the timing of physiological events is carefully controlled so that incompatible processes do not compete and complementary functions coincide. Given the pivotal role of the hypothalamus in homeostatic regulation, the discovery that a master circadian pacemaker resides in this region was not surprising. Soon after the discovery in 1972 that the suprachiasmatic nucleus (SCN) is indispensable for circadian rhythmicity (18), investigation of its anatomical organization intensified. A heterogeneous distribution of neuropeptides became apparent, with parvicellular vasopressinergic neurons concentrated in the dorsomedial region and cells containing vasoactive intestinal polypeptide (VIP) in the ventrolateral portion (14).
Over the past decade, remarkable progress has been made in uncovering the molecular mechanisms that govern pacemaker function (for a review, see Ref. 7). At the core of the mammalian circadian oscillator lies an intricate arrangement of feedback loops. In constant conditions, the transcription of the core clock genes Period and Cryptochrome is periodically activated by BMAL1:CLOCK dimers that bind E-box motifs. The protein products of Per and Cry act after an interval of several hours to block this transcriptional activation. Retinoic orphan receptors regulate Bmal1 expression both positively and negatively, and thus participate in the determination of the amplitude, phase, and period of circadian rhythms. Posttranslational events, including phosphorylation and ubiquitylation of the protein products of the core clock genes, also contribute to regulation of rhythmicity. Nevertheless, efforts to understand pacemaker function of the SCN have focused on operation of the core transcriptional-translational loop. Rhythmic Per expression in constant conditions is most prominent in the vasopressinergic cells of the dorsal SCN (5). In the VIP-rich ventral region, Per expression is regulated primarily by retinal input; circadian phase is set through activation at cAMP response element rather than E-box sequences. Although free-running rhythms of clock gene expression are not evident in the ventrolateral “core,” elimination of either VIP or its receptor VPAC2 severely compromises locomotor rhythmicity and circadian rhythms of electrical activity in the SCN (1, 13). This has drawn attention to VIP as a critical intra-SCN signal that maintains phase coherence of intrinsically rhythmic pacemaker neurons (Fig. 1) . Vasopressinergic cells in the dorsal SCN have received less of the glory; they have been regarded as downstream from the critical core of the pacemaker, rather than integral to coherence of cellular oscillations.
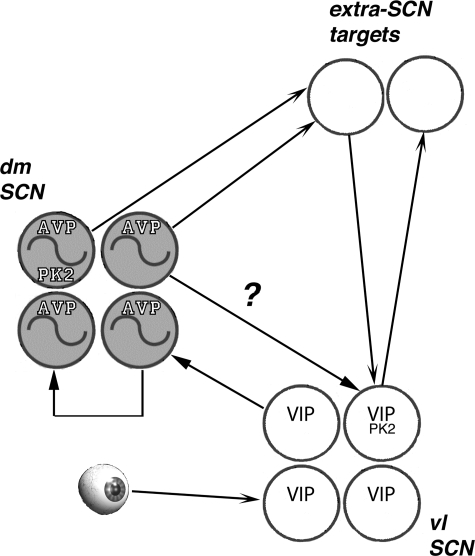
Fig. 1.
The retinorecipient ventrolateral core of the suprachiasmatic nucleus (SCN) contains a high concentration of neurons that express vasoactive intestinal polypeptide (VIP). These neurons have a critical role in coordinating rhythms of clock gene expression in the dorsomedial, vasopressin-rich shell of the SCN (dmSCN). About half of the VIP neurons and half of the arginine vasopressin (AVP) neurons also express prokineticin 2 (PK2), and all 3 of these peptides comprise SCN outputs that govern circadian rhythmicity in other brain regions through axonal projections, humoral release, or some combination of both of these routes. Extra-SCN structures may project back to the pacemaker to regulate its function. The gradual loss of circadian rhythms of locomotor behavior in V1a−/− mice reported by Li et al. (9) may reflect V1a-mediated action of AVP upon VIP neurons within the SCN (indicated by the ?), vasopressinergic regulation of PK2 production and release, and/or a role of V1a in receipt of vasopressinergic signals by extra-SCN targets. V1a, vasopressin receptor; Vl SCN, ventrolateral SCN.
In order for an animal to benefit from its accurate clock, the pacemaker must communicate with its targets. Although understanding of SCN outputs has advanced, attention to this question has been less intense than the focus on the core loops. Both vasopressinergic and VIPergic efferents have been described, and there is evidence for regulation of endocrine and temperature rhythms by axonal projections, including those that express these peptides (3, 6, 11, 15, 20). However, the outputs of the pacemaker may not be exclusively axonal. Silver et al. (17) found that encapsulated SCN grafts that do not send fibers to the host brain are competent to reinstate locomotor rhythms in animals previously made arrhythmic by lesions of the SCN. This evidence prompted a search for humoral outputs of the SCN. Rhythmic SCN-dependent release of vasopressin into the cerebrospinal fluid had already been demonstrated (16). Prokineticin 2 (PK2), which like VIP was originally described as a gut hormone, was shown to be produced largely during the subjective day and to suppress locomotor activity when administered intraventricularly (2). This prompted the hypothesis that PK2 might serve as a secretory signal of the SCN. Indeed, knockout of either PK2 or the PK2 receptor produced alterations in the activity pattern: although circadian rhythms persist and rhythmic expression of core clock genes continues, mice deficient in PK2 or PK2r lack the bout of activity normally evident in the early subjective night (10, 13). It may be significant that PK2 receptors are concentrated in regions (including the paraventricular thalamus, dorsomedial hypothalmus) that receive SCN efferent projections. Thus PK2, like vasopressin, may not act exclusively as a humoral signal. Other peptides (including cardiotrophin-like cytokine and TGF-α) may also play a role as SCN outputs; the extent to which they are delivered humorally vs. by axonal projections is unknown. In addition, the possibility of paracrine coordination of pacemaking function within the SCN is raised by the observation that circadian rhythmicity begins prior to the development of synapses in this structure (19).
The paper by Li et al. (9) may contribute to a renaissance of interest in vasopressin and help to establish its role not only as an output signal of the SCN, in its own right and in combination with PK2, but also as an important influence within the SCN. The authors first characterize vasopressin receptor (V1a) expression in wild-type mice, showing that the abundance of the mRNA free runs (interestingly, 180° out of phase with its ligand) and i.e., it is under the control of a circadian oscillator. V1a expression may also be regulated by light independently of the circadian clock; although its nighttime peak is blunted in Bmal1−/− mice, rhythms of V1a mRNA are not entirely absent when these mice are maintained on a light-dark cycle. Li et al. (9) next examined mice lacking V1a. These knockouts entrain to light-dark cycles in a normal fashion, and light presentation during the night suppresses (masks) their locomotor activity as it does in wild types. Rhythmic expression of the core clock genes is little altered in V1a-deficient mice on the third free-running cycle. After several weeks of transfer to constant darkness, however, fascinating effects of the elimination of V1a become apparent. Free runs of wheel running and home cage activity deteriorate to arrhythmicity in about 25% of the knockout mice. The reason for the variability in the effect of the knockout is not clear, but may be clarified when further inbreeding is done to obtain the deletion on a pure C57 background. Even V1a-deficient mice that do not become arrhythmic show increased cycle-to-cycle variability in the timing of activity onset, and the duration of the active phase (α) expands. The expansion of α in Li's knockout mice suggests that V1a may function in the coupling of substituent coupled circadian oscillators whose expression is normally restricted to the early or late subjective night. Behavioral arrhythmicity most likely results from gradual loss of oscillating expression of core clock genes, but this cannot be documented because of drift from zeitgeber time and the lack of phase reference points to determine circadian time by the time 4 wk have elapsed since transfer to constant darkness. Such findings have been reported in mice lacking genes regarded as more central to the circadian oscillator than is V1a; rhythmicity fades away only over the course of many cycles after Clock mutant mice are placed in constant darkness, and, as in the V1a knockouts, this is preceded by a lengthening of the free-running period. Loss of rhythmicity is also not immediate after transfer to constant conditions of animals deficient in either of the orthologs of Period or Cryptochrome. Taken together, the data of Li et al. (9) suggest that rather than serving exclusively as an output signal, vasopressin may play a role over the long term in coordinating oscillations, either of individual SCN cells or of cell populations. Significantly, Li et al. illustrate a distribution of V1a in wild-type mice that extends widely within the SCN, including the ventral region. Vasopressinergic input to VIP cells may thus contribute to their synchronizing effect within the SCN.
The findings of Li et al. (9) also suggest that, in addition to serving as an SCN output in its own right, vasopressin acts through the V1a receptor to boost transcription of PK2 and hence the availability of its peptide product to serve as an output signal. In the rat SCN, PK2 is expressed in about half of both the AVP and the VIP cells, although PK2r mRNA is found in neither (12). Thus the loss of precision, expansion of α, and arrhythmicity of the V1a knockout could result from altered output of PK2, VIP, or both. The nature of the action of any of these peptides on extra-SCN targets that regulate clock-controlled functions including (but perhaps not restricted to) locomotor activity is little understood; circadian oscillations certainly occur elsewhere in the brain (4) and the function of SCN signals may be to regulate their phase and period. Inputs to the SCN, perhaps representing reciprocation from clock-driven targets, may modulate circadian function (21). The role in such feedback of SCN target cells that make vasopressin or respond to it via the V1a receptor is unknown. Thus the extent to which deterioration of rhythmicity described by Li et al. (9) in V1a-deficient mice depends upon intra-SCN actions, as opposed to effects on targets of vasopressin that are addressed by humoral or axonal pathways, requires investigation. New tools that would allow targeted deletion of V1a in specific brain regions or cell types may prove useful in answering this question. Also to be determined is the extent to which these effects of V1a knockout are mediated by loss of rhythmic production of other output signals, including, but not necessarily restricted to, PK2.
The versatility of vasopressin continues to astound physiologists. In addition to its importance in regulation of hydration, blood pressure, body temperature, corticotropin release, memory, and sociosexual behavior, we may find that its role in circadian timing is more than merely to serve as an output of the clock.
GRANTS
Funding supporting preparation of this article was provided by National Institutes of Health Grants RO1-MH-070019 and R21-HL-086828.
REFERENCES
- 1.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 8: 476–483, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417: 405–410, 2002. [DOI] [PubMed] [Google Scholar]
- 3.de la Iglesia HO, Meyer J, Schwartz WJ. Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci 23: 7412–7414, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci 25: 3195–3216, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Hamada T, Antle MC, Silver R. Temporal and spatial expression patterns of canonical clock genes and clock-controlled genes in the suprachiasmatic nucleus. Eur J Neurosci 19: 1741–1748, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalsbeek A, Verhagen LA, Schalij I, Foppen E, Saboureau M, Bothorel B, Buijs RM, Pévet P. Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species. Eur J Neurosci 27: 818–827, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 15, Spec No 2: R271–R277, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signalling. Novartis Found Symp 253: 250–62, 2003. [PubMed] [Google Scholar]
- 9.Li JD, Burton KJ, Zhang C, Hu SB, Zhou QY. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am J Physiol Regul Integr Comp Physiol (December 3, 2008). doi: 10.1152/ajpregu.90463.2008. [DOI] [PMC free article] [PubMed]
- 10.Li JD, Hu WP, 1 Boehmer L, Cheng MY, Lee AG, Jilek A, Jerome Siegel JM M, Zhou QY. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci 26: 11615–11623, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci 21: 4864–4874, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masumoto KH, Nagano M, Takashima N, Hayasaka N, Hiyama H, Matsumoto S, Inouye ST, Shigeyoshi Y. Distinct localization of prokineticin 2 and prokineticin receptor 2 mRNAs in the rat suprachiasmatic nucleus. Eur J Neurosci 23: 2959–2970, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Maywood ES, O'Neill JS, Reddy AB, Chesham JE, Prosser HM, Kyriacou CP, Godinho SI, Nolan PM, Hastings MH. Genetic and molecular analysis of the central and peripheral circadian clockwork of mice. Cold Spring Harb Symp Quant Biol 72: 85–94, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Morin LP, Shivers KY, Blanchard JH, Muscat L. Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience 137: 1285–1297, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience 93: 659–666, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz WJ, Reppert SM. Neural regulation of the circadian vasopressin rhythm in cerebrospinal fluid: a pre-eminent role for the suprachiasmatic nuclei. J Neurosci 5: 2771–2778, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382: 810–813, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69: 1583–1586, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Pol AN, Dudek FE. Cellular communication in the circadian clock, the suprachiasmatic nucleus. Neuroscience 56: 793–811, 1993. [DOI] [PubMed] [Google Scholar]
- 20.van der Beek EM, van Oudheusden HJ, Buijs RM, van der Donk HA, van den Hurk R, Wiegant VM. Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-releasing hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology 134: 2636–2644, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Vansteensel MJ, Yamazaki S, Albus H, Deboer T, Block GD, Meijer JH. Dissociation between circadian Per1 and neuronal and behavioral rhythms following a shifted environmental cycle. Curr Biol 13: 1538–1542, 2003. [DOI] [PubMed] [Google Scholar]