Abstract
We hypothesize that A2A adenosine receptors (A2A AR) promote aortic relaxation in mice through cytochrome P450 (CYP)-epoxygenases and help to avoid salt sensitivity. Aortas from male mice maintained on a high-salt (HS; 7% NaCl) or normal-salt (NS; 0.45% NaCl) diet for 4–5 wks were used. Concentration-response curves (10−11–10−5 M) for 5′-N-ethylcarboxamidoadenosine (NECA; a nonselective adenosine analog) and CGS 21680 (A2A AR agonist) were obtained with different antagonists including ZM 241385 (A2A AR antagonist; 10−6 M), SCH 58261 (A2A AR antagonist; 10−6 M), Nω-nitro-l-arginine methyl ester (l-NAME; endothelial nitric oxide synthase inhibitor; 10−4 M) and inhibitors including methylsulfonyl-propargyloxyphenylhexanamide (MS-PPOH; CYP epoxygenases inhibitor; 10−5M), 14,15-epoxyeicosa-5(z)-enoic acid (14,15-EEZE; EET antagonist; 10−5M), dibromo-dodecenyl-methylsulfimide (DDMS; CYP4A inhibitor; 10−5M), and HET0016 (20-HETE inhibitor; 10−5M). At 10−7 M of NECA, significant relaxation in HS (+22.58 ± 3.12%) was observed compared with contraction in NS (−10.62 ± 6.27%, P < 0.05). ZM 241385 changed the NECA response to contraction (P < 0.05) in HS. At 10−7 M of CGS 21680, significant relaxation in HS (+32.04 ± 3.08%) was observed compared with NS (+10.45 ± 1.34%, P < 0.05). SCH 58261, l-NAME, MS-PPOH, and 14,15-EEZE changed the CGS 21680-induced relaxation to contraction (P < 0.05) in HS. Interestingly, DDMS and HET0016 changed CGS 21680 response to relaxation (P < 0.05) in NS; however, there was no significant difference found between DDMS, HET0016-treated HS and NS vs. nontreated HS group (P > 0.05). CYP2C29 protein was 55% and 74% upregulated in HS vs. NS (P < 0.05) mice aorta and kidney, respectively. CYP4A protein was 30.30% and 35.70% upregulated in NS vs. HS (P < 0.05) mice aorta and kidneys, respectively. A1 AR was downregulated, whereas A2A AR was upregulated in HS compared with NS. These data suggest that HS may activate CYP2C29 via A2A AR, causing relaxation, whereas NS may contribute to the upregulation of CYP4A causing contraction.
Keywords: vasodilation, vasoconstriction
several lines of evidence suggest that adenosine is cardioprotective (29–31). This nucleoside is present in nearly every tissue and organ (17, 29–31) and is involved in modulation of various physiological activities (31–34, 40). Furthermore, the production of adenosine is also increased under stressful conditions such as hypoxia, ischemia, and inflammation and in response to pathological events (27, 38).
The regulation of vascular tone by adenosine involves activation of four receptor subtypes: A1, A2A, A2B, and A3. Adenosine A1 and A2A receptors are widely distributed throughout the peripheral vasculature (24, 45, 46). Adenosine-induced vasodilation is primarily caused by the activation of A2A receptors in the coronary and renal arteries and other vessels (1, 2, 15, 28, 43).
There is a correlation between adenosine and salt intake; switching rats from a normal-salt (NS) diet to a high-salt (HS) diet leads to increased adenosine levels in the renal cortex and medulla and increased urinary adenosine levels (41). Through the activation of A2A receptors, this increase in renal adenosine concentration could possibly contribute to a reduction in macula densa-mediated renin secretion, dilation of pre- and postglomerular vessels, and inhibition of tubular sodium reabsorption (22, 42), leading to enhanced sodium excretion to maintain the constancy of body fluid volume and arterial pressure (3, 42, 44). In contrast, stimulation of A1 receptors produces preglomerular vasoconstriction, activation of tubuloglomerular feedback response, increased cortical and medullary tubular sodium reabsorption and consequently a reduction of sodium excretion (39, 42).
Cytochrome P450 (CYP) enzymes are found in human and mouse heart and also in the endothelium and smooth muscle of blood vessels (13, 18, 48). In previous studies, investigators have found that the endothelium produces metabolites of arachidonic acid, commonly referred to as endothelium-derived hyperpolarizing factors (EDHFs), which elicit hyperpolarization and relaxation of the underlying smooth muscle. Cheng et al. (7) have uncovered a close relationship between the stimulation of vascular A2A receptors and increased activity of CYP epoxygenase in rat model. 11,12-Epoxyeicosatrienoic acid (11, 12-EET) has been identified as the likely mediator of preglomerular microvascular dilation triggered by the activation of A2A receptors (7, 20). Capdevila et al. (5) have shown that a salt-inducible renal epoxygenase protects against hypertension. Inhibition of epoxygenase with clotrimazole produces an elevation of blood pressure in rats maintained on an HS diet, which by itself does not increase blood pressure, thus rendering the rats salts-sensitive (23). Decreased CYP epoxygenase activity and blockade of EET formation is associated with salt-sensitive hypertension (14, 23, 50). Furthermore, CYP4A enzymes synthesize 20-HETE, which is a potent vasoconstrictor in small arteries through depolarization of the smooth muscle membrane (10, 11). The production of 20-HETE through CYP4A is lower in glomeruli isolated from kidneys of rats fed an HS diet than in kidneys of rats fed a low-salt diet (16). Furthermore, the expression of CYP4A protein in glomeruli and cortex of kidneys of rats fed an HS diet is lower than in kidneys of rats fed low-salt diet (16).
There is little information regarding the effects of HS intake on CYP epoxygenase/CYP ω-hydroxylase activity in rat but not in the mouse or whether these changes affect vascular responses through adenosine. Furthermore, there is no information regarding the role of HS on mouse aortic vascular response; usually, hypertension or salt-sensitive hypertension also affects thoracic and abdominal aorta and, can lead to the development of aortic aneurysm.
MATERIALS AND METHODS
All animal care and experimentation protocols were approved and carried out in accordance with the West Virginia University Institutional Animal Care and Use Committee and were in accordance with the principles and guidelines of the Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals. Ten-week-old inbred male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were placed on a whole-grain diet containing either 0.45% NaCl (NS) or 7% NaCl (HS) (TD88311 and TD92100 diets; Teklad, Madison, WI). Nurkiewicz and Boegehold (37) previously showed that these mice remain normotensive when placed on an HS diet. All mice were studied 4–5 wk after assignment to either the NS or HS group.
Preparation of mouse aortic rings.
Mice were killed with pentobarbital sodium (100 mg/kg ip). After thoracotomy, the aorta was gently removed, cleaned of fat and connective tissue, and cut transversely into rings of 3–4 mm in length. Extreme care was taken not to damage the endothelium. The rings were mounted vertically between two stainless steel wire hooks. Two rings were suspended in 10-ml organ baths containing modified Krebs-Henseleit buffer (in mM: 118 NaCl, 4.8 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11 glucose, and 2.5 CaCl2). The pH of the buffer was adjusted and maintained at 7.4 by equilibration with 95% O2-5% CO2 at 37°C. Aortic rings were equilibrated for 90 min with a resting force of 1 g, with changes of the bathing solution at 15-min intervals, according to our previously described protocol (43, 47). At the end of equilibration period, tissues were contracted with KCl (50 mM) to check the viability of the tissue. Aortic rings were then constricted with phenylephrine (PE, 10−7 M), and changes in tension were monitored continuously with a fixed-range precision force transducer (model 125 C; Biopac Systems) connected to a differential amplifier (model 100B; Biopac systems). Data were recorded using model MP100 WSW Biopac digital acquisition system and analyzed using Acknowledge 3.5.7 software (Biopac systems).
The integrity of the vascular endothelium was verified pharmacologically by acetylcholine (ACh; 10−7 M)-induced relaxation of PE-precontracted aortic rings. Preparations were then washed several times with Krebs-Henseleit solution and allowed to equilibrate for 30 min before the experimental protocol began. For all tests, the contraction and relaxation responses are expressed as %decrease or increase of PE-induced precontraction.
NECA concentration-response curve in HS and NS mouse aorta.
To determine the responsiveness of the precontracted aortic rings to the nonselective adenosine analog, 5′-N-ethylcarboxamidoadenosine (NECA), the concentration-response curve was obtained by cumulative addition of NECA to the organ bath in one-log increments. One NECA concentration-response curve was constructed for each ring, and all concentration-response determinations were run in parallel on pairs of rings from either HS or NS in the same organ bath.
Effect of A2A receptor antagonist on NECA concentration-response curve in HS and NS mouse aorta.
Concentration-response curves for aortic responses to NECA were obtained as described above, either with or without ZM 241385 (1 μM), a selective A2A receptor antagonist. ZM 241385 was added 30 min before contraction of the tissue with PE and was present throughout the experiment. These experiments were performed in parallel on four rings from the same aorta with two serving as control and two treated with ZM 241385.
CGS 21680 concentration-response curve in HS and NS mouse aorta.
To determine responsiveness of the precontracted aortic rings to the selective A2A receptor agonist CGS 21680, the concentration-response curve was obtained by cumulative addition of CGS 21680 to the organ bath in one-log increments. One concentration-response curve was constructed for each ring, and all concentration-response determinations were made in parallel on pairs of rings from both HS and NS mice in the same organ bath.
Effect of another A2A receptor antagonist on CGS 21680 concentration-response curve in HS and NS mouse aorta.
Concentration-response curves for aortic responses to CGS 21680 were obtained as described above, either with or without SCH 58261 (1 μM), a selective A2A receptor antagonist. SCH 58261 was added 30 min before contraction of the tissue with PE and was present throughout the experiment. These experiments were performed in parallel on four rings from the same aorta with two serving as control and two treated with SCH 58261.
Effect of endothelial nitric oxide synthase inhibitor on CGS 21680 concentration-response curves in HS and NS mouse aorta.
Nω-nitro-l-arginine methyl ester (l-NAME; 100 μM), an endothelial nitric oxide synthase inhibitor, was added 30 min before contraction of the tissue with PE and was present throughout the experiment. These experiments were performed in parallel on four rings from the same aorta with two serving as control and two treated with l-NAME.
Effect of CYP epoxygenase inhibitor on CGS 21680 concentration-response curves in HS and NS mouse aorta.
Methylsulfonyl-propargyloxyphenylhexanamide (MS-PPOH; 10 μM), a selective CYP epoxygenase inhibitor, was added 30 min before contraction of the tissue with PE and was present throughout the experiment. These experiments were performed in parallel on four rings from the same aorta with two serving as control and two treated with MS-PPOH.
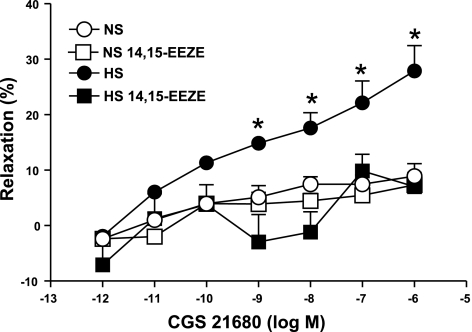
Effect of EETs inhibitor on CGS 21680 concentration-response curves in HS and NS mouse aorta.
14,15-epoxyeicosa-5(z)-enoic acid (14,15-EEZE; 10 μM), an EETs inhibitor, was added 30 min before contraction of the tissue with PE and was present throughout the experiment. These experiments were performed in parallel on four rings from the same aorta with two serving as control and two treated with 14,15-EEZE.
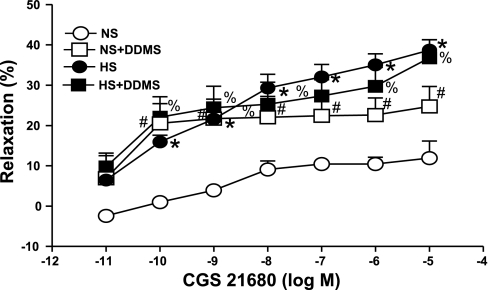
Effect of CYP ω-hydroxlase inhibitor on CGS 21680 concentration-response curves in HS and NS mouse aorta.
Dibromo-dodecenyl-methylsulfimide (DDMS; 10 μM), a selective CYP ω-hydroxylase inhibitor, was added 30 min before contraction of the tissue with PE and was present throughout the experiment. These experiments were performed in parallel on four rings from the same aorta with two serving as control and two treated with DDMS.
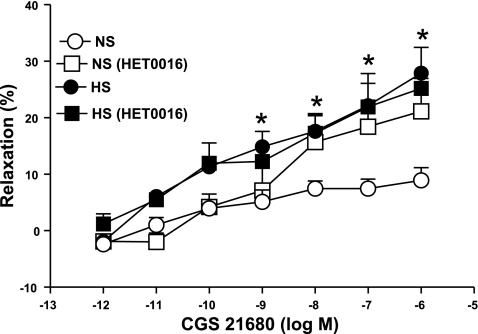
Effect of 20-HETE blocker on CGS 21680 concentration-response curves in HS and NS mouse aorta.
HET0016 (10 μM), a 20-HETE blocker, was added 30 min before contraction of the tissue with PE and was present throughout the experiment. These experiments were performed in parallel on four rings from the same aorta with two serving as control and two treated with HET0016.
Protein extraction, gel electrophoresis, and Western blot analysis.
In brief, aorta, kidneys, and liver from both HS and NS mice were isolated, and each sample was treated with 1 ml of lysis buffer (50 mM Tris·HCl, pH 7.4, 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 1 mM PMSF, 0.25% sodium deoxycholate, 1 μg/ml aprotinin, 1 μg /ml pepstatin, 1 μg/ml leupeptin, 1 mM Na3VO4, 1 mM NaF) and homogenized on wet ice. The samples were transferred to dry ice for 5 min and then thawed on wet ice. After thawing, the samples were vortexed and centrifuged for 5 min at 12,000 rpm at 4°C. Supernatants were sonicated and stored at −80°C. Protein was measured using Bio-Rad assay based on the Bradford dye binding procedure with bovine serum albumin as a standard. The protein mixture was divided into aliquots and stored at −80°C. At the time of analysis, samples were thawed and ∼40 μg of total protein per lane was loaded on a slab gel. Proteins were separated by SDS-PAGE using 10% acrylamide gels (1-mm thick). After electrophoresis, the proteins on the gel were transferred to nitrocellulose membrane (Hybond-ECL) by electroelution. Protein transfer was confirmed by employing prestained molecular weight markers (Bio-Rad Laboratories, Hercules, CA). Following blocking with nonfat dry milk, the nitrocellulose membranes were incubated with monoclonal and polyclonal antibodies cross-reacting with primary antibody for CYP2C29 No. 202, made against the peptide, GRGSFPMAEKMIKGC, specific to CYP2C29 [Dr. Zeldin, National Institute of Environmental Health Sciences/National Institutes of Health, Research Triangle Park, NC (NIEHS/NIH)], CYP4A (polyclonal, Affinity Bio-Reagents), A1 AR (Sigma, St. Louis, MO ), A2A AR [Dr. Mustafa, West Virginia University, Morgantown, WV (WVU)]; β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used to detect as housekeeping proteins for proper loading of the samples in each lane. The secondary antibody was a horseradish peroxidase-conjugated anti-rabbit IgG. The membranes were developed using enhanced chemiluminescence (Amersham BioSciences) and exposed to X-ray film for the appropriate time.
Chemicals, drugs, and antibodies.
Phenylephrine and acetylcholine were dissolved in distilled water. NECA and CGS 21680 (Sigma) were dissolved in 100% DMSO as 10 mM stock solutions, which were followed by serial dilutions in distilled water. MS-PPOH and 14,15-EEZE (Dr. Falck), and DDMS and HET0016 from Cayman (Ann Arbor, Michigan) were dissolved in 100% ethanol; CYP2C29 (polyclonal, Dr. Zeldin, NIEHS/NIH), CYP4A antibody (polyclonal, Affinity BioReagents, Golden, CO), A1 AR (Sigma), and A2A AR (Dr. Mustafa, WVU) were used for Western blot experiments.
Statistical analysis.
Data are reported as means ± SE. One-way ANOVA was used to compare difference among groups; and two-way ANOVA for repeated measure, followed by Tukey post hoc test was used to compare the vascular responses to antagonist (ZM 241385, SCH 58261, MS-PPOH, 14,15-EEZE, DDMS, HET0016). Differences were considered significant if P < 0.05. Furthermore, densitometry of Western blot analysis was expressed as means ± SE in arbitrary units. All of the statistical analyses were performed using Graph Pad Prism statistical package.
RESULTS
Responses to ACh.
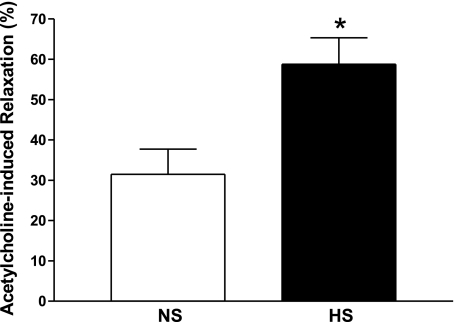
Relaxation to 10−7M ACh was significantly greater in PE-precontracted HS (58.8 ± 6.6%) compared with NS (31.5 ± 6.3%) aorta (P < 0.05, Fig. 1). MS-PPOH (10 μM), a selective CYP epoxygenase inhibitor, was able to reduce ACh-dependent relaxation in HS aortas significantly (30.3 ± 4.0% vs. 60.0 ± 3.2% for untreated controls, P < 0.05). No significant difference was found between MS-PPOH treated and nontreated NS aortas.
Fig. 1.
Acetylcholine (10−7M)-dependent responses of aortas from mice fed normal-salt (NS) and high-salt (HS) diets. Values are means ± SE. *P < 0.05 compared with NS, n = 6.
Responses to NECA before and after A2A receptor antagonism.
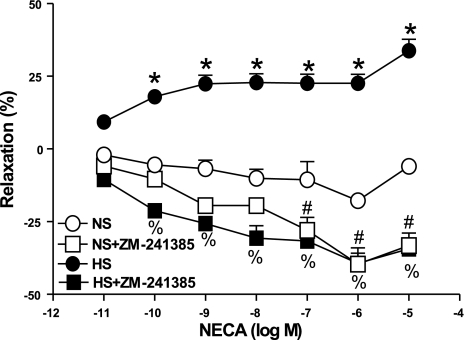
NECA produced a concentration-dependent relaxation in aorta from HS as opposed to contraction in NS (Fig. 2). For example, the response to 10−7 M NECA in HS aorta was 22.6 ± 3.1% relaxation, while in NS aorta, 10.6 ± 6.3% contraction was produced. Responses in HS vs. NS were significantly different from each other at each concentration of NECA (10−10–10−5 M, P < 0.05). In HS aorta, the selective A2A receptor antagonist ZM 241385 (1 μM) transformed the relaxation response to NECA into significant contraction at all concentrations. In NS aorta, ZM 241385 enhanced NECA-induced contraction, with this effect becoming significant at the higher concentrations of NECA (10−7–10−5 M). SCH 58261 (1 μM), another selective A2A AR antagonist, was also found to block NECA-induced relaxation in HS aorta (P < 0.05, data not shown).
Fig. 2.
Effect of ZM 241385 (1 μM) on 5′-N-ethylcarboxamidoadenosine (NECA)-induced vascular responses in HS and NS mouse aortic rings. Values are means ± SE. *P < 0.05 compared with NS, n = 6; %P < 0.05 between HS and HS+ZM 241385; #P < 0.05 between NS and NS+ZM 241385, n = 6.
Effects of A2A receptor antagonism, eNOS, CYP epoxygenase, EETs, CYP ω-hydroxylase, and 20-HETE inhibition on CGS 21680 concentration-response.
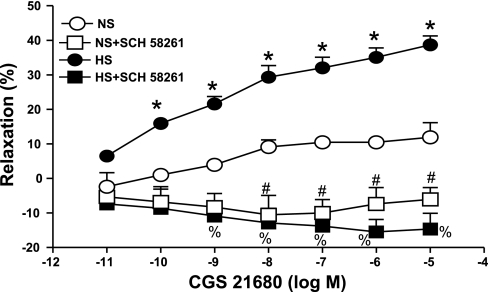
CGS 21680 produced a concentration-dependent relaxation (P < 0.05) in aorta from HS and NS mice, but the relaxation response was significantly greater for HS at all concentrations (10−10–10−5 M, P < 0.05) (Fig. 3). For example, at 10−7 M CGS 21680, the relaxation response was 32.0 ± 3.1% in HS compared with 10.4 ± 1.3% in NS. Furthermore, a complete blockade (P < 0.05) of CGS 21680-induced relaxation was obtained with the selective A2A AR antagonist SCH 58261 (1 μM) in HS. In NS aorta, SCH 58261 tended to enhance contraction, with this effect becoming significant at higher concentrations of CGS 21680 (10−8–10−5 M). ZM 241385 (1 μM), another selective A2A AR antagonist, was also able to significantly block CGS 21680-induced relaxation in HS aorta (P < 0.05, data not shown). Similarly, in NS aorta, ZM 241385 tended to enhance contraction, with this effect becoming significant at higher concentrations of CGS 21680 (10−7–10−5 M). l-NAME (100 μM) did not alter vascular responses in both the treated (+24.32 ± 3.75% at 10−7 CGS 21680) and the control HS (+22.13 ± 3.96%, P > 0.05) tissues. There was no significant difference observed in concentration-response curves between treated (l-NAME) and control NS aorta (+6.53 ± 2.28 vs. +6.45 ± 1.69 at 10−7 CGS 21680).
Fig. 3.
Effect of SCH 58261 (1 μM) on CGS 21680-induced vascular responses in HS and NS mouse aortic rings. Values are means ± SE. *P < 0.05 between HS and NS, n = 6; %P < 0.05 between HS and HS+SCH 58261; #P < 0.05 between NS and NS+SCH 58261, n = 6.
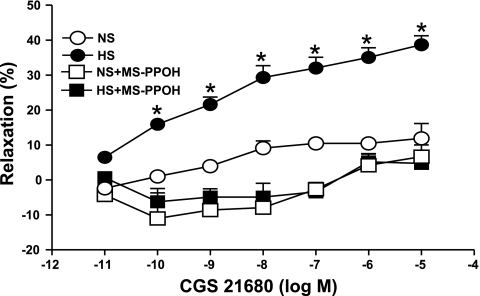
Complete abolition of the CGS 21680-induced relaxation response in HS was also achieved with MS-PPOH (10 μM), a highly selective CYP epoxygenase inhibitor and with 14,15-EEZE (10 μM), an EETs inhibitor. Use of MS-PPOH caused 3.4 ± 2.7% relaxation, while in the absence of MS-PPOH, 32.0 ± 3.1% relaxation was observed (P < 0.05, at 10−7 M CGS 21680, Fig. 4) in HS. No significant effect of MS-PPOH was observed on CGS 21680-induced responses in NS mice aorta (P > 0.05, Fig. 4). A significant blockade was also found in concentration-dependent response (P < 0.05) with CGS 21680-induced relaxation in the presence of 14,15-EEZE (10 μM; an EET nonselective antagonist) compared with control in HS aorta. At 10−7 M of CGS 21680, 14,15-EEZE changed the relaxation response in HS (7.86 ± 3.01%) compared with controls (22.13 ± 3.96%, P < 0.05, Fig. 5). However, the CGS 21680 concentration-response did not change between the 14,15-EEZE-treated HS and NS and control (P > 0.05, Fig. 5).
Fig. 4.
Effect of methylsulfonyl-propargyloxyphenylhexanamide (MS-PPOH; 10 μM) on CGS 21680-induced vascular response in HS and NS mice aortic rings. Values are means ± SE. *P < 0.05 between HS vs. NS, HS+MS-PPOH, and NS+MS-PPOH, n = 6.
Fig. 5.
Effect of 14,15-EEZE (10 μM) on CGS 21680-induced vascular responses in HS and NS mouse aortic rings. Values are means ± SE. *P < 0.05, between treated NS and HS vs. control HS groups, n = 6.
DDMS (10 μM), a ω-hydroxylase (CYP4A) inhibitor, produced significantly higher relaxation (22.42 ± 4.3%, P < 0.05) in the CGS 21680-induced vascular response in NS aorta as opposed to control (10.4 ± 1.3%; Fig. 6). In contrast, DDMS had no significant effect on CGS 21680-mediated relaxation in HS aorta. There were no significant differences in responses to CGS 21680 among NS aorta treated with DDMS, treated HS, and untreated HS mice aorta (P > 0.05). Similarly, the 20-HETE inhibitor, HET0016 (10 μM) also significantly increased relaxation at 10−7 M of CGS 21680 from 6.45 ± 1.69% to 18.43 ± 4.22% (P < 0.05, Fig. 7) in the NS group. No significant change was observed between the control and HET0016-treated HS groups at 10−6 M of CGS 21680 (Fig. 7).
Fig. 6.
Effect of dibromo-dodecenyl-methylsulfimide (DDMS; 10 μM) on CGS 21680-induced vascular response in HS and NS mice aortic rings. Values are means ± SE. *P < 0.05, between HS and NS; #P < 0.05, between NS and NS+DDMS; %P < 0.05, between NS and HS+DDMS, n = 6.
Fig. 7.
Effect of HET0016 (10 μM) on CGS 21680-induced vascular responses in of HS and NS mouse aortic rings. Values are means ± SE. *P < 0.05, between treated NS and HS vs. control HS groups, n = 6.
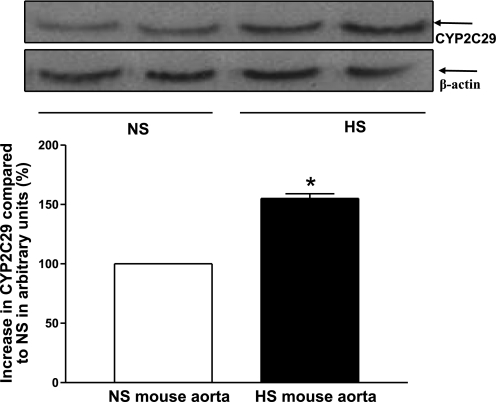
Expression of CYP2C29 and CYP4A in HS and NS mouse aorta and kidney.
Western blot analysis for CYP2C29 (∼50 kDa) protein showed 55 ± 4% more CYP2C29 protein in HS mouse aorta than in NS mouse aorta (P < 0.05, Fig. 8). Western blot analysis also revealed that the amount of CYP2C29 protein was increased by 74 ± 4% in HS mouse kidney compared with NS mouse kidney (P < 0.05).
Fig. 8.
Representative Western blots and densitometric data for CYP2C29 (∼50 kDa) protein in HS and NS mouse aortas. Values are means ± SE. *P < 0.05, compared with NS aortas, n = 4.
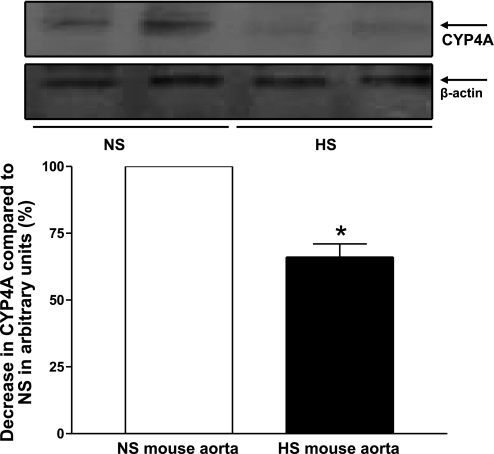
Figure 9 shows the Western blot analysis for CYP4A (∼50 kDa) protein. The amount of CYP4A protein in HS mouse aorta was reduced by 30.3 ± 3% compared with NS mouse aorta (P < 0.05, Fig. 9). Western blot analysis also revealed that CYP4A protein was decreased by 35.7 ± 5% in HS mouse kidneys compared with NS mouse kidneys (P < 0.05).
Fig. 9.
Representative Western blots and densitometric data for CYP4A (∼50 kDa) protein in HS and NS mouse aortas. Values are means ± SE. *P < 0.05, compared with NS aortas, n = 3.
Expression of A1 AR and A2A AR in HS and NS mouse aorta.
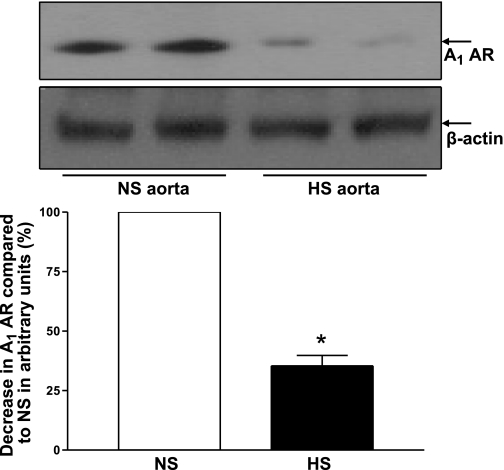
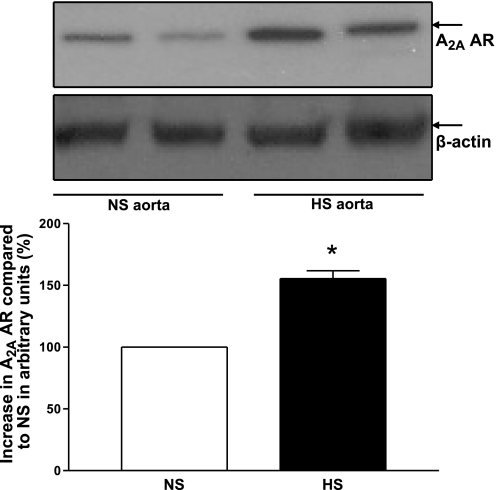
Western blot analysis for A1 AR (∼37 kDa) protein showed 64 ± 4% less A1 AR protein in HS mouse aorta than in NS mouse aorta (P < 0.05, Fig. 10). Figure 11 shows the Western blot analysis for A2A AR (∼45 kDa) protein. The amount of A2A AR protein in HS mouse aorta was increased by 55 ± 69 compared with NS mouse aorta (P < 0.05, Fig. 11).
Fig. 10.
Representative Western blots and densitometric data for A1 AR (∼37 kDa) protein in HS and NS mouse aortas. Values are means ± SE. *P < 0.05, compared with NS aortas, n = 4.
Fig. 11.
Representative Western blots and densitometric data for A2A AR (∼45 kDa) protein in HS and NS mouse aortas. Values are means ± SE. *P < 0.05, compared with NS aortas, n = 3.
DISCUSSION
There are a number of important findings in this study. We found HS enhanced acetylcholine-dependent relaxation compared with NS. The nonselective adenosine analog NECA dilated aortas from mice fed an HS diet, while it had no significant effect on aortas from mice fed an NS diet. In the presence of the selective A2A receptor antagonist ZM 241385, the NECA-induced dilation of aortas from HS mice was converted to a concentration-dependent contraction. Furthermore, the selective A2A receptor agonist CGS 21680 caused greater dilation of aortas from HS mice than those from NS mice, and these responses were abolished by the selective A2A receptor antagonist SCH 58261 in these aortas. The expression of A1 adenosine receptors was lower in HS compared with NS, while the A2A receptor expression was higher in HS compared with NS. Taken together, these findings suggest that high dietary salt intake leads to an increase in the density of aortic A2A and a decrease in the density of A1 receptors. As a matter of fact, the role of A1 AR in vascular contraction and the role of A2A AR in vascular relaxation are well established in mice (on NS diet) aortas (32, 34, 45). Our data also show that l-NAME was unable to block the CGS 21680-induced relaxation in HS mouse aorta, indicating that nitric oxide does not contribute to A2A AR-induced relaxation in HS mouse aorta, similar to findings we reported recently in A2A AR+/+ mice (on NS diet) aorta (32, 34). The responses in our present study are similar to studies in which NO-independent adenosine-induced vascular relaxation in rat preglomerular microvessels was observed (6, 7). The CGS 21680-induced dilation of vessels from HS mice was inhibited by the CYP 450 epoxygenase inhibitor MS-PPOH. We were also able to abolish CGS 21680-induced vasodilation through the use of 14,15-EEZE, a blocker of EET formation in HS mouse aorta. Our data suggest that the endothelium-dependent CGS 21680-induced vascular relaxation is mediated by CYP epoxygenases and EETs in HS mouse aorta. Notably, at the concentration used in the current study, MS-PPOH and 14,15-EEZE do not have any effect on pathways other than the CYP pathway (4, 25, 26).
In contrast, the CYP 450 ω-hydroxylase inhibitor DDMS enhanced dilation in NS vessels. Furthermore, HET0016, which inhibits 20-HETE formation, was also able to block vascular contraction in NS mouse aorta. No significant difference was observed between either DDMS- or HET0016-treated and control HS tissues. This suggests that the CYP4A enzyme and 20-HETE formation induced vascular contraction in NS. These findings also suggest that the activation of A2A receptors increases the activity of CYP 450 epoxygenase and decreases CYP 450 ω-hydroxylase in aorta from HS mice. Furthermore, a decrease in the activity of A2A receptors appears to lower CYP 450 epoxygenase and increase CYP 450 ω-hydroxylase activities in aorta from NS mice. We conclude that an increase in the production of CYP epoxygenases, A2A AR, and a decrease in the activity of A1 AR contribute to NECA and CGS 21680-induced dilation of HS aorta, whereas the action of CYP 450 ω-hydroxylase and A1 AR products predominate when NS aorta are challenged with NECA and CGS 21680, producing a net constriction.
The finding that NECA and CGS 21680 cause concentration-dependent vasorelaxation in HS aorta but only minor relaxation or a net contraction in NS aorta are similar to an earlier report of an exaggerated vasodilator response to adenosine in kidneys from HS-fed rats (20). Furthermore, Zou et al. (52) reported that rats maintained on an HS diet showed an increase in adenosine receptor activity in renal cortical and medulla regions and also the activation of A2A ARs dilated pre- and postglomerular vessels in salt-loaded rats (42).
Data in this study also suggest that an HS diet can lead to a change in adenosine receptor activity, not only in the rat kidney and renal vasculature (20, 45, 46, 56), but also in extrarenal conduit arteries of the mouse. The thoracic and abdominal aorta may also be prone to aneurysm due to the hypertensive or salt-sensitive hypertensive conditions. Our data imply that salt induced increases in vascular adenosine A2A receptor activity and decreases in A1 receptor activity is a systemic phenomenon rather than limited to the kidneys.
Also, we have found that the selective A2A antagonist ZM 241385 was able to enhance NECA-induced contraction in NS and block NECA-induced relaxation in HS group. Another selective A2A antagonist, SCH 58261, was able to abolish the small CGS 21680-induced relaxation of NS aorta and the larger CGS 21680-induced relaxation of HS aorta. These experiments further confirmed that there is an important role of A2A AR in aortic relaxation in HS-fed mice.
The relationship between the activity of CYP epoxygenases and the generation of endothelium-derived hyperpolarization factor (EDHF) has been investigated over the last several years in different vascular tissues by others in different animal models (8, 12, 35, 49). EDHF, a substance released from endothelial cells through CYP epoxygenases activity, stimulates hyperpolarization in smooth muscle cells and thus induces vasodilation (9). Luo et al. (21) demonstrated that CYP2C29 and CYP2C39 are involved in the production of 14,15-EET in murine brain, kidney, lung, heart, intestine, and liver. In the present study, significant expression of the CYP2C family of proteins, including CYP2C29, in HS compared with NS mice aorta was observed in Western blot data. This suggests that an HS diet enhances CYP epoxygenase activity not only in mouse kidneys but also in mouse aorta. The present findings are in agreement with those reported earlier in renal microvessels of HS fed rats (20). Also, the vascular responses seen in our study were similar to those reported earlier by others in rat kidneys, where CYP epoxygenases played a role in the generation of EDHFs leading to vasorelaxation in preglomerular microvessels from rats fed an HS diet (6, 7). We believe that the involvement of CYP epoxgenase in the enhanced-vascular relaxation associated with HS diet is not limited to kidneys but also extends to large vessels like aorta (in mouse). When mice are maintained on an HS diet for 4–5 wk, increases in CYP epoxyganase activity are possibly a systemic phenomenon and not limited to a specific organ. Also, the functional data are further supported by the Western blot data for CYP2C family proteins, including CYP2C29 as well as expression of A2A AR in HS compared with NS mice aorta.
Interestingly, we found the involvement of ω-hydroxylase (CYP4A) in aorta from mice fed the NS diet, where NECA induced contraction and CGS 21680 induced no response. We also observed a higher CYP2C29 enzyme expression and lower CYP4A protein in aorta and kidneys from mice fed HS than those from mice fed NS. Similar findings have been reported in rat kidneys (16) and renal microvessels (50, 51). 20-HETE, an arachidonic acid-derived metabolite synthesized through CYP4A, elicits vasoconstriction in rat renal tubules (19, 36). In this study, we found significant downregulation of CYP4A and A1 AR in HS compared with NS mouse aorta. This conclusion is further supported by the fact that NECA elicited enhanced relaxation in HS mouse aorta compared with NS mouse aorta. Also, greater availability of adenosine A2A receptors in HS compared with NS mouse aorta is supported by the observations that CGS 21680 induced an enhanced relaxation-response, and there was decreased expression of CYP4A protein. More interestingly, DDMS (a CYP4A blocker) and HET0016 (a 20-HETE inhibitor) did not change HS response but significantly changed the contraction response into relaxation in NS with CGS 21680. These data suggest that adenosine-mediated contraction in the NS diet-maintained mice is mediated through CYP4A and 20-HETE generation and indicate the possibility of a link between the activation or availability of A2A ARs, functional capacity of endothelium, upregulation of CYP2C29, generation of EETs, and NECA and CGS 21680-enhanced vasodilation in HS diet-fed mouse aorta. Conversely, there also appears to be a link between less availability of A2A ARs, upregulation of CYP4A, generation of 20-HETE, NECA-induced vasoconstriction, and CGS 21680-induced response in NS diet fed mouse aorta.
Perspectives and Significance
The present data provide evidence that NECA and CGS 21680-induced relaxation was significantly higher in HS than in NS mice aorta. As reported in the literature (5, 6, 7, 20), this phenomenon is not limited to kidneys but also extends to larger vessels like aorta as seen in this study. The thoracic and abdominal aorta may also be prone to aneurysm due to the hypertensive or salt-sensitive hypertensive conditions. These data elucidate the relationship between the activation and availability of A2A ARs, functional capacity of endothelium, upregulation CYP2C29 and A2A AR, generation of EETs, downregulation of CYP4A, NECA, and CGS 21680-enhanced relaxation in HS mouse aorta. In contrast, the NS data provide evidence that there may be a link between the less availability of A2A AR, less functional capacity of endothelium, less expression of CYP2C29, upregulation of CYP4A, A1 AR generation of 20-HETE with NECA-induced vasoconstriction, and CGS 21680-induced nonresponsiveness in mouse aorta. Further studies will be necessary to unravel the mechanism(s) by which adenosine A2A and A1 receptors play a role with CYP epxygenases and CYP4A in the regulation of vascular tone through HS/NS diet in mouse aorta.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-027339 and HL-094447, American Heart Association Great Rivers Affiliate Grant-in-Aid 0755264B, National Institutes of Health Grant GM-31278, and the Intramural Research Program of the National Institute of Environmental Health Sciences Grant z01-ES-025034.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abebe W, Makujina SR, Mustafa SJ. Adenosine receptor-mediated relaxation of porcine coronary artery in presence and absence of endothelium. Am J Physiol Heart Circ Physiol 266: H2018–H2025, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Belardinelli L, Shryock JC, Snowdy S, Zhang Y, Monopoli A, Lozza G, Ongini E, Olsson RA, Dennis DM. The A2A adenosine receptor mediates coronary vasodilation. J Pharmacol Exp Ther 284: 1066–1073, 1998. [PubMed] [Google Scholar]
- 3.Biaggioni I Contrasting excitatory and inhibitory effects of adenosine in blood pressure regulation. Hypertension 20: 457–465, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Blanton A, Nsaif R, Hercule H, Oyekan A. Nitric oxide/cytochrome P450 interactions in cyclosporin A-induced effects in the rat. J Hypertens 24: 1865–1872, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Capdevila JH, Wei S, Yan J, Karara A, Jacobson HR, Falck JR, Guengerich FP, DuBois RN. Cytochrome P-450 arachidonic acid epoxygenase. Regulatory control of the renal epoxygenase by dietary salt loading. J Biol Chem 267: 21720–21726, 1992. [PubMed] [Google Scholar]
- 6.Carroll MA, Doumad AB, Li J, Cheng MK, Falck JR, McGiff JC. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol 291: F155–F161, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol 141: 441–448, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Fleming I Cytochrome p450 and vascular homeostasis. Circ Res 89: 753–762, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Frisbee JC, Falck JR, Lombard JH. Contribution of cytochrome P-450 ω-hydroxylase to altered arteriolar reactivity with high-salt diet and hypertension. Am J Physiol Heart Circ Physiol 278: H1517–H1526, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Frisbee JC, Lombard JH. Parenchymal tissue cytochrome P450 4A enzymes contribute to oxygen-induced alterations in skeletal muscle arteriolar tone. Microvasc Res 63: 340–343, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Fulton D, McGiff JC, Quilley J. Pharmacological evaluation of an epoxide as the putative hyperpolarizing factor mediating the nitric oxide-independent vasodilator effect of bradykinin in the rat heart. J Pharmacol Exp Ther 287: 497–503, 1998. [PubMed] [Google Scholar]
- 13.Granberg AL, Brunstrom B, Brandt I. Cytochrome P450-dependent binding of 7,12-dimethylbenz[a]anthracene (DMBA) and benzo[a]pyrene (B[a]P) in murine heart, lung, and liver endothelial cells. Arch Toxicol 74: 593–601, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int 71: 1105–1115, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hein TW, Belardinelli L, Kuo L. Adenosine A2A receptors mediate coronary microvascular dilation to adenosine: role of nitric oxide and ATP-sensitive potassium channels. J Pharmacol Exp Ther 291: 655–664, 1999. [PubMed] [Google Scholar]
- 16.Ito O, Roman RJ. Regulation of P-450 4A activity in the glomerulus of the rat. Am J Physiol Regul Integr Comp Physiol 276: R1749–R1757, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol 283: F41–F51, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Kerzee JK, Ramos KS. Constitutive and inducible expression of Cyp1a1 and Cyp1b1 in vascular smooth muscle cells: role of the Ahr bHLH/PAS transcription factor. Circ Res 89: 573–582, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Belusa R, Nowicki S, Aperia A. Arachidonic acid metabolic pathways regulating activity of renal Na+-K+-ATPase are age dependent. Am J Physiol Renal Physiol 278: F823–F829, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Liclican EL, McGiff JC, Pedraza PL, Ferreri NR, Falck JR, Carroll MA. Exaggerated response to adenosine in kidneys from high salt-fed rats: role of epoxyeicosatrienoic acids. Am J Physiol Renal Physiol 289: F386–F392, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Luo G, Zeldin DC, Blaisdell JA, Hodgson E, Goldstein JA. Cloning and expression of murine CYP2Cs and their ability to metabolize arachidonic acid. Arch Biochem Biophys 357: 45–57, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Ma H, Ling BN. Luminal adenosine receptors regulate amiloride-sensitive Na+ channels in A6 distal nephron cells. Am J Physiol Renal Fluid Electrolyte Physiol 270: F798–F805, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest 94: 2414–2420, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCoy DE, Bhattacharya S, Olson BA, Levier DG, Arend LJ, Spielman WS. The renal adenosine system: structure, function, and regulation. Semin Nephrol 13: 31–40, 1993. [PubMed] [Google Scholar]
- 25.Michaelis UR, Falck JR, Schmidt R, Busse R, Fleming I. Cytochrome P4502C9-derived epoxyeicosatrienoic acids induce the expression of cyclooxygenase-2 in endothelial cells. Arterioscler Thromb Vasc Biol 25: 321–326, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Michaelis UR, Fleming I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: Epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol Ther 111: 584–595, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Miller WL, Thomas RA, Berne RM, Rubio R. Adenosine production in the ischemic kidney. Circ Res 43: 390–397, 1978. [DOI] [PubMed] [Google Scholar]
- 28.Morrison RR, Talukder MA, Ledent C, Mustafa SJ. Cardiac effects of adenosine in A2A receptor knockout hearts: uncovering A2B receptors. Am J Physiol Heart Circ Physiol 282: H437–H444, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Nayeem MA, Matherne GP, Mustafa SJ. Ischemic and pharmacological preconditioning induces further delayed protection in transgenic mouse cardiac myocytes over-expressing adenosine A1 receptors (A1AR): role of A1AR, iNOS and KATP channels. Naunyn Schmiedebergs Arch Pharmacol 367: 219–226, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Nayeem MA, Mustafa SJ. Mechanisms of delayed preconditioning with A1 adenosine receptor activation in porcine coronary smooth muscle cells. Pol J Pharmacol 54: 443–453, 2002. [PubMed] [Google Scholar]
- 31.Nayeem MA, Mustafa SJ. Protein kinase C isoforms and A1 adenosine receptors in porcine coronary smooth muscle cells. Vascul Pharmacol 39: 47–54, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. Role of CYP-epoxygenases in A2A adenosine receptor-mediated relaxation using A2AAR-null and wild-type mice. Am J Physiol Heart Circ Physiol 295: H2068–H2078, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nayeem MA, Boegehold Mustafa MA, SJ. Adenosine A2A receptor mediated aortic relaxation in mice fed high salt: role of CYP epoxygenase. FASEB J 21: A899–A900, 2007. [Google Scholar]
- 34.Nayeem MA, Falck Ledent JR, C, et al. Absence of adenosine-induced relaxation in A2A adenosine receptor-KO mouse aorta: role of CYP2C9 and CYP 4A. FASEB J 21: A1381–A1381, 2007. [Google Scholar]
- 35.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nowicki S, Chen SL, Aizman O, Cheng XJ, Li D, Nowicki C, Nairn A, Greengard P, Aperia A. 20-Hydroxyeicosa-tetraenoic acid (20 HETE) activates protein kinase C. Role in regulation of rat renal Na+,K+-ATPase. J Clin Invest 99: 1224–1230, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 292: R1550–R1556, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Okusa MD A2A adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol 282: F10–F18, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Pawlowska D, Granger JP, Knox FG. Effects of adenosine infusion into renal interstitium on renal hemodynamics. Am J Physiol Renal Fluid Electrolyte Physiol 252: F678–F682, 1987. [DOI] [PubMed] [Google Scholar]
- 40.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998. [PubMed] [Google Scholar]
- 41.Siragy HM, Linden J. Sodium intake markedly alters renal interstitial fluid adenosine. Hypertension 27: 404–407, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Spielman WS, Arend LJ. Adenosine receptors and signaling in the kidney. Hypertension 17: 117–130, 1991. [DOI] [PubMed] [Google Scholar]
- 43.Talukder MA, Morrison RR, Mustafa SJ. Comparison of the vascular effects of adenosine in isolated mouse heart and aorta. Am J Physiol Heart Circ Physiol 282: H49–H57, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Taubes G The (political) science of salt. Science 281: 898–901, 903–897, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol 288: H1411–H1416, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Tawfik HE, Teng B, Morrison RR, Schnermann J, Mustafa SJ. Role of A1 adenosine receptor in the regulation of coronary flow. Am J Physiol Heart Circ Physiol 291: H467–H472, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Teng B, Ansari HR, Oldenburg PJ, Schnermann J, Mustafa SJ. Isolation and characterization of coronary endothelial and smooth muscle cells from A1 adenosine receptor-knockout mice. Am J Physiol Heart Circ Physiol 290: H1713–H1720, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thum T, Borlak J. Gene expression in distinct regions of the heart. Lancet 355: 979–983, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Wu S, Chen W, Murphy E, Gabel S, Tomer KB, Foley J, Steenbergen C, Falck JR, Moomaw CR, Zeldin DC. Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J Biol Chem 272: 12551–12559, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension 41: 709–714, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Zhao X, Pollock DM, Zeldin DC, Imig JD. Salt-sensitive hypertension after exposure to angiotensin is associated with inability to upregulate renal epoxygenases. Hypertension 42: 775–780, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Zou AP, Wu F, Li PL, Cowley AW Jr. Effect of chronic salt loading on adenosine metabolism and receptor expression in renal cortex and medulla in rats. Hypertension 33: 511–516, 1999. [DOI] [PubMed] [Google Scholar]