Abstract
In rats selectively bred to develop diet-induced obesity (DIO) 3 wk of postweaning exercise reduces weight and adipose regain for 10 wk after exercise cessation, despite intake of 31% fat high-energy (HE) diet. To test the hypothesis that this effect is due to increased central leptin sensitivity, 4-wk-old DIO rats were fed the HE diet and left sedentary (Sed), exercised for 3 wk, and then remained sedentary for 10 additional weeks (Ex/Sed) or continued exercise for a full 13 wk (Ex). After 3 wk, leptin (5 mg/kg ip) induced a 36% decrease in 24-h food intake in Ex rats, while Sed rats had no change in 24-h intake. Ex rats also had 23% more leptin-induced phospho-STAT3 (pSTAT3)-expressing neurons in the arcuate nucleus (ARC) and 95% and 68% higher 125I-labeled leptin receptor binding in the ventromedial and dorsomedial nuclei than did Sed rats, respectively. At 7 wk after onset, leptin decreased 24-h intake by 20% in Ex and 24% in Ex/Sed rats without altering Sed intake. After a total of 13 wk, compared with Sed rats, Ex and Ex/Sed rats had 58% and 38% less fat, respectively, but leptin failed to decrease food intake in any group. Nevertheless, Ex, but not Ex/Sed rats, still had 32% more ARC leptin-induced pSTAT3-expressing neurons than Sed rats. These data suggest that brief postweaning exercise in DIO rats that are inherently leptin resistant causes a sustained resistance to obesity on HE diet, which is, in part, due to increased central leptin sensitivity.
Keywords: hypothalamus, caloric restriction, development, leptin receptor, arcuate nucleus
central leptin resistance occurs during the development of obesity in rodents (37) and probably humans (33). Such central resistance can either be caused by reduced leptin transport across the blood-brain barrier (4, 35) or by reduced activation of leptin receptor signaling (34). As opposed to most forms of rodent obesity, rats that are selectively bred to develop diet-induced obesity (DIO) when placed on a 31% fat, high-energy (HE) diet have an apparently inborn reduction in their sensitivity to the anorectic and thermogenic effects of leptin (14, 20, 22). These deficits are associated with significant decreases in hypothalamic mRNA expression of the long form of the leptin receptor Lepr-b (20, 23), binding to the leptin receptor (17), and leptin-induced phosphorylation of signal transducer and activator of transcription (pSTAT3) (7, 23). This inherent leptin resistance and resultant reduction in leptin's trophic effects appear to impede the outgrowth of axons from neurons within the hypothalamic arcuate nucleus (ARC), which are critical mediators of energy homeostasis (7). On the other hand, manipulation of the perinatal environment can enhance leptin sensitivity in prepubertal DIO rats (14). Such findings suggest that leptin resistance in DIO rats contributes to their development of obesity on HE diet and that early interventions that improve leptin sensitivity during the critical postnatal period might prevent their obesity on HE diets.
We have shown that exercise in the postweaning period produces long-term lowering of the body weight set point in DIO rats (29). In fact, only 3 wk of running wheel exposure is sufficient to inhibit the development of obesity for an additional 10 wk of sedentary existence, despite the continued intake of HE diet (29). Given their inherent leptin resistance, we hypothesized that this long-lasting resistance to obesity was due to a persistent exercise-induced increase in leptin sensitivity. Since DIO rats display normal blood-brain barrier leptin transport compared with diet-resistant (DR) rats (22) and our previous studies demonstrated no differences in hypothalamic Lepr-b mRNA expression between sedentary and exercised juvenile DIO rats on an HE diet (29), we predicted that exercise would improve central leptin sensitivity by increasing the binding of leptin to its receptor and/or signaling downstream of the receptor. In addition, given leptin's trophic actions (8), we also postulated that early onset exercise might correct the inborn defects in ARC-paraventricular nucleus (PVN) axonal projections seen in selectively bred DIO rats (7).
Similar to our previous studies (29), we demonstrate here that, compared with sedentary DIO rats, 3 wk of postweaning exercise significantly reduced the long-term body weight gain and adiposity of DIO rats maintained on a HE diet, even 10 wk after exercise termination. This exercise-induced resistance to obesity was accompanied by enhanced sensitivity to the anorectic and thermogenic effects of leptin in association with increased leptin-receptor binding and increased leptin-induced downstream activation of pSTAT3. Most importantly, while exercise did not enhance ARC-PVN pathway development, it did increase the anorectic effect of leptin for several weeks beyond exercise termination, suggesting that increased leptin sensitivity contributes to the resistance to weight regain in juvenile DIO rats following exercise cessation.
MATERIALS AND METHODS
Animals and diet.
The experiments were approved by the Institutional Animal Care and Usage Committee of the East Orange Veterans Affairs Medical Center and are in compliance with the guidelines of the American Physiological Society (1). Male rats bred selectively to express the DIO trait when fed a diet moderately high in fat and calories (HE diet) (21) were raised in our in-house colony and used for all studies. To avoid potential litter effects, 6–8 litters were culled to 10 pups per dam on postnatal day 2 (P2) and weaned at P28 onto the HE diet and water ad libitum, unless otherwise noted. The HE diet is a defined diet (cat. no. C11024F; Research Diets, New Brunswick, NJ) containing 4.47 kcal/g with 21% of the metabolizable energy content as protein, 31% as fat, and 48% as carbohydrate, 50% of which is sucrose (24). All rats were individually housed from weaning at 23–24°C on a reversed 12:12-h light-dark cycle (lights off at 0900 unless otherwise noted). During the course of all experiments cumulative food intake and body weight measurements were obtained on a weekly basis.
Experiment 1: long-term effects of 3-wk postweaning exercise on sensitivity of rats to the anorectic, thermogenic, and activity-related effects of leptin.
Selectively bred DIO rats (n = 18) were weaned, given ad libitum access to the HE diet and, under light isofluorane anesthesia, were implanted intraperitoneally with temperature and activity telemetry probes (Mini-Mitter, Bend, OR). They were then randomly divided into three groups of six rats each: 1) sedentary (Sed) rats were sedentary for 13 wk; 2) exercised (Ex) rats were given free access to a running wheel (Mini-Mitter) for 13 wk; and 3) exercised/sedentary (Ex /Sed) rats were given free access to a running wheel for 3 wk and following the 3rd wk, their wheels were removed and rats remained sedentary for an additional 10 wk. In the middle of the 3rd, 7th, and 13th wk, 2 h prior to dark onset (0700), food was removed, running wheels were locked, and 0.5 ml of blood was obtained via tail nick for plasma leptin and insulin immunoassay. At dark onset (0900), all rats received saline or murine leptin (5 mg/kg ip in 0.5 ml of PBS) (National Hormone and Peptide Program, Torrance, CA), and food intake was monitored at 4 and 24 h postinjection. Core temperature and activity were monitored every 5 min for 24 h postinjection from the intraperitoneal telemetry probes. Running wheels were unlocked for exercising rats 24 h after injection. At 3 days following the final injection (13th wk), wheels were locked and food was removed 2 h prior to dark onset. At dark onset, rats were injected with 5 mg/kg leptin ip and transcardially perfused 45 min later with 0.9% NaCl followed by 4% paraformaldehyde in 0.1 M PBS. Brains were removed from the skull and postfixed at 4°C for 24 h. The brains were then sunk in 20% sucrose in PBS overnight at 4°C and then frozen with 2-methylbutane cooled to −50°C with dry ice. Brains were then stored at −70°C until 35-μm serial sections were cut on a cryostat and processed by pSTAT3 and phospho-ERK (pERK) immunocytochemistry. Due to inconsistent temperature readings from telemetry probes, core temperature following leptin treatment was not analyzed at 7 and 13 wk.
Experiment 2: effect of 3-wk postweaning exercise and caloric restriction on hypothalamic pSTAT3 and pERK expression following leptin administration and leptin receptor binding.
Selectively-bred male DIO rats (n = 36) were weaned onto HE diet and randomized into three groups of 12 rats each: 1) sedentary (Sed) rats remained sedentary for 3 wk with ad libitum access to HE diet; 2) exercise (Ex) rats had free access to a running wheel for 3 wk with ad libitum access to HE diet; and 3) restricted (Rstr) rats remained sedentary and were food restricted to 85% of the daily caloric intake of the Sed animals for 3 wk. At the end of 3 wk, six rats per group were fasted 2 h prior to dark onset and were given an injection of murine leptin (5 mg/kg ip in 0.5 ml of PBS) at the onset of dark. After 30 min, they were anesthetized with chloropent and then rapidly perfused with ice-cold saline for 1 min, followed by ice-cold 4% paraformaldehyde for 10 min. Brains were treated as those in experiment 1. Previous studies from our lab reported low and equal baseline pSTAT3 immunoreactivity following saline injection in DIO and DR rats (22). Therefore, for the sake of economy, no saline-injected controls were utilized in this experiment.
Also following 3 wk of experimental manipulations, the remaining six rats/group were fasted 2 h prior to dark onset when they were decapitated and their brains were frozen and stored at −80°C for subsequent leptin receptor-binding autoradiography.
Experiment 3: effect of 3 wk of postweaning exercise and caloric restriction on the development of the ARC NPY/AgRP and α-MSH projections to the PVN in DIO rats.
We assessed the effect of exercise on the density of agouti-related peptide (AgRP) and proopiomelanocortin pathways projecting from the ARC to their targets in the PVN. Selectively bred DIO rats (n = 19) were weaned onto the HE diet and randomly divided into three groups of six to seven rats each: 1) sedentary (Sed) rats were sedentary for 3 wk with ad libitum access to HE diet; 2) exercised (Ex) rats were given free access to a running wheel (Mini-Mitter) for 3 wk with ad libitum access to HE diet; 3) restricted (Rstr) rats were sedentary and calorically restricted to 85% of the daily caloric intake of the Sed 3 wk rats for 3 wk. Following 3 wk, rats had their wheels locked 4 h prior to dark onset, food was removed 2 h prior to dark onset, and all rats were transcardially perfused at the initiation of the dark cycle with 0.9% NaCl followed by 4% paraformaldehyde in borate buffer. Brains were then postfixed in perfusion solution at 4°C overnight, cryoprotected with 20% sucrose, and 35-μm serial sections were cut on a cryostat, collected, and processed for AgRP and α-MSH immunocytochemistry. Brains were then analyzed for relative fiber density in the PVN using confocal microscopy according to previously established methods (7).
Plasma insulin, glucose, and leptin levels.
Blood was collected in EDTA-coated tubes and plasma insulin, and leptin levels were analyzed by radioimmunoassay utilizing antibodies authentic to rat insulin and leptin (Linco, St. Charles, MO). Plasma glucose was measured using an Analox glucometer (San Diego, CA).
Telemetry probes.
Selected rats were implanted intraperitoneally with a 23 × 8-mm telemetry transponder (Mini Mitter) under isofluorane anesthesia. Core temperature and activity were recorded from a receiver under the home cage every 5 min using Mini Mitter software on a central computer (14).
Immunocytochemistry for pSTAT3 and pERK.
Immunocytochemistry for pSTAT3 was performed to assess downstream Lepr-b signaling as previously established in our laboratory (7, 20). Serial 35-μm brain sections were cut through the hypothalamic ARC, ventromedial (VMN), and dorsomedial (DMN) nuclei. The first series of sections was floated in potassium PBS (KPBS) buffer with gentle agitation and washed three times. They were incubated for 20 min in KPBS containing 1% NaOH and 1% H2O2 and then washed in KPBS two times for 5 min each. Sections were then placed in fresh KPBS containing 0.15% SDS for 10 min and then washed three times for 10 min in KPBS. Blocking was done with 4% horse serum in KPBS containing 0.4% Triton X-100 for 2 h, and sections were incubated overnight in rabbit anti-pSTAT3 antibody (Cell Signaling Technologies, Danvers, MA) at a 1:1,000 dilution in KPBS containing 4% horse serum and 0.4% Triton X-100. The following day, sections were warmed to room temperature for 1 h, rinsed six times for 10 min each in KPBS containing 1% horse serum and 0.02% Triton X-100, and incubated for 1 h with 1:250 biotinylated goat anti-rabbit antibody in KPBS containing 1% horse serum. They were then washed three times for 10 min each in KPBS containing 1% horse serum and incubated for 1 h with ABC reagent (Vector Labs, Burlingame, CA) prepared 30 min before in KPBS containing 1% horse serum. Following six washes in KPBS, the sections were reacted with diaminobenzidine for 5 min, washed three times, mounted on slides, and dehydrated.
Immunocytochemistry for pERK was used to assess MAP kinase activation following leptin administration. A second series of 35-μm sections was floated in 0.02 M KPBS buffer with gentle agitation and washed 10 times for 6 min each. Blocking was done with 2% goat serum in KPBS containing 0.3% Trition X-100 overnight at 4°C. The following day, sections were incubated for 48 h in rabbit anti-pERK antibody (Cell Signaling) at a 1:1,000 dilution in blocking solution. Sections were then rinsed eight times for 5 min each in KPBS followed by a 1-h incubation in goat anti-rabbit Alexa Fluor 568 antibody (Molecular Probes, Carlsbad, CA) at a 1:200 dilution in blocking solution. Following four rinses for 5 min each in KPBS, sections were counterstained with bis-benzamide (Invitrogen, Carlsbad, CA) for 3 min. Sections were then rinsed three times for 5 min each in KPBS and mounted and coverslipped slightly wet with buffered glycerol mountant.
Quantitative analysis of immunolabeled cells.
The number of cells expressing pSTAT3 or pERK were counted in three consecutive sections per rat in the ARC, VMN, DMN, and PVN by an observer blinded to the experimental groups using a Bioquant computerized image analysis system (Nashville, TN) (7). Each hypothalamic nucleus was visualized at low power (×10), and individual nuclei were outlined on the screen and defined using anatomical landmarks. Cells were then counted in the entire defined nucleus at high power (×20) in a single focal plane using intensities set by the experimental observer. Cells that appeared twice when moving the visual field through the nucleus were automatically rejected by the system. The average number of cells counted in the three sections per area in each rat was taken for statistical comparisons.
Quantitative leptin receptor autoradiography.
Leptin receptor binding autoradiography was used to assess binding of leptin to its receptor by methods previously established in our laboratory (17). Rat brains were quick frozen on dry ice and then cut using a cryostat in six sets of 10-μm sections, which were then freeze thawed onto gel-coated slides and dried. They were then incubated with radiolabeled 125I-labeled leptin (PerkinElmer Life Sciences, Boston, MA), with or without an excess of unlabeled competing ligand for the receptor to assess specific binding. Slides were then dried and apposed to X-ray film. Binding of 125I-leptin was carried out with 0.25 nM murine 125I-leptin, Tris·HCl (pH 7.2), 1% BSA (Sigma, St. Louis, MO), 0.05% leupeptin (Sigma), and 0.001% pepstatin (Sigma). Nonspecific binding was defined as that seen in the presence of a 1,000-fold excess of unlabeled murine leptin (National Hormone and Peptide Program) and accounted for < 30% of total binding (17). The resulting autoradiograms were quantitated by an experimentally blinded observer using computer-assisted densitometry (Drexel) by outlining the area to be analyzed on a computer image of the cresyl violet-stained section used to generate the autoradiogram and reading the exposed autoradiogram density from the overlaid image. Film density was equated to density of known radiolabeled standards placed on each film, and binding was calculated directly from density (11, 19, 25).
Immunocytochemistry for AgRP and α-MSH.
AgRP immunocytochemistry was performed to assess the density of AgRP projections to the PVN from the ARC. Serial 35-μm brain sections were cut through the hypothalamic ARC, VMN, DMN, and PVN. Sections were floated in 0.02 M KPBS buffer with gentle agitation and washed 10 times for 6 min each. Blocking was done with 2% goat serum in KPBS containing 0.3% Triton X-100 overnight at 4°C. The following day, sections were incubated for 72 h in rabbit anti-human AgRP antibody (Phoenix Pharmaceuticals, Burlingame, CA) at a 1:4,000 dilution in blocking solution. Sections were then rinsed 10 times for 5 min each in KPBS followed by a 1-h incubation in biotin-goat anti-rabbit IgG antibody (Vector Labs) at a 1:5,000 dilution in KPBS + 0.4% Triton X-100. Following eight rinses for 5 min each in KPBS, sections were incubated for 30 min in ABC reagent (Vector Labs) prepared 30 min before use in KPBS + 0.4% Triton X-100. Sections were then rinsed eight times for 5 min each and then reacted for 20 min with biotinylated tyramide (Perkin Elmer Life Sciences) in KPBS and 0.005% H2O2. Following eight rinses for 5 min each in KPBS, sections were incubated for 1 h with streptavidin-Alexa Fluor 568 at a 1:1,000 dilution in KPBS + 0.4% Triton X-100. Sections were then rinsed eight times for 5 min in KPBS and then counterstained with green Nissl stain in KPBS for 5 min. Following four rinses in KPBS for 5 min each, sections were mounted and coverslipped slightly wet with buffered glycerol mountant.
Immunocytochemistry for α-MSH was performed to assess the density of α-MSH axons projecting to the PVN from the ARC. Serial 35-μm brain sections were cut through the ARC, VMN, DMN, and PVN. Sections were floated in 0.02 M KPBS buffer with gentle agitation and washed 10 times for 6 min each. Blocking was done with 2% donkey serum in KPBS containing 0.3% Triton X-100 overnight at 4°C. The following day, the sections were incubated for 72 h in sheep anti-α-MSH antibody (Chemicon, Temecula, CA) at a 1:40,000 dilution in blocking solution. Sections were then rinsed 10 times for 5 min each in KPBS followed by 1-h incubation in Alexa Fluor 568 donkey anti-sheep antibody (Molecular Probes) at a 1:200 dilution in blocking solution. Following eight rinses for 5 min each in KPBS, sections were counterstained with bis-benzamide (Invitrogen) for 3 min. Sections were then rinsed three times for 5 min each in KPBS and mounted and coverslipped slightly wet with buffered glycerol mountant.
Quantification of PVN fiber density.
The density of AgRP and α-MSH innervation of the PVN was determined by quantitative confocal microscopy using previously published methods (7). For each animal, two sections through the PVN were acquired using a Leica SP confocal microscope equipped with a ×10 objective (numerical aperture, 0.40; working distance, 360 μm). Image analysis was performed using Metamorph image analysis software (Universal Imaging, Downingtown, PA). Each image plane was binarized, so as to isolate labeled fibers from background, as well as to compensate for differences in fluorescence intensity, and was then skeletonized so that each fiber segment was 1-pixel thick. The integrated intensity was then calculated for each image, which reflects the total number of pixels in the skeletonized image and was proportional to the total length of labeled fibers in the image. To avoid overcounting fibers that were thicker than the distance between planes, each skeletonized image was compared with the preceding one and pixels appearing in the previous image were rejected automatically. This procedure was carried out on each image plane in the stack, and the values for all image planes in a stack were summed. The resulting value is an accurate index of fiber density in the volume sampled (i.e., entire PVN, medial parvocellular division of the PVN, or posterior magnocellular division of the PVN).
Statistics.
Groups were compared by Student's t-test, one-way and repeated-measures ANOVA. Post hoc assessment was by Bonferroni correction for multiple comparisons. Correlations between body weight gain and number of leptin-induced pSTAT3-positive cells, between leptin-induced pSTAT3-positive cells and food intake, and between leptin receptor binding and plasma leptin were carried out using Pearson's correlation coefficient.
EXPERIMENTAL RESULTS
Effect of postweaning exercise on body weight gain, plasma hormones, and fat pad mass.
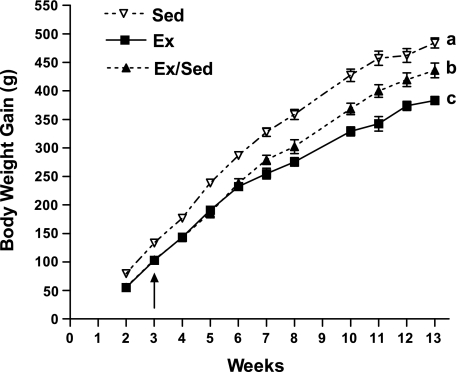
Following weaning onto the HE diet at 4 wk of age, sedentary rats steadily gained weight over the following 13 wk. During the first 3 wk, exercised rats gained 23% less weight than the Sed rats (Table 1, Fig. 1). This reduced weight gain was associated with a 71% reduction in plasma insulin and an 18% reduction in plasma glucose. Unlike previous studies (29), although they tended to be lower, leptin levels were not significantly reduced after 3 wk of exercise (Table 1).
Table 1.
Experiment 1
| Sed | Ex | Ex/Sed | ||||
|---|---|---|---|---|---|---|
| Weeks 0–3 | ||||||
| Initial body weight, g | 73±4 | 63±6 | ||||
| Body weight week 3, g | 206±6a | 166±7b | ||||
| Body weight gain weeks 1–3, g | 133±4a | 103±3b | ||||
| 3-wk 24-h food intake, g | 22±1.4 | 19±1 | ||||
| 3-wk glucose, mg/dl | 118±3.7a | 97±2.9b | ||||
| 3-wk leptin, ng/ml | 3.34±0.3 | 2.36±0.3 | ||||
| 3-wk insulin, ng/ml | 1.79±0.2a | 0.51±0.2b | ||||
| Weeks 3–7 | ||||||
| Body weight week 7, g | 401±10a | 319±12b | 341±11b | |||
| Body weight gain weeks 1–7, g | 328±8a | 255±9b | 279±8b | |||
| Body weight gain weeks 3–7, g | 195±7a | 152±9b | 175±6ab | |||
| 7-wk 24-h food intake, g | 26±0.6 | 23±0.5 | 27±1.1 | |||
| 7-wk glucose, mg/dl | 120±7.0a | 108±6.8b | 120±3.1a | |||
| 7-wk leptin, ng/ml | 9.6±1.8a | 3.7±0.4b | 6.3±0.3ab | |||
| 7-wk insulin, ng/ml | 1.0±0.3 | 0.8±0.3 | 1.0±0.3 | |||
| Weeks 3–13 | ||||||
| Final body weight, g | 556±11a | 447±12b | 498±16c | |||
| Body weight gain weeks 1–13, g | 484±9a | 383±7b | 436±13c | |||
| Body weight gain weeks 3–13, g | 351±9a | 280±5b | 332±12a | |||
| 13 wk 24 h food intake, g | 24±1.1 | 24±0.8 | 23±0.8 | |||
| Total fat pads, g | 44.8±3.0a | 14.3±0.7b | 24.3±1.7c | |||
| Total fat pads/body weight, % | 8.02±0.42a | 3.32±0.2b | 4.94±0.24c | |||
| 13-wk glucose, mg/dl | 107±5.8 | 111±7.9 | 107±8.1 | |||
| Final leptin, ng/ml | 8.0±0.8 | 10.1±1.7 | 7.0±0.7 | |||
| Final insulin, ng/ml | 0.8±0.1 | 0.4±0.1 | 0.5±0.1 | |||
Values are means ± SE. Sedentary (Sed) rats were left sedentary for 13 wk (Sed; n = 6). Exercised (Ex) rats had free access to a running wheel for 13 wk (Ex; n = 6). Following 3 wk, one-half of the Ex rats had their running wheels removed and were allowed to remain sedentary for 10 wk more (Ex/Sed; n = 6). Rats were evaluated after 3, 7, and 13 wk of experimental manipulation. Total fat pads = sum of epididymal (EPI), retroperitoneal (RP), perirenal (PERI), mesenteric (MES), and inguinal (ING) pads.
Parameters with differing superscripts differ from each other at the P < 0.05 level by post hoc Bonferroni adjustment after significant intergroup differences were found by 1-way ANOVA.
Fig. 1.
Experiment 1: body weight gain of male diet-induced obesity (DIO) rats that were weaned onto high-energy (HE) diet and allowed to remain sedentary for 13 wk (Sed; n = 6), were given access to a running wheel for 13 wk (Ex; n = 6), or were given access to a running wheel for only 3 wk and then left sedentary for an additional 10 wk (Ex/Sed; n = 6). Values are means ± SE. a,b,cParameters with differing superscripts differ from each other at the P ≤ 0.05 level when analyzed by repeated-measures ANOVA.
After the initial 3 wk period, those rats that continued to exercise for 4 wk more gained 22% less body weight and their plasma leptin levels were reduced by 61% and plasma glucose by 10%. However, insulin levels were not different compared with Sed rats (Table 1). At this time, the additional group of rats that had their running wheels removed after 3 wk of exercise but continued HE diet intake (Ex/Sed) maintained a lowered body weight comparable to continuously exercising rats. However, over the 4 wk after the wheels were removed their body weight gain was intermediate between the Sed and Ex groups (Table 1, Fig. 1). In parallel, the Ex/Sed rats had plasma leptin levels which were in between those of the Sed and Ex rats at this time (Table 1). On the other hand, glucose and insulin levels of the Ex/Sed rats were not different from those levels of the Sed rats at this time (Table 1).
At the end of the 13 wk experimental period, rats that continued to exercise (Ex) gained 21% less body weight than Sed controls (Table 1, Fig. 1). Although 13 wk of exercise was not associated with reduced plasma leptin, insulin, or glucose levels, Ex rats had a 68% reduction in total fat pad mass and a 58% reduction in fat pad mass as a percent of body weight compared with sedentary controls (Table 1). Those rats that had their wheels removed after 3 wk (Ex/Sed) had a 10% reduction in body weight and an overall 13-wk weight gain that was 10% lower than the Sed controls (Table 1, Fig. 1). However, their relatively modest reduction in weight gain was far exceeded by a 46% reduction in fat pad mass and 38% reduction in fat pad mass as a percent of body weight compared with Sed rats. Therefore, even 10 wk after their wheels were removed, previously exercised rats maintained a reduced level of adiposity compared with Sed rats, despite their continued intake of HE diet. On the other hand, despite their reduced adiposity, Ex/Sed rats had comparable plasma leptin, glucose, and insulin levels to Sed rats (Table 1).
Effect of 3-wk postweaning exercise on sensitivity of DIO rats to the anorectic, thermogenic, and activity-related effects of leptin.
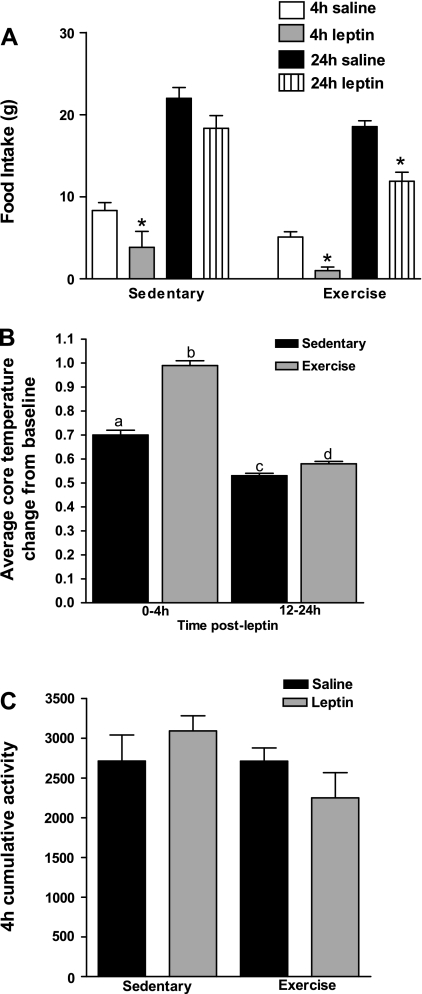
To test the hypothesis that the sustained lowering of body weight set point seen previously after 3 wk of exercise was due to increased leptin sensitivity, rats were first tested with leptin after 3 wk. Baseline 24-h food intake did not differ between Sed and Ex rats during the 3rd wk (Table 1; Fig. 2A). At 4 h following leptin administration, absolute intake was reduced by 54% in Sed but by 80% in Ex rats compared with their saline-treated baselines (Fig. 2A). Importantly, Sed rats compensated for their early leptin-induced decrease in intake by 24 h, while leptin-injected Ex rats still had a 36% reduction in 24-h intake compared with their baseline levels (Fig. 2A). Leptin administration increased core temperature of both Sed and Ex rats from 0–4 h and 12–24 h after leptin administration. However, this effect was 30% greater over 4 h and 9% greater over 12–24 h in Ex rats compared with Sed rats (Fig. 2B), while there was no effect of leptin on activity in either group (Fig. 2C). Thus, 3 wk of running wheel exercise potently increased both the anorectic and thermogenic effects of leptin in juvenile DIO rats, despite their continued intake of HE diet.
Fig. 2.
Experiment 1: male DIO rats were weaned onto HE diet and randomized into 3 groups as in Fig. 1. A: at 3 wk, rats received 5 mg/kg ip leptin, and food intake was monitored at 4 and 24 h and compared with their saline-injected intake. B: average change in baseline core temperature 0–4 h and 12–24 h following leptin administration at 3 wk. C: cumulative activity measured in arbitrary units from intraperitoneal telemetry probe during the 4 h following saline or leptin administration at 3 wk. Data are means ± SE. *P ≤ 0.05 when food intake following leptin treatment was significantly reduced compared with food intake following saline administration.
Effect of 3-wk postweaning exercise on leptin signaling in DIO rats.
Since 3-wk postweaning exercise led to an increase in the anorectic and thermogenic effects of leptin, we postulated that this would be associated with an increase in leptin signaling in the hypothalamus. To assess this, a separate group of juvenile DIO rats fed HE diet remained sedentary, had voluntary access to a running wheel, or were calorically restricted for 3-wk postweaning (experiment 2). Following 3 wk, half of each group was injected with leptin (5 mg/kg ip) to assess the ability of exogenous leptin to activate the leptin signaling cascade and 125I-leptin receptor binding was assessed in the remaining rats. In this study, rats exercised for 3 wk postweaning reduced their cumulative body weight gain by 12% compared with the Sed rats (Table 2). This reduced body weight gain was associated with a 44% reduction in plasma leptin (Table 2), suggesting that 3 wk of exercise reduced total carcass adiposity (29). A separate group of DIO rats, which were calorically restricted to 85% of the daily caloric intake of the Sed rats, lost 25% more weight than did Sed rats. However, despite the chronic caloric restriction, and unlike the Ex rats, this reduction in body weight was not associated with a significant decrease in plasma leptin levels (Table 2), as would usually be expected with weight loss (38). Thus, these results suggest that caloric restriction may have produced a preferential loss of lean rather than adipose mass in these rapidly growing animals.
Table 2.
Experiment 2: body weight and plasma leptin of rats assessed for leptin-induction of hypothalamic phospho-STAT3 and leptin receptor binding
| Sed | Ex | Rstr | |
|---|---|---|---|
| Initial body weight, g | 63±4 | 66±3 | 65±3 |
| Body weight 3 wk, g | 213±6a | 196±7b | 175±4c |
| Body weight gain 3 wk, g | 149±5a | 131±6b | 111±2c |
| Plasma leptin, ng/ml | 5.8±1.2a | 3.3±0.8b | 4.4±0.4ab |
Values are means ± SE. Male DIO rats were weaned onto HE diet and then remained sedentary for 3 wk (Sed; n = 12), were given access to a running wheel for 3 wk (Ex; n = 12), or were calorically restricted (Rstr) to 85% of the daily caloric intake of Sed rats for 3 wk (Rstr; n = 12). Leptin levels are from those rats assessed for leptin receptor binding.
Parameters with differing superscripts differ from each other at the P < 0.05 level by post hoc Bonferroni adjustment after significant intergroup differences were found by 1-way ANOVA.
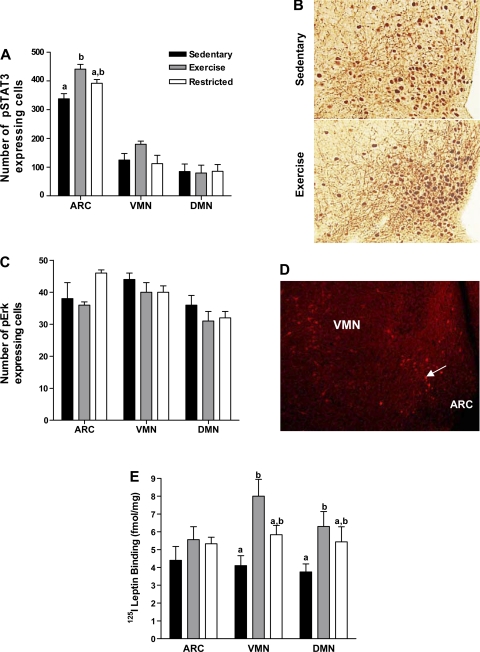
Following leptin administration, there was significant expression of pSTAT3 immunoreactivity in neurons within the ARC, VMN, and DMN of Sed, Ex, and Rstr rats. However, no immunoreactivity was present in the lateral hypothalamus. As previously reported, pSTAT3 expression was most prominent in the medial portion of the ARC where Lepr-b are localized (22). Exercise for 3-wk postweaning significantly increased the number of ARC pSTAT3 immunoreactive cells by 23% compared with those rats which remained sedentary (Fig. 3, A and B). Rstr rats had intermediate ARC pSTAT3 immunoreactivity following leptin administration compared with the Sed and Ex rats. Furthermore, there was no correlation between 3-wk body weight gain and the number of ARC pSTAT3 positive cells in any of the rats. Although there was moderate pSTAT3 expression of leptin-induced pSTAT3 within the VMN and DMN, the amount of this expression did not differ among Sed, Ex, and Rstr rats (Fig. 3A). Expression of pERK was also found in the ARC, VMN, and DMN following leptin administration; however, the quantity of this expression was similar among all of the groups (Fig. 3, C and D).
Fig. 3.
Experiment 2: male DIO rats that were weaned onto HE diet and remained sedentary (Sed; n = 6) were given access to a running wheel (Ex; n = 6) or calorically restricted to 85% of the daily caloric intake of the sedentary rats (Rstr; n = 6) for 3 wk. After a 2-h fast, rats were injected with leptin (5 mg/kg ip) at dark onset and perfused 30 min later. Brains were processed for hypothalamic phospho-STAT3 (pSTAT3) and phospho-ERK (pERK) immunocytochemistry. A: number of pSTAT3 positive cells within the hypothalamic arcuate nucleus (ARC), ventromedial nucleus (VMN), and dorsomedial nucleus (DMN) of Sed, Ex, and Rstr rats. B: pSTAT3 immunocytochemistry in the ventrobasalar hypothalamus of a Sed and Ex DIO rat viewed at magnification, ×20. C: number of pERK positive cells in the hypothalamic ARC, VMN, and DMN of Sed, Ex, and Rstr rats. D: pERK immunoreactivity in the ventrobasalar hypothalamus viewed at magnification, ×10. Arrow indicates pERK-positive cell. E: at the same time, an additional 6 Sed, Ex, and Rstr rats were decapitated at dark onset and their brains processed for 125I-labeled leptin receptor binding autoradiography. a,bBars for each given brain area with differing superscripts are significant from one another at P ⩽ 0.05 by post hoc analysis after intergroup differences were found by ANOVA. Data represent rats from Table 2.
Also following 3 wk, the brains from Sed, Ex, and Rstr rats were processed for 125I-leptin receptor binding autoradiography to assess the effects of exercise and caloric restriction on the ability of leptin to bind to its receptor. Overall, as we have previously found in adult rats (17), leptin binding was present in the ARC, VMN, and DMN, as well as in the ventral tegmental area, the substantia nigra pars compacta and the choroid plexus. While 125I-leptin receptor binding in the ARC did not differ among the groups, it was 95% and 68% higher in the VMN and DMN of Ex vs. Sed rats, respectively (Fig. 3E). Despite their decreased weight gain compared with both the Sed and Ex rats, VMN and DMN 125I-leptin binding in Rstr rats was intermediate between these groups and not significantly different from either (Fig. 3E). Furthermore, plasma leptin levels did not correlate with 125I-leptin binding in any of the rats, indicating that reduced leptin levels in Ex rats alone were not responsible for increased binding in the VMN and DMN.
Long-term effects of 3-wk postweaning exercise on sensitivity of rats to the anorectic, thermogenic, and activity-related effects of leptin.
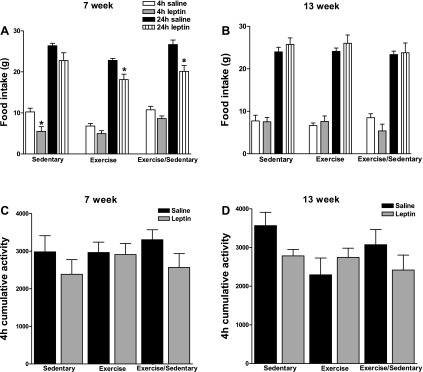
Given the sustained effect on lowering body weight gain and adiposity for up to 10 wk after 3 wk of exercise, we postulated that the enhanced leptin sensitivity seen at 3 wk would also be prolonged for up to 10 wk. Thus, during the 7th wk of postweaning exercise (4-wk postexercise termination in Ex/Sed rats), baseline 24-h food intake was similar among Sed, Ex, and Ex/Sed rats (Table 1; Fig. 4A). Whereas only the Sed rats decreased their food intake (by 46%) relative to their saline-treated baseline over the first 4 h, after leptin administration they compensated for this early decrease and had no overall reduction in food intake over 24 h following leptin administration. On the other hand, leptin produced a 20% reduction in 24-h intake in Ex rats and a 24% reduction in Ex/Sed rats compared with their respective saline-treated baselines (Fig. 4A). Similar to the results at 3 wk, there was no effect of leptin on motor activity in any of the groups (Fig. 4C). Of note were the findings that Ex and Ex/Sed rats weighed the same amount at 7 wk, gained the same amount of weight from 3–7 wk, and both of these parameters were significantly less than those in Sed rats (Table 1). However, after this 7-wk time point, Ex/Sed rats began to gain weight more rapidly than Ex rats but still maintained lower weight gain than Sed rats (Table 1, Fig. 1).
Fig. 4.
Experiment 1: male DIO rats were weaned onto HE diet and randomized into 3 groups as in Fig. 1. At 7 (A) and 13 wk (B), rats received 5 mg/kg ip leptin, and food intake was monitored at 4 and 24 h and compared with their saline-injected intake. Cumulative activity measured in arbitrary units from intraperitoneal telemetry probe during the 4 h following saline or leptin administration at 7 (C) and 13 wk (D). Data are means ± SE. *P ≤ 0.05 when food intake following leptin treatment was significantly reduced compared with food intake following saline administration. Data represent rats from Table 1.
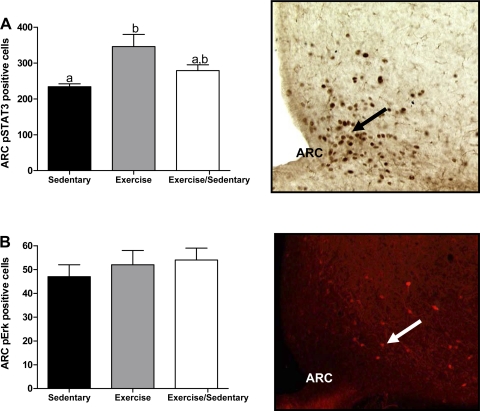
All rats were reevaluated again after 13 wk of experimental manipulations (10-wk postexercise termination in Ex/Sed rats). At this time, baseline 24-h food intake was similar among Sed, Ex, and Ex/Sed groups, and all of the rats were unresponsive to the anorectic effects of leptin (Fig. 4B), whereas activity remained unaffected by leptin in any of the groups (Fig. 4D). Despite this insensitivity to leptin's anorectic and activity-related effects, all rats displayed both pSTAT3 and pERK immunoreactivity in neurons of the hypothalamic ARC after leptin injection (Fig. 5, A and B). As at 3 wk, leptin produced 32% more pSTAT3-expressing neurons in the ARC of Ex vs. Sed rats (Fig. 5A). ARC pSTAT3 induction in Ex/Sed rats was intermediate between these two groups. The number of leptin-induced ARC pSTAT3 immunoreactive cells did not correlate with food intake at either 4 or 24 h following leptin administration in any of the groups. However, activation of pSTAT3 was greatly reduced and in many cases undetectable in the VMN and DMN of rats at 13 wk in all three groups. Given the small number of rats that did display pSTAT3 immunoreactivity in these nuclei, we could not statistically analyze these data. Finally, pERK expression following leptin-injection was almost entirely restricted to the ARC, and, contrary to leptin-induced pSTAT3, much of this immunoreactivity was visualized in the lateral portions of the nucleus (Fig. 5B). Also in contrast to hypothalamic leptin-induced pSTAT3, there were no differences among Sed, Ex, and Ex/Sed rats in pERK expression in the ARC following leptin treatment at 13 wk (Fig. 5B).
Fig. 5.
Experiment 1: following 13 wk, Sed, Ex, and Ex/Sed rats were injected with 5 mg/kg leptin ip and perfused 45 min later. A: hypothalamic ARC cells expressing immunoreactive pSTAT3. B: hypothalamic ARC cells expressing immunoreactive pERK. Cell number is expressed as means ± SE. a,bGroups with differing superscripts differ from each other at the P ≤ 0.05 level by post hoc Bonferroni adjustment after significant intergroup differences were found by one-way ANOVA. Data are from rats represented in Table 1.
Effect of 3 wk of postweaning exercise and caloric restriction on the development of the ARC neuropeptide Y/AgRP and α-MSH projections to the PVN in DIO rats (experiment 3).
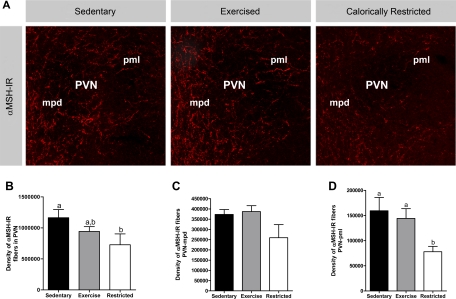
The inherent reduction in the signaling effects of leptin appears to play a role in the reduced innervation of the PVN by ARC neuropeptide Y (NPY)/AgRP and α-MSH neurons in DIO rats (7). Therefore, we postulated that the increased leptin sensitivity associated with postweaning exercise in DIO rats might correct these deficits. To test this hypothesis, groups of Sed, Ex, and Rstr rats were assessed after 3 wk as in the previous studies. Following 3 wk, all rats were killed, and their brains were processed for AgRP and α-MSH immunocytochemistry to determine the effect of postweaning exercise and caloric restriction on the development of these pathways extending from the ARC to PVN. AgRP (data not shown) and α-MSH (Fig. 6) fiber density did not differ between Sed and Ex rats. However, despite a lack of differences in PVN AgRP fiber density, rats calorically restricted for 3 wk postweaning had a 51% decrease in PVN α-MSH fiber density specifically within the posterior magnocellular subdivision of the PVN, compared with Sed controls (Fig. 6).
Fig. 6.
Experiment 3: Sed, Ex, and Rstr (n = 6–7/group) DIO rats were assessed after 3 wk on their respective treatments for AgRP and α-MSH immunocytochemistry. A: confocal images of α-MSH immunoreactive (α-MSH-IR) fibers innervating the paraventricular nucleus (PVN) of a Sed, Ex, and Rstr rat. Quantitative comparisons between groups were made of α-MSH immunoreactive fibers innervating the entire PVN (B), the medial parvocellular PVN (PVNmpd; C), and the posterior magnocellular (PVNpml; D). Values are means ± SE. a,bParameters with differing superscripts differ from each other at the P ≤ 0.05 level by post hoc Bonferroni adjustment after significant intergroup differences were found by 1-way ANOVA.
DISCUSSION
We originally postulated that our previous demonstration of the sustained effects of early onset exercise on obesity resistance after exercise cessation (29) was due to enhanced leptin sensitivity and/or correction of the inborn deficits in ARC-PVN pathway development (7). We found here that, while there was no correction of pathway development, the reduced body weight and adipose gain following 3 wk of exercise was associated with increased sensitivity to leptin's anorectic and thermogenic effects. These behavioral and physiologic improvements were associated with increased VMN and DMN leptin receptor binding and increased leptin-induced pSTAT3 expression in the ARC. While these data confirm the increased leptin sensitivity conferred by exercise (13), the most important finding is that the increased anorectic effect of leptin lasted for at least 4 wk after exercise cessation in association with a reduction in body weight gain comparable to that seen in rats that continued to exercise. However, by 10 wk after exercise cessation, neither the previously exercised, nor the continuously sedentary, or continuously exercising rats responded to leptin by reducing their food intake. Such leptin resistance is expected after prolonged exposure to a high-fat diet, even in obesity-resistant rats (36), and may be, in part, due to reduced leptin transport across the blood-brain barrier associated with the development of obesity on an HE diet in DIO rats (22). Interestingly, despite this resistance to the anorectic effects of leptin, all groups displayed leptin-induced hypothalamic pSTAT3 expression that was greater than the constitutive expression found in the absence of leptin stimulation (22). In addition, the continuously exercising rats maintained a significantly higher leptin-induced ARC pSTAT3 expression than did sedentary rats, while the Ex/Sed rats were intermediate in expression. Thus, while the increased sensitivity to the anorectic effects of a bolus injection of leptin associated with 3 wk of exercise was sustained for at least 4 wk after exercise cessation, this effect disappeared after 10 wk even though the body weight and adipose gain of these rats was still attenuated. Given these findings, one might predict that the gradual loss of enhanced leptin sensitivity over the postexercise period would eventually permit regain of body weight and adiposity to the level of sedentary rats if the Ex/Sed rats were kept on HE diet for long enough. In fact, this seems likely, since the previously exercised rats had regained more carcass adiposity by 10 wk than the continuously exercising rats. Such a regain, and our failure to demonstrate an exercise-induced correction of the inborn reduction of AgRP and α-MSH fibers innervating the PVN in selectively bred DIO rats (7), also speaks against any changes in neuronal circuitry that might perpetuate the lowering of body weight set point seen in continuously exercising DIO rats.
The finding that enhanced sensitivity to leptin's anorectic and thermogenic effects was associated with an increase in leptin binding to its receptor in the VMN and DMN, but increased leptin-induced pSTAT3 expression only in the ARC without enhanced binding in that nucleus, suggests that exercise might have differential effects on various aspects of leptin signaling in these neighboring hypothalamic nuclei. Obviously, there are multiple regulatory steps involved in leptin signaling that might be affected by a variety of manipulations or genetic differences. For example, we previously showed that preobese DIO rats have reduced Lepr-b mRNA expression in the ARC, but not the VMN or DMN, despite having significantly lower leptin-induced pSTAT3 expression (22) and leptin receptor binding in all three nuclei compared with DR rats (17). Similarly, we found no effect of 3 wk of exercise on hypothalamic Lepr-b mRNA expression in any of these nuclei (29), even though exercise did increase leptin-induced pSTAT3 expression and leptin binding here. One possibility for the apparent discrepancies between Lepr-b mRNA expression and leptin binding is that exercise might differentially affect receptor trafficking. Another is that 125I-leptin binds to all splice variants of the leptin receptor and is not selective for the long-form (Lepr-b) (18). As for differences in leptin binding and leptin-induced pSTAT3 expression, this could result from exercise-induced interleukin-6 enhancement of pSTAT3 expression without necessarily altering the number of cell surface leptin receptors or Lepr-b mRNA expression (13). Exercise might also improve leptin sensitivity by improving leptin blood-brain barrier transport, signaling through the phosphoinositol-3 kinase pathway (27) or by downregulating negative regulators of leptin signaling such as suppressor of cytokine signaling 3, protein tyrosine phosphatase-1B (3, 9), or OB-R gene-related protein that inhibits trafficking of Lepr-b to the cell surface (10). Our studies do suggest that exercise did not affect leptin-induced activation of the ERK-MAP kinase pathway. Finally, since we used only a single dose of leptin, it is possible that a higher dose might have revealed intergroup differences in the anorectic and downstream signaling effects of leptin.
Postweaning caloric restriction to 85% of the intake of sedentary rats on HE diet reduced body weight and body weight gain to a greater extent over 3 wk than did exercise. But this enhanced weight loss was not associated with significant improvement in leptin-induced hypothalamic pSTAT3 activation or 125I-leptin binding compared with sedentary rats. These results, and the lack of exercise-induced change in Lepr-b mRNA expression (29), differ from studies showing that fasting or caloric restriction improves leptin-signaling and increases Lepr-b mRNA expression in rats and mice (5, 6, 39). But caloric restriction in those studies was carried out in adult animals and was associated with reduced plasma leptin levels, which did not occur in the rapidly growing juvenile rats in the present study. Since we only assessed pSTAT3 and pERK immunoreactivity, we cannot rule out the possibility that postweaning caloric restriction increased leptin-sensitivity though the activation or suppression of a different intracellular signaling pathway of leptin. Along these lines, we previously showed that 3 wk of caloric restriction postweaning is associated with a reduction in suppressor of cytokine signaling 3 mRNA expression in the VMN and DMN that would be expected to increase leptin sensitivity (28). However, unlike exercise, caloric restriction for 3 wk postweaning in the current studies led to a decrease in α-MSH fiber innervation of the PVN that should produce a net increase in anabolic tone. These data, along with the fact that 3-wk postweaning caloric restriction did not reduce plasma leptin levels to the same extent as exercise, suggests that exercise acts in a unique, yet unidentified way, to modulate leptin signaling in selectively bred DIO rats fed a moderate fat diet from weaning.
There are some caveats to these findings that must be addressed. One is that there were differences in body weight and leptin levels between the experiments presented here and our previous studies (28) that we cannot fully explain. In that first study (28), Ex/Sed rats gained comparable amounts of body weight and adiposity to continuously exercising rats at 10 wk after the first 3 wk of exercise. It is likely that some of these differences could be due to the degree of exercise and sympathetic activation that occurred in each group and the fact that rats used for different experiments were not from the same generation. Although the selectively bred DIO rats used in these studies have maintained the same general body weight gain phenotype on HE diet for over 40 generations, we do see some differences in the rate and degree of weight and adipose gain on HE diet among generations. Furthermore, we have consistently found that DIO rats display a much greater spread in body weight gain and adiposity on an HE diet compared with selectively bred DR rats (21), and this variability may be the cause of some of the discrepancies presented here. Sed and Ex rats here also displayed inconsistencies in leptin levels. However, fat pad mass is likely to be a more reliable indicator of adiposity in exercising rats that may have had decreased leptin production due to exercise-induced sympathetic activation. Regardless of these differences, the overall effect of 3 wk of exercise in reducing weight and adipose gain between experiments followed the same overall pattern of protecting DIO rats consuming HE diet from becoming as obese as sedentary rats for up to 10 wk after exercise cessation.
A second issue was the surprising short-term (4 h) anorectic response to leptin of Sed rats after both 3 and 7 wk on HE diet, while neither Ex nor Ex/Sed rats demonstrated this short-term leptin-induced anorexia at 7 wk. On the other hand, only Ex and Ex/Sed rats had significant anorectic responses to leptin over 24 h at 7 wk. Arguably, this sustained anorectic response is likely to be more relevant to the physiological effects of leptin in regulating long-term energy homeostasis. At least after 3 wk of exercise, the increased 24 h anorectic response was associated with increases in parameters associated with enhanced leptin signaling, i.e., leptin-induced ARC pSTAT3 and VMN and DMN 125I-leptin receptor binding. In addition, an enhanced 24 h, but not 4-h leptin anorectic response was our primary finding under other circumstances associated with increased leptin sensitivity in postweaning DIO rats (14). This raises the important question of whether such enhanced leptin sensitivity is responsible for the obesity-resistance of the leptin-resistant DIO rats (7, 14, 17, 20, 22, 23) that were fed HE diet over the full 13 wk of these studies. The data suggest, but do not prove a direct link between leptin sensitivity and obesity resistance. Thus, Ex/Sed rats maintained the same rate of body weight gain as Ex rats for 4 wk after exercise cessation, and this was associated with comparably enhanced anorectic responses to leptin at that time. Unfortunately, we did not assess leptin signaling at this time so that there is no direct link of this in vivo response to leptin signaling. However, it was after that 7-wk time point that the body weight gain curves of Ex and Ex/Sed rats began to diverge. By 13 wk, when Ex/Sed rats had heavier carcass and adipose depots than Ex rats, they had mostly lost the greater leptin-induced ARC pSTAT3 expression seen in Ex compared with Sed rats at 3 wk. Therefore, the parallel temporal patterns suggest, but do no prove a causal link between a gradual loss of increased leptin sensitivity and partial regain of adiposity by 10 wk after exercise cessation. It is still possible that the Ex/Sed rats were more sensitive to the thermogenic effects of leptin than were Sed rats to explain their persistently reduced, although attenuated rate of body weight and adipose gain. Unfortunately, we could not test these responses because of technical problems.
In conclusion, even when fed a moderate fat diet, postweaning exercise attenuates the development of obesity in genetically predisposed DIO rats in association with increased leptin sensitivity. Importantly, both the obesity-resistance and enhanced leptin sensitivity persist for several weeks after exercise cessation, although the obesity resistance was attenuated and the anorectic response to leptin was eventually lost after 10 wk. This sustained effect is very different from what occurs in adult rats that rapidly regain lost weight following exercise cessation (2). Since the obesity-resistance in juvenile DIO rats outlived their enhanced leptin sensitivity, it may be that other factors associated with early onset exercise are responsible for the long-lasting improvement in energy homeostasis conferred by brief exercise exposure. While such exercise did not appear to correct their inborn defect in ARC-PVN neural pathway development (7), it is certainly possible that it might have altered other pathways involved in the regulation of energy homeostasis that we did not examine here. Additionally, even though there are important differences in the temporal patterns of hypothalamic pathway development between rats and humans (8, 15, 16, 30, 31), it is possible that early onset exercise in human beings might have a similar effect on inhibiting the development of obesity in obesity-prone children.
Perspectives and Significance
As we have previously demonstrated (29), 3 wk of postweaning exercise in rats selectively bred to develop DIO causes a sustained reduction in adiposity, despite cessation of exercise and continued intake of a 31% fat HE diet. Here we demonstrate that this 3 wk of exercise is associated with enhanced anorectic responsiveness and molecular signaling in response to peripherally administered leptin and that this increased anorectic sensitivity persists for several weeks beyond exercise termination. We postulate that this increase in exercise-induced leptin sensitivity provides initial protection against weight and adipose gain that is attenuated over time and likely involves other factors liberated by exercise, such as cytokines or fatty acids. Although the studies presented here indicate that postweaning exercise does not modify the development of terminal density of ARC AgRP or α-MSH fibers innervating the PVN, it is possible that such exercise might influence the formation of a particular subtype of glia or modulate the innervation of the DMN, the lateral hypothalmus, or other extrahypothalamic brain regions, from anabolic and catabolic axons originating the the ARC leading to a net increase in catabolic balance. Although it is tempting to extrapolate the prolonged increase in leptin sensitivity and protection from obesity found in our rats to the human situation, such comparisons are difficult because of the marked differences in the temporal patterns of brain development between humans and rodents (8, 15, 16, 30, 31). Although some brain development occurs during human childhood, the human hypothalamus is fully formed at birth. For this reason, even if early onset exercise might prevent the development of obesity in children, it is difficult to know at what age humans would have to begin exercising to obtain similar benefits, or even if such benefits are possible in humans as they are in rats. However, a few human studies have shown an effect of increased childhood activity on reduced propensity of later life obesity (12, 32). Even if the effects of exercise in rodents do not directly parallel the effects of exercise in humans, future identification of exercise-induced central and peripheral factors and how they interact to modulate energy homeostasis might aid in the identification of new targets for the pharmacological treatment of human obesity.
GRANTS
This work was supported by National Institutes of Health Grants DK-30066 (to B. E. Levin) and F31-NS-050903 (to C. M. Patterson) and by the Research Service of the Veterans Administration (to B. E. Levin and A. A. Dunn-Meynell).
Acknowledgments
We thank Charlie Salter, Sunny Park, and Antoinette Moralishvilli for expert technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American Physiological Society. Guiding principals for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Applegate EA, Upton DE, Stern JS. Exercise and detraining: effect on food intake, adiposity and lipogenesis in Osborne-Mendel rats made obese by a high fat diet. J Nutr 114: 447–459, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Banks AS, Davis SM, Bates SH, Myers MG Jr. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275: 14563–14572, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides 17: 305–311, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Baskin DG, Breininger JF, Bonigut S, Miller MA. Leptin binding in the arcuate nucleus is increased during fasting. Brain Res 828: 154–158, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle DS, Erickson JC, Palmiter RD, Schwartz MW. Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting. Diabetes 47: 538–543, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Bouret S, Gorski J, Patterson C, Chen S, Levin B, Simerly R. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7: 179–185, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304: 108–110, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell 2: 497–503, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Couturier C, Sarkis C, Seron K, Belouzard S, Chen P, Lenain A, Corset L, Dam J, Vauthier V, Dubart A, Mallet J, Froguel P, Rouille Y, Jockers R. Silencing of OB-RGRP in mouse hypothalamic arcuate nucleus increases leptin receptor signaling and prevents diet-induced obesity. Proc Natl Acad Sci USA 104: 19476–19481, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn-Meynell AA, Levin BE. Location and effect of obesity on putative anorectic binding sites in the rat brain. Obes Res 5: 201–207, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Epistien L Exercise in the treatment of childhood obesity. Int J Obes Relat Metab Disord 19: S117–S121, 1995. [PubMed] [Google Scholar]
- 13.Flores MB, Fernandes MF, Ropelle ER, Faria MC, Ueno M, Velloso LA, Saad MJ, Carvalheira JB. Exercise improves insulin and leptin sensitivity in hypothalamus of wistar rats. Diabetes 55: 2554–2561, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gorski JN, Dunn-Meynell AA, Levin BE. Maternal obesity increases hypothalamic leptin receptor expression and sensitivity in juvenile obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 292: R1782–R1791, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Grove KL, Allen S, Grayson BE, Smith MS. Postnatal development of the hypothalamic neuropeptide Y system. Neuroscience 116: 393–406, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav 79: 47–63, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Irani BG, Dunn-Meynell AA, Levin BE. Altered hypothalamic leptin, insulin and melanocortin binding associated with moderate fat diet and predisposition to obesity. Endocrinology 148: 310–316, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Levin BE, Dunn-Meynell AA. Maternal obesity alters adiposity and monoamine function in genetically predisposed offspring. Am J Physiol Regul Integr Comp Physiol 283: R1087–R1093, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283: R941–R948, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling prior to obesity onset. Am J Physiol Regul Integr Comp Physiol 286: R143–R150, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Levin BE, Dunn-Meynell AA, Ricci MR, Cummings DE. Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am J Physiol Endocrinol Metab 285: E949–E957, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Levin BE, Hogan S, Sullivan AC. Initiation and perpetuation of obesity and obesity resistance in rats. Am J Physiol Regul Integr Comp Physiol 256: R766–R771, 1989. [DOI] [PubMed] [Google Scholar]
- 25.Levin BE, Israel PA, Figlewicz Lattemann DP. Insulin selectively downregulates α2-adrenoceptors in the arcuate and dorsomedial nucleus. Brain Res Bull 45: 179–181, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413: 794–795, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Patterson C, Levin B. Role of exercise in the central regulation of energy homeostasis and in the prevention of obesity. Neuroendocrinology 87: 65–70, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol 294: R290–R301, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Rinaman L Postnatal development of catecholamine inputs to the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol 438: 411–422, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Rinaman L Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiol Behav 79: 65–70, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Schultz Y Role of physical inactivity in the etiology of obesity. Ther Umsch 281–290, 1989. [PubMed]
- 33.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte JD. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nature Med 2: 589–593, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarity A, Moore KJ, Smutko JS, Mays GG, Woolf EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell 83: 1263–1271, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Thomas SA, Preston JE, Wilson MR, Farrell CL, Segal MB. Leptin transport at the blood–cerebrospinal fluid barrier using the perfused sheep choroid plexus model. Brain Res 895: 283–290, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Tulipano G, Vergoni AV, Soldi D, Muller EE, Cocchi D. Characterization of the resistance to the anorectic and endocrine effects of leptin in obesity-prone and obesity-resistant rats fed a high-fat diet. J Endocrinol 183: 289–298, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR Jr. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 99: 385–390, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weigle DS, Duell PB, Connor WE, Steiner RA, Soules MR, Kuijper JL. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab 82: 561–565, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Wilsey J, Scarpace PJ. Caloric restriction reverses the deficits in leptin receptor protein and leptin signaling capacity associated with diet-induced obesity: role of leptin in the regulation of hypothalamic long-form leptin receptor expression. J Endocrinol 181: 297–306, 2004. [DOI] [PubMed] [Google Scholar]