Abstract
Fluorescence in situ hybridization (FISH) testing is used to detect bladder cancer in urine specimens. The purpose of this study was to determine whether there are associations between the percentage of chromosomally abnormal cells by FISH and time to bladder cancer recurrence and progression to metastasis. Clinical records were searched to identify patients with urine FISH results, history of non-invasive bladder cancer, and at least one follow-up pathological diagnosis. Covariates analyzed included age, gender, smoking status, treatment after FISH, cystoscopy result, and prior stage of bladder cancer. The percentage of abnormal cells (hazard ratio [HR] 1.03, 95% CI 1.02–1.03; P < 0.001), age (HR 1.02, 95% CI 1.00–1.03; P = 0.033), male gender (HR 0.60, 95% CI 0.41−0.87; P < 0.001), treatment (HR 0.37, 95% CI 0.25−0.55; P < 0.001), and history of TIS/T1-stage tumors (HR 1.66, 95% CI 1.23–2.24; P = 0.001) were significantly associated with time to cancer recurrence. Time to invasive cancer was significantly associated with the percentage of abnormal cells (HR 1.02, 95% CI 1.01, 1.03; P < 0.001), history of TIS/T1 tumor (HR 3.73, 95% CI 1.88, 7.38; P = 0.001), and treatment (HR 0.33, 95% CI 0.13, 0.83; P = 0.019), suggesting that the percentage of abnormal cells independently predicts cancer recurrence and progression to invasive disease in patients with a history of non-invasive bladder cancer.
Of the estimated 67,160 newly diagnosed bladder cancers in 2007, approximately 70% were non-muscle invasive tumors.1,2 Unfortunately, up to 70% of patients with a history of non-muscle invasive bladder cancer will have recurrent tumor(s) after initial treatment. In addition, approximately 10% to 30% of these tumors will progress to muscle invasive disease, which significantly increases the risk of cancer-related death.1,3
Fluorescence in situ hybridization (FISH) is a technique that utilizes fluorescently labeled DNA probes to detect chromosomal abnormalities in cells. Studies have shown that FISH, using the UroVysion probe set, has similar specificity to and better sensitivity than routine cytology for detecting bladder cancer in urine.4,5,6,7,8 However, almost all studies to date have only qualitatively assessed (positive or negative) the ability of FISH to detect tumor and predict tumor recurrence. Limited data suggest that assessing FISH quantitatively may help predict which patients will recur and progress to muscle-invasive disease.9 However, there are no studies that have assessed a large cohort of patients to determine whether a relationship exists between the percentage of abnormal cells by FISH and the likelihood of bladder cancer recurrence and progression to muscle-invasive disease. If a relationship between percent abnormal cells and tumor recurrence and muscle-invasive disease is observed, the percentage of abnormal cells by FISH may help clinicians determine which patients to treat more aggressively and which patients to treat with more conservative therapies.
Materials and Methods
A total of 303 patients with FISH diagnoses of negative (n = 100) or positive (n = 203) with a mean and median follow-up time of 450 and 189 days (range, 1 to 2166 days) were included in this study. Demographic and clinical features of patients based on FISH groupings are shown in Table 1.
Table 1.
Patient Data by FISH Groupings
| Percent abnormal FISH groupings |
||||||
|---|---|---|---|---|---|---|
| Variables | 0%* | 1%–4% | 5%–10% | 11%–30% | ≥31% | All patients |
| Number of patients (%) | 100 (33) | 44 (15) | 46 (15) | 46 (15) | 67 (22) | 303 (100.0) |
| Sex N (%) | ||||||
| Male | 79 (79) | 37 (84) | 39 (85) | 42 (91) | 61 (91) | 258 (85) |
| Female | 21 (21) | 7 (16) | 7 (15) | 4 (9) | 6 (9) | 45 (15) |
| Age (Years) | ||||||
| Mean | 67 | 71 | 71 | 74 | 72 | 70 |
| Median | 69 | 72 | 74 | 76 | 73 | 72 |
| Range | 33, 94 | 53, 86 | 50, 86 | 51, 90 | 49, 92 | 33, 94 |
| Smoking | ||||||
| Unknown | 1 (1) | 0 (0) | 0 (0) | 2 (4) | 0 (0) | 3 (1) |
| Current | 10 (10) | 4 (9) | 9 (20) | 4 (9) | 10 (15) | 37 (12) |
| Previous | 53 (53) | 27 (61) | 25 (54) | 22 (48) | 38 (57) | 165 (54) |
| Nonsmoker | 36 (36) | 13 (30) | 12 (26) | 18 (39) | 19 (28) | 98 (32) |
| Therapy† | ||||||
| Yes | 26 (26) | 15 (34) | 11 (24) | 8 (17) | 10 (15) | 70 (23) |
| No | 74 (74) | 29 (66) | 35 (76) | 38 (83) | 57 (85) | 233 (77) |
| Cystoscopy‡ | ||||||
| Positive | 12 (12) | 9 (20) | 6 (13) | 13 (28) | 20 (30) | 60 (20) |
| Negative/equivocal | 60 (60) | 25 (57) | 24 (52) | 15 (33) | 26 (39) | 150 (49) |
| No Result | 28 (28) | 10 (23) | 16 (35) | 18 (39) | 21 (31) | 93 (31) |
| Prior stage | ||||||
| Ta | 69 (69) | 26 (59) | 25 (54) | 13 (28) | 26 (39) | 159 (52) |
| CIS | 15 (15) | 6 (14) | 12 (26) | 20 (43) | 19 (28) | 72 (24) |
| T1 | 14 (14) | 9 (20) | 8 (17) | 10 (22) | 15 (22) | 56 (18) |
| Unknown§ | 2 (2) | 3 (7) | 1 (2) | 3 (7) | 7 (10) | 16 (5) |
| Cancer on FU | 34 (34) | 21 (48) | 32 (70) | 38 (83) | 63 (94) | 188 (62) |
| Muscle-Invasive cancer on FU | 5 (5) | 5 (11) | 4 (9) | 9 (20) | 24 (36) | 47 (16) |
Negative FISH cases.
Therapy after FISH and prior to recurrent bladder cancer detection (eg, maintenance BCG, fulguration of erythematous lesions, etc.).
Same day as FISH cystoscopy result.
Patients with medical record stating history of non-muscle invasive bladder cancer but no prior stage listed in records.
All patients selected for this study were originally evaluated using the commercially available UroVysion probe set (Abbott Molecular Inc., Des Plaines, IL), which consists of fluorescently labeled DNA probes to the pericemtromeric regions of chromosome 3 (red), 7 (green), 17 (aqua), and to the 9p21 band (gold), location of the P16 tumor suppressor gene. The protocol used in our clinical laboratory to identify polysomic cells (ie, cells with gains of two or more of the four probes) with the UroVysion probe set utilizes the test package insert method. This retrospective cohort study was approved by the Mayo Clinic Institutional Review Board.
Positive FISH Population
Medical records were reviewed to identify patients that had: 1) a positive urine FISH result (ie, ≥4 or more cells demonstrating a polysomic signal pattern) diagnosed between January 2001 and December 2005; 2) a history of non-muscle invasive bladder cancer and no pathological evidence of muscle-invasive disease before their FISH analysis; 3) a minimum of one pathological (cytology or histopathology) diagnosis assessing for bladder cancer recurrence at the time of the initial FISH result or on subsequent patient follow-up; and 4) institutional research authorization. Some patients had more than one FISH analysis during the time frame of the study. However, this study only assessed associations between the first FISH result and time to tumor recurrence and progression to muscle-invasive disease.
Percentage of Polysomic Cells by FISH
All patients with a polysomic FISH result at our institution also have a percentage of abnormal cells by FISH included in their clinical report. The percentage of abnormal cells reported is the percentage of 100 consecutive urothelial cells with polysomic signal patterns (ie, cells with gains of two or more of the four chromosomal targets). Non-urothelial cells (eg, squamous cells or neutrophils) are not included in the denominator of this calculation. In rare situations when the number of total urothelial cells on the slide is less than 100, the percentage of positive cells among the total number of urothelial cells identified is recorded. It should be noted that the percent abnormal and the actual number of abnormal cells within the specimen are different. It is the absolute number of cells not a percentage that determines if a case is positive. Thus, if four or more polysomic cells are found on the slide, regardless of the total number of cells on the slide, the case is considered positive. However, among cases that are considered positive (ie, have four or more abnormal cells), the fraction of cells that show FISH abnormality can range from 1% to 100%. Patients with a negative FISH result are recorded as having 0% abnormal cells by FISH.
Negative FISH Population
The patients with positive FISH results, who fulfilled the criteria described above (n = 203), were entered into an Excel database. In addition, 100 patients with negative FISH results during this same period were also entered into this database to serve as a negative control group. The patients with negative FISH results were selected in an attempt to match the amount of clinical follow-up for patients with negative and positive FISH results.
Statistics
Bladder cancer and muscle invasive bladder cancer free survival were estimated using the Kaplan-Meier method.10 The primary end point for recurrent bladder cancer was cytologic or histopathological detection of bladder cancer (any stage or grade) on the same day as the FISH result or on subsequent follow-up. The primary end point for muscle invasive bladder cancer was pathological evidence of a stage ≥T2 tumor of the bladder or upper urinary tract or metastatic bladder cancer (eg, nodal or distant metastases). Possible factors affecting outcomes (ie, bladder cancer-free survival) that were assessed included the percentage of abnormal cells by FISH, age, race, smoking status (nonsmoker, previous, current), treatment following FISH and before pathological detection of cancer (yes or no), highest stage of bladder cancer before FISH result (TIS, Ta, or T1), and the same day as FISH cystoscopy result (negative, equivocal, or positive). Over 99% of patients were Caucasian. Therefore, race was excluded from further statistical analyses because the population was almost entirely homogeneous for that variable. The percentage of abnormal cells was assessed both as a continuous variable and as a grouped variable (0%, 1% to 4%, 5% to 10%, 11% to 30%, and 31% to 100% abnormal). The four pre-determined positive FISH groupings (1% to 4%, 5% to 10%, 11% to 30%, and 31% to 100%) were selected based on the fact that historical data at our institution showed that approximately 25% of all patients with polysomic results had abnormal percentages that fell within one of these four percent abnormal categories. Univariate relationships between potential prognostic factors and outcome were assessed using a log rank or Cox proportional hazards models, as appropriate. After assessment of univariate relationships, multiple Cox proportional hazard modeling was performed. Potential predictive factors were considered significant at P ≤ 0.05.
Results
Descriptive Statistics
The pathological and clinical follow-up of patients is shown in Table 1. One-hundred eighty-eight of the 303 (62%) patients were diagnosed with recurrent bladder cancer. The percentage of patients that were diagnosed with bladder cancer appeared to be related to the increase in the percentage of abnormal cells with only 34% and 48% of patients in the 0% and 1% to 4% grouping eventually having recurrent tumor compared with 70%, 83%, and 94% of patients in the 5% to 10%, 11% to 30%, and 31+% groupings, respectively. Similar results were observed in the diagnosis of muscle invasive bladder cancer in which only 5%, 11% and 9% of patients in the 0%, 1% to 4%, and 5% to 10% groupings were eventually diagnosed with muscle invasive disease compared with 20% and 36% of patients in the 11% to 30% and ≥31% categories, respectively. Two-hundred ten of the 303 (69%) patients had a same day as FISH cystoscopy result with the majority (150/210, 71%) of those cases being diagnosed as negative or equivocal for tumor. Seventy of the 303 patients (23%) had treatment (intravesical/chemotherapy, n = 34; fulguration, n = 25; or intravesical/chemotherapy and fulguration, n = 11) after the FISH result and before pathological detection of bladder cancer.
Assessment of Recurrent Bladder Cancer
Kaplan-Meier analysis revealed a significant association between percentage abnormal cell groupings and time to tumor recurrence (P < 0.001; Figure 1A) with higher tumor percentage groupings showing a shorter time to tumor recurrence. For example, at 1 year post-FISH, we see that the percentage of patients with a cancer diagnosis ranged from approximately 25% in the 0% FISH abnormal category to 90% in the >30% abnormal category. Kaplan-Meier analysis also revealed a decreased time to tumor recurrence in patients that were female (P = 0.046; Figure 1B), had a positive same day as FISH cystoscopy result (P < 0.001; Figure 1C), had no therapy after FISH (P < 0.001), or had a history of a CIS or T1 stage tumor before FISH (P = 0.007; Figure 1D). A univariate Cox proportional hazards model also demonstrated that there was a significant association between time to tumor recurrence and increased age (HR 1.02; P = 0.002). There was not a significant difference (P = 0.851) in the time to tumor recurrence based on smoking status of the patient.
Figure 1.
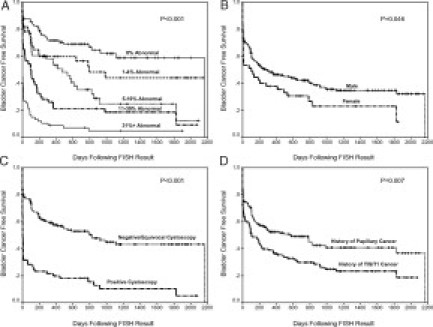
Kaplan-Meier Estimates of Bladder Cancer Free Survival by (A) FISH groupings, (B) gender, (C) same day as FISH cystoscopy result, and (D) bladder cancer stage before FISH result.
A Cox proportional hazard test was performed to determine whether the percentage of abnormal cells (continuous variable) was predictive of bladder cancer recurrence. The cystoscopy result was removed from this analysis due the large number of patients (n = 93) that did not have a same-day-as-FISH cystoscopy result. The final model revealed that the percentage of abnormal cells by FISH, age, female gender, absence of treatment after FISH, and history of a TIS or T1 tumor before FISH were all associated with recurrence of bladder cancer (Table 2). The percent abnormal by FISH was one of the most significant variables (HR 1.026, P < 0.001) for predicting recurrent bladder cancer with a 2.6% increased chance of having cancer for every 1% increase in the percentage of abnormal cells by FISH.
Table 2.
Cox Proportional Hazard Analysis for Predicting Recurrent Bladder
| Cancer (cystoscopy variable excluded; N = 287) | |||
|---|---|---|---|
| Variable | Hazard ratio | 95% C.I. for hazard ratio | P |
| Percent abnormal cells (actual percent) | 1.026 | 1.020, 1.031 | <0.001 |
| Age (Year) | 1.015 | 1.001, 1.029 | 0.033 |
| Male gender | 0.595 | 0.408, 0.869 | 0.007 |
| Treatment* | 0.368 | 0.245, 0.553 | <0.001 |
| History of TIS or T1 tumors prior to FISH | 1.657 | 1.225, 2.240 | 0.001 |
Treatment post-FISH and prior to pathologic evidence of bladder cancer.
Assessment of Muscle-Invasive Bladder Cancer
Kaplan-Meier analysis revealed a significant association between increasing percentage of abnormal cells by FISH and the eventual development of muscle-invasive bladder cancer (P < 0.001; Figure 2A). Kaplan-Meier analysis also revealed a decreased time to the development of muscle-invasive bladder cancer in patients that did not have therapy after FISH (P = 0.028; Figure 2B), had a positive cystoscopy result at the time of FISH (P = 0.009; Figure 2C), or had a history of TIS or T1 stage tumor before FISH (P < 0.001; Figure 2D). There was not a significant difference in the number of patients that developed muscle invasive bladder cancer based on age (P = 0.612), gender (P = 0.396), or smoking status (P = 0.702).
Figure 2.
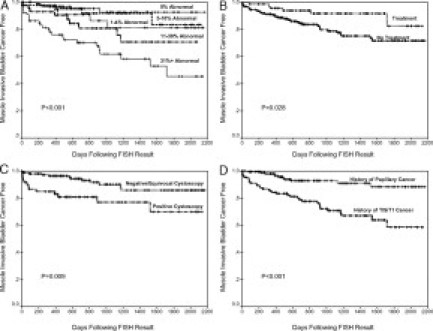
Kaplan-Meier estimates of progression to muscle-invasive bladder cancer by (A) FISH groupings, (B) treatment post FISH and before pathological detection of bladder cancer, (C) same day as FISH cystoscopy result, and (D) bladder cancer stage before FISH result.
A Cox proportional hazard test was performed to assess if the percentage of abnormal cells (continuous variable) was predictive of muscle-invasive bladder cancer. The final model revealed that the percentage of abnormal cells by FISH, absence of treatment after FISH, and history of a TIS or T1 tumor before FISH were all associated with the development of muscle-invasive bladder cancer (Table 3). Again, the percentage of abnormal cells was one of the most significant (P < 0.001) variables for identifying disease with a 1.8% increased risk of having muscle-invasive cancer for every 1% increase in the percentage of abnormal cells by FISH.
Table 3.
Cox Proportional Hazard Analysis for Predicting Muscle-Invasive Bladder Cancer (Cystoscopy Variable Excluded; N = 287)
| Variable | Hazard ratio | 95% C.I. for hazard ratio | P |
|---|---|---|---|
| Percent abnormal cells | 1.018 | 1.009, 1.028 | <0.001 |
| Treatment* | 0.325 | 0.128, 0.830 | 0.019 |
| History of TIS or T1 tumors prior to FISH | 3.727 | 1.883, 7.379 | 0.001 |
Treatment after FISH and prior to pathologic evidence of bladder cancer.
Discussion
The results of this study demonstrate that among patients with a history of non-muscle-invasive bladder cancer there is a positive correlation between the percentage of polysomic cells found in the urine by FISH and bladder cancer recurrence and progression to muscle-invasive disease. In this study, the likelihood of patients being diagnosed with recurrent bladder cancer increased as the percentage of abnormal cells by FISH increased. Multiple Cox proportional hazards analysis identified a 2.6% increased chance of having cancer for every 1% increase in the percentage of abnormal cells by FISH. Therefore, a patient with a 10% abnormal has a 26% increased risk of bladder cancer recurrence over a patient with 1% of abnormal cells.
Another finding of this study is that the percent abnormal cells by FISH are predictive for a follow-up diagnosis of muscle-invasive bladder cancer. Forty-seven of the 303 (16%) patients in this study were diagnosed with muscle-invasive cancer and the largest proportion of patients that developed muscle-invasive cancer (36%) were those in the ≥31% percentage abnormal cell grouping (Table 1). A Cox proportional hazard model that assessed associations between the percentage of abnormal cells and an eventual diagnosis of muscle-invasive disease while controlling for covariates found that the percentage of abnormal cells by FISH was the most significant independent predictor of muscle-invasive cancer (P < 0.001; HR 1.018; Table 2).
One of the dilemmas urologists face is how to treat patients with negative cystoscopy findings but a positive FISH result. Previous studies suggest that the FISH assay is quite sensitive and can frequently detect recurrent bladder cancer before it is clinically evident by cystoscopy.4,7,9,11 There were 66 patients in our study with a positive FISH and same-day-as-FISH negative cystoscopy result. Thirty-two (48%) of these patients had cancer on follow-up. The likelihood that a patient with a negative same-day-as-FISH cystoscopy result would be diagnosed with cancer was dependent on the percent abnormal grouping and increased from 29% in the 1% to 4% grouping to 39%, 64%, and 75%, in the 5% to 10%, 11% to 30%, and 31+% groupings, respectively. In addition, 7 of the 66 patients with a positive FISH result and same-day-as-FISH negative cystoscopy result had muscle-invasive cancer on follow-up of which 6/7 had ≥5% or more abnormal cells by FISH (actual percentages, 4%, 5%, 11%, 13%, 32%, 56%, and 73%). These data, along with the data shown in Figure 1, suggest that patients with ≥5% abnormal cells should possibly be evaluated for recurrent tumor (eg, by random biopsies) even in the absence of clinical or cystoscopic evidence of tumor since FISH can detect tumors before they are clinically evident by cystoscopy.
The data from this study also show that, especially among patients in the 1% to 4% group, a proportion of positive FISH results appear to be true false-positive FISH results since only about 50% of the patients in the 1% to 4% group were eventually diagnosed with recurrent tumor. False-positive FISH results can lead to costly, invasive, and potentially dangerous evaluation and treatment in patients that do not have tumor. As can be seen from this study, the majority of the apparent false-positive cases are in the 1% to 4% group. Thirty nine of the 49 “false-positive” FISH specimens in this study were available for retrospective review by a lead FISH technologist who routinely performs UroVysion testing. The results of this review suggest that 23 specimens (47%) may have been misdiagnosed for varying reasons such as: (1) cells appearing polysomic due to signal splitting, (2) the presence of a few tetrasomic cells (cells with four copies for each of the four probes) that may have been consistent with normal cells in the G2 or M phase of the cell cycle, or (3) unrecognized overlapping cells.12 At our institution, all low-level positive (1% to 4% abnormal) FISH specimens are now reviewed by a second technologist to decrease the chance for a false-positive diagnosis. Another possible explanation for some of the apparent false-positive results is that some patients had treatment after their initial positive FISH result because of a clinical suspicion of tumor and it is possible that the treatment eradicated the tumor. Cox proportional hazard analyses (Tables 2 and 3) show that treatment following FISH was an independent predictor of a decreased chance of bladder cancer recurrence and progression to muscle-invasive disease. Therefore, FISH may have detected some cancers that were subsequently treated and cured before they could be pathologically detected on follow-up. Taken together, the findings suggest that patients with FISH results in the 1% to 4% range should, in the absence of other signs of recurrent tumor, be followed expectantly.
Another challenging task for urologists is predicting which patients with a history of non-muscle-invasive bladder cancer will progress to muscle-invasive bladder cancer. The data from this study suggests that the percentage of abnormal cells observed by FISH can help stratify the risk of progression to muscle-invasive disease. Figure 2 illustrates that patients in the 11% to 30% and ≥31% abnormal groupings are almost twice as likely to have muscle-invasive disease on follow-up as compared with the 1% to 4% and 5% to 10% groupings. This was also true in patients with a history of T1 tumors where 1/14 (7%), 2/17 (12%), and 10/25 (40%) of patients progressed to muscle-invasive cancer in the negative, 1% to 10%, and 11+% abnormal categories, respectively. Therefore urologists should consider patients with ≥11% abnormal cells at high risk for developing muscle-invasive disease.
Although the data from this study is informative, not all patients in this study were clinically followed and treated exactly the same since this was a retrospective cohort study. For example, some patients with a positive FISH, cystoscopy or cytology result may have received immediate treatment by tumor fulguration and/or BCG therapy based on a clinical suspicion of malignancy, whereas another patient with the same results may have not received treatment for clinical reasons (eg, elderly or poor health). We tried to control for this limitation by controlling for treatment in the multiple Cox proportional hazards model. Secondly, this study consisted of a heterogeneous group of patients with different size tumors, tumor grades, tumor multiplicity, number of previous occurrences, and different therapies before FISH, which was not separately analyzed due to the large number of patients it would take to individually assess each of these variables in addition to the variables collected. Further prospective studies that assess for each of these predictive variables are needed to further define the value of interpreting the percentage of abnormal cells by FISH. Homogeneity of the population was a limitation in that Caucasians accounted for over 99% of the population, and 85% of the population was male. Further studies are needed to determine whether the outcomes of our study would differ significantly with a non-Caucasian or female population. Lastly, this study assumes that the percentages of abnormal cells diagnosed by FISH are reproducibly diagnosed by different technologists. A recent study within our laboratory has addressed various aspects of the qualitative and quantitative reproducibility of the UroVysion assay.13 That study suggests that the quantitative reproducibility of the assay would lead to a reasonably accurate categorization of patients if 1% to 4%, 5% to 10%, 11% to 30%, and >30% groupings were used for risk stratification.
In conclusion, the results of this study demonstrate that the percentage of abnormal cells detected by FISH in urine specimens is associated with bladder cancer recurrence and progression to muscle-invasive cancer. These data can help urologists identify patients that are at higher risk for bladder cancer recurrence and progression to muscle-invasive disease. Further prospective studies are needed to determine whether improved treatment algorithms can be instituted based on the information from this study. The ability to aggressively follow and treat patients at higher risk will hopefully decrease the risk that a patient will progress to muscle-invasive cancer and ultimately improve survival in patients being monitored for recurrent bladder cancer.
Footnotes
K.H. is listed as a co-inventor on a patent and he and the Mayo Clinic receive royalties from the sale of the FISH probe set used in this study. In addition, K.H. receives grant support from Abbott Molecular Inc. to develop FISH assays for the detection of malignant cells in cytologic specimens.
References
- 1.Clark PE. Bladder cancer. Curr Opin Oncol. 2007;19:241–247. doi: 10.1097/CCO.0b013e3280ad43ac. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thon MJ. Cancer Statistics, CA Cancer. J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Parekh DJ, Bochner BH, Dalbagni G. Superficial and muscle-invasive bladder cancer: principles of management for outcomes assessments. J Clin Oncol. 2006;24:5519–5527. doi: 10.1200/JCO.2006.08.5431. [DOI] [PubMed] [Google Scholar]
- 4.Sarosdy MF, Schellhammer P, Bokinsky G, Kahn P, Chao R, Yore L, Zadra J, Burzon D, Osher G, Bridge JA, Anderson S, Johansson SL, Lieber M, Soloway M, Flom K. Clinical evaluation of a multi-target fluorescent in situ hybridization assay for detection of bladder cancer. J Urol. 2002;168:1950–1954. doi: 10.1016/S0022-5347(05)64270-X. [DOI] [PubMed] [Google Scholar]
- 5.Yoder BJ, Skacel M, Hedgepeth R, Babineau D, Ulchaker JC, Liou LS, Brainard JA, Biscotti CV, Jones JS, Tubbs RR. Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: a prospective study with focus on the natural history of anticipatory positive findings. Am J Clin Pathol. 2007;127:295–301. doi: 10.1309/ADJL7E810U1H42BJ. [DOI] [PubMed] [Google Scholar]
- 6.Varella-Garcia M, Akduman B, Sunpaweravong P, Di Maria MV, Crawford ED. The UroVysion fluorescence in situ hybridization assay is an effective tool for monitoring recurrence of bladder cancer. Urol Oncol. 2004;22:16–19. doi: 10.1016/S1078-1439(03)00098-X. [DOI] [PubMed] [Google Scholar]
- 7.Skacel M, Fahmy M, Brainard JA, Pettay JD, Biscotti CV, Liou LS, Procop GW, Jones JS, Ulchaker J, Zippe CD, Tubbs RR. Multitarget fluorescence in situ hybridization assay detects transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. J Urol. 2003;169:2101–2105. doi: 10.1097/01.ju.0000066842.45464.cc. [DOI] [PubMed] [Google Scholar]
- 8.Skacel M, Pettay JD, Tsiftsakis EK, Procop GW, Biscotti CV, Tubbs RR. Validation of a multicolor interphase fluorescence in situ hybridization assay for detection of transitional cell carcinoma on fresh and archival thin-layer, liquid-based cytology slides. Anal Quant Cytol Histol. 2001;23:381–387. [PubMed] [Google Scholar]
- 9.Kipp BR, Karnes RJ, Brankley SM, Harwood AR, Pankratz VS, Sebo TJ, Blute MM, Lieber MM, Zincke H, Halling KC. Monitoring intravesical therapy for superficial bladder cancer using fluorescence in situ hybridization. J Urol. 2005;173:401–404. doi: 10.1097/01.ju.0000149825.83180.a4. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 11.Bubendorf L, Grilli B, Sauter G, Mihatsch MJ, Gasser TC, Dalquen P. Multiprobe FISH for enhanced detection of bladder cancer in voided urine specimens and bladder washings. Am J Clin Pathol. 2001;116:79–86. doi: 10.1309/K5P2-4Y8B-7L5A-FAA9. [DOI] [PubMed] [Google Scholar]
- 12.Del Rosario KM, Stevens CL, Kipp BR, Halling KC. Retrospective review of positive fluorescence in situ hybridization specimens from patients without pathologic evidence of a urinary tract cancer. Cancer Cytopathology. 2007;111:s422. (Abstract) [Google Scholar]
- 13.Brankley SM, Adams EK, Christensen MR, Everts CR, Lund JD, Oberg TN, Plagge AM, Zieman AH, R. KB, Halling KC. A study of the reproducibility of the UroVysion fluorescence in situ hybridization bladder cancer detection assay. Analytical and Quantitative Cytology and Histology. 2008;30:145–151. [PubMed] [Google Scholar]


