Abstract
BRAF V600E is the predominantly occurring mutation of the cytoplasmic kinase BRAF, and, in colorectal cancer, its determination provides a diagnostic exclusion criterion for hereditary nonpolyposis colorectal cancer. The aim of our study was to develop a sensitive BRAF V600E high resolution melting (HRM) assay. We first established and optimized the BRAF HRM assay using a cell line dilution model, enabling us to detect 1% mutant DNA in a background of wild-type DNA. In a comparison, DNA sequencing and real-time allele-specific PCR in the cell line dilution model HRM assay proved to be more sensitive than DNA sequencing and denaturing high performance liquid chromatography, retaining the same sensitivity as real-time allele-specific PCR. In a learning set of 13 patients with known BRAF V600 status, the mutation was detected with high concordance by all four methods. Finally, we validated the HRM assay on 60 formalin-fixed, paraffin-embedded colorectal cancer samples. Although all mutated samples were correctly identified by HRM, the detection limit of the HRM assay decreased when using low-quality DNA derived from formalin-fixed, paraffin-embedded samples. In conclusion, HRM analysis is a powerful diagnostic tool for detection of BRAF V600E mutation with a high sensitivity and high-throughput capability. Despite the expected decrease in sensitivity, HRM can reliably be applied in archival formalin-fixed, paraffin-embedded samples tissues.
The cytoplasmic localized serine/threonine kinase BRAF is a part of the RAS/RAF/MAPK signal transduction pathway, which acts as a major mediator for cell growth in different human cells. An oncogenic hotspot mutation in the BRAF gene, the BRAF V600E mutation in exon 15, has been described in a variety of human cancers, especially melanoma, thyroid cancer, and colorectal cancer.1,2 In colorectal cancer, mutated BRAF kinase contributes to carcinogenesis by increasing resistance to apoptotic stimuli and promoting development of invasive phenotype.3 BRAF mutations have been associated with a poor survival in colon cancer.4,5 Given that the BRAF V600E mutation is exclusively found in malignant cells, this alteration provides a novel target for anticancer therapeutics, and BRAF inhibitors are currently under clinical investigation.6,7 In addition to the use of BRAF V600E as a potential predictive factor, detection of BRAF V600E mutation has been proposed as a diagnostic marker to distinguish sporadic microsatellite instable colon cancer from hereditary non-polyposis colorectal cancer (Lynch syndrome). The presence of BRAF V600E suggests a sporadic origin of microsatellite instable colorectal cancer and detection of this mutation, therefore has a potential to be used before time-consuming and expensive hereditary non-polyposis colorectal cancer testing.8,9 Until now, a variety of methods for detection of this mutation in colorectal cancer has been described, including single-strand conformation analysis, DNA sequencing, TaqMan-based real-time PCR, real-time allele-specific PCR, pyrosequencing, and oligonucleotide microarray.9,10,11,12,13,14,15 DNA sequencing, currently the gold standard for mutational analysis, is limited by high cost and low sensitivity. Furthermore, most of the mentioned assays are time-consuming and require manipulation of amplified PCR products, which is a common source of sample contamination. Pyrosequencing offers a sensitive alternative method, but the equipment is expensive and may not be economical for low-throughput laboratories. For Taqman-based real-time PCR analysis the major disadvantage is the need for expensive fluorescence-labeled probes.
High resolution melting (HRM) analysis is a recently developed molecular technique proved to be applicable for detection of various clinically relevant human mutations.16,17,18,19,20,21 It is a cost-efficient, closed-tube system that allows high-throughput analysis without any post-PCR processing, features that are desired characteristics for research and clinical application. The aim of our study was to develop a clinically useful BRAF V600E HRM assay and to critically compare the HRM-based assay with three commonly used mutation detection techniques. We used a cell line-based dilution model to establish and optimize a BRAF V600E HRM assay and, in a second step, compared the sensitivity of HRM with denaturing high performance liquid chromatography (DHPLC), real-time allele-specific PCR, and DNA sequencing. Finally, we validated the HRM assay using a collection of archival formalin-fixed paraffin-embedded (FFPE) colorectal cancer samples.
Materials and Methods
Cell Lines and Patient Samples
For optimization of the four different mutation detection methods, we used a cell line model system consisting of two human colon cancer cell lines (HT29 and SW480). The HT29 cell line harbors the heterozygous BRAF V600E mutation, and SW480 is homozygous for the wild-type allele. Cell lines were grown under standard conditions and genomic DNA was extracted using the Blood and Cell Culture DNA Midi Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. To optimize the sensitivity of the BRAF HRM assay, we used DNA extracted from HT29 mixed with DNA obtained from SW480 at the following dilutions: 100%, 20%, 10%, 5%, 1%, and 0% of tumor cell line DNA known to harbor BRAF mutation. For comparison of the analytical sensitivity of all four methods, the same dilutions were used. Results obtained by each method were assessed independently by three different investigators in a blinded fashion.
After completing the cell line experiments, we validated the BRAF HRM assay for its ability to detect the BRAF V600E mutation on routinely used FFPE blocks. Ethical approval for this research was obtained from the local ethical committee. DNA analysis was performed in two independent cohorts. The learning set consisted of 13 FFPE colon cancer tissues showing negative immunohistochemical staining for hMLH1 where 11 tissues were known to harbor BRAF V600E mutation. The training set involved 60 randomly selected colorectal carcinoma patients, ages 41 to 87 years (median 67), with histologically confirmed colorectal cancer. In the training set staging had been performed according to the International Union Against Cancer guidelines. There were 17 stage I, 11 stage II, 20 stage III, and 12 stage IV colorectal carcinomas. In all of the cases tumor areas were marked on the H&E-stained slides and corresponding unstained slides were microdissected so that samples contained at least 50% tumor cells. DNA was isolated from scraped material as described by Wu et al.22 DNA quantity was assessed spectrophotometrically and quality of genomic DNA was confirmed by agarose gel electrophoresis.
Where corresponding fresh frozen samples were available, we additionally analyzed DNA from cryopreserved tissue. DNA was extracted according to the QIAamp DNA micro kit protocol (Qiagen) and isolated DNA was quantified spectrophotometrically and stored at −20°C.
In an additional step, we evaluated the sensitivity of the HRM in FFPE samples. After the analysis of the training set was completed, we selected one sample where the BRAF V600E mutation was detected, and two independent wild-type samples. We prepared the same dilutions as in the cell lines. The BRAF V600E positive sample was mixed with the wild-type DNA at 100%, 20%, 10%, 5%, 1%, and 0%, respectively.
High Resolution Melting Analysis
PCR and HRM were consecutively done on a LightCycler 480 (Roche Diagnostics, Vienna, Austria) in one single run, and all reactions were performed in triplicate. Primers were selected to flank the BRAF V600E mutation. We evaluated two different amplicons for the ability to detect BRAF mutation. Primers for the 238 bp amplicon were Fw 5′-CATAATGCTTGCTCTGATAGGAAA-3′ and Rv 5′-TCAGCACATCTCAGGGCCAAA-3′ (primer sequences were obtained at May 2, 2008 from http://www.mutationdiscovery.com/md/MD.com/home_page.jsp) and for the shorter 147 bp amplicon were Fw 5′-GGTGATTTTGGTCTAGCTACAG-3′ and Rv 5′-AGTAACTCAGCAGCATCTCAGG -3′ (primers were designed with Primer3 software23). The melting behavior of the smaller 147-bp amplicon was designed to contain only a single melting domain by using Poland software.24 Each reaction mixture contained 1 μl of DNA solution (50 ng), 200 nmol/L of each primer, 10 μl of LightCycler LC480 High Resolution Melting Master (Roche), 3 mmol/L MgCl2, and water to a final volume of 20 μl. PCR conditions were: 95°C for 10 minutes, followed by 45 cycles of 10 seconds at 95°C, a touchdown of 64°C to 54°C for 10 seconds (1°C/cycle), and 20 seconds at 72°C. After amplification, the PCR product was denatured at 95°C for 1 minute and cooled down to 40°C to allow heteroduplex formation. The final HRM step was performed from 75°C to 95°C with an increase of 1°C per second with 25 acquisitions per degree. The HRM curve analysis was performed using the Gene Scanning Software (Roche). For sample analysis, melting curves were normalized, temperature-adjusted and, finally, a difference plot was generated. A wild-type control (ie, DNA from the wild-type cell line SW480) was used to normalize melting profiles of the other samples against this predefined horizontal baseline. Samples were considered mutated when significant difference of fluorescence level for all triplicates fell outside of the range of variation detected for the wild-type control.
To evaluate the influence of poor quality DNA from FFPE samples on the melting behavior of the amplicon, we compared the conventional Taq polymerase with a high fidelity proofreading polymerase (Optimase, Transgenomic). For this purpose, we performed for both enzymes a preamplification PCR followed by the above described HRM analysis. We used 100 ng of FFPE-derived genomic DNA in a 50 μl reaction volume for the two different preamplification approaches. For the preamplification experiment using Optimase, the reaction mixture contained 1 μl polymerase (2.5 U/μl Transgenomic Optimase Polymerase, Glasgow, UK), 5 μl reaction buffer (10× reaction buffer containing 15 mmol/L MgSO4, Transgenomic), 5 μl dNTPs (final concentration 0.2 mmol/L), and 5 μl (final concentration 0.5 μmol/L) of each primer (primers were the same as described above for the short amplicon). For the preamplification experiment using a commercially available TaqDNA polymerase, the reaction mixture contained 1.3 μl Taq polymerase (1 U/μl Fermentas TaqDNA polymerase, Fermentas GmbH, St. Leon-Rot, Germany), 5 μl reaction buffer (10× reaction buffer, Fermentas), 5 μl dNTPs (final concentration 0.2 mmol/L), and 5 μl (final concentration 0.5 μmol/L) of each primer (primers were the same as described above for the short amplicon). The incubation for both preamplifications was performed in a standard thermocycler at 95°C for 3 minutes, followed by 30 cycles of 95°C for 30 seconds, a touchdown of 64°C to 55°C (0.5°C/cycle) for 30 seconds, 72°C for 30 seconds, and a final extension step at 72°C for 7 minutes. The amplicon was checked on an agarose gel for unspecific bands, diluted 100,000 in distilled water and then subjected to the PCR and HRM as described above.
DHPLC
DNA from cell mixtures and patients were amplified before performing DHPLC analysis with the same primers as described for the 238-bp amplicon. A total of 50 ng of genomic DNA was amplified in a 50 μl reaction volume, containing 1 μl polymerase (2.5 U/μl Transgenomic Optimase Polymerase, Glasgow, UK), 5 μl reaction buffer (10× reaction buffer containing 15 mmol/L MgSO4, Transgenomic), 0.5 μl dNTPs (20 μmol/L stock solution), and 0.5 μl (50 μmol/L stock solution) of each primer. The reaction mixture was incubated in a standard thermocycler at 95°C for 5 minutes, followed by 37 cycles of 95°C for 30 seconds, a touchdown of 64°C to 50°C (1°C/cycle) for 30 seconds, 72°C for 30 seconds, and a final extension step at 72°C for 7 minutes. After amplification, the quality of the PCR product was confirmed on the agarose gel. To form heteroduplexes, the PCR product was heated to 95°C and cooled down slowly (−1°C/min) to 65°C followed by a fast cooling down to 4°C. Finally, the amplicon was analyzed by DHPLC (Transgenomic WAVE system 3500 with DNASep cartridge). Navigator software (Version 1.6.4, Transgenomic) was used to determine the optimal run conditions (melting temperature: 56.4°C, flow rate: 0.9 ml/min, no time shift) and empirical optimization was performed. Samples were considered mutated when an additional peak before the wild-type signal was detected.
DNA Sequencing
For sequence analysis, genomic DNA isolated from the identical samples as described above was initially amplified by a conventional PCR reaction. Briefly, primers described above for HRM and DHPLC were used to amplify the 238bp fragment. A 5-μl aliquot was analyzed on an agarose gel to verify the specificity of the product, and the remaining material was used for sequencing. All PCR products were purified with a PCR purification Kit (Qiagen) and directly sequenced from both sides using the BigDye terminator chemistry 3.1 (Applied Biosystems, Foster City, CA). Sequences were run on an ABI3730 automated sequencer (Applied Biosystems) and data were manually edited using SeqScape v.1.0 (Applied Biosystems).
Real-Time Allele-Specific PCR
Real-time allele-specific PCR was slightly modified from the previously published protocol by Jarry et al.12 Amplification conditions were adapted to the LightCycler 480 instrument (Roche Diagnostics, Vienna, Austria). Briefly, real-time PCR was performed in a final reaction volume of 20 μl containing 10 μl of SYBR Green I Master Mix (Roche), 1 μl of each primer (0.25 μmol/L each) and 1 μl of DNA solution (50 ng genomic DNA). Amplification was performed in a 96-well plate. Reaction conditions were 5 minutes at 95°C, followed by 50 cycles of 10 seconds at 95°C for denaturation, 20 seconds at 68°C for annealing, and 15 seconds at 72°C for extension. After the cycling process, a melting curve analysis was performed to exclude unspecific PCR products. Analysis of real-time allele-specific PCR was done as described in Jarry et al.12
Results
For establishment and optimization of the HRM methodology, we first used colon cancer cell lines with known BRAF V600E mutational status. The HT29 cell line, which carries a heterozygous 1799T>A (V600E) mutation, was mixed with wild-type DNA from SW480 cells in a serial dilution as described in the Materials and Methods section. Figure 1, A and C shows a normalized plot and a difference plot for the 238-bp amplicon. The HRM assay was able to reproducibly distinguish 5% of cell line DNA known to harbor BRAF V600E in a background of wild-type DNA. As the normalized plot shows, the melting curve of the 238-bp amplicon was biphasic, suggesting that there were two independent melting domains. To evaluate if this melting behavior influences the sensitivity of the HRM assay, we designed additional primers to generate a shorter amplicon that contained only one homogeneous melting domain (see Figure 1B) as predicted by the melting profile using Poland software.24 To test and compare the sensitivity for both 238-bp and 147-bp HRM assays we analyzed a dilution series of 100%, 20%, 10%, 5%, and 1% of cell line DNA known to harbor BRAF V600E in the background of wild-type DNA. Using the longer amplicon, we reliably detected as few as 5% of DNA from mutated cells in wild-type DNA, whereas using the shorter amplicon with only one melting domain, sensitivity increased to 1% mutated DNA in the background of 99% wild-type DNA (Figure 1C and 1D). To assess the performance of our BRAF HRM assay against DHPLC, DNA sequencing and real-time allele-specific PCR, we compared the results obtained from the analysis of the cell line dilutions with 100%, 50%, 20%, 10%, 5%, and 1%, as depicted in Figure 2A–C. In our comparison, DNA sequencing displayed the lowest sensitivity. Only 20% of cell line DNA known to harbor BRAF V600E could be detected with confidence by the presence of a small additional peak. Using DHPLC, the detection limit improved down to 10% mutated cell DNA in a background of 90% wild-type DNA, however, 5% mutated DNA could not be detected with this method (as indicated in Figure 2A). Real-time allele-specific PCR gave a positive result when 1% of the mutated cell line was analyzed (data not shown).
Figure 1.

Comparison of 238 bp (A and C) and 147 bp (B and D) amplicons containing the BRAF V600E mutation. Melting curves are shown in A and B difference plots are shown in C and D.
Figure 2.

Comparison of the sensitivities of DHPLC (A), DNA sequencing (B), and HRM assay (C) for the detection of the BRAF V600E mutation.
To evaluate the HRM assay on FFPE colorectal carcinoma tissues, we first used a learning set of 13 samples with a high frequency of the BFAF V600E mutation. By means of HRM, 11 BRAF V600E mutated samples could clearly be distinguished from the two wild-type samples by a prominent melting peak. The BRAF V600E mutation was detected in those 11 samples also by real-time allele-specific PCR and DNA sequencing, whereas two samples were wild-type tissues. In one case there was a discrepancy between DHPLC and the other three methods. While DHPLC categorized the sample as wild-type, it was classified as BRAF V600E mutant by HRM, real-time allele-specific PCR and DNA sequencing (see Table 1). The high concordance encouraged us to screen a larger data set of 60 FFPE colorectal cancer samples of unknown mutation status using the BRAF HRM assay. In 2 (3.3%) out of 60 patients the BRAF V600E mutation was detected for each triplicate by the Genescan software. Based on the results of our cell line dilution experiments and FFPE learning set, we decided to evaluate the results obtained with the HRM assay using the highly sensitive real-time allele-specific PCR assay and obtained identical results for samples containing the BRAF mutation. The two mutant BRAF V600E samples were detected within the range of 50% to 100% of diluted mutant DNA of our cell line model. This is depicted in Figure 3A together with wild-type samples from FFPE colorectal tissues. Figure 3A shows that variations of wild-type melting curves were detectable up to the melting curve of 20% mutant cell line DNA. When we compared the results of corresponding wild-type FFPE and fresh frozen samples, we also observed a higher degree of variation in the melting plots within FFPE tissues (Figure 3B). Thus, we additionally investigated whether a preamplification PCR with a proofreading polymerase could influence the HRM analysis on FFPE tissues. In comparison with the conventional Taq polymerase, we noticed a decrease in the spread of wild-type melting curves (Figure 3C). Figure 3A-C further indicates that not only the elevation of the curve discriminates the BRAF V600E mutant DNA from wild-type DNA, but also the position and the shape of the curve, since the peak of the curve generated from BRAF V600E mutant DNA is shifted to the right.
Table 1.
Comparison of HRM, DHPLC, Sequencing and Real-Time Allele-Specific PCR for the Detection of the BRAF V600E Mutation in the Learning Set of Archival Colorectal Cancer Samples
| Sample ID | HRM | DHPLC | Sequencing | Real-time allele-specific PCR |
|---|---|---|---|---|
| P2 | mt | mt | mt | mt |
| P13 | mt | mt | mt | mt |
| P15 | mt | mt | mt | mt |
| P16 | mt | mt | mt | mt |
| P17 | mt | mt | mt | mt |
| P19 | mt | wt | mt | mt |
| P20 | wt | wt | wt | wt |
| P22 | mt | mt | mt | mt |
| P23 | mt | mt | mt | mt |
| P24 | mt | mt | mt | mt |
| P26 | wt | wt | wt | wt |
| P30 | mt | mt | mt | mt |
| P45 | mt | mt | mt | mt |
mt = BRAF V600E mutant; wt = wild-type.
Figure 3.
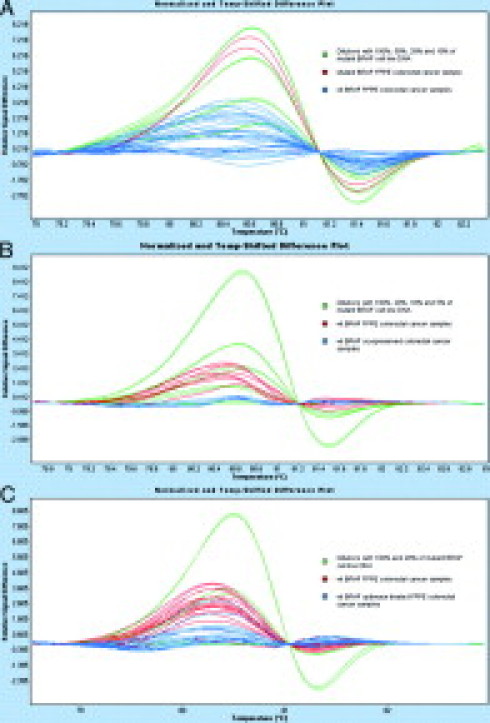
Influence of formalin-fixation and paraffin-embedding on HRM analysis. Green melting curves represent different dilutions of mutant DNA in wild-type cell line DNA. All samples and dilutions are displayed in duplicates. In (A) melting curves of 10 wild-type FFPE samples (blue) and one BRAF V600E mutant FFPE sample (red) are depicted. B: Comparison of four corresponding BRAF wild-type cryopreserved (blue) and FFPE tissues (red). C: Preamplification step for FFPE derived DNA with the proofreading polymerase Optimase versus preamplifcation of FFPE samples by conventionally used Taq polymerase.
To approximate the sensitivity of the HRM assay in FFPE tissues, we finally performed a dilution experiment where DNA from FFPE wild-type tissues was mixed with the DNA from samples where the BRAF V600E mutation was detected by HRM. In these experiments we were able to reproducibly detect 20%, 50%, and 100% of DNA with the BRAF V600E mutation in the background of wild-type DNA. There was a high degree of variation below the 20% of mutant BRAF V600E DNA, similar to what we already observed in FFPE samples without the proofreading polymerase.
Discussion
In the era of targeted therapy for cancer, molecular diagnosis of particular genetic markers in tumors enables a more individualized treatment of patients, as was recently shown for the KRAS mutation status and response rate to anti-EGFR therapy in colorectal cancer.25,26 Given the impact of BRAF mutation detection for cancer diagnosis,8 prognosis,4,5 and the potential predictive value,27 a reliable diagnostic test may affect clinical diagnosis and future therapeutic decisions. In this study, we demonstrate the utility of HRM analysis as a reliable and sensitive technology for the detection of the BRAF V600E mutation in colorectal cancer. First, we used a cell line-based model system to establish and optimize a BRAF V600E HRM assay using a 238-bp amplicon that contains two melting domains. According to previous studies, amplicon length could influence the sensitivity of genotyping, since short amplicon lengths have given better resolution of genotypes and increased the sensitivity of mutation detection.28,29 To design an amplicon with one single melting domain, we compared the melting curves of several amplicons of exon 15 in the BRAF gene by applying in silico DNA melting simulations using the program Poland before empirical testing. Based on this approach, we designed a shorter 147-bp amplicon and tested sensitivity for both amplicons in the cell line-based dilution experiment. Consistent with previously published reports, the shorter amplicon increased the sensitivity of mutation detection down to 1% mutated DNA in the background of wild-type DNA. Therefore, for development of optimal HRM assays, short amplicons and in silico melting experiments may be a useful strategy. In the comparison of HRM with DNA sequencing and DHPLC, respectively, neither sequencing nor DHPLC were able to detect 5% mutated cell DNA. The advantage of HRM to detect even 1% of cell line DNA known to harbor BRAF V600E may be of importance when only tissues with a low proportion of tumor cells are available. The determination of the significance of low level mutated DNA detection, however, was not the subject of the present study. In a comparative study, Chou et al30 have already shown that the diagnostic sensitivity of HRM is better than DHPLC. In our experience, DHPLC is a more expensive technique and requires more technical expertise and staff training for routine implementation. In addition to the higher sensitivity, another advantage of HRM is the closed-tube technique. The sensitivity of HRM was comparable to real-time allele-specific PCR,12 both methods being able to detect 1% of mutated DNA.
One critical issue for the HRM assays in the detection of mutations from tumor tissues is the optimization of the PCR reaction. For the clear discrimination of mutated samples from wild-type samples it is important to melt a highly specific PCR product. The presence of primer dimers, unspecific PCR products or variations in salt concentrations, and the presence of inhibitors may contribute to aberrations in melting curve behavior. This is critical for the analysis of FFPE tissues, in which DNA is often chemically modified by the formalin fixation process.31 Do et al have recently shown false positive results by performing HRM analysis of KRAS and epidermal growth factor receptor mutations in FFPE lung cancer samples.32 Their results have underlined the importance of using shorter amplicons for FFPE tissue analysis to enhance reliability of HRM assays. To address the question whether formalin fixation of tissue influences the results of our BRAF assay, the performance of HRM was tested in comparison with the real-time allele-specific PCR, DNA sequencing and DHPLC in the learning set, and showed consistent results. Consequently, we selected the real-time allele-specific PCR12 as the reference method for the analysis on further FFPE colorectal cancer samples. Both methods identified the BRAF V600E mutation in the same two samples in our training set (3.3% of all samples). Although this percentage is low, we are confident that HRM detected all samples with BRAF mutation, since tissue samples contained at least 50% tumor cells and the sensitivity of real-time allele-specific PCR is reported to be 2%.12 Also, the comparison of our result with previously published reports is limited due to the heterogeneity of cohorts and methodological differences.1,33 Based on our long-term experience, microsatellite instability is present in less than 10% of regionally diagnosed colorectal cancers (unpublished data). Thus, the number of patients with BRAF V600E mutation in the collection of patients analyzed in the present study is also expected to be lower. We, therefore, consider 2/60 patients within the margin of error, given the low total number of patients. However, the determination of the number of cases carrying the BRAF V600E mutation was not the scope of our study.
For analyzing HRM data, we used difference plots to visualize melting profiles. We have chosen one sample from the center of the distribution of the wild-type curves to construct a horizontal baseline as a standard for melting curve analysis. Interestingly, when we assayed FFPE tissue and the corresponding fresh frozen samples, we observed a higher degree of variation of the melting curves of the formalin-fixed wild-type samples, as compared with those of the cryopreserved samples. As a consequence, sensitivity of mutation detection in FFPE samples decreases as the risk of false positive results rises. This confounding effect seen for FFPE samples may be due to the failure of Taq polymerase to recognize modified bases and therefore amplify more artifacts than true mutations.34 We tested this hypothesis by performing a short preamplification step with a high fidelity proofreading polymerase (Optimase). Muhr et al showed that using Optimase for DHPLC led to a significant reduction of background heteroduplices, thus facilitating interpretation of elution profiles.35 As a result of this experiment, the spread of the melting curves in the group of wild-type samples decreased, so that discrimination between wild-type and mutated samples improved. However, as we observed from the analysis of normalized melting curves of individual samples even without proofreading polymerase, the curves from samples with the BRAF V600E mutation differed from the wild-type curves and the position of the peak was shifted to the right. This feature represents the additional discriminating power of the normalized melting curves.
In conclusion, we have developed a BRAF V600E mutation HRM assay as a sensitive and specific diagnostic tool. We demonstrated that the HRM method is more sensitive than DNA sequencing and DHPLC for the detection of the BRAF mutational status, and comparable to real-time allele-specific PCR. The validation in the FFPE colorectal cancer tissues showed accurate mutation detection when compared with real-time allele-specific PCR, although not being as sensitive as compared with results obtained for high-quality DNA from fresh-frozen samples. As a low cost, fast, closed-tube method, it represents a compelling alternative to established mutation detection techniques.
Footnotes
Supported by the Austrian National Bank Fund (grant 12680 to N.D.) and by the Austrian Ministry for Health, Family and Youth (project GZ 70420/0134-IV/B/12/2006 to G.H.).
M.P. and M.B. contributed equally to this work.
References
- 1.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Ueda M, Toji E, Nunobiki O, Izuma S, Okamoto Y, Torii K, Noda S. Mutational analysis of the BRAF gene in human tumor cells. Hum Cell. 2008;21:13–17. doi: 10.1111/j.1749-0774.2008.00046.x. [DOI] [PubMed] [Google Scholar]
- 3.Preto A, Figueiredo J, Velho S, Ribeiro AS, Soares P, Oliveira C, Seruca R. BRAF provides proliferation and survival signals in MSI colorectal carcinoma cells displaying BRAF(V600E) but not KRAS mutations. J Pathol. 2008;214:320–327. doi: 10.1002/path.2295. [DOI] [PubMed] [Google Scholar]
- 4.Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, Wolff RK, Slattery ML. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 5.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, Giovannucci EL, Fuchs CS. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oikonomou E, Pintzas A. Cancer genetics of sporadic colorectal cancer: BRAF and PI3KCA mutations, their impact on signaling and novel targeted therapies. Anticancer Res. 2006;26:1077–1084. [PubMed] [Google Scholar]
- 7.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, Sproesser K, Li L, Smalley KS, Fong D, Zhu YL, Marimuthu A, Nguyen H, Lam B, Liu J, Cheung I, Rice J, Suzuki Y, Luu C, Settachatgul C, Shellooe R, Cantwell J, Kim SH, Schlessinger J, Zhang KY, West BL, Powell B, Habets G, Zhang C, Ibrahim PN, Hirth P, Artis DR, Herlyn M, Bollag G. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domingo E, Laiho P, Ollikainen M, Pinto M, Wang L, French AJ, Westra J, Frebourg T, Espin E, Armengol M, Hamelin R, Yamamoto H, Hofstra RM, Seruca R, Lindblom A, Peltomaki P, Thibodeau SN, Aaltonen LA, Schwartz S., Jr BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J Med Genet. 2004;41:664–668. doi: 10.1136/jmg.2004.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domingo E, Niessen RC, Oliveira C, Alhopuro P, Moutinho C, Espin E, Armengol M, Sijmons RH, Kleibeuker JH, Seruca R, Aaltonen LA, Imai K, Yamamoto H, Schwartz S, Jr, Hofstra RM. BRAF-V600E is not involved in the colorectal tumorigenesis of HNPCC in patients with functional MLH1 and MSH2 genes. Oncogene. 2005;24:3995–3998. doi: 10.1038/sj.onc.1208569. [DOI] [PubMed] [Google Scholar]
- 10.Benlloch S, Paya A, Alenda C, Bessa X, Andreu M, Jover R, Castells A, Llor X, Aranda FI, Massuti B. Detection of BRAF V600E mutation in colorectal cancer: comparison of automatic sequencing and real-time chemistry methodology. J Mol Diagn. 2006;8:540–543. doi: 10.2353/jmoldx.2006.060070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikehara N, Semba S, Sakashita M, Aoyama N, Kasuga M, Yokozaki H. BRAF mutation associated with dysregulation of apoptosis in human colorectal neoplasms. Int J Cancer. 2005;115:943–950. doi: 10.1002/ijc.20957. [DOI] [PubMed] [Google Scholar]
- 12.Jarry A, Masson D, Cassagnau E, Parois S, Laboisse C, Denis MG. Real-time allele-specific amplification for sensitive detection of the BRAF mutation V600E. Mol Cell Probes. 2004;18:349–352. doi: 10.1016/j.mcp.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Kim IJ, Kang HC, Jang SG, Ahn SA, Yoon HJ, Park JG. Development and applications of a BRAF oligonucleotide microarray. J Mol Diagn. 2007;9:55–63. doi: 10.2353/jmoldx.2007.060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogino S, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: possible associations with male sex and KRAS mutations. J Mol Diagn. 2006;8:582–588. doi: 10.2353/jmoldx.2006.060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spittle C, Ward MR, Nathanson KL, Gimotty PA, Rappaport E, Brose MS, Medina A, Letrero R, Herlyn M, Edwards RH. Application of a BRAF pyrosequencing assay for mutation detection and copy number analysis in malignant melanoma. J Mol Diagn. 2007;9:464–471. doi: 10.2353/jmoldx.2007.060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandersteen JG, Bayrak-Toydemir P, Palais RA, Wittwer CT. Identifying common genetic variants by high-resolution melting. Clin Chem. 2007;53:1191–1198. doi: 10.1373/clinchem.2007.085407. [DOI] [PubMed] [Google Scholar]
- 17.Holden JA, Willmore-Payne C, Coppola D, Garrett CR, Layfield LJ. High-resolution melting amplicon analysis as a method to detect c-kit and platelet-derived growth factor receptor alpha activating mutations in gastrointestinal stromal tumors. Am J Clin Pathol. 2007;128:230–238. doi: 10.1309/7TEH56K6WWXENNQY. [DOI] [PubMed] [Google Scholar]
- 18.Kennerson ML, Warburton T, Nelis E, Brewer M, Polly P, De Jonghe P, Timmerman V, Nicholson GA. Mutation scanning the GJB1 gene with high-resolution melting analysis: implications for mutation scanning of genes for Charcot-Marie-Tooth disease. Clin Chem. 2007;53:349–352. doi: 10.1373/clinchem.2006.080010. [DOI] [PubMed] [Google Scholar]
- 19.Willmore-Payne C, Holden JA, Layfield LJ. Detection of epidermal growth factor receptor and human epidermal growth factor receptor 2 activating mutations in lung adenocarcinoma by high-resolution melting amplicon analysis: correlation with gene copy number, protein expression, and hormone receptor expression. Hum Pathol. 2006;37:755–763. doi: 10.1016/j.humpath.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Willmore-Payne C, Holden JA, Tripp S, Layfield LJ. Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol. 2005;36:486–493. doi: 10.1016/j.humpath.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Simi L, Pratesi N, Vignoli M, Sestini R, Cianchi F, Valanzano R, Nobili S, Mini E, Pazzagli M, Orlando C. High-resolution melting analysis for rapid detection of KRAS. BRAF, and PIK3CA gene mutations in colorectal cancer. Am J Clin Pathol. 2008;130:247–253. doi: 10.1309/LWDY1AXHXUULNVHQ. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Patten N, Yamashiro CT, Chui B. Extraction and amplification of DNA from formalin-fixed, paraffin-embedded tissues. Appl Immunohistochem Mol Morphol. 2002;10:269–274. doi: 10.1097/00129039-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Rozen S, Skaletsky HJ. Primer 3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa, NJ: 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 24.Steger G. Thermal denaturation of double-stranded nucleic acids: prediction of temperatures critical for gradient gel electrophoresis and polymerase chain reaction. Nucleic Acids Res. 1994;22:2760–2768. doi: 10.1093/nar/22.14.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 26.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 27.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krypuy M, Newnham GM, Thomas DM, Conron M, Dobrovic A. High resolution melting analysis for the rapid and sensitive detection of mutations in clinical samples: kRAS codon 12 and 13 mutations in non-small cell lung cancer. BMC Cancer. 2006;6:295. doi: 10.1186/1471-2407-6-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liew M, Pryor R, Palais R, Meadows C, Erali M, Lyon E, Wittwer C. Genotyping of single-nucleotide polymorphisms by high-resolution melting of small amplicons. Clin Chem. 2004;50:1156–1164. doi: 10.1373/clinchem.2004.032136. [DOI] [PubMed] [Google Scholar]
- 30.Chou LS, Lyon E, Wittwer CT. A comparison of high-resolution melting analysis with denaturing high-performance liquid chromatography for mutation scanning: cystic fibrosis transmembrane conductance regulator gene as a model. Am J Clin Pathol. 2005;124:330–338. doi: 10.1309/BF3M-LJN8-J527-MWQY. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002;161:1961–1971. doi: 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Do H, Krypuy M, Mitchell PL, Fox SB, Dobrovic A. High resolution melting analysis for rapid and sensitive EGFR and KRAS mutation detection in formalin fixed paraffin embedded biopsies. BMC Cancer. 2008;8:142. doi: 10.1186/1471-2407-8-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 34.Williams C, Ponten F, Moberg C, Soderkvist P, Uhlen M, Ponten J, Sitbon G, Lundeberg J. A high frequency of sequence alterations is due to formalin fixation of archival specimens. Am J Pathol. 1999;155:1467–1471. doi: 10.1016/S0002-9440(10)65461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muhr D, Wagner T, Oefner PJ. Polymerase chain reaction fidelity and denaturing high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;782:105–110. doi: 10.1016/s1570-0232(02)00695-5. [DOI] [PubMed] [Google Scholar]


