Abstract
Maternal obesity accentuates offspring obesity in dams bred to develop diet-induced obesity (DIO) on a 31% fat, high energy (HE) diet but has no effect on offspring of diet-resistant (DR) dams. Only DIO dams became obese on HE diet when they and DR dams were fed 5% fat chow or HE diets throughout gestation and lactation. Leptin sensitivity of dissociated arcuate (ARC) and ventromedial (VMN) hypothalamic nucleus neurons from the 3- to 4-wk-old offspring was assessed using fura-2 calcium imaging to monitor leptin-induced changes in intracellular calcium ([Ca2+]i) as an index of neuronal activity. At 0.1, 1, 10 fmol/l leptin, ∼4 times more VMN and ARC neurons were excited than inhibited by leptin. In the VMN, leptin excited up to 41% fewer neurons, and these excited neurons were less sensitive to increasing doses of leptin in DIO compared with DR offspring. Also, maternal HE diet intake decreased the percentage of leptin-excited VMN neurons in both DIO and DR offspring and decreased the percentage of leptin-inhibited VMN neurons by 36% only in DIO offspring. In the ARC, there were no genotype or maternal diet effects on the percentage of ARC neurons excited by leptin. However, those DR neurons that were leptin excited were more sensitive to leptin than were those from DIO offspring. These data suggest that reduced responsiveness of DIO VMN neurons to leptin's excitatory effects may be an important contributing factor to the reduced anorectic and thermogenic leptin responsiveness of DIO rats in vivo.
Keywords: body weight, neuropeptide Y, Agouti-related peptide, development
epidemiological studies in human beings suggest that maternal obesity is associated with a higher risk of obesity in offspring (2, 3, 33). Because such studies rarely provide clues regarding the underlying factors promoting offspring obesity, we have used a rat model in which rats are selectively bred to produce polygenically inherited diet-induced obesity (DIO) or diet-resistance (DR) when fed a high-energy (HE), 31% fat diet (21, 24) to identify factors that promote obesity in offspring of obese mothers. Our prior studies showed that maternal obesity in DIO dams throughout gestation and lactation selectively promotes increased obesity in their adult offspring (27). On the other hand, offspring of DR dams do not become more obese as adults, even when their dams are fed a highly palatable diet to make them obese. Furthermore, the increased obesity in offspring of obese DIO dams is associated with abnormal development of central monoamine pathways involved in regulation of energy homeostasis (18). Therefore, the combination of genetic background, maternal diet, and obesity has differential effects on offspring obesity and the development of neural pathways, which play an important role in the regulation of energy homeostasis.
An important feature of selectively bred DIO rats is their reduced sensitivity to the anorectic (19, 22), thermogenic (12), and neurotrophic (4) effects of leptin, which precedes the development of obesity. These behavioral and physiological findings are accompanied by decreased leptin receptor mRNA expression, leptin receptor binding and downstream signaling in hypothalamic arcuate (ARC) and ventromedial (VMN) nuclei (4, 14, 22, 25). Given the importance of leptin as a regulator of energy homeostasis acting in both these nuclei (7, 30), it is likely that an inborn reduction in leptin sensitivity is a major contributing factor to the development of obesity when the fat and caloric density of their diet are increased. However, neither baseline sensitivity to leptin nor the effect of maternal diet, obesity, or genetic background on this sensitivity has ever been studied at the level of the individual neuron in these areas. Therefore, we undertook the current studies to assess the leptin sensitivity of individual dissociated ARC and VMN neurons of offspring of DIO and DR dams fed chow or HE diet throughout gestation and lactation.
Assessment of leptin sensitivity of individual hypothalamic neurons was carried out using calcium imaging to assess leptin-induced alterations in intracellular calcium ([Ca2+]i) oscillations as an index of neuronal activity (8, 16, 17). We previously used this method to demonstrate that the ARC and VMN contain populations of neurons, which are excited (LepE), inhibited (LepI), or exhibit a bimodal response to increasing concentrations of leptin (15). Our hypothesis was that DIO ARC and VMN neurons would have reduced sensitivity to leptin and that maternal obesity would further impair this sensitivity in DIO offspring and that maternal intake of HE diet would also alter the sensitivity of these neurons to leptin. We found that a complex interaction among genotype, maternal diet, and obesity led to alterations in the proportions of LepE vs. LepI neurons, which occurred predominantly in the VMN, with a lesser effect in the ARC. In addition, we found that ARC neurons from DIO rats expressed more NPY mRNA than did those from DR rats and that offspring of dams fed the HE diet expressed less Agouti-related peptide (AgRP) mRNA expression compared with offspring of dams fed chow.
METHODS
Dams and breeding.
Animal usage was in compliance with and approved by the Animal Care and Use Committee of the East Orange Veterans Administration Medical Center and the guidelines of the American Physiological Society (1). All breeding pairs were derived from our in-house colonies of rats bred selectively for their propensity to develop DIO or DR (21). All rats were housed at 23–24°C on 12:12-h light-dark cycle (light off at 1800) with food (Purina rat chow #5001) and water available ad libitum. Dams were housed in cages in which the food was placed in bins in the wire cage tops to limit access to food to the 2–3-wk old pups. One month prior to breeding DIO and DR dams were divided into four groups: 1) DR chow (n = 8) were fed Purina rat chow ad libitum which contains 3.30 kcal/g with 23.4% as protein, 4.5% as fat and 72.1% as carbohydrate (29); 2) DR HE diet (n = 6) were fed HE diet (no. C11024F; Research Diets, New Brunswick, NJ), which contains 4.47 kcal/g with 21% of the metabolizable energy as protein, 31% as fat, and 48% as carbohydrate, 50% of which is sucrose (29). 3) DIO chow (n = 8) were fed Purina rat chow; and 4) DIO HE diet (n = 6) were fed HE diet. After 1 mo on their respective diets, DR HE and DIO HE dams underwent tail bleeding to obtain plasma glucose, insulin, and leptin levels. Because we have previously shown that DIO rats do not develop obesity until the caloric density and fat content of their diet are increased, we took blood samples only from dams fed an HE diet (18). All dams were then mated with males of the same genotype and were kept on their respective diets throughout gestation and weaning. A second blood sample was drawn on the 2nd wk of gestation for all dams on the HE diet. At birth, all litters were adjusted to 10 pups (5 male: 5 female). The final blood drawing was carried out in DR and DIO HE dams at 2 wk into the lactation period.
Plasma glucose, leptin, and insulin levels.
Glucose was analyzed by automated glucose oxidase method (Beckman Coulter, Fullerton, CA). Insulin and leptin levels were analyzed by RIA (Linco, St. Charles, MO) using antibodies to authentic rat insulin and leptin, respectively.
Pups and diet manipulations.
At 3 wk of age, all male pups were weaned to the diet groups of their respective dams. After 2–3 days on their respective diets, 1 male pup/dam was used to assess effects of increasing concentrations of leptin on VMN and ARC neurons. Pups might also have access to food crumbs of food dropped by their dams so, although we refer to the effects of “maternal diet” below, it is likely that pups did have some intake of the same diets as their dams for a week or so prior to being studied.
Measurement of leptin-induced intracellular Ca2+ concentration responses in dissociated VMN and ARC neurons.
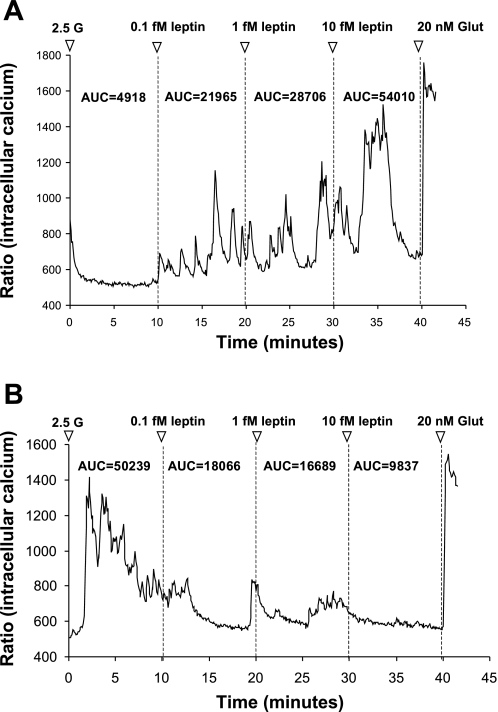
Single VMN and ARC neurons were prepared as described previously (15, 17), and leptin-responsive neurons were classified as described previously (15). Briefly, all experiments began with neurons held at 2.5 mM glucose, since we have previously shown that the responses to leptin are independent of ambient glucose levels between 0.5 and 2.5 mM (15), and 2.5 mM is equivalent to brain levels in the postprandial state (36). Neurons were next exposed to sequential addition of 0.1, 1, and 10 fmol/l. of leptin and were observed for ∼10 min after addition. Significant changes in intracellular calcium ([Ca2+]i) fluctuations were determined by first calculating the integrated area under the curve (AUC) for every 10-min period for a given concentration of leptin or glucose using Origin 7.0 software (OriginLab) (15, 17). The neurons were then classified as leptin excited (LepE; Fig. 1A), leptin inhibited (LepI; Fig. 1B), or leptin unresponsive. LepE neurons were defined as those which increased their AUC for [Ca2+]i oscillations by >30% of baseline levels at 2.5 mM glucose. LepI neurons decreased their AUC for [Ca2+]i oscillations after the addition of leptin by >30% of baseline levels. Neurons not meeting the minimal AUC criteria (30% increase or decrease) were classified as leptin-unresponsive neurons (15).
Fig. 1.
Representative changes in [Ca2+]i oscillations after exposure to incremental doses of leptin in freshly dissociated ventromedial hypothalamic nucleus (VMN) neurons from 23-day-old male rats. All recordings were performed in 2.5 mM glucose (2.5 G) followed by three doses of leptin (0.1, 1, and 10 fM). Neurons were tested terminally with 20 nM glutamate (Glut) to ascertain viability. A: leptin-excited (LepE) neuron showing increased [Ca2+]i oscillations at 0.1, 1, and 10 fM leptin. B: Leptin-inhibited (LepI) neuron showing decreased [Ca2+]i oscillations at 0.1, 1, and 10 fM leptin. Area under the curve (AUC) for each leptin dose is given above each tracing.
Quantitative PCR methods.
After completion of calcium imaging recordings, coverslips were gently rinsed in 2.5 mM glucose solution, and cells were harvested by adding lysis buffer (MagMax -96; Ambion, Austin, TX). Quantitation of mRNA was carried out by quantitative PCR as previously described (22) using GenBank. Primers and their sequence-specific 6-carboxyfluorescein-labeled probes were prepared by Applied Biosystems (Foster City, CA), as described previously (12). Results were calculated relative to cyclophilin mRNA levels as the constitutive gene standard in each sample.
Statistical analysis.
Comparisons of body weight, plasma hormones, and glucose for the dams were carried out by two-way ANOVA (genotype, diet). In offspring, the % LepE, % LepI neurons, % change AUC (an index of leptin sensitivity) and mRNA expression levels were compared by two-way ANOVA (genotype, maternal diet). When significant differences were found by two-way ANOVA, further comparisons were made by one-way ANOVA with post hoc Bonferroni corrections (P ≤ 0.05). All data are expressed as means ± SE.
RESULTS
Dam body weight gain and plasma hormone levels.
As we have previously shown (11), DIO dams were heavier than DR dams before they were put on HE diet. After 1 mo on HE diet, DIO dams gained 367% more weight, had 126% higher plasma leptin and 258% higher insulin levels than DR dams (Table 1). At 2 wk of gestation, DIO dams gained comparable amount of weight, had 84% higher plasma leptin and 201% higher insulin levels than DR dams. Two weeks after delivery, DIO dams lost 190% more body weight than DR rats. However, DIO dams remained significantly heavier and had 125% higher plasma leptin but not insulin levels than DR rats. Glucose levels did not differ significantly between the two groups during gestation or lactation (Table 1).
Table 1.
Body weights, plasma glucose, leptin and insulin levels of DR and DIO dams fed HE diet during gestation and lactation
| DR HE | DIO HE | ||
|---|---|---|---|
| Initial body weight, g | 243±7.00 | 315±7.05* | |
| One month of HE diet | |||
| Body weight, g | 261±10.0 | 385±9.45* | |
| Body weight gain, g | 15.0±3.60 | 69.7±4.06* | |
| Glucose, mg/dl | 124±3.70 | 132±5.49 | |
| Leptin, ng/ml | 4.38±1.40 | 9.90±1.88* | |
| Insulin, ng/ml | 0.91±0.18 | 3.26±0.22* | |
| Second week of gestation | |||
| Body weight, g | 307±11.0 | 433±15.9* | |
| Body weight gain, g | 45.0±5.40 | 48.8±4.95 | |
| Glucose, mg/dl | 121±8.50 | 133±4.82 | |
| Leptin, ng/ml | 5.00±1.02 | 9.22±0.86* | |
| Insulin, ng/ml | 1.79±0.45 | 5.39±0.17* | |
| Second week postgestation | |||
| Body weight, g | 293±5.90 | 350±8.03* | |
| Body weight gain, g | −20.0±5.80 | −58.0±2.00* | |
| Glucose, mg/dl | 125±6.30 | 135±9.21 | |
| Leptin, ng/ml | 1.28±0.23 | 2.88±0.59* | |
| Insulin, ng/ml | 0.43±0.08 | 0.51±0.07 | |
Values are presented as means ± SE. Diet-resistant dams (DR) and diet-induced obesity (DIO) dams were fed high-energy (HE) diet (n = 6/group) for 1 mo before breeding and continued on HE diet during gestation and lactation. Body weight gain represents the changes in body weight during 1 mo on HE diet, the first 2 wk of gestation, and from gestation to second week of lactation, respectively.
P < 0.05 when data from DIO dams were compared to that of DR dams.
Differences in leptin-induced changes in [Ca2+]i oscillations in VMN neurons of 3- to 4-wk-old DIO vs. DR offspring.
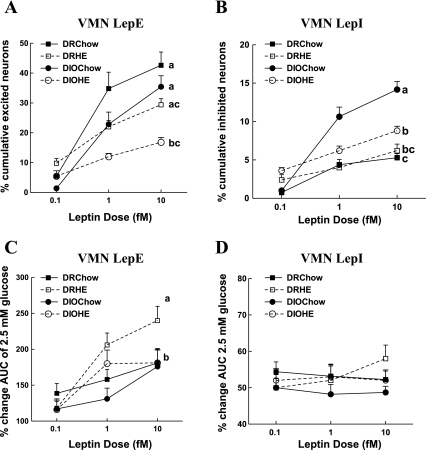
Of 1,412 VMN neurons assessed in offspring of DR and DIO dams fed chow or HE diet during gestation and weaning, almost four times as many neurons were excited (30%; Fig. 2A) as inhibited (8%; Fig. 2B), irrespective of the offspring genotype. However, there were significant effects of both genotype and maternal diet on the percent of VMN neurons, which were excited by leptin (Fig. 2A). DIO offspring had fewer total LepE neurons, regardless of their maternal diet (19% on chow, 41% on HE diet) than did DR offspring [F(1,21) = 6.83; P = 0.02]. Also, offspring of dams fed HE diet had fewer (33% for DR, 51% for DIO) LepE neurons than did offspring of chow-fed dams [F(1,21) = 17.7; P = 0.001]. There was also a genotype × maternal diet interaction effect. When the total cumulative percentage of neurons excited by leptin across all three doses was compared (Fig. 2A), maternal intake of HE diet (and its attendant maternal obesity) led to a significant reduction in the percentage of LepE neurons only in offspring of DIO dams. This suggests that the combination of the DIO genotype and maternal obesity had a cumulative inhibitory effect on the number of demonstrable VMN neurons. When comparing the percent increase in [Ca2+]i AUC fluctuations across the three concentrations of leptin as an index of leptin sensitivity of individual neurons, there was a significant genotype [F(1,8) = 6.2; P = 0.038] and maternal diet [F(1,8) = 6.2; P = 0.038] effect (Fig. 2C). Overall, VMN LepE neurons from DIO offspring were less sensitive to leptin than were those from DR offspring. This genotype effect was mainly due to the reduced sensitivity of DIO LepE neurons to leptin at the 1 fM dose. On the other hand, maternal intake of HE diet was associated with an unexpected increase in leptin sensitivity in LepE neurons. This was first seen at the 1 fM dose, whereas, at the highest dose of leptin (10 fM), leptin sensitivity for all groups was similar except for offspring of DR dams on HE diet, which had significantly (P = 0.01) increased leptin sensitivity (Fig. 2C).
Fig. 2.
Effects of genotype and maternal diet on leptin-induced changes in [Ca2+]i oscillations. VMN neurons of offspring of diet-resistant (DR; n = 8) and diet-induced obesity (DIO; n = 8) dams fed chow, and DR (n = 5) and DIO (n = 5) dams fed high-energy (HE) diet during gestation and lactation were exposed to increasing doses of leptin (0.1, 1.0, and 10 fM). Total numbers of neurons analyzed for a given group are as follows: DR chow = 286, DR HE = 429, DIO chow = 292, DIO HE = 405. A: cumulative percentage of LepE neurons at the three increasing leptin doses. B: cumulative percentage of LepI neurons at the three increasing leptin doses. C: percent increase in AUC on the addition of increasing doses of leptin compared with 2.5 mM glucose. D: percent decrease in AUC on addition of leptin compared with 2.5 mM glucose. Data are expressed as means ± SE. Parameters with differing superscripts differ from each other by P < 0.05 by post hoc Bonferroni tests after intergroup differences were found by ANOVA.
Whereas leptin excited fewer VMN neurons in DIO rats, it inhibited more VMN neurons in DIO than DR rats [F(1,19) = 53.5; P = 0.00]. There was also a maternal diet effect [F(1,19) = 8; P = 0.01], which was predominantly seen in DIO rats where leptin inhibited 180% more VMN neurons in DIO offspring of chow-fed dams and 50% more neurons in offspring of DIO dams fed an HE diet compared with respective DR offspring (Fig. 2B). This selective effect of HE diet on DIO offspring was most likely due to maternal obesity since only DIO dams fed an HE diet became obese. Unlike the effects on LepE neurons, there was no dose-related decrease in [Ca2+]i AUC fluctuations across the three concentrations of leptin for LepI neurons, and there were no intergroup genotype or maternal diet effects for sensitivity to leptin inhibition (Fig. 2D). In summary, given the 4:1 preponderance of LepE:LepI neurons in the VMN, the major differences in leptin's effects were reflected in a large reduction in the number and sensitivity of neurons excited by leptin in DIO offspring. This reduced sensitivity in DIO rats was partially offset by the fact that maternal intake of HE diet made VMN neurons slightly more sensitive to the excitatory effects of leptin, but only at a single dose.
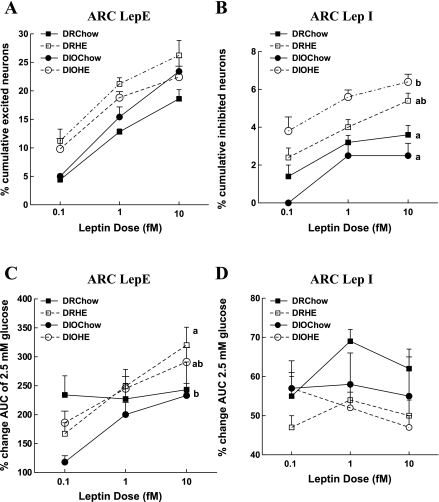
Differences in leptin-induced changes in [Ca2+]i oscillations in ARC neurons of 3- to 4-wk-old DIO vs. DR offspring.
Similar to the VMN, more than four times as many of 1,926 ARC neurons assessed were excited (22%; Fig. 3A) as inhibited (5%; Fig. 3B) by leptin, regardless of genotype or maternal diet. As opposed to the VMN, there were no significant dose-related differences by genotype or maternal diet in the percent of neurons excited by leptin in the ARC (Fig. 3A). On the other hand, there was both a genotype [F(1,8) = 5.2; P = 0.05] and maternal diet effect [F(1,8) = 5.95; P = 0.04] on sensitivity to leptin as assessed by changes in [Ca2+]i AUC fluctuations (Fig. 3C). Overall, neurons of DR offspring were more sensitive to leptin's excitatory effects than were those from DIO offspring. Leptin caused a dose-related increase in [Ca2+]i AUC fluctuations across three concentrations of leptin in all neurons except those from DR offspring of chow-fed dams (Fig. 3C). Despite their lack of a dose-response curve, neurons from these DR offspring were the most sensitive to leptin at the lowest doses used here. Thus, it is possible that they would have demonstrated a dose-responsiveness had lower leptin doses been used. Similar to VMN LepE neurons, maternal intake of HE diet increased the sensitivity to the excitatory effects of leptin selectively in DR offspring neurons (Fig. 3C).
Fig. 3.
Effects of genotype and maternal diet on leptin-induced changes in [Ca2+]i oscillations. Hypothalamic arcuate nucleus (ARC) neurons of DR and DIO offspring as described in Fig. 2. Total numbers of neurons analyzed for a given group are as follows: DR chow = 448, DR HE = 474, DIO chow = 465, DIO HE = 539. A: cumulative percentage of LepE neurons at the three increasing leptin doses. B: cumulative percentage of LepI neurons at the three increasing leptin doses. C: percent increase in AUC on the addition of leptin compared with 2.5 mM glucose. D: percent decrease in AUC on the addition of leptin compared with 2.5 mM glucose Data are expressed as means ± SE. Parameters with differing superscripts differ from each other by P < 0.05 by post hoc Bonferroni tests after intergroup differences were found by ANOVA.
There was a significant effect of maternal diet but not genotype on ARC LepI neurons. Offspring of dams fed HE diet had greater (50% for DR, 100% for DIO) total percentage of LepI neurons (Fig. 3B) than chow-fed offspring [F(1,15) = 34.5; P = 0.001].While maternal intake of HE diet was associated with an increase in the percentage of total neurons inhibited by leptin in the ARC (Fig. 3B), maternal intake of HE diet actually led to decreased leptin sensitivity ([Ca2+]i AUC fluctuations) in individual neurons of offspring (Fig. 3D) [F(1,8) = 7.1; P = 0.03]. Thus, the effects of maternal intake of an HE diet tended to counteract each other by increasing the percentage of ARC neurons inhibited by leptin but decreasing the sensitivity of these neurons to the inhibitory effects of leptin. This may well account for the only modest overall differences between ARC LepE neurons from DIO vs. DR offspring.
ARC neuropeptide and receptor mRNA expression in 3- to 4-wk-old offspring.
By two-way ANOVA, there was a main genotype, but no diet effect on NPY expression in dissociated ARC neurons by which neurons from DIO offspring expressed 70–75% more NPY mRNA than did those from DR offspring (Table 2) [F(1,14) = 8.81; P = 0.01]. On the other hand, there was a main diet effect for AgRP. Offspring of dams fed an HE diet had 40% (DR) and 17% (DIO) less AgRP mRNA expression compared with offspring of dams fed chow (Table 2) [F(1,14) = 4.96; P = 0.04].
Table 2.
Arcuate mRNA expression in 23-day-old offspring of DR and DIO dams fed chow or HE diet during gestation and lactation
| DR Chow | DIO Chow | DR HE | DIO HE | |
|---|---|---|---|---|
| NPY | 0.85±0.22a | 1.48±0.19b | 0.71±0.17a | 1.21±0.17b |
| AgRP | 1.03±0.21 | 1.12±0.11 | 0.62±0.12 | 0.93±0.11 |
| POMC | 1.32±0.22 | 0.93±0.13 | 0.90±0.10 | 0.97±0.14 |
| Lepr-b | 0.90±0.20 | 0.83±0.20 | 0.57±0.05 | 0.42±0.02 |
Values are expressed as means ± SE for ratios of the mRNA of interest relative to cyclophilin mRNA. Parameters with different superscripts differ from each other by P < 0.05 by Bonferroni post hoc test after intergroup differences were found by one-way ANOVA; n = 5 per group.
DISCUSSION
We previously showed that, prior to the onset of obesity, selectively bred DIO rats are less sensitive to the anorectic and thermogenic effects of leptin (12, 22, 25) and that they also have reduced Lepr-b mRNA expression, leptin-induced pSTAT3 expression, and leptin binding in both the VMN and ARC (4, 14, 22, 25). The current studies extend these previous ones by showing that 3- to 4-wk-old DIO rats have fewer VMN neurons that are excited by leptin and that these neurons are also less sensitive to the excitatory effects of leptin than are those from DR rats. On the other hand, leptin inhibited a higher percentage of VMN neurons in DIO than DR rats. However, since ∼75% of leptin-responsive neurons in the VMN were LepE in type, it is likely that the previously demonstrated reduction in leptin signaling and sensitivity in the VMN of DIO rats (4, 12, 14, 22, 25) is a reflection of the reduced sensitivity of their LepE neurons.
The differences in leptin responsiveness between ARC neurons from DIO and DR rats were much more modest than were those in the VMN and were reflected primarily as a small reduction in sensitivity to the excitatory effects of leptin in DIO vs. DR ARC neurons. In the ARC, proopiomelanocortin (POMC) neurons are excited by leptin (6) via a phosphoinositide 3 kinase-dependent (13, 39), AMP kinase-independent (5) mechanism. Although we did not phenotype the ARC neurons tested here, the fact that DIO ARC LepE neurons were less sensitive to leptin suggests that these rats would have a reduced activation of their catabolic POMC neurons as one cause of their propensity to become obese on high-fat diets. On the other hand, although leptin either inhibits (31, 34, 39) or has no effect (5) on the activity of ARC NPY/AgRP neurons, the acute effects of leptin on these neurons may not participate in the increased responsiveness of DIO rats to obesity on HE diets since the sensitivity of their LepI neurons was comparable to those of DR rats. However, the fact that DIO rats had higher levels of ARC NPY mRNA expression does suggest that these neurons are, in fact, less sensitive to the inhibitory effects of leptin on NPY transcription, a process that is not necessarily linked to neuronal activity. Although our results suggest that there are no major differences in the leptin responses in the overall population of ARC neurons, it is still possible that there might be a specific reduction in leptin responsiveness in one or more of the neuronal subtypes in this critical nucleus.
As opposed to the ARC, little is known about the phenotypes of VMN neurons that respond to leptin other than the fact that, at supraphysiological concentrations of glucose, leptin activates neurons expressing steroidogenic factor-1 (SF-1) (7). Although the neuropeptide and transmitter phenotypes of SF-1 neurons are unknown, these neurons are important mediators of leptin signaling since selective deletion of Lepr-b in them decreases (7), while deletion of suppressor of cytokine signaling 3 increases leptin signaling and obesity resistance in mice (40). Also, whereas an association with leptin has not been examined, there are a large number of glutamatergic neurons in the VMN that selectively excite catabolic ARC POMC neurons (37). Thus, if these VMN glutamatergic neurons are LepE in type, it might explain why DIO rats, with their smaller complement of VMN LepE neurons, overeat for several weeks and become obese when first exposed to HE diet (25).
Diet composition is an important determinant of both the development of obesity and central leptin sensitivity. Intake of high-fat diets leads to central leptin resistance, but usually after the animals have developed obesity (9, 10, 32). In addition, maternal intake of HE diet has a major impact on the propensity of DIO offspring to become obese as adults (12). Intake of HE diet during gestation and lactation causes DIO but not DR dams to become obese and their adult offspring become more obese than all other groups when fed either chow or HE diet from weaning (18, 27). Part of this effect is due to the postnatal exposure to an “obesogenic” environment since offspring of lean DR dams cross fostered to obese DIO dams eating HE diet develop diet-induced obesity as adults and have reduced VMN Lepr-b expression (11). On the other hand, the inherently reduced Lepr-b expression of lean DIO rats (22, 25) is further reduced in their VMN when they are cross-fostered to obese DIO dams at birth (11). Most of the maternal dietary effects on VMN and ARC neuronal leptin sensitivity seen in the current studies were probably due to maternal intake. However, despite the fact that food was placed overhead in the cages, pups could have had significant access to the maternal diets by crumbs left over from maternal ingestion. Thus, it is possible that such exposure might have had effects independent of their exposure to maternal milk. Whatever the origin, because intake of a high-fat diet reduces central leptin signaling (9, 22, 25, 38), we expected that maternal intake of an HE diet would similarly reduce the sensitivity of individual hypothalamic neurons to leptin. In fact, there were fewer VMN LepE neurons in both DIO and DR offspring of dams fed an HE diet, but this reduction was more marked in DIO offspring. Although this was somewhat offset by a small increase in leptin sensitivity of VMN LepE neurons in offspring of dams fed an HE diet, the predominant effect was a decrease in the overall percentage of neurons responding to the excitatory effects of leptin, especially in DIO offspring. This suggests that maternal intake of an HE diet, especially when combined with the maternal obesity of DIO dams, can reduce leptin signaling of offspring in a large proportion of leptin-responsive VMN neurons. Given the reduced anorectic and thermogenic effects of leptin in DIO rats (12, 20, 23), our data support an important role for VMN LepE neurons in the control of energy homeostasis.
Perspectives and Significance
As with many types of obesity in rodents and humans, the DIO rat has reduced sensitivity to the anorectic and thermogenic effects of leptin (4, 12, 14, 20, 23, 26). Depending upon the age, perinatal environment, and brain area assessed, this resistance antedates the development of obesity in the DIO rats and is largely due to a primary reduction in the number of leptin receptors available for binding leptin at the cell surface in the ARC and VMN neurons (14). Here, we delve into the neuronal sensitivity of ARC and VMN neurons to leptin in DIO and DR rats as a function of maternal diet and obesity. Interestingly, the reduced in vivo sensitivity of DIO rats to leptin is paralleled by a reduction primarily in both the number and sensitivity of VMN in DIO rats. Leptin-excited neurons make up ∼75% of the leptin-responsive neurons in both the VMN and ARC. Since the much less numerous leptin-inhibited neurons in these areas were, if anything, more sensitive to the leptin in DIO rats, it is likely that a major cause of reduced leptin sensitivity in DIO rats is the decreased responsiveness of their leptin-excited hypothalamic neurons.
In addition to this genotype effect, maternal intake of an HE diet was associated with a reduced number of VMN neurons, which were excited by leptin, and this effect was most marked in DIO offspring. This is in keeping with our previous studies, in which HE diet produced obesity only in DIO dams, and only the offspring of these obese DIO dams became more obese as adults, even when fed only a low-fat diet from weaning (28). Thus, the interaction of an obese genotype and an “obesogenic” maternal environment has a combined, adverse effect on leptin sensitivity at the level of individual VMN neurons. The fact that the most consistent effects of genotype and diet were on VMN rather than ARC neurons reinforces the importance of the VMN as a major effect of leptin's regulatory role in energy homeostasis (7). Perhaps the most important message of the current studies is that leptin resistance in DIO rats and the adverse effects of maternal HE diet and obesity on leptin signaling do not equally affect all ARC and VMN neurons. Such diversity might explain the selective leptin resistance seen in obese animals with regard to energy homeostasis vs. cardiovascular control (35).
GRANTS
This work was supported by the Research Service of the Department of Veterans Affairs (to B. E. Levin and A. A. Dunn-Meynell), National Institute of Diabetes, Digestive and Kidney Diseases (Grant DK 30066 to B. E. Levin) and by an award from the American Heart Association (B. G. Irani).
Acknowledgments
We thank Sunny Park and Antoinette Moralishvilli for their expert technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ Intrauterine programming of adult disease. Mol Med Today 1: 418–423, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proc Biol Sci 262: 37–43, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7: 179–185, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117: 2325–2336, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S Jr, Elmquist JK, and Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49: 191–203, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 51: 2056–2065, 2002. [DOI] [PubMed] [Google Scholar]
- 9.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105: 1827–1832, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Gorski J, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol 291: R768–R778, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Gorski JN, Dunn-Meynell AA, Levin BE. Maternal obesity increases hypothalamic leptin receptor expression and sensitivity in juvenile obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 292: R1782–R1791, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118: 1796–1805, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irani BG, Dunn-Meynell AA, Levin BE. Altered hypothalamic leptin, insulin, and melanocortin binding associated with moderate-fat diet and predisposition to obesity. Endocrinology 148: 310–316, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Irani BG, Le Foll C, Dunn-Meynell A, Levin BE. Effects of leptin on rat ventromedial hypothalamic neurons. Endocrinology 149: 5146–5154, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eikis J, Zhang BB, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55: 412–420, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53: 549–559, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Levin BE, Dunn-Meynell AA. Maternal obesity alters adiposity and monoamine function in genetically predisposed offspring. Am J Physiol Regul Integr Comp Physiol 283: R1087–R1093, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283: R941–R948, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 286: R143–R150, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Levin BE, Dunn-Meynell AA, McMinn JE, Alperovich M, Cunningham-Bussel A, Chua SC Jr. A new obesity-prone, glucose-intolerant rat strain (F. DIO). Am J Physiol Regul Integr Comp Physiol 285: R1184–R1191, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Levin BE, Dunn-Meynell AA, Ricci MR, Cummings DE. Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am J Physiol Endocrinol Metab 285: E949–E957, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol Regul Integr Comp Physiol 275: R1374–R1379, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Levin BE, Hogan S, Sullivan AC. Initiation and perpetuation of obesity and obesity resistance in rats. Am J Physiol Regul Integr Comp Physiol 256: R766–R771, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Morton GJ, Niswender KD, Rhodes CJ, Myers MG Jr, Blevins JE, Baskin DG, Schwartz MW. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fa(k)/fa(k)) rats. Endocrinology 144: 2016–2024, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Mountjoy PD, Bailey SJ, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia 50: 168–177, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Ounsted M, Sleigh G. The infant's self-regulation of food intake and weight gain. Difference in metabolic balance after growth constraint or acceleration in utero. Lancet 1: 1393–1397, 1975. [DOI] [PubMed] [Google Scholar]
- 34.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304: 110–115, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 54: 2012–2018, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Silver IA, Erecinska M. Extracellular glucose concentrations in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci 14: 5068–5076, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH→arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci 8: 1356–1363, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR Jr. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 99: 385–390, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang JH, Wang F, Yang MJ, Yu DF, Wu WN, Liu J, Ma LQ, Cai F, Chen JG. Leptin regulated calcium channels of neuropeptide Y and proopiomelanocortin neurons by activation of different signal pathways. Neuroscience 156: 89–98, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Zhang R, Dhillon H, Yin H, Yoshimura A, Lowell BB, Maratos-Flier E, Flier JS. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology 149: 5654–5661, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]