial (LPB) region outlined in A is magnified for improved visibility of the H129 infection. C: ventral subcoeruleus nucleus (SubCV) region outlined in A is magnified. HSV-ir cells:brown; cresyl violet:blue. 4n, trochlear nerve, or root; Aq, aqueduct; ml, medial lemniscus.
Fig. 7.
A: photomicrograph illustrating H129 infection in the forebrain at 114 h after inoculation unilaterally into the hamster IWAT. B: portion of the paraventricular hypothalamus (PVH) outlined in A is magnified. HSV-ir cells:brown; cresyl violet:blue. OC, optic chiasm; Re, reuniens thalamus; SCH, suprachiasmatic hypothalamus; ZI, zona incerta.
Table 1.
Means ± SE herpes simplex virus-1 (HSV-1)-immunoreactive cells after injection of the H129 strain of the HSV-1 into IWAT and EWAT
| IWAT (n = 5) | EWAT (n = 4) | |||
|---|---|---|---|---|
| Forebrain | ||||
| Hypothalamic | ||||
| A14 | 2.80±1.96 | 1.00±1.00 | ||
| AH/LA | 15.4±5.80 | 18.00±11.02 | ||
| Arc | 4.2±4.20 | 2.25±1.93 | ||
| DM | 33.2±24.44 | 25.75±14.62 | ||
| DTM | 0.80±0.80 | 0.00 | ||
| LH | 26.20±12.31 | 34.25±22.80 | ||
| Pe/AVPe | 73.60±33.04 | 155.75±86.20 | ||
| PH | 27.80±18.30 | 4.25±3.92 | ||
| POA | 15.00±11.56 | 10.25±7.81 | ||
| PVH | 676.00±199.51 | 736.75±370.59 | ||
| PaAP | 52.80±21.34 | 92.50±67.22 | ||
| PaDC | 35.80±23.61 | 31.00±14.08 | ||
| PaLM | 16.00±6.55 | 40.00±21.15 | ||
| PaMM | 49.40±32.09 | 50.00±34.51 | ||
| PaMP | 222.80±85.67 | 181.50±106.06 | ||
| PaPo | 229.20±59.41 | 183.00±114.64 | ||
| PaV | 70.00±41.29 | 158.75±119.06 | ||
| RCh | 2.20±1.56 | 2.00±2.00 | ||
| SON | 7.00±7.00 | 0.00 | ||
| Spa | 16.20±8.94 | 42.25±27.47 | ||
| SCH | 5.60±3.44 | 5.75±3.33 | ||
| VMH | 3.00±1.84 | 1.00±0.71 | ||
| Thalamic | ||||
| CM | 1.60±0.75 | 0.25±0.25 | ||
| MDM | 2.60±1.47 | 0.00 | ||
| PV | 11.20±5.27 | 5.25±4.92 | ||
| Re/Vre/Xi | 58.60±20.01 | 129.50±81.47 | ||
| Rt | 5.00±2.45 | 8.75±6.37 | ||
| Other forebrain | ||||
| AC | 5.80±5.55 | 1.00±0.71 | ||
| Amygdala/slea | 17.60±17.35 | 7.25±4.57 | ||
| BST | 2.60±1.69 | 2.00±1.68 | ||
| Fascicular | 47.00±27.48 | 32.25±16.62 | ||
| ns | 3.80±2.65 | 3.5±3.50 | ||
| PeF/f | 6.40±2.60 | 22.50±20.23 | ||
| PS | 2.00±2.00 | 0.00 | ||
| SI | 0.00 | 0.50±0.50 | ||
| SubI | 18.40±11.12 | 0.25±0.25 | ||
| Subzi | 6.80±3.97 | 16.50±13.09 | ||
| TC | 3.00±1.41 | 5.33±4.19 | ||
| ZI | 47.00±22.56 | 35.25±17.84 | ||
| Midbrain | ||||
| Collicular | 10.40±4.34 | 2.00±1.22 | ||
| CnF | 4.80±3.57 | 0.00 | ||
| Dk | 2.60±1.44 | 0.25±0.25 | ||
| DpMe | 11.20±3.73 | 9.75±8.17 | ||
| DMTg | 7.40±4.38 | 0.00 | ||
| ERS | 0.80±0.80 | 0.00 | ||
| EW | 0.40±0.40 | 0.25±0.25 | ||
| IF | 0.20±0.20 | 0.50±0.50 | ||
| InC | 2.40±1.75 | 0.50±0.50 | ||
| LDTg | 17.80±8.42 | 12.33±5.51 | ||
| Lemniscus | 20.6±15.99 | 8.25±4.97 | ||
| Mammillary | 0.04±0.04 | 2.00±2.00 | ||
| MiTg | 2.00±1.30 | 0.50±0.29 | ||
| ml/lfp | 18.40±18.40 | 10.25±8.97 | ||
| Oculomotor | 10.20±7.33 | 9.00±6.14 | ||
| Olivary | 119.20±83.99 | 139.00±78.70 | ||
| PAG | 55.60±24.52 | 25.75±15.26 | ||
| Pontine | 62.40±26.41 | 55.50±33.09 | ||
| PPTg | 7.20±4.32 | 6.50±4.09 | ||
| pv | 19.00±8.94 | 28.25±20.03 | ||
| Rubral areas | 119.40±52.21 | 72.00±26.82 | ||
| RtTg/P | 2.60±1.21 | 0.00 | ||
| PN | 1.40±1.40 | 0.00 | ||
| SN | 9.40±5.47 | 10.50±6.16 | ||
| SPTg | 0.00 | 0.00 | ||
| VLTg | 13.60±9.91 | 2.25±2.25 | ||
| VTA | 7.00±5.83 | 0.50±0.50 | ||
| Xscp | 1.40±1.40 | 0.00 | ||
| scp | 7.00±3.59 | 4.50±2.72 | ||
| Brain stem | ||||
| 12/12N | 16.80±8.07 | 14.50±11.14 | ||
| 10 (DMV) | 333.2±122.41 | 807.00±458.47 | ||
| A1/Cl | 267.20±84.02 | 14.00±9.06 | ||
| A2 | 3.00±2.76 | 8.75±8.75 | ||
| A5 | 267.60±51.43 | 106.75±53.98 | ||
| A7 | 4.00±4.00 | 0.00 | ||
| Amb | 102.80±57.54 | 54.00±44.15 | ||
| Abducens | ||||
| 6N | 5.00±5.00 | 0.00 | ||
| Pa6 | 12.00±12.00 | 0.00 | ||
| AP | 35.40±22.31 | 249.50±177.52 | ||
| Bar | 17.20±15.74 | 1.00±1.00 | ||
| CeCv | 10.20±4.63 | 0.00 | ||
| C3 | 21.20±14.94 | 16.75±11.26 | ||
| Cu/Ecu | 26.4±12.30 | 2.75±2.75 | ||
| Facial regions | 324.20±90.71 | 167.75±113.03 | ||
| DPGi | 94.80±43.11 | 42.25±29.01 | ||
| LPGi | 842.4±217.32 | 502.25±246.14 | ||
| Gi | 224.2±97.00 | 167.75±96.73 | ||
| GiA | 144.00±67.05 | 176.75±101.55 | ||
| GiV | 381.80±156.73 | 329.75±210.05 | ||
| KF | 11.60±7.74 | 8.50±8.17 | ||
| LC | 93.8±64.75 | 4.50±2.63 | ||
| Li | 28.8±13.67 | 7.50±5.95 | ||
| Parabrachial areas | ||||
| LPB | 187.80±77.76 | 46.00±30.20 | ||
| MPB | 40.80±29.00 | 10.25±5.15 | ||
| PPy | 9.00±9.00 | 19.00±12.11 | ||
| Pr | 169.20±68.03 | 123.67±73.47 | ||
| Raphe areas | ||||
| DR | 11.20±5.54 | 6.75±5.79 | ||
| RC | 58.80±33.15 | 40.25±29.00 | ||
| RMg | 150.00±37.65 | 137.00±82.01 | ||
| Rob | 195.20±78.53 | 103.50±70.84 | ||
| Rpa | 219.00±79.36 | 243.25±154.78 | ||
| Reticular regions | ||||
| IRt | 1131.40±448.91 | 772.25±538.40 | ||
| LRt | 411.60±132.69 | 247.50±152.79 | ||
| MdD-/V | 224.40±105.37 | 160.50±108.17 | ||
| PCRt | 19.60±8.52 | 11.00±9.41 | ||
| PCRtA | 68.80±28.93 | 56.25±40.58 | ||
| PMn | 122.20±75.84 | 54.75±43.17 | ||
| RVL | 618.20±193.63 | 303.25±160.66 | ||
| Ro | 3.60±2.40 | 3.00±1.91 | ||
| Gr/gr | 11.40±3.06 | 10.75±8.82 | ||
| Sol | 541.60±188.50 | 1010.25±640.78 | ||
| SubCD/V | 261.40±71.59 | 36.00±19.40 | ||
| trigeminal areas | 136.80±59.52 | 63.25±40.26 | ||
| Tz/tz | 12.40±12.15 | 18.50±11.30 | ||
| vestibular areas | 72.40±36.82 | 43.25±28.93 | ||
| vsc | 1777.80±582.86 | 1067.75±596.69 | ||
| X | 2.80±1.71 | 6.25±3.35 | ||
IWAT, inguinal white adipose tissue; EWAT, epididymal white adipose tissue. Forebrain. Hypothalamic: AH, anterior hypothalamus; Arc, arcuate nucleus; DM, dorsomedial nucleus; DTM, dorsal tuberomammillary; LH, lateral hypothalamus; Pe/AVPe, periventricular/anteroperiventricular; PH, posterior hypothalamus; POA, preoptic area; PVH, paraventricular hypothalamus; PaAP, paraventricular hypothalamic anterior, parvicellular; PaDC, parventricular hypothalamic, dorsal cap; PaLM, parventricular hypothalamic, lateral magnocellular; PaMM, paraventricular hypothalamic nucleus, medial magnocellular; PaMP, paraventricular hypothalamic, medial parvocellular; PaPo, paraventricular hypothalamic, posterior; PaV, paraventricular hypothalamic, ventral part; RCh, retrochiasmatic area; SON, supraoptic nucleus; Spa, subparaventricular nucleus; SCH, suprachiasmatic nucleus; VMH, ventromedial hypothalamus. Thalamic: CM, central medial thalamic nucleus; MDM, mediodorsal thalamic nucleus, medial part; PV, parventricular, thalamus; Re/Vre/Xi, reuniens/reuniens, ventral/xiphoid. Other forebrain: AC, anterior commissural nucleus; BST, bed nucleus of the stria terminalis; ns, nigrostriatal bundle; PeF/f, perifornical nucleus/fornix; PS, parastrial nucleus; SI, substantia innominata; SLEA, sublenticular extended amygdala; SubI, subincertal nucleus; subZI, sub zona incerta; TC, Tuber cinerium; ZI, zona incerta. Midbrain. CnF, cuneiform; Dk, darkschewitsch; DMTg, dorsomedial tegmental area; DpMe, deep mesencephalic nucleus; DTg, dorsal tegmental nuclei; ERS, epirubrospinal nucleu; EW, Edinger Westphal; IF, interfascicular nucleus; InC, interstitial nucleus of Cajal; LDTg, laterodorsal tegmental; MiTg, microcellular tegmental nucleus; ml/lfp, medial lemniscus/longitudinal fasciculus of the pons; PAG, periaqueductal gray; PPTg, pedunculopontine nuclei; pv, periventricular fiber system; RtTg/P, reticulotegmental nucleus of the pons/pericentral; PN, paranigral nucleus; SN, substantia nigra; SPTg, subpeduncular tegmental nucleus; VLTg, ventrolateral tegmental nucleus; VTA, ventral tegmental area; xscp, decussation of the superior cerebellar peduncle; scp, superior cerebellar peduncle. Brain stem: 12, hypoglossal nucleus; 10 (DMV), dorsal motor nucleus of the vagus nerve; A1/C1, noradrenaline cells/adrenaline cells; A5, noradrenergic cell field; A7, A7 noradrenaline cells; Amb, ambiguous nucleus; Pa6, paraabducens; AP, area postrema; Bar, Barrington's nucleus; CeCv, central cervical nucleus; C3, adrenergic cell field; Cu/Ecu, cuneate/external cuneate; DPGi, dorsal paragigantocellular nucleus; Gi-A/V, gigantocellular reticular nucleus-alpha/ventral; KF, Kolliker-Fuse nucleus; LC, locus coeruleus; Li, linear nucleus of the medulla; LPGi, lateral paragigantocellular nucleus; LPB, lateral parabrachial nucleus; MPB, medial parabrachial nucleus; PPy, peripyramidal nucleus; Pr, prepositus hypoglossal nucleus; DR, dorsal raphe; RC, raphe cap; RMg, raphe magnus nucleus; Rob, raphe obscurus; Rpa, raphe pallidus; Irt, intermediate reticular nucleus; LRt, lateral reticular nucleus; MdD-/V, medullary nucleus dorsal-/ventral; PCRt-/A, parvicellular reticular nucleus; PMn, paramedian reticular nucleus; RVL, rostroventrolateral reticular nucleus; Ro, nucleus of Roller; Gr/gr, gracile nucleus/fasciculus; Sol, solitary tract nucleus; SubCD/-V, subcoeruleus nucleus, dorsal/-ventral; Tz/tz, trapezoid nucleus/body; vsc, ventral spinocerebellar tract; X, nucleus X.
EWAT.
When H129 was injected into EWAT, HSV-1-ir cells were found in a very similar distribution throughout the brain to that seen after IWAT injections 114 h after injection. Specifically, infected cells were most notable in the vsc, Sol, DMV, RVL, IRt, RPa, and LPGi. Intense labeling also was found in the area postrema. Similar to the infection pattern found with IWAT injections, HSV-1-ir cells were scant in the midbrain, but they were still identifiable in the PAG, pontine, olivary, and rubral regions. Forebrain infections included thalamic sites (Re/VRe/Xi) and hypothalamic sites (PVH, Pe, and a few cells in the LH; see Table 1).
Effects of systemic 2DG-induced glucoprivation on multiunit neurophysiological action potential recordings from IWAT sensory afferents.
Systemic injection of 2DG triggered a marked and significant increase in multiunit sensory action potentials in anesthetized Siberian hamsters with ∼5- and ∼19-fold increases in spikes at 5 and 10 min postinjection, respectively (P < 0.05, Fig. 8).
Fig. 8.
Effects of intraperitoneal administration of 500 mg/kg of 2-deoxy-d-glucose. Intraperitoneal saline is without effect; n = 4. Ketamine/xylazine anesthetized nonfasted male Siberian hamster with right inguinal fat nerve exposed, and isolated in situ inguinal fat pad. Inguinal fat sensory fiber bundles were isolated and transected, and the distal endings, still attached to adipose tissue, were placed on paired Teflon-insulated tungsten electrodes for bipolar multiunit neurophysiological action potential recordings from sensory afferents arising from inguinal fat depot. Representative tracings from one animal is shown.
DISCUSSION
The central and peripheral sensory afferent projections from IWAT and EWAT to the brain were identified for the first time in any species using a transneuronal anterograde viral tract tracer, the H129 strain of HSV-1. In doing so, we labeled the spinal sensory afferents, as evidenced by DRG infections. In addition, as an entry into functional tests of WAT sensory afferents, we found increases in neural activity of decentralized sensory nerve fibers from IWAT in response to systemic 2DG-induced glucoprivation. This increase in multiunit sensory afferent firing rate may be due to sympathetic nerve-driven lipolysis, because this same dose of 2DG given to awake, freely moving Siberian hamsters markedly increases IWAT NETO, accompanied by significant increases in serum free fatty acids (FFAs; a lipolytic product) (11). Collectively, we present for the first time the central targets of WAT sensory afferents and demonstrate a condition that stimulates these afferents. Therefore, these data reinforce and greatly extend the seminal finding of the sensory innervation of WAT demonstrated over 20 years ago in a more limited manner when a traditional retrograde tract tracer injected into WAT-labeled DRG pseudounipolar neurons (18).
While the present work was in progress, sparse labeling in the Gr was reported after the conventional monosynaptic retrograde tract tracer CTb was injected into laboratory rat retroperitoneal WAT, which is suggestive of sensory afferents from WAT terminating centrally (26). We confirmed that the Gr receives WAT sensory input using the H129 transneuronal tract tracing method; moreover, we defined more fully the extent of central afferent circuitry from WAT.
There are several parallels between labeling of central sensory inflow input circuits from WAT with H129 and labeling of central sympathetic outflow circuits with PRV. For example, in the present study using H129, we found infection in the ipsilateral DRG beginning at 48 h postinjection, as well as in the ipsilateral T13 sympathetic ganglion. H129 is most useful because of its strictly anterograde travel through neural circuits (6, 43, 50). The labeling of T13 postganglionic SNS cells may have occurred because they also are first-order neurons, and H129 initially infects all first-order neurons, as was shown with stomach injections of H129 (33). Further progression into the CNS via the WAT SNS circuits would require retrograde spread of the virus across synapses, which the H129 is not capable of doing (17, 33, 50). In addition, the labeling of the ipsilateral T13 sympathetic ganglion may be due to sensory nerves from WAT, impinging on this sympathetic ganglion, as others have noted previously (for a review, see Ref. 12). Indeed, DRG pseudounipolar neurons send projections to the superior cervical ganglia [a sympathetic ganglion; (47)], possibly forming short SNS-sensory feedback loops. T13 could then be similarly connected and therefore be subject to infection via sensory DRG neurons.
At 72 h postinjection, we found HSV-1-ir cells in the spinal cord lower thoracic and upper lumbar dorsal horn, in the ipsilateral ventral white matter region (ascending ventral spinocerebellar tract), as well as the occasional fibers projecting to the contralateral dorsal horn and ascending spinothalamic tract. Information relayed through the ascending spinothalamic and spinocerebellar circuits are traditionally thought to relay pain and proprioceptive information, respectively (45). SNS afferent soma reside in the DRG of the thoracic and more rostral lumbar spinal levels (12), as we found, and evidence exists that they contain non-nociceptive (i.e., nonpain) visceral receptors involved in the reflexive control or homeostasis (12). In the present context, these visceral afferents may impart sensory information associated with lipolysis, as our 2DG electrophysiological evidence would suggest.
At 96 h post injection, we found HSV-ir cells in the IML. The IML is composed of sympathetic preganglionic cells that could be subject to sensory feedback. Support for such sensory-sympathetic interplay at the level of the spinal cord comes from the expression of substance P receptors by sympathetic preganglionic IML neurons (22), which is highly suggestive of direct sensory input onto these cells. We believe that this is what we captured in Fig. 2B, a clearly labeled HSV-ir dorsal horn sensory fiber projecting into the ipsilateral IML. We found that the ipsilateral DRG is infected first at 48 h followed by infection in the dorsal horn at 72 h, and finally in the IML at 96 h—suggestive of the progression of H129 anatomically from IWAT into IML.
In addition to HSV-1-ir cells in the lower brainstem Gr and Cu at 96 h postinjection, H129 infected cells occurred in several other brainstem sites, including the raphe areas (e.g., RMg, RPa), LPGi, DPGi, GiA, reticular regions (Irt, LRt, RVL), Pr, vsc, A5, SubCD/-V, Sol, and lateral edges of the DMV, collectively demonstrating extensive afferent inputs to the brainstem. Midbrain structures also received some sensory inputs (e.g., PAG, olivary, and rubral regions; see Table 1 for complete list). Also occurring at 96 h postinoculation were HSV-1-ir cells in several forebrain areas known to be involved in energy balance (e.g., PVH, LH, Pe, DM, PH, and POA), as well as in the thalamus (e.g., Re/VRe/Xi; see Table 1 for the complete list). This thalamic infection, however, was minor compared with that of the brainstem and hypothalamic structures, and the role of these thalamic sites in energy balance is unknown.
Notable at 96 h were HSV-1-ir cells in the nodose ganglia predominantly ipsilateral to the site of injection, with a few, infrequently infected cells also in the contralateral side. The overwhelming ipsilateral pattern of nodose infection is consistent with the general view that the distal process, soma, and proximal process of pseudounipolar cells of the nodose ganglia remain uncrossed, and thus ipsilateral to the tissue they innervate (and their origin in the nodose ganglia) in rodents (e.g., 19). The nodose labeling, although suggestive of parasympathetic (PSNS) afferents, is instead more likely secondary to infection of WAT spinal afferents projecting to the dorsal vagal complex of the brainstem, an area where we saw HSV-1-ir at 96 h, which, in turn, have descending projections to the nodose ganglia (e.g., 23). The late infection of the nodose ganglia, compared with DRG-infections, supports this circuitous route of nodose ganglia labeling. To test this notion definitively, extensive studies combining surgical deafferentation with viral labeling will be required. Our interpretation here is consistent with our previous finding that there is no efferent PSNS innervation of WAT (20, 25), and, thus, no nodose-based PSNS afferents. The conclusion that WAT does not have PSNS efferent projections is based on PRV tract tracing methodology and the immunohistochemical absence in WAT of well-established PSNS markers found in other tissues [e.g., vasoactive intestinal peptide, neuronal nitric oxide, vesicular acetylcholine transporter; (20)], as well as the lack of cholinergic-associated biochemicals in this tissue (2).
By 114 h postinjection, our longest postinjection time before the onset of adverse effects, such as malaise and labored breathing, H129 infection progressed more robustly into brain regions that showed infections at the 96 h postinoculation time. Although there is some overlap in the areas receiving afferent information from WAT with those areas involved in the sympathetic outflow to this tissue at this and other times, there also are clear differences. In general, H129 appears to cause greater infection in the forebrain. For example, H129 produces greater infection in the Pe at 114 h (4.75 days) postinoculation than does PRV at 6 days when PRV is injected into WAT (3, 36, 39, 41) or BAT (4, 42). By contrast, H129 causes little infection in the SCH and Arc at its maximal postinoculation time (4.75 days) than does PRV at its maximal postinoculation time [6 days (3, 39, 41)]. Furthermore, the SCH and Arc are readily observable as components of the SNS outflow from brain to WAT using PRV, even at shorter postinoculation intervals (3, 36, 39, 41). We cannot, however, be certain that these are actual differences in the circuitries labeled by the two viruses because it is possible that the apparent discrepancies could result from the different survival times used for each virus, as well as potential differential rates for transmission of the virus through the two circuitries. We have observed, however, cells in forebrain areas such as the PVH and LH that are dually labeled with PRV and with H129 when both viruses are injected into the same WAT pad (Song, Smith and Bartness, unpublished observations) or into interscapular BAT (42). This suggests that the viruses, although progressing through their respective circuits, reached these neurons at about the same time, given that infection of cells by one virus will inhibit subsequent infection by another virus (for a review, see Ref. 40).
As a beginning toward understanding the function of these WAT afferents, we made electrophysiological recordings from pseudounipolar nerves innervating IWAT pad by creating 2DG-induced glucoprivation as the stimulus to trigger the increases in sympathetic drive to WAT, as we demonstrated previously in this species (11), and presumably stimulate lipolysis via activation of WAT β-adrenoceptors (e.g., 28); indeed, 2DG increased circulating concentrations of the lipolytic product, FFAs (11). We duplicated that glucoprivic condition here in anesthetized Siberian hamsters, in which some of the fiber bundles from the major nerve supply to IWAT (13) were cut (decentralized), enabling electrophysiological recording of only sensory activity from some of these IWAT afferents and leaving the remaining afferents and efferents intact. 2DG significantly increased multiunit neurophysiological action potentials in these neurons (up to ∼19-fold) in a time-dependent manner. Although we cannot unequivocally state that the increased electrophysiological activity of the IWAT sensory afferents was a direct result of sympathetic-stimulated lipolysis elicited by 2DG, it inferentially appears that some factor associated with the increased SNS drive to WAT ultimately increased the activity of these WAT-associated afferents. The precise mechanism of that event remains to be elucidated. In one scenario, WAT could be sensing products of sympathetic nerve-driven lipolysis such as FFAs or glycerol. Such a process seems feasible because neural receptive elements in the gastrointestinal system exist with afferents that respond to FFAs [in sheep (14), rats (27), and cats (29)]. In addition, the cat gastrointestinal system has two types of receptors—one activated by short-chain FFAs and by glycerol and another by long-chain FFAs (29), suggesting that such receptors are physiological realities, but perhaps only occurring in the gut. Alternatively, an associated factor with sympathetic nerve discharge, such as prostaglandin E2 secretion (35), could be sensed via WAT sensory afferents, as receptors for PGE2 are found on sensory neurons (38). Obviously, the function of WAT sensory neurons is not clear, but they may serve as feedback to the CNS origins of the SNS drive to WAT, thereby modulating lipolysis so as to match the need for lipid-derived fuels to energy needs, such as would occur with food deprivation or cold exposure. Indeed, preliminary data show dually infected cells in the PVH when PRV (to label sympathetic outflow) and H129 (to label sensory afferents) were both injected into IWAT. It seems likely that similar WAT SNS-sensory nerve feedback circuits exist in other brain sites, which would help explain why many of the areas we previously identified as components of the sympathetic circuit to WAT using PRV (e.g., A5 and Sol) also were labeled by H129 in the present study (see Table 1).
In summary, we report for the first time the sensory afferents from WAT from its origins in the periphery to its projection sites in the CNS, including all major subdivisions of the ascending neuraxis (brainstem, midbrain, and forebrain). We find substantial overlap of the CNS sites in the present study with the central origins of the sympathetic outflow to WAT detailed in previous work (3, 36, 39, 41), suggesting possible SNS-sensory feedback circuitry that could function to regulate SNS drive to WAT. Indeed, consistent with this notion, is the increase in sensory nerve multiunit firing rates with glucoprivation, a condition that produces a robust increase in WAT sympathetic drive (NETO) in these animals (11). Collectively, these data serve as a beginning toward understanding the structural and functional basis of the sensory innervation of WAT.
Perspectives and Significance
The prevalence of obesity worldwide (e.g., 44) suggests that if physiological systems exist to control total body fat, that these systems are relatively easily overridden by environmental factors (e.g., nonexercise promoting environments, readily available inexpensive foods). Alternatively, such a negative feedback system may not exist. On a moment-to-moment basis, however, it would seem reasonable to hypothesize the existence of a negative feedback system for the short-term control of lipid energy reserves, in this case, the control of lipolysis. A relatively precise control of lipid mobilization would allow enough lipid fuels to be released via lipolysis to meet energy oxidation needs but not so much as to exceed these needs, thereby overdepleting lipid energy reserves (31). The present data suggest that such short-term control may be achieved by complete SNS-sensory neural loops from WAT that would function in a way analogous to other neural feedback loop systems involved in blood pressure regulation. For example, the neural control of blood pressure appears to involve short-term alterations in arterial blood pressure responded to by sinoaortic barorecptors to maintain the constancy of arterial pressure despite altered behavior/physiology: they are considered incapable, however, of adjusting atrial pressure under chronic stimuli due to their rapid resetting with the prevailing arterial blood pressure (15). In terms of lipid mobilization, on a moment-to-moment basis, a neural negative feedback loop/system could serve short-term energy needs from WAT lipid fuels such as mediating intermeal lipolysis or exercise-induced lipolysis, rather than as a more sustained negative feedback loop that might come into play to control body fat reserves.
GRANTS
This research was supported by National Institutes of Health (NIH) Research Grant R0-1 DK-35254 to T. J. Bartness, the National Science Foundation Center for Behavioral Neuroscience Viral Tract Tracing Core through the Science Technology Center Program of National Science Foundation under agreement No. IBN-987654 and by NIH R0-1 DK 47208, NY Obesity Research Center DK 026687 and the Skirball Institute for Nutrient Sensing to G. J. Schwartz.
Acknowledgments
The authors thank Bruce James Smith and Maisie Adiviani for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arch JR Central regulation of energy balance: inputs, outputs and leptin resistance. Proc Nutr Soc 64: 39–46, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne B Histochemical and biochemical aspects of cholinesterase activity of adipose tissue. Arch Int Pharmacodyn Ther 173: 343–350, 1968. [PubMed] [Google Scholar]
- 3.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol Regul Integr Comp Physiol 275: R291–R299, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R1569–R1578, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA, Kastin AJ, Huang WT, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides 17: 305–311, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Barnett EM, Evans GD, Sun N, Perlman S, Cassell MD. Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurosci 15: 2972–2984, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: Implications for the regulation of total body fat. Am J Physiol Regul Integr Comp Physiol 275: R1399–R1411, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Bartness TJ, Song CK. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res 48: 1655–1672, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Baskin DG, Lattemann DF, Seeley RJ, Woods SC, Porte D Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res 848: 114–123, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148: 5339–53347, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Integr Comp Physiol 294: R1445–R1452, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Cervero F, Foreman RD. Sensory innervation of the viscera. In: Central Regulation of Autonomic Functions, edited by Loewy AD and Spyer KM. New York: Oxford University Press, 1990, p. 104–125.
- 13.Cinti S The Adipose Organ. Milano, Italy: Editrice Kurtis, 1999.
- 14.Cottrell DF, Iggo A. Tension receptors with vagal afferent fibres in the proximal duodenum and pyloric sphincter of sheep. J Physiol 354: 457–475, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowley AW Long-term control of arterial blood pressure. Physiol Rev 72: 231–300, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Dark J, Miller DR, Zucker I. Reduced glucose availability induced torpor in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 267: R496–R501, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Dix RD, McKendall RR, Baringer JR. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun 40: 103–112, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishman RB, Dark J. Sensory innervation of white adipose tissue. Am J Physiol Regul Integr Comp Physiol 253: R942–R944, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Fox EA, Powley TL. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res 341: 269–282, 1985. [DOI] [PubMed] [Google Scholar]
- 20.Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, Bartness TJ. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol 291: R1243–R1255, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Havel PJ Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med (Maywood ) 226: 963–977, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Helke CJ, Charlton CG, Wiley RG. Studies on the cellular localization of spinal cord substance P receptors. Neuroscience 19: 523–533, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol 211: 248–265, 1982. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy GC The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B 140: 578–592, 1953. [DOI] [PubMed] [Google Scholar]
- 25.Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen CL, Sluiter AA, Mettenleiter TC, Romijn JA, Sauerwein HP, Buijs RM. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat-functional implications. J Clin Invest 110: 1243–1250, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreier F, Kap YS, Mettenleiter TC, van HC, van d V, Kalsbeek A, Sauerwein HP, Fliers E, Romijn JA, Buijs RM. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology 147: 1140–1147, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol 281: G907–G915, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Langin D, Portillo MP, Saulnier-Blache JS, Lafontan M. Coexistence of three beta-adrenoceptor subtypes in white fat cells of various mammalian species. Eur J Pharm 199: 291–301, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Melone J Vagal receptors sensitive to lipids in the small intestine of the cat. J Auton Nerv Syst 17: 231–241, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Naito C, Yoshitoshi Y, Higo K, Ookawa H. Effects of long-term administration of 2-deoxy-d-glucose on food intake and weight gain in rats. J Nutr 103: 730–737, 1973. [DOI] [PubMed] [Google Scholar]
- 31.Newsholme EA, Leech AR. Biochemistry for the Medical Sciences. Chichester, UK: John Wiley, 1983.
- 32.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2001.
- 33.Rinaman L, Schwartz GJ. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci 24: 2782–2786, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz GJ, McHugh PR, Moran TH. Pharmacological dissociation of responses to CCK and gastric loads in rat mechanosensitive vagal afferents. Am J Physiol Regul Integr Comp Physiol 267: R303–R308, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Shaw JE, Ramwell PW. Release of prostaglandin from rat epididymal fat pad on nervous and hormonal stimulation. J Biol Chem 243: 1498–1503, 1968. [PubMed] [Google Scholar]
- 36.Shi H, Bartness TJ. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain Res Bull 54: 375–385, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Shi H, Song CK, Giordano A, Cinti S, Bartness TJ. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am J Physiol Regul Integr Comp Physiol 288: R1028–R1037, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Smith JA, Amagasu SM, Eglen RM, Hunter JC, Bley KR. Characterization of prostanoid receptor-evoked responses in rat sensory neurones. Br J Pharmacol 124: 513–523, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song CK, Bartness TJ. CNS sympathetic outflow neurons to white fat that express melatonin receptors may mediate seasonal adiposity. Am J Physiol Regul Integr Comp Physiol 281: R666–R672, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Song CK, Enquist LW, Bartness TJ. New developments in viral tracings of neural circuits. Virus Res 11: 235–249, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Song CK, Jackson RX, Harris RBS, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol 289: R1467–R1476, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: Neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol 295: R417–R428, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun N, Cassell MD, Perlman S. Anterograde, transneuronal transport of herpes simplex virus type 1 strain H129 in the murine visual system. J Virol 70: 5405–5413, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes 1: 11–25, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. New York: Plenum Press, 1991.
- 46.Wirtshafter D, Davis JD. Set points, settling points, and the control of body weight. Physiol Behav 19: 75–78, 1977. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto K, Senba E, Matsunaga T, Tohyama M. Calcitonin gene-related peptide containing sympathetic preganglionic and sensory neurons projecting to the superior cervical ganglion of the rat. Brain Res 487: 158–164, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Youngstrom TG, Bartness TJ. Catecholaminergic innervation of white adipose tissue in the Siberian hamster. Am J Physiol Regul Integr Comp Physiol 268: R744–R751, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Youngstrom TG, Bartness TJ. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am J Physiol Regul Integr Comp Physiol 275: R1488–R1493, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Zemanick MC, Strick PL, Dix RD. Direction of transneuronal transport of Herpes simplex virus 1 in the primate motor system is strain-dependent. Proc Natl Acad Sci USA 88: 8048–8051, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]