Abstract
As central nervous system residents, mast cells contain many cytokines and are localized primarily near large blood vessels in the diencephalon and within the leptomeninges, making them candidates for immune to neural “cross talk.” Using mast cell-deficient KitW-sh/W-sh mice, we assessed the role of these cells in the thermoregulatory component of the immune response to lipopolysaccharide (LPS). KitW-sh/W-sh and wild-type (WT) mice differed in several respects in response to injection of a high dose of LPS (1 mg/kg ip). Core temperature (Tc) of WT mice decreased by ∼3°C, whereas KitW-sh/W-sh mice did not become hypothermic but instead exhibited pronounced low-frequency Tc oscillations around their baseline temperature. In addition, KitW-sh/W-sh mice had lower levels of whole brain TNF-α but no differences in IL-1β, IL-6, IFN-γ, or histamine compared with WT mice following injection of the high dose of LPS, consistent with the role of TNF-α in sepsis. KitW-sh/W-sh mice had increased resistance to LPS, and some survived a dose of LPS that was lethal in littermate controls. In contrast, KitW-sh/W-sh and WT mice were similar in other aspects, namely, in the hyperthermia following injection of TNF-α (1.5 μg icv), reduced nighttime Tc and locomotor activity (to 1 mg/kg LPS), response to a low dose of LPS (10 μg/kg ip), and response to subcutaneous turpentine injection. These results indicate that mast cells play a role in the regulation of thermoregulatory responses and survival following sepsis induction and suggest a brain site of action.
Keywords: tumor necrosis factor-α, interleukins, interferon-γ, histamine, mast cell-deficient sash mice, hypothermia
mast cells are implicated in the mediation of sepsis in a cecal ligation puncture model in mice (7, 24). Sepsis is characterized by an extreme bacterial infection leading to overstimulation of the immune system, which ultimately causes vasoconstriction leading to organ failure (2, 31). The response includes many effector systems, particularly those controlling energy homeostasis. In fact, the thermoregulatory response is a component of immune reactivity (33), and hypothermia is a reliable predictor of septic lethality (41).
Injection of gram-negative bacterial lipopolysaccharide (LPS) is commonly used to induce immune responses, which at high doses results in a septic response. LPS binds LPS-binding protein (LPB), forming a LPS-LPB complex. This complex then binds to Toll-like receptors (TLR) and complement (C′) receptors on cells of the innate immune system (5, 22). NF-κB-dependent transcription is subsequently upregulated, increasing production of cytokines and other immune effectors, including C′-mediated lysis for pathogen clearance (2). Unlike the hyperthermia induced by low doses of LPS, the mechanisms and physiological role of the hypothermia resulting from LPS-induced sepsis remain unclear (19). This hypothermic response to LPS, however, is dependent on ambient temperature (3). A dose of LPS that causes hypothermia at room temperature (∼21°C) elicits fever when injected in animals housed within their thermoneutral zone (∼31°C for C57BL/6 mice) (34). The decline in core body temperature (Tc) that occurs at room temperature, known as anapyrexia, is a regulated response involving the establishment of a new, lower set point in Tc (4) rather than an uncontrolled loss of body heat due to decreased metabolic heat production or hypotension or organ failure. Overproduction of cytokines [such as tumor necrosis factor (TNF)-α, interleukins (IL), and interferons (IFN), among many others], contribute to sepsis-induced lethality (31). In addition, the reduction in Tc further stimulates NF-κB-dependent cytokine production, thereby increasing the severity of sepsis (8).
Mast cells are part of the innate immune system and have long been implicated in allergy and local inflammation (27). It has been shown more recently that mast cells also have a critical and protective role in the initiation of an immune response to pathogens (reviewed in Ref. 21). In response to bacterial infection, mast cells increase the recruitment of neutrophils and aid in bacterial clearance (23). In mounting a host response against LPS, mast cells bind the LPS-LPB complex to their surface TLR-4 (39). Upon activation, mast cells synthesize and release mediators including amines, cytokines, and lipid-derived factors, many of which are implicated in the thermoregulatory response to LPS (20, 30, 37).
Mast cells are found at host-environment interfaces and in many vascularized tissues in the body (27). They also are resident in the brain and are localized to the diencephalon, hippocampus, and surrounding leptomeninges (14, 17, 37, 38). Specifically, many mast cells are found on the brain side of blood vessels adjacent to astrocytic and neuronal processes (15). Electrophysiological evidence suggests that mast cell mediators can influence neuron firing (16).
A link has been made between brain mast cells and the thermoregulatory response to LPS. Blocking the degranulation of brain mast cells with intracerebroventricular administration of disodium cromoglycate (cromolyn, a mast cell stabilizer) reduces the hyperthermia to a low dose of LPS in the rat (28). Because cromolyn also affects eosinophils and neutrophils and disrupts the signaling and chemotaxis of other immune cells (29), we sought to clarify the involvement of brain mast cells in the response to LPS-induced sepsis and to further investigate the underlying mechanisms.
In the present study we have used mast cell-deficient KitW-sh/W-sh “sash” mice, which serve as a powerful model for the study of mast cell function in vivo (42). KitW-sh/W-sh mice carry an inversion mutation upstream of the promoter region reducing c-kit receptor function (6, 18). The reduction in c-kit expression on stem cells in the bone marrow inhibits the myeloid progenitor from differentiating into mast cell precursors. This results in a mast cell deficiency but no other known disruptions in the development of hematopoietic stem cell derivatives (12). In addition, c-kit receptor mRNA and protein expression are normal in other tissues, including brain, liver, thymus, and primordial germ cells (6, 12). Thus the KitW-sh/W-sh mouse permits investigation of the role of mast cells in thermoregulatory responses to endotoxemia. We compared Tc, activity, cytokine profiles, and lethality responses of KitW-sh/W-sh and wild-type or littermate control mice to systemic and localized injection of three doses of LPS and turpentine.
METHODS
Animals and Housing
Homozygous male mast cell-deficient KitW-sh/W-sh mice, C57BL/Ka-Thy1.1-CD45.1 (Ly5.2) (Jackson Laboratory, Bar Harbor, ME) were bred to establish a colony at the Columbia University Animal Facility. For experiments 1 and 2, wild-type (WT) C57BL/6 mice (Charles River Breeding Facility, Charles River, MA) served as controls. For experiments 3–5, heterozygous and WT littermates of KitW-sh/W-sh mice served as controls.
All mice were housed individually from 10 wk of age in a 12:12-h light-dark cycle. Ambient temperature was 21 ± 0.5°C during the experiment. Purina rodent chow (5001) and water were available ad libitum. All husbandry procedures and experimental methods were reviewed and approved by the Columbia University Institutional Animal Care and Use Committee.
Transponder Implantation
Transponders (models ER-4000 or XM-FH; MiniMitter, Bend, OR) measuring Tc and gross motor activity were implanted surgically according to the manufacturer's directions. For implantation, mice were anesthetized with a ketamine (100 mg/kg)-xylazine (13 mg/kg) mixture administered by intraperitoneal (i.p.) injection. Buprenephrine (0.1 mg/kg) was also given intraperitoneally as an analgesic. The peritoneal cavity was opened and the transponder positioned within the cavity. The body wall was sutured closed, and the skin was autoclipped. Animals were monitored and allowed to recover for 1 wk before the start of the experiments.
Temperature and Activity Measurements
Activity and Tc data were collected every 2 min from each mouse. Activity and Tc data collected over the 3 days before experimental manipulations established baseline responses for within-animal comparisons. All mice were habituated to the handling and injection process with an intraperitoneal injection of pyrogen-free saline (PFS) at 2 h after lights on. Activity was measured as an index of sickness behavior. Furthermore, because activity itself raises body temperature, this measure provided assessment of thermal effects that are not activity dependent. Data were collected continuously starting from the preinjection baseline through 6 days following injection.
Intracerebroventricular Cannulations
For examination of the response to TNF-α, animals were allowed to recover from transponder implantation for 1 wk and then anesthetized and implanted stereotaxically (David Kopf Instruments, Tujunga, CA) with chronic indwelling cannulas (Plastics One, Roanoke, VA). The tip of the cannula was positioned 0.2 mm dorsal to the right lateral ventricle (coordinates: AP, 0.5 mm from bregma; ML, 1.2 mm; DV, 2.5 mm from skull). Screws (McMaster Carr, Princeton, NJ) were anchored to the skull, and dental cement (Jet Brand Acrylics; Lang Dental, Wheeling, IL) was used to secure the guide cannula to the skull. Animals were allowed to recover for 1 wk following cannulation.
Injections of TNF-α (1.5 μg/2 μl; Sigma Aldrich, St. Louis, MO) were made with a 10-μl Hamilton (Reno, NV) syringe attached to polyethylene-50 tubing. The injector cannula was 0.2 mm longer than the guide cannula. Cannula placement was verified following the experiment by injection of india ink.
Assays
Trunk blood was collected in K2EDTA tubes (BD, Franklin Lakes, NJ). Whole blood was spun at 3,000 g for 15 min, and plasma was collected into centrifuge tubes and stored at −80°C until processed. The brains were rapidly removed from the crania following decapitation and placed on ice for dissection. For assay of TNF-α, the cerebellum and cortex were removed. For all other assays, whole brains were used. The tissue was weighed, put in a solution of phosphate buffer with complete mini protease inhibitor tablets (Roche Diagnostics), homogenized using an electronic pestle, and then centrifuged at 7,500 g for 20 min at 4°C. The supernatant was stored at −80°C until processed.
TNF-α, interleukin (IL)-1β, IL-6, interferon (IFN)-γ, and histamine levels in plasma and central nervous system homogenates were analyzed using commercially available ELISA kits according to the manufacturer's directions (Ready-SET-Go ELISA; eBioscience, San Diego, CA for cytokines; Cayman Chemicals, Ann Arbor, MI for histamine). Plates were read on a Dynex spectrophotometer. Dynex Revelation software (v4.06) was used to fit optical densities to the curve generated by the standards provided by the manufacturer. Samples were diluted so that they fell within the linear portion of the standard curve. Brain cytokine and histamine levels are reported per gram of brain weight.
Experimental Design
Experiment 1: Systemic immune response.
To assess systemic responses, LPS was administered intraperitoneally at two doses. WT (n = 7) and KitW-sh/W-sh (n = 9) mice weighing 26.0 ± 0.4 and 26.6 ± 0.6 g, respectively, each received one intraperitoneal injection of PFS and 1 mg/kg LPS from Escherichia coli 0111:B4 (lot no. 104K4109; Sigma Aldrich) in a PFS vehicle in counterbalanced fashion. Injections were given 2 h after lights on. Three days intervened between the injections. A second group of WT (n = 9) and KitW-sh/W-sh (n = 10) mice weighing 23.2 ± 0.6 and 20.9 ± 0.3 g, respectively, were similarly injected with PFS and 10 μg/kg LPS.
Experiment 2: Local immune challenge.
After an interval of 2 wk, animals that had been given a low dose of LPS in experiment 1 were tested to assess their ability to respond systemically to a local immune challenge. Mice of each genotype, WT (n = 6) and KitW-sh/W-sh (n = 6), were given a subcutaneous injection of 0.15 ml of turpentine in the right posterior thigh. WT (n = 3) and KitW-sh/W-sh (n = 4) mice received an injection of the equivalent volume of saline. Activity and Tc were measured for 6 days following injection.
Experiment 3: LPS effects on cytokines and histamine.
To evaluate the levels of TNF-α, IL-1β, IL-6, IFN-γ, and histamine, KitW-sh/W-sh mice and littermate controls were injected with LPS (1 mg/kg) or saline 2 h after lights on. Two hours following injection, mice were rapidly decapitated and trunk blood and brains were collected for cytokine and histamine assays. For TNF-α assays, 4 KitW-sh/W-sh mice and 5 littermate controls were used; for IL-1β, IL-6, and IFN-γ assays, 13 KitW-sh/W-sh mice and 13 littermate controls were used; and for histamine assays, 9 KitW-sh/W-sh mice and 8 littermate controls were used.
Experiment 4: Central TNF-α administration.
To assess their ability to respond to TNF-α, KitW-sh/W-sh mice (n = 3) and their littermate controls (n = 3) received a 2-μl injection of PFS for habituation to injection. Three days later, each mouse was injected with 2 μl of PFS and TNF-α (1.5 μg/2 μl) in a counterbalanced order with a 3-day intercondition interval. All injections took place 2 h after lights on. Tc and activity were monitored as previously described.
Experiment 5: LPS-induced lethality.
For analysis of tolerance to septic lethality, KitW-sh/W-sh mice (n = 16) and littermate controls (n = 14) were injected intraperitoneally with LPS (15 mg/kg) or PFS (n = 6 mice per genotype) at 2 h after lights on. The mice were run in two cohorts, and the data from both runs were combined following statistical verification of no between-cohort differences. The status of the mice was monitored continuously for the first 8 h and thereafter as necessary at a minimum of 8-h intervals by using a clinical checklist that included parameters such as movement and posture as well as symptoms of illness such as mucus discharge or respiratory and gastrointestinal distress.
Data Analysis
Tc over the experimental period was quantified by calculating the area under the curve (AUC) for each animal's record. Two-way analysis of variance (ANOVA; 2 × 2: treatment × genotype) was performed on activity measures in the form of average counts per 15-min bin (cpb) and on AUC indices of Tc. Post hoc comparisons were done using Tukey's honestly significant difference, as appropriate. Oscillations in Tc were quantified using the spectrum function in the R statistical language. This calculates the power of the fast-Fourier transform (FFT) based on residuals from a regression line fit to the data. The maximum power and the frequency of that power were noted and compared using a two-way ANOVA (2 × 2: treatment × genotype). Survival curves from the LPS lethality experiment were compared between genotypes with a Mantel-Cox log-rank test. Differences were considered statistically significant when P < 0.05. Data are presented as means ± SE.
RESULTS
Experiment 1: Systemic Immune Response
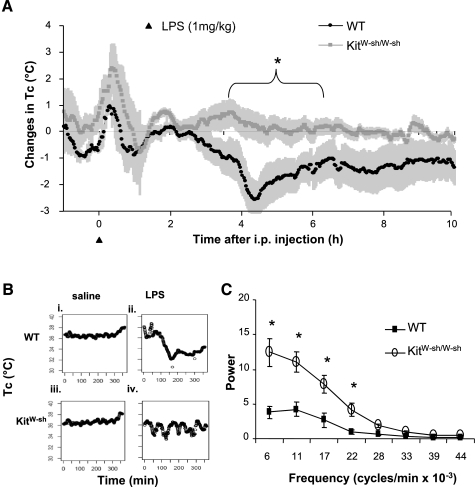
The response to an injection of LPS at 1 mg/kg ip for the 10 h following injection is shown in Fig. 1. Initially, all mice showed an injection stress-induced hyperthermia. Thereafter, WT mice exhibited a hypothermic response that was significantly different from baseline by ∼3.5 h after treatment, peaking at 2.3°C below baseline at 4.5 h (Fig. 1A; 34.3 ± 1.2 vs. 36.6 ± 0.3°C; P < 0.01). In contrast, KitW-sh/W-sh mice did not exhibit a hypothermic change in Tc, but rather maintained a mean Tc that was not significantly different from their preinjection baseline (36.1 ± 0.5 vs. 36.6 ± 0.2°C; P > 0.05).
Fig. 1.
A: effects of LPS-induced sepsis on core body temperature (Tc) in wild-type (WT) and homozygous mast cell-deficient (KitW-sh/W-sh) mice. Results are shown as change from preinjection baseline, using within-animal comparisons. Shaded regions represent SE. LPS (1 mg/kg) intraperitoneal (i.p.) injection (at time 0) induced an initial injection stress-related hyperthermia in all mice, with a return to baseline by ∼2 h. Subsequently, WT but not KitW-sh/W-sh mice showed a septic hypothermia peaking at ∼4.5 h. *P < 0.01. B: Tc of representative WT (i and ii) and KitW-sh/W-sh mice (iii and iv) following injection of saline (i and iii) or LPS (1 mg/kg; ii and iv). Low-frequency, high-amplitude oscillations are illustrated in a LPS-injected KitW-sh/W-sh mouse (iv). C: effects of LPS on the power of frequency bandwidths in the Tc response in WT and KitW-sh/W-sh mice. *P < 0.05.
FFT analysis of temperature profiles of individual mice revealed fluctuations in Tc of KitW-sh/W-sh but not in WT mice. As shown in representative records, the WT mouse exhibited a stable Tc profile following injection of saline (Fig. 1Bi) and a hypothermic response following LPS (Fig. 1Bii) as indicated above in the group data. The KitW-sh/W-sh mouse also showed a stable Tc profile following injection of saline (Fig. 1Biii). In contrast, following LPS, the KitW-sh/W-sh mouse showed high-amplitude (0.75°C), low-frequency (0.44 cycles/h) fluctuations of Tc around the baseline (Fig. 1Biv). The group means for the power of these low-frequency oscillations reveal that the Tc of KitW-sh/W-sh mice oscillated significantly more at lower frequencies than in WT mice (Fig. 1C).
Tc and activity levels in response to the same dose of LPS above, are shown over a 72-h interval in Fig. 2. Baseline conditions were recorded continuously for 3 consecutive days before injections; there were no significant differences between WT and KitW-sh/W-sh mice in Tc or locomotor activity, and data for the two groups were combined. Both groups showed disrupted long-term regulation of Tc and activity compared with baseline over 3 days following LPS injection. Specifically, WT and KitW-sh/W-sh mice lacked the normal nightly rise in Tc for 2 and 3 nights, respectively, following treatment (P < 0.01; Fig. 2A). Also, activity was reduced for 3 nights after injection in both WT and KitW-sh/W-sh mice (P < 0.01; Fig. 2B).
Fig. 2.
Effects of LPS injection (1 mg/kg ip) on Tc (A) and activity (B) in WT and KitW-sh/W-sh mice compared with baseline over 3 days. Both genotypes show attenuated nighttime rises in Tc and activity. *P < 0.01 vs. baseline in WT mice. †P < 0.01 vs. baseline in KitW-sh/W-sh mice.
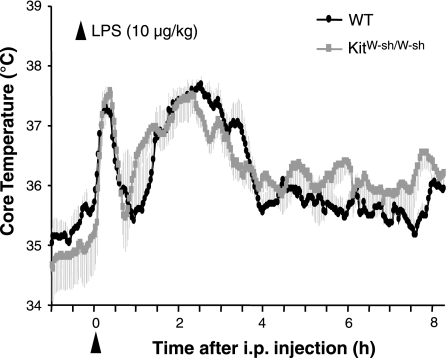
When injected with a low dose of LPS (10 μg/kg), there were no significant differences in Tc between WT and KitW-sh/W-sh mice (Fig. 3). Both groups showed injection-stress induced short-latency, short-duration hyperthermia and then a return to baseline within 1 h following injection. Subsequently, they exhibited an average increase in Tc of 1.2°C lasting 1–4 h after injection (P < 0.05; Fig. 3).
Fig. 3.
Tc in WT and KitW-sh/W-sh mice following LPS injection (10 μg/kg ip, at time 0). Shaded regions represent SE. Injection stress-induced hyperthermia is evident in both groups, followed by a return to baseline by 1 h. LPS results in a moderate hyperthermia peaking ∼2.5 h postinjection. The Tc response to this low dose of LPS is not different between genotypes (P > 0.05).
Experiment 2: Local Immune Challenge
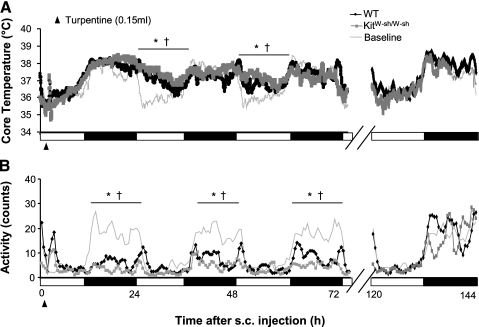
Examination of the systemic response to local immune challenge allowed assessment of other thermoregulatory immune mechanisms. There were no significant differences in Tc or locomotor activity between WT and KitW-sh/W-sh mice following subcutaneous turpentine administration for the 3 days after injection, in either the light or dark phase (Fig. 4A; range over days: P > 0.18 to 0.11). Both genotypes showed normal baseline circadian fluctuation in Tc (low in day and high at night) over the first day following injection. In the next 48-h interval, they showed a low-grade, long-term fever with elevated Tc during the light phase rather than their normal daytime troughs (range over days: P < 0.001 to .04 for WT and P < 0.001 to .002 for KitW-sh/W-sh). Both genotypes recovered to baseline Tc levels by the sixth day following turpentine injection (P > 0.05 for WT and KitW-sh/W-sh).
Fig. 4.
Responses induced by subcutaneous injection of turpentine (at time 0) in WT and KitW-sh/W-sh mice. Disruptions in Tc (A) and activity (B) are evident in both genotypes compared with baseline over the 3 days shown. By the 6th day, Tc and activity in WT and KitW-sh/W-sh mice returned to normal levels and were not different from baseline conditions. * P < 0.001–0.04 ( range over days) vs. baseline in WT mice. †P < 0.001–0.002 (range over days) vs. baseline in KitW-sh/W-sh mice.
All mice showed reduced nighttime activity, with a 3-day average of 7.9 ± 1.0 and 4.8 ± 0.6 cpb compared with baseline averages of 26.1 ± 0.9 and 21.3 ± 2.3 cpb for WT and KitW-sh/W-sh mice, respectively (Fig. 4B). Nighttime activity levels returned to normal on the sixth dark cycle following injection (P > 0.05 for WT and KitW-sh/W-sh).
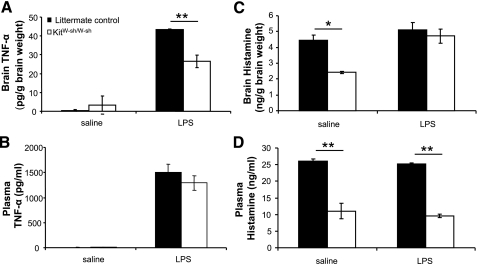
Experiment 3: LPS Effects on TNF-α, IL-1β, IL-6, IFN-γ, and Histamine Levels
Because of the observed differences in septic hypothermia, we measured brain and plasma levels of major mast cell derivatives known to mediate hypothermia (19, 20, 40) in littermate controls and KitW-sh/W-sh mice following LPS or saline injection (Fig. 5). For TNF-α, there were significant differences between groups: KitW-sh/W-sh mice had an attenuated rise in brain TNF-α levels 2 h after 1 mg/kg ip LPS injection (Fig. 5A; 43.4 ± 0.2 vs. 26.5 ± 3.2 pg/g of brain tissue for littermate controls and KitW-sh/W-sh, respectively; P < 0.01). There were no significant differences between littermate controls and KitW-sh/W-sh mice in brain TNF-α levels following injection of saline (0.3 ± 0.5 and 3.4 ± 4.8 pg/g of tissue, respectively). Examination of levels of plasma TNF-α revealed no significant differences between littermate control and KitW-sh/W-sh mice following saline or LPS injection (Fig. 5B; saline: 3.2 ± 4.5 and 9.5 ± 2.0 pg/ml; LPS: 1,506.5 ± 161.4 and 1,291.8 ± 142.0 pg/ml for littermate controls and KitW-sh/W-sh, respectively).
Fig. 5.
Effects of LPS (1 mg/kg) on brain and plasma levels of TNF-α (A and B) and histamine (C and D) in littermate control and KitW-sh/W-sh mice. For brain TNF-α (A), 2 h following injection of LPS, control mice showed a larger increase TNF-α compared with KitW-sh/W-sh mice. There was no difference in levels of brain TNF-α following saline injection (P > 0.05). Levels of plasma TNF-α (B) were not different between genotypes following saline or LPS injection. For histamine (C), KitW-sh/W-sh mice had lower brain levels following saline injection compared with littermate controls, as expected. However, following LPS injection, there were no significant differences in brain levels between KitW-sh/W-sh and control mice (P > 0.05). Plasma levels of histamine (D) were lower in KitW-sh/W-sh mice compared with littermate controls following saline and LPS injections. *P < 0.05; **P < 0.01.
Of the other cytokines measured, IL-1β, IL-6 and IFN-γ, none were different (P > 0.05) between genotypes in either brain or plasma following saline or LPS treatment (Table 1). For IL-1β and IL-6, there was a main effect of LPS treatment (P < 0.01) for both brain and plasma samples. There were no main effects of LPS treatment on plasma levels of IFN-γ (P > 0.05). However, there was a main effect of LPS treatment (P < 0.05) on brain levels of IFN-γ.
Table 1.
Cytokines in plasma and brain following saline or LPS administration in KitW-sh/W-sh and littermate control mice
| n |
IL-1β |
IL-6
|
IFN-γ
|
||||
|---|---|---|---|---|---|---|---|
| Plasma† | Brain† | Plasma† | Brain† | Plasma | Brain† | ||
| Control mice | |||||||
| Saline | 5 | 72.5±7.2 | 74.5±9.8 | 310.8±42.2 | 84.8±6.9 | 175.0±15.6 | 107.3±10.0 |
| LPS | 8 | 111.8±15.7* | 134.9±12.1* | 87,918.5±9,781.1* | 3,163.5±278.9* | 247.6±41.3 | 438.3±108.4 |
| KitW-sh/W-sh mice | |||||||
| Saline | 5 | 62.6±6.7 | 73.1±11.9 | 211.4±76.7 | 97.2±0.2 | 160.8±39.3 | 122.4±18.2 |
| LPS | 8 | 111.4±14.4* | 163.6±21.1* | 73,228±9,447.6* | 3,582.6±412.7* | 260.8±65.2 | 395.3±89.5 |
Values are means ± SE of ELISA determinations of cytokines in plasma (pg/ml) and brain samples (pg/g brain weight) following saline or LPS (1 mg/ kg) administration in mast cell-deficient (KitW-sh/W-sh) and littermate control mice.
P < 0.05, LPS vs. saline within genotype.
P < 0.05, main effect of LPS treatment within the measure.
As expected, brain and plasma levels of histamine were higher in control than in sash mice following saline injection (Fig. 5, C and D; P < 0.05 for brain, P < 0.01 for plasma). After LPS treatment, however, there were no differences in brain levels of histamine between genotypes (P > 0.05), although plasma levels were lower in sash mice (P < 0.01).
Experiment 4: Central TNF-α Administration
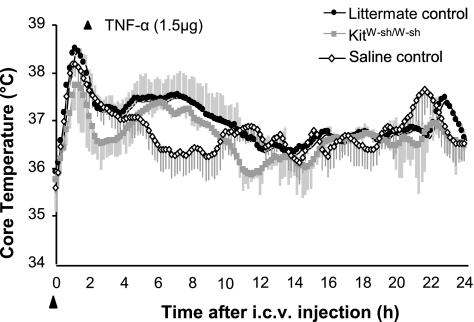
Given the differences in brain TNF-α levels between KitW-sh/W-sh mice and controls following LPS injection, we next examined the response to centrally injected TNF-α. There were no significant differences between littermate controls and KitW-sh/W-sh mice in the Tc responses to intracerebroventricular injection of TNF-α (Fig. 6). Both genotypes had a short latency rise in Tc, peaking at ∼1 h due to injection-induced hyperthermia (Fig. 6); this also was seen in saline-injected animals. Subsequently, both littermate control and KitW-sh/W-sh mice showed a TNF-α-induced rise in Tc, beginning ∼3 h following injection, with a return to baseline temperature by 12 h (P < 0.01 and 0.03 for control and KitW-sh/W-sh mice, respectively).
Fig. 6.
Tc following intracerebroventricular (i.c.v.) injection of TNF-α (1.5 μg) in littermate controls and KitW-sh/W-sh mice. Shaded regions represent SE. Both genotypes showed first an initial injection stress-related hyperthermia and then a moderate hyperthermia, peaking around 7 h postinjection (P < 0.01 for controls; P < 0.03 for KitW-sh/W-sh mice). There were no significant differences between control and KitW-sh/W-sh mice in the thermal response to TNF-α.
Experiment 5: LPS-Induced Lethality
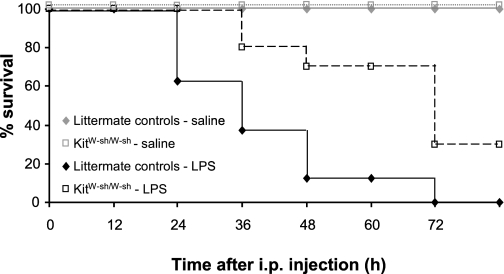
Given evidence showing that a reduction in core temperature is an indicator of mortality (41), we measured lethality to a 15 mg/kg dose of LPS in littermate control and KitW-sh/W-sh mice. Mast cell-deficient mice have increased resistance to LPS-induced septic lethality compared with their littermate controls (Fig. 7). Significantly more KitW-sh/W-sh mice survived (30%) than littermate controls (0% survival rate; P < 0.01). All mice injected with saline survived regardless of genotype.
Fig. 7.
LPS induced septic lethality rates in littermate control and KitW-sh/W-sh mice. Percent survival is plotted for both groups every 12 h following injection of saline or a high dose of LPS (15 mg/kg, at time 0) known to cause lethality. By 72 h postinjection, no WT mice survived compared with 30% survival of KitW-sh/W-sh mice (P < 0.01). There was no further change in survival after 72 h.
DISCUSSION
The differences between WT and KitW-sh/W-sh mast cell-deficient mice revealed in the present study implicate mast cells in the thermoregulatory response to LPS-induced sepsis, highlighting a novel element that contributes to the immune response during endotoxic shock. Specifically, we have shown that mast cells mediate the hypothermic response following induction of LPS-induced sepsis. KitW-sh/W-sh mice fail to develop the profound hypothermia seen in WT mice; instead, they show large Tc fluctuations around baseline. These fluctuations of Tc are not present in the control conditions in KitW-sh/W-sh mice. This hysteresis-like variability represents thermoregulatory competence. The results also show that in response to LPS-induced sepsis, KitW-sh/W-sh mice, like their WT counterparts, have reduced nighttime Tc and locomotor activity. In addition, KitW-sh/W-sh mice show an intact systemic immune response following either a low dose of LPS (10 μg/kg) or local turpentine injection. Together, these data demonstrate that KitW-sh/W-sh mice can detect LPS as a pathogen and mount appropriate responses. KitW-sh/W-sh mice have a smaller increase in brain TNF-α compared with littermate controls following high-dose injection of LPS. Nonetheless, their thermal response to central injection of TNF-α is not different from controls, indicating that they have the ability to respond appropriately to TNF-α. Finally, lethality caused by LPS-induced sepsis is attenuated in the absence of mast cells. Therefore, it is possible that both the lack of hypothermia and the smaller elevation in brain TNF-α is beneficial to the survival of KitW-sh/W-sh mice.
Mast cells produce numerous amine-, cytokine-, and lipid-derived mediators implicated in inflammation (25). We assayed TNF-α, IL-1β, IL-6, IFN-γ, and histamine and found differences between KitW-sh/W-sh and littermate controls in response to LPS in TNF-α alone. Not surprisingly, histamine levels were lower in KitW-sh/W-sh than in control mice following saline but did not differ between these groups in brain or plasma following LPS. The rise in brain histamine in KitW-sh/W-sh mice following LPS indicates involvement of a non-mast cell-derived source (14). It is unlikely that this rise in histamine mediates the thermoregulatory differences seen between genotypes because central histamine causes hypothermia (11), a response that is absent in the KitW-sh/W-sh mice.
Although it is improbable that TNF-α alone mediates the response observed, previous studies have implicated TNF-α in the cytokine storm that mediates the hypothermic response in sepsis (20). High circulating levels of TNF-α are correlated with reduction in Tc (20). Treatment with a soluble TNF-α receptor or TNF binding protein (which reduces the availability of circulating TNF-α) increases survival and attenuates the hypothermia following induction of sepsis (31, 40). However, these studies did not differentiate between central and peripheral sources and actions of TNF-α.
The present results point to the possibility of a role for mast cell-derived TNF-α in the brain, rather than in periphery, given that KitW-sh/W-sh mice show an attenuated rise in brain, but not plasma, TNF-α levels (Fig. 5A). This hypothesis is supported by evidence that mast cells in the brain upregulate TNF-α synthesis following immune activation (1) and that TNF-α injection in brain produces changes in Tc (see Fig. 6; reviewed in Ref. 19). Although the results do not identify the source of TNF-α, mast cell granules contain preformed TNF-α, enabling immediate release (10), and the timing of the response supports the possibility of a mast cell source. Other cells such as microglia or macrophages that produce TNF-α do so upon activation, requiring de novo synthesis and therefore resulting in longer response latency (36). Finally, the lack of differences in the plasma between genotypes does not point to a role of TNF-α in the periphery. In summary, the data support the hypothesis that mast cell-derived mediators are involved in LPS-induced septic hypothermia.
Although the lack of hypothermia in KitW-sh/W-sh mice may, in part, be attributed to reduced brain TNF-α levels, the hysteresis-like regulation of Tc around baseline likely reflects other thermoregulatory mechanisms. The high-amplitude, low-frequency oscillations in the Tc of KitW-sh/W-sh mice following LPS compared with saline controls could be mediated by various changes in the thermoregulatory system, both central and peripheral. One possibility is that KitW-sh/W-sh mice fail to monitor Tc normally, in that the temperature range in which thermal effectors are activated is enlarged (32). Given this scenario, it is plausible that a lack of mast cells is influencing peripheral and/or central thermosensors, thereby disrupting afferent information to central thermoregulatory centers (35). Another possibility is that KitW-sh/W-sh mice have disruptions in autonomic effectors, like blood vessel dilation, brown adipose tissue activation, or skeletal muscle shivering. If this is the case, imprecise effectors would lead to disruption of normal Tc regulation. Finally, firing of warm- and cold-sensitive neurons in the brain mediating autonomic responses required for precise thermoregulation around a set point could be altered by a lack of mast cell mediators (26). In this case, altered processing in thermoregulatory control centers would compromise efferent signals to thermal effectors of Tc. We favor this last hypothesis given both our results from brain TNF-α measures and the ability of mast cells to release other mediators implicated in central thermoregulation, such as serotonin and histamine (13, 37). Therefore, the Tc oscillations in KitW-sh/W-sh mice following LPS may be ascribed to altered efferent commands mediated by the lack of mast cells in the brain.
KitW-sh/W-sh mice have higher survival rates compared with littermate controls, supporting the conclusion that mast cells play a detrimental role during sepsis. However, the role of mast cells in sepsis is controversial (9). Our data are consistent with several converging lines of evidence supporting mast cells' cytotoxic role in host defense. One study showed that in the absence of a mast cell-derived cysteine protease (dipeptidyl peptidase I), mice are more likely to survive following onset of sepsis induced by cecal ligation and puncture (24). Other work suggests a detrimental, rather than protective, role of mast cells in sepsis using the W/Wv mast cell-deficient mouse, which differs from the KitW-sh/W-sh mouse in that it has other immune abnormalities and is anemic and infertile (12). Echtenacher et al. (7) reported that the W/Wv mouse is more susceptible than WT mice to the lethality caused by sepsis induced by cecal ligation and puncture. Reconstitution of these mice with mast cells from a WT donor provides protection for the host and reduces fatality.
Perspectives and Significance
These data support a broader role of mast cells in physiology beyond immediate hypersensitivity. Mast cells constitute a previously unknown agent in thermoregulatory control following an extreme bacterial infection. In addition, our results showing increased resistance to LPS-induced sepsis in KitW-sh/W-sh mice provide a potential therapeutic target in the clinical treatment of sepsis. Overall, these results contribute to the currently expanding known physiological functions of mast cells.
GRANTS
This work was supported by National Science Foundation Grant IOB-05-54514 (to R. Silver) and National Institute of Mental Health Grant MH067782 and MH075045 (to A. J. Silverman and R. Silver).
Acknowledgments
We thank Drs. Dave Krantz and Norma Graham for help with analysis of the temperature oscillations and Charles Liu for technical assistance. We also thank the late Dr. Evelyn Satinoff for advice and guidance throughout the project.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Cocchiara R, Bongiovanni A, Albeggiani G, Azzolina A, Geraci D. Evidence that brain mast cells can modulate neuroinflammatory responses by tumour necrosis factor-alpha production. Neuroreport 9: 95–98, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J The immunopathogenesis of sepsis. Nature 420: 885–891, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Derijk RH, Van Kampen M, Van Rooijen N, Berkenbosch F. Hypothermia to endotoxin involves reduced thermogenesis, macrophage-dependent mechanisms, and prostaglandins. Am J Physiol Regul Integr Comp Physiol 266: R1–R8, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Dogan MD, Ataoglu H, Akarsu ES. Characterization of the hypothermic component of LPS-induced dual thermoregulatory response in rats. Pharmacol Biochem Behav 72: 143–150, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Dunzendorfer S, Lee HK, Soldau K, Tobias PS. TLR4 is the signaling but not the lipopolysaccharide uptake receptor. J Immunol 173: 1166–1170, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Duttlinger R, Manova K, Chu TY, Gyssler C, Zelenetz AD, Bachvarova RF, Besmer P. W-sash affects positive and negative elements controlling c-kit expression: ectopic c-kit expression at sites of kit-ligand expression affects melanogenesis. Development 118: 705–717, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381: 75–77, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Fairchild KD, Singh IS, Patel S, Drysdale BE, Viscardi RM, Hester L, Lazusky HM, Hasday JD. Hypothermia prolongs activation of NF-κB and augments generation of inflammatory cytokines. Am J Physiol Cell Physiol 287: C422–C431, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Galli SJ, Wershil BK. The two faces of the mast cell. Nature 381: 21–22, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med 174: 103–107, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green MD, Cox B, Lomax P. Histamine H1- and H2-receptors in the central thermoregulatory pathways of the rat. J Neurosci Res 1: 353–359, 1975. [DOI] [PubMed] [Google Scholar]
- 12.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 167: 835–848, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjorth S Hypothermia in the rat induced by the potent serotoninergic agent 8-OH-DPAT. J Neural Transm 61: 131–135, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Hough LB Cellular localization and possible functions for brain histamine: recent progress. Prog Neurobiol 30: 469–505, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Khalil M, Ronda J, Weintraub M, Jain K, Silver R, Silverman AJ. Brain mast cell relationship to neurovasculature during development. Brain Res 1171: 18–29, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalil MH, Silverman AJ, Silver R. Mast cell mediators alter electrophysiological activity or rat thalamic neurons. In: Mast Cells in Physiology, Host Defense and Disease: Beyond IgE (C5). Silverthorne, CO: Keystone Symposia, 2004, p. 57.
- 17.Kiernan JA A comparative survey of the mast cells of the mammalian brain. J Anat 121: 303–311, 1976. [PMC free article] [PubMed] [Google Scholar]
- 18.Kitamura Y Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol 7: 59–76, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Leon LR Hypothermia in systemic inflammation: role of cytokines. Front Biosci 9: 1877–1888, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol Regul Integr Comp Physiol 275: R269–R277, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Leslie M Mast cells show their might. Science 317: 614–616, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Holers VM, Boackle SA, Blatteis CM. Modulation of mouse endotoxic fever by complement. Infect Immun 70: 2519–2525, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381: 77–80, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Mallen-St. Clair J, Pham CT, Villalta SA, Caughey GH, Wolters PJ. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J Clin Invest 113: 628–634, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall JS Mast-cell responses to pathogens. Nat Rev Immunol 4: 787–799, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda T, Hori T, Nakashima T. Thermal and PGE2 sensitivity of the organum vasculosum lamina terminalis region and preoptic area in rat brain slices. J Physiol 454: 197–212, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 77: 1033–1079, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Nava F, Caputi AP. Central effects of cromoglycate sodium salt in rats treated with lipopolysaccharide. Eur J Pharmacol 367: 351–359, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Norris AA Pharmacology of sodium cromoglycate. Clin Exp Allergy 26, Suppl 4: 5–7, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J Physiol 551: 945–954, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remick DG, Call DR, Ebong SJ, Newcomb DE, Nybom P, Nemzek JA, Bolgos GE. Combination immunotherapy with soluble tumor necrosis factor receptors plus interleukin 1 receptor antagonist decreases sepsis mortality. Crit Care Med 29: 473–481, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Romanovsky AA Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 292: R37–R46, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 10: 2193–2216, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol 289: R1244–R1252, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Satinoff E Neural organization and evolution of thermal regulation in mammals. Science 201: 16–22, 1978. [DOI] [PubMed] [Google Scholar]
- 36.Sawada M, Kondo N, Suzumura A, Marunouchi T. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res 491: 394–397, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Silver R, Silverman AJ, Vitkovic L, Lederhendler II. Mast cells in the brain: evidence and functional significance. Trends Neurosci 19: 25–31, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci 20: 401–408, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Supajatura V, Ushio H, Nakao A, Okumura K, Ra C, Ogawa H. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J Immunol 167: 2250–2256, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Tollner B, Roth J, Storr B, Martin D, Voigt K, Zeisberger E. The role of tumor necrosis factor (TNF) in the febrile and metabolic responses of rats to intraperitoneal injection of a high dose of lipopolysaccharide. Pflügers Arch 440: 925–932, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Vlach KD, Boles JW, Stiles BG. Telemetric evaluation of body temperature and physical activity as predictors of mortality in a murine model of staphylococcal enterotoxic shock. Comp Med 50: 160–166, 2000. [PubMed] [Google Scholar]
- 42.Wolters PJ, Mallen-St. Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient KitW-sh/KitW-sh sash mice. Clin Exp Allergy 35: 82–88, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]