Abstract
Responses to focal cerebral ischemia by neurons and adjacent microvessels are rapid, simultaneous, and topographically related. Recent observations indicate the simultaneous appearance of proteases by components of nearby microvessels that are also expressed by neurons in the ischemic territory, implying that the events could be coordinated. The structural relationship of neurons to their microvascular supply, the direct functional participation of glial cells, and the observation of a highly ordered microvessel-neuron response to ischemia suggest that these elements are arranged in and behave in a unitary fashion, the neurovascular unit. Their roles as a unit in the stimulation of cellular inflammation and the generation of inflammatory mediators during focal cerebral ischemia have not been explored yet. However, components of the neurovascular unit both generate and respond to these influences under the conditions of ischemia. Here we briefly explore the potential inter-relationships of the components of the neurovascular unit with respect to their potential roles in ischemia-induced inflammatory responses.
Keywords: microvessels, microvessel-neuron distribution, astrocytes, endothelial cells, integrins, dystroglycan
1.0 Introduction
Inflammatory cell-related responses of cerebral tissue to an inciting injury, whether infectious, immune, or ischemic, entail the generation of chemoattractants, cytokines, and chemokines. Those substances lead to the incursion of cells of the leukocyte lineage into the injured territory, but appear also to alter resident cell function and viability. Some or all of the processes initiated by ischemic injury within the central nervous system (CNS), for instance, can amplify the injury. In the maturation of the lesion initiated by ischemia, caused by occlusion of one or more brain-supplying arteries to a cerebral hemisphere, both cellular and humoral inflammatory elements are involved. Some products of the maturation of the ischemic lesion, among them cytokines and chemoattractant elements, can affect gene expression within the contralateral hemisphere, and can have systemic effects (particularly in small animal models). Yet the mature lesion, the infarction, is confined to the territory-at-risk that is supplied by the occluded vessel.
The processes or events that affect a particular region within the ischemic cerebral vascular territory can be categorized as vascular, non-vascular, and modulatory: i) Among the processes that target or involve the microvasculature are those that underlie the adherence of leukocytes to the endothelium and their transmigration, complement activation, and cytokine-mediated cell activation responses (e.g. endothelium, astrocytes). ii) Among the cellular and humoral inflammatory events occurring outside the microvasculature are those affecting the glia, and those augmenting neuron injury, recovery, or replacement. Still poorly understood are events and processes that can promote white matter (axonal) injury directly, including leukocyte and glial-dependent events. iii) Modulatory processes include those that initiate “pre-conditioning,” tolerization, and/or sensitization of the cerebral parenchymal responses to ischemia. These processes also include the impact of microglial activation on the evolution of tissue injury and the development of neuron progenitors; the possible effects of concomitant infection on injury maturation; and, the diverse effects of proteases due to the responses of immune-related cells during injury development. Descriptive studies have identified or confirmed that alterations in subsets of circulating lymphocytes and PMN leukocytes occur during focal ischemia; that the injury zones are infiltrated by leukocytes; that the evolving injury can be modulated by immune suppression and that recognized immune responses of neurovascular cells can be altered by ischemia; and that innate and adaptive immunity can modulate injury. The course of many of these events, summarized in papers in this volume, suggest that the processes highlighted here are not necessarily confined only to the vascular or to the extravascular compartments. They suggest that events that are initiated within the microvasculature often extend to other elements of the neuropil and involve glial and neuronal elements.
2.0 Microvessel-neuron relationships within cerebral tissue
Within the territory downstream from the arterial occlusion(s), the development of the ischemic injury is heterogeneous. Neuron dysfunction and demise varies within the territory with the time from the onset of ischemia.(Tagaya et al., 1997; Abumiya et al., 1999; Heo et al., 1999) This is consistent with the variable microvascular supply to neurons,(Bär, 1983; del Zoppo and Mabuchi, 2003) known differences in neuron vulnerability,(Nyberg and Waller, 1989; Gonzales et al., 1992) and the yet unclear differences in microvessel-neuron functions.
The roles of the (micro)vascular supply and the effects of alterations in its integrity on neuron viability have not received much attention. In both the cortex and corpus striatum the vascular beds are arranged geometrically in a consistent fashion.(Bär, 1978; Bär, 1983) For the cortex, Bär described the hierarchical organization of the arterial supply of the cerebral gray matter as a descending series of stacked hexagonal arrays from the penetrating arteries of the pial supply to the white matter border.(Bär, 1978; Bär, 1980) Within the white matter, capillaries are arranged in line with axons, and comprise ~10% of the density of capillaries found within the gray matter.(del Zoppo and Mabuchi, 2003) Within the striatum, where the microvessels (< 100 μm diameter vessels) and (inter)neurons are distributed more or less homogeneously throughout the tissue, the relationship of the neuron to its proximate microvessels is highly ordered and consistent.(Mabuchi et al., 2005)
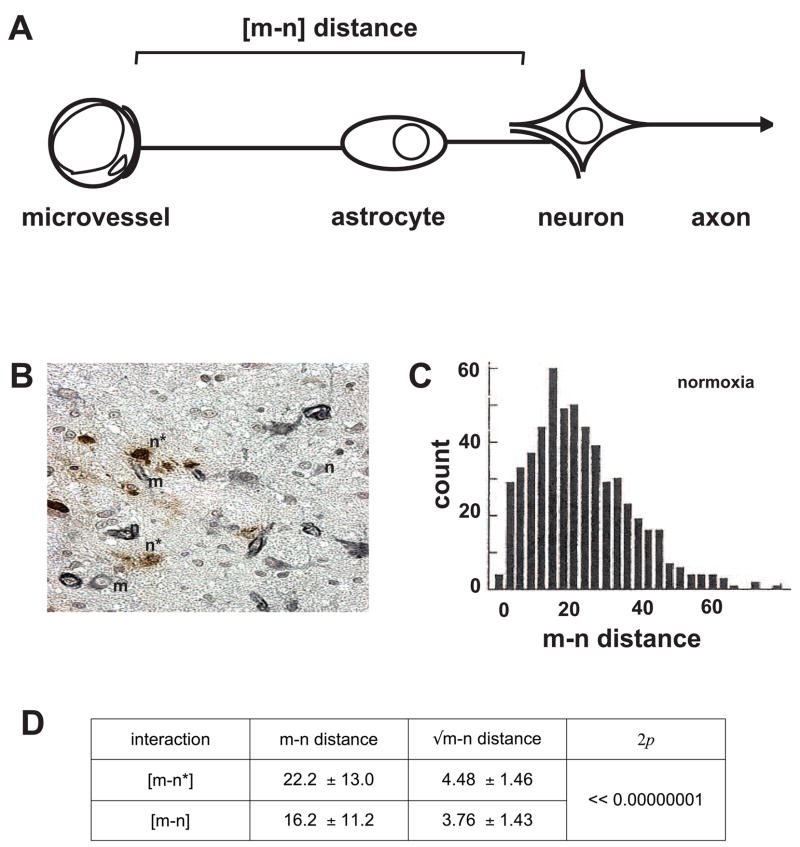
Importantly, the response to focal ischemia (initiated by middle cerebral artery occlusion, MCA:O) is highly ordered and consistent. In a study of the course of neuron injury following MCA:O in the non-human primate, at 2 hours following ischemia onset neurons furthest from their most proximate microvessel (within a ~30 μm radius) were most likely to display injury (Figure 1).(Mabuchi et al., 2005) In this period there was no distortion of tissue due to ischemic injury (e.g. edema) or evidence of hemorrhage. Injured neurons [n*] were scattered among neurons not displaying evidence of injury (by dUTP incorporation into nuclear DNA). Therefore the foci of injury were heterogeneously distributed within the ischemic territory. The observation of neurons most distant from their nearest microvessel preferentially displaying dUTP incorporation compared to uninjured neurons is not easily explained by the expected distribution of O2 diffusion, however.(Mabuchi et al., 2005) Regarding selective vulnerability, the subgroup of neurons that do not express glutamic acid decarboxylase (GAD−) displayed the same injury distance [m–n*] distribution as the overall group, but this was not true the response of the entire neuron population. Neurons identified by other neurotransmitters did not display selective injury in this fashion. The [m–n*] distribution could not be explained as either a consequence of leukocyte invasion or cytokine effects, because the former would have been expected to injure neurons closest to their nearest microvessels, and cytokine generation would have been expected to affect all neurons in a region no matter the distance from their supply microvessel(s) if they induced neuron degeneration equally.
Figure 1.
Microvessel-neuron (m–n) inter-relationships based upon [m–n] distance distributions. A, the neurovascular unit and definition of [m–n] distance. B, indication of positions of microvessels (m) and neurons with evidence of dUTP incorporation (n*) and neurons without evidence of DNA scission (n) at 2 hours following middle cerebral occlusion (MCA:O) in the striatum of the non-human primate. C, untransformed data of [m–n] distances in the non-ischemic striatum of the non-human primate (in μm). D, neurons with evidence of injury (n*) at 2 hours MCA:O are at significantly greater distance from their nearest neighboring microvessel ([m–n*]) than those without evidence of injury ([m–n]). Data and figures from Mabuchi et al.(Mabuchi et al., 2005)
While inflammatory responses to ischemia within the target territory have been attributed to neurons (e.g. “neurodegeneration”), all cells within the territory are susceptible to ischemia alone, and each cell type can mount a response to one or more inflammatory stimuli (e.g. the cytokine TNF-α). A consistent hierarchy of responses to ischemia is now evident: neurons are most sensitive, whereas astrocytes, microglia, and endothelial cells less so in descending order.(Tagaya et al., 1997; Milner et al., 2008a; Milner et al., 2008b) Endothelial cells of cerebral microvessels are particularly resistant to ischemia in vivo, accounting for less than 2% of cells in the ischemic core displaying evidence of scission of nuclear DNA by 24 hours after MCA:O.(Tagaya et al., 1997) A similar observation has been made of murine primary cerebral microvascular endothelial cells in culture.(Milner et al., 2008a) As neurons comprise less than 5% of the cellular content of the brain, and there is repeated evidence that glial cells display injury to focal ischemia in concert with the neurons they support, the impact of ischemia and its inflammatory consequences on the glial compartment must be taken into account.
Overall, the microvessel-neuron relationships within the striatum are highly ordered and consistent, as are their responses to ischemia.
3.0 Neuron-microvessel relationships within the central nervous system
Pressure to examine the relationship of neuron injury to the microvascular supply during focal ischemia comes from clinical observations. Neuronal control of local cerebral blood flow (CBF) (“neurovascular coupling”) engages signaling within and among astrocytes in the abluminal part of cerebral microvessels as they support neuron function and relay information to microvessels. The control of regional and local flow in the absence of ischemic injury depends upon this neurovascular coupling.(Iadecola, 2004) Here, neuron activation can promote arteriolar tonal change via the intervening astrocytes.(Zonta et al., 2003; Iadecola, 2004)
But, “neuron protectant” agents have been singularly ineffective in the acute treatment of ischemic stroke, perhaps because they do not address glial or axonal injury. Only strategies intended to preserve microvessel patency have been effective in preserving neuron function, or decreasing the region of injury.(The National Institutes of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995; Young et al., 1997; Choudhri et al., 1998) The primacy of microvascular integrity and flow in the maintenance of neuron function, under conditions of focal ischemia, is very relevant to the impact of both humoral and cellular inflammation on the injury maturation.
The cerebral microvasculature is a low pressure high flow bed. During CNS development the vascular architectural arrangement is determined by the regional location of the microvasculature, as in the anterior cerebral circulation, while microvessel and neuron positions develope relative to one another along lines of extracellular matrix (ECM) (e.g. laminin).(Liesi, 1985; Engvall et al., 1986; Herken et al., 1990; David et al., 1995; Grant and Kleinman, 1997) Within the corpus striatum (of primates), capillaries are located within a mean of 30 μm from the nearest neighboring neuron.(Mabuchi et al., 2005) This capillary arrangement also has branch points at approximately 30 μm intervals.(Mabuchi et al., 2005) The branching arrangement of the capillaries allows diversion of flow to patent capillaries/microvessels and thereby around occluded vessels, preserving flow in the vicinity of neurons that sit within a 30 μm distance.(Mabuchi et al., 2005) Region-specific arrangements of the microvasculature accord with differences in the rCBF such that flow is lowest in the striatum and highest in the gray matter.(Fenstermacher et al., 1991) The hierarchy of vessels within the CNS implies that while ischemia may initiate similar processes that injure larger arteries and microvessels alike, the consequences are likely to be different.
4.0 The Neurovascular Unit
In the cerebral tissue, microvessels are an integral part of the neuropil. The close proximity of endothelial cells to astrocyte end-feet within microvessels and the support of astrocytes for neurons suggests that communication could also be directed from microvessels to the neurons they supply.(del Zoppo and Mabuchi, 2003) This hypothesizes a conceptual “neurovascular unit” that consists of microvessels (endothelial cells-basal lamina matrix-astrocyte end-feet, and pericytes), astrocytes, neurons and their axons, in addition to other supporting cells (e.g. microglia and oligodendroglia) (Figure 1). The implication is that information is transmitted in both directions. The resilience of the “unit” to reductions in flow or to flow cessation is unclear, but is likely to be complex as adjacent units would be connected through their common microvessels and through dendritic connections. The syncytial arrangement of astrocytes also allows for extension of inter-communication.(Nedergaard et al., 2003) This provides for intercommunication and protected perfusion at the same time. Furthermore, cell-cell interactions may be different than those perceived from isolated cell cultures.
Alterations in microvessel integrity could have other follow-on effects within the neurovascular unit that impact on neuronal function. For instance, it is possible that sustained hypertension, hyperglycemia, amyloid deposition, and other processes that could injure the endothelium also affect microvessel integrity, and hence neuron function at a distance through non-inflammatory mechanisms.
5.0 Microvascular cell components
Microvessel integrity depends upon the interactions of astrocyte end-feet with the endothelium: both are required for the formation of the basal lamina matrix, and for the formation of the endothelial permeability barrier (“blood-brain barrier”).(Risau et al., 1986) Within capillaries, astrocytes and endothelial cells interact to form the intervening basal lamina barrier and the inter-endothelial tight junctions that constitute the permeability barrier.(Bernstein et al., 1985; Webersinke et al., 1992; Nagano et al., 1993; Furuse et al., 1993; Itoh et al., 1993; Furuse et al., 1994; Furuse et al., 1999) Elegant xenograft experiments have demonstrated that the permeability barrier phenotype can be transplanted, and that its integrity requires the close interaction of endothelial cells with astrocyte end-feet.(Hurwitz et al., 1993) Inter-endothelial tight junctions contribute to the vascular portion of the blood-brain barrier.(Furuse et al., 1993; Itoh et al., 1993; Furuse et al., 1994; Furuse et al., 1999) The matrix also provides a barrier to the transmigration or leakage of erythrocytes during hemorrhage and leukocyte subsets (e.g. PMN leukocytes and cells of the monocyte/macrophage lineage) in response to inflammatory stimuli (e.g. the inflammatory phase of ischemia).(Hamann et al., 1996) The integrity of the microvasculature is also affected by the presence of pericytes within the matrix or vessel wall histiocytes within larger vessels.(Dore-Duffy, 2003) The ultrastructure and integrity of cerebral microvessels depend upon their location and regional tissue composition.
6.0 Inflammation and the components of the neurovascular unit
Inasmuch as focal ischemia of the brain is a pro-inflammatory stimulus, it is of interest to consider how the hypothetical “neurovascular unit” and its component elements might respond separately and in concert. The responses of the “unit” to inflammatory modulators generated early during focal ischemia or from other processes that affect individual component of the “unit” have not been established; although, the responses of some of the individual “unit” components have been detailed in specific experimental settings. For instance, during focal ischemia the cytokines TNF-α and IL-1β are generated and secreted by cells within and around the injured territory. Their expression response profiles depend upon both temporal and topographical factors.(Sirén et al., 1992; Wang et al., 1994; Yang et al., 1998) Among the known interactions of inflammatory cytokines with the proposed neurovascular components and elements are:
6.1 Endothelium
Under normoxic conditions the intact resting endothelium preserves the anti-thrombotic and anti-inflammatory characteristics of the cerebral microvasculature, and contributes to the permeability barrier through its inter-endothelial cell cohesion receptors, components of the tight junctions, and adhesion to the subjacent basal lamina matrix. The anti-thrombotic milieu most probably involves mechanisms similar to the endothelium in other vascular beds, including the generation of adenosine, production and secretion of PGI2, and the glycocalyx surface. The anti-inflammatory properties derive from storage of leukocyte adhesion receptors (e.g. P-selectin), and the requirement for synthesis of other receptors necessary for cell-matrix adhesion. Typically, thrombosis and inflammation are linked.
Endothelial cells, within cerebral capillaries, and especially within the post-capillary venules, respond to TNF-α and IL-1β by translocation of P-selectin (stored in Weibel-Palade bodies) to the luminal surface, the synthesis of ICAM-1 and its expression on the luminal surface, and the subsequent appearance of E-selectin on this surface.(Okada et al., 1994b; Haring et al., 1996b) These adhesion receptors facilitate firm adhesion of PMN leukocytes to the local endothelium in preparation for their transmigration into the injured neuropil.(Granger et al., 1989; Bevilacqua et al., 1989; McEver, 1991)
Both cytokines have been shown to down-regulate the integrin α6β1 found on endothelial cells.(Defilippi et al., 1992) How this impacts microvessel integrity is not known, but could augment the processes initiated by focal ischemia that lead to the rapid loss in β1-integrin expression observed in vivo,(Tagaya et al., 2001) and in the down-regulation of matrix-dependent integrins by experimental ischemia in vitro.(Milner et al., 2008a)
We have hypothesized that these events could contribute to the development of edema during focal ischemia, in addition to recognized changes in the inter-endothelial tight junction proteins (known to occur later).(del Zoppo and Milner, 2006) Focal ischemia stimulates loss in the solute permeability barrier established by the endothelium, allowing leakage of electrolytes and plasma proteins as large as fibrinogen (~ 360 kDa). Kogure and colleagues have postulated that IL-1β facilitates edema formation in the initial moments following the onset of focal ischemia.(Yamasaki et al., 1992) Others have suggested that edema is associated with leukocyte infiltration.(Stamatovic et al., 2006) Furthermore, immunologic blockade of the functional β1-integrin subunit, expressed on epithelial cells of the dermis, produces significant local edema within the skin, consistent with the events noted above.(Reed et al., 1992) The fact that the endothelium does not detach from the basement membrane during focal ischemia implies that changes in these receptors does not fully explain the adhesion of the endothelium to the underlying matrix.(Tagaya et al., 2001)
6.2 Astrocytes
There is considerable evidence that astrocytes can serve immune function, can potentially present antigens, have phagocytic properties, and generate cytokines and chemokines in response to a variety of stimuli.(Dong and Benveniste, 2001) The latter include TNF-α and IL-1β in addition to other cytokines. In culture, microglia and astrocytes maintain a close interrelationship. Astrocytes for the most part are capable of generating the latent metalloproteinase (MMP)-2 under a number of conditions. In contrast, in the circulation PMN leukocytes release pro-MMP-8 and pro-MMP-9, while cells of the monocyte/macrophage lineage generate pro-MMP-9. Transmigration of these cells is accompanied by myeloperoxidase (MPO) deposition in the tissue through which they transit (e.g. the ischemic regions). How these processes and the pro-MMP-8 and-9 release affect astrocyte-endothelial cell relationships has not been carefully studied in this setting.
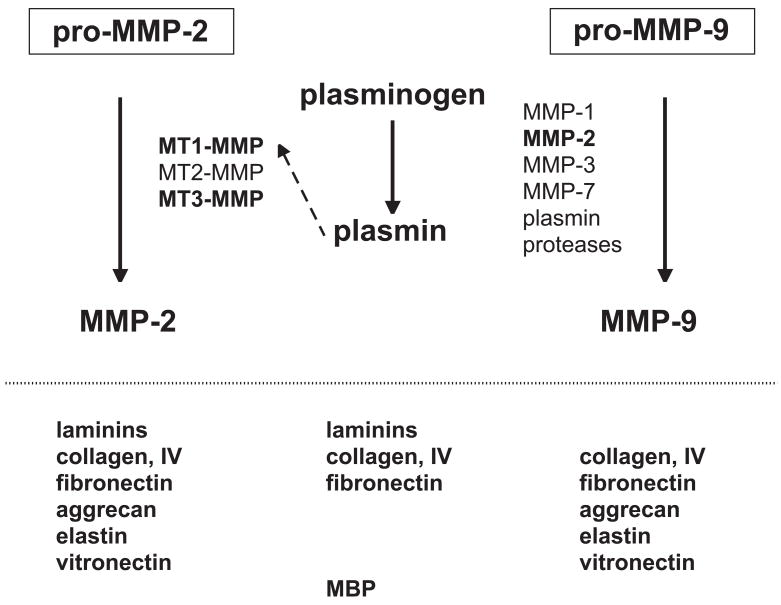
Both pro-MMP-2 and pro-MMP-9 are generated in the ischemic core of the evolving lesion within 1–2 hours following the onset of focal ischemia.(Heo et al., 1999) Activation systems for pro-MMP-2 also appear during this time in the same regions (Figure 2).(Chang et al., 2003)
Figure 2.
Schema indicating the potential interactions of pro-MMP-2 within 2 hours MCA:O in the ischemic striatum of the non-human primate, the appearance of pro-MMP-9, and their respective activations systems. Proteases identified within this system are noted in bold and in boxes. The known matrix substrates of possibly active proteases are indicated below each system. From data presented in (Heo et al., 1999; Hosomi et al., 2001; Chang et al., 2003).
6.3 Extracellular matrix (ECM)
The vascular basal lamina and the extravascular intercellular space contain glycoproteins of the ECM. ECM components found in the cerebrovascular basal lamina matrix include laminins, type IV collagen, fibronectin of cellular origin, thrombospondin, heparan sulfate proteoglycans (HSPGs) including perlecan, nidogen, entactin, and other minor components. In contrast, the intercellular matrix of the extravascular space contains chondroitin sulfate proteoglycans (CSPGs) CAT-301pg, neurexin, and other proteoglycans, although some of those gylcoproteins found in the lamina have been noted in this space in small amounts (Table).(Carlson and Hockfield, 1996)
TABLE.
A partial list of the differential content of extracellular matrix proteins within cerebral microvessels and the extravascular intercellular space of cerebral tissues.
| Microvessel basal lamina |
| laminins |
| collagen, type IV |
| fibronectin (cellular origin) |
| thrombospondin |
| heparan sulfate proteoglycans (HSPGs) |
| nidogen |
| entactin |
| others |
| Extravascular matrix (intercellular spaces) |
| heparan sulfate proteoglycans (HSPGs) |
| chondroitin sulfate proteoglycans (CSPGs) |
| CAT-301pg |
| other proteoglycans |
| minor amounts(*) of |
| laminins |
| collagen, type IV |
| fibronectin |
| thrombospondin |
Endothelial cells and astrocytes adhere firmly to matrix proteins that are ligands for adhesion receptors of the integrin and dystroglycan families. Cellular matrix receptors are central to the integrity of the microvasculature. Integrin and the dystroglycan receptors are positioned to bind endothelial cells and astrocyte end-feet to the intervening matrix components of the basal lamina (e.g. fibronectin, laminins, collagen type IV, and perlecan for the β1-integrins, laminin and perlecan for αβ-dystroglycan).(Milner et al., 2008a; Milner et al., 2008b) αβ-dystroglycan is expressed by astrocyte end-feet on all cerebral vessels, while integrin α6β4 is expressed on astrocytes around all microvessels in the white matter and large penetrating vessels of the gray matter. Cerebral endothelial cells express integrin α1β1, α3β1, and α6β1 under conditions of normoxia.(Haring et al., 1996a; Tagaya et al., 2001; Milner et al., 2008a) β1-integrins are expressed by the endothelium of all cerebral microvessels in gray and white matter.(Tagaya et al., 2001) Disruption of the permeability barrier has been associated with changes in tight junction protein expression, although those changes are not rapid.
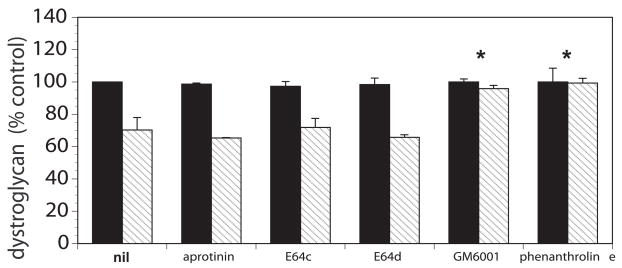
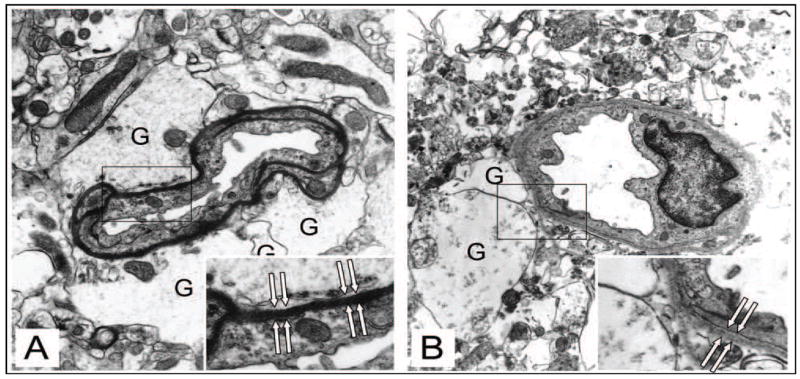
The cerebral microvessel wall displays rapid dynamic changes in response to focal ischemia that precede the incursion of inflammatory cells. Significant changes in the matrix integrity of the basal lamina and in matrix receptors occur simultaneously with neuron injury. The expression of the matrix constituents of the basal lamina, including laminin-1 and -5, collagen IV, cellular fibronectin, and perlecan, decrease substantially following the onset of focal ischemia.(Hamann et al., 1995) The endothelial cell β1-integrin receptor and integrin α6β4 on astrocyte end-feet decrease substantially in the first 60 minutes following MCA occlusion.(Wagner et al., 1997; Tagaya et al., 2001) The alternate receptor family, dystroglycan, is lost from the end-feet in the same timeframe (Figure 3).(Milner et al., 2008b) It is notable that aquaporin-4 is co-distributed with dystroglycan in the luminal membrane of astrocyte end-feet.(Neely et al., 2001; Warth et al., 2005; Milner et al., 2008b) Separation of the end-feet from the matrix of select microvessels occurs in this period (Figure 4).(Heo et al., 2005) The separation coincides with the accumulated fluid within the extravascular space.
Figure 3.
Complete inhibition of the loss of β-dystroglycan immunoreactivity from primary murine astrocytes cultured on laminin under conditions of experimental ischemia (hatched bars) compared to normoxia (solid bars) by blockade of MMP-like activity (with GM 6001 and with 1, 10-phenanthroline, *). Inhibition of serine proteases (aprotonin) and some cathepsins (E64c and E64d) had no effect on the loss of β-dystroglycan immunoreactivity during experimental ischemia.(Milner et al., 2008b)
Figure 4.
Separation of the end-feet of astrocytes from the abluminal surface of the basal lamina matrix of select microvessels very early following MCA:O in the rat (B) compared to normoxia (A). Note swelling of the astrocytes (G), but no swelling of the endothelium. Used with permission of the principal author.(Heo et al., 2005)
Proteases that could process matrix are generated rapidly within the core under the conditions of focal ischemia,(Fukuda et al., 2004) and belong to four families: the MMPs (e.g. pro-MMP-2 and -9, and their MMP activators), serine proteases (e.g. urokinase plasminogen activator, u-PA), cysteine proteases (e.g. cathepsin L), and heparanase. Members of all four families appear within 1–2 hours after MCA:O in the ischemic core.
Degradation of some matrix proteins have been shown to have cytokine activity, and it is possible that some of these products can also affect the integrity of the neuropil. For instance, in the process of PMN leukocyte activation and migration protease degradation of specific ECM substrates occurs with the generation of chemotactic products. Elastase (NE) released upon degranulation of PMN leukocytes can cleave laminin-5 to generate a peptide capable of promoting granulocyte chemotaxis.(Mydel et al., 2008) Similarly MMP-8 (also released by PMN leukocytes upon activation and degranulation) can generate a Pro-Gly-Pro-containing peptide from collagen that is chemotactic for granulocytes.(Lin et al., 2008) While not yet tested in the CNS, it is likely that activation and migration of PMN leukocytes through the cerebral microvasculature can generate substances from the basal lamina matrix (which contain both laminin-5 and collagen (IV)) that promote the progress of cellular inflammation post-ischemia.
6.4 Pericytes
Pericytes are pluripotential cells located within the basal lamina matrix of microvessels. These cells become activated and can migrate in response to specific stimuli including focal ischemia and inflammation.(Dore-Duffy, 2008) Pericytes also have been shown to have some properties found in select inflammatory cells.(Balabanov et al., 1996; Dore-Duffy, 2008)
6.5 Neurons and axons
The impact of inflammation initiated by focal ischemia on neuron and axon viability has been debated. The concordance of cytokine generation and leukocyte infiltration in the early hours following ischemia onset has suggested that both events can contribute to neuron injury. The cytokines TNF-α and IL-1β can derive from endothelial cells, astrocytes, microglia, and neurons themselves. Recent work has indicated that in isolated conditions, dorsal root ganglia (DRG) neurons can stimulate isolated PMN leukocytes to initiate their respiratory burst with free radical release leading to an increase in Ca+2 transients in the neurons.(Shaw et al., 2008) One consequence is that PMN leukocyte infiltration could aggravate neuron injury. While IL-1β expression has been associated with neuron injury, a recent report suggests that it could stimulate neurite outgrowth from DRG neurons, and could support nerve regeneration.(Temporin et al., 2008) However, events in the peripheral circulation are not uniformly recapitulated in the CNS. The manner in which IL-1β could mediate neuron injury in the CNS has been the subject of vigorous discussion. One possibility is that enzymes with matrix protease activity, generated during ischemia, could promote neuron injury,(Thornton et al., 2008) either directly or indirectly. This hypothesis has been proposed recently in conjunction with microvascular events.(Heo et al., 1999; Chang et al., 2003)
6.6 Other cells that can modulate function
Among other cells that are found in the neurovascular unit, and respond to inflammatory stimuli, microglia (in the neuropil) and mast cells (in select microvessels) have quite interesting properties.
6.6.1 Microglia
Resident inflammatory cells of the CNS, the microglia derive from bone marrow stem cells, evolving from cells of the monocyte/macrophage lineage.(Hoogerbrugge et al., 1988) Microglia can play a number of roles in inflammatory responses of the CNS. While normally quiescent, these cells have Fc receptors, complement receptors, receptors for a number of cytokines, chemokine receptors, CD40, Fas and Fas ligand.(Aloisi, 2001) Microglia can also generate TNF-α and IL-1β in addition to other cytokines, and are known to regulate T-cell mediated immune processes.(Aloisi, 2001) In response to activators microglia can express pro-MMP-9.
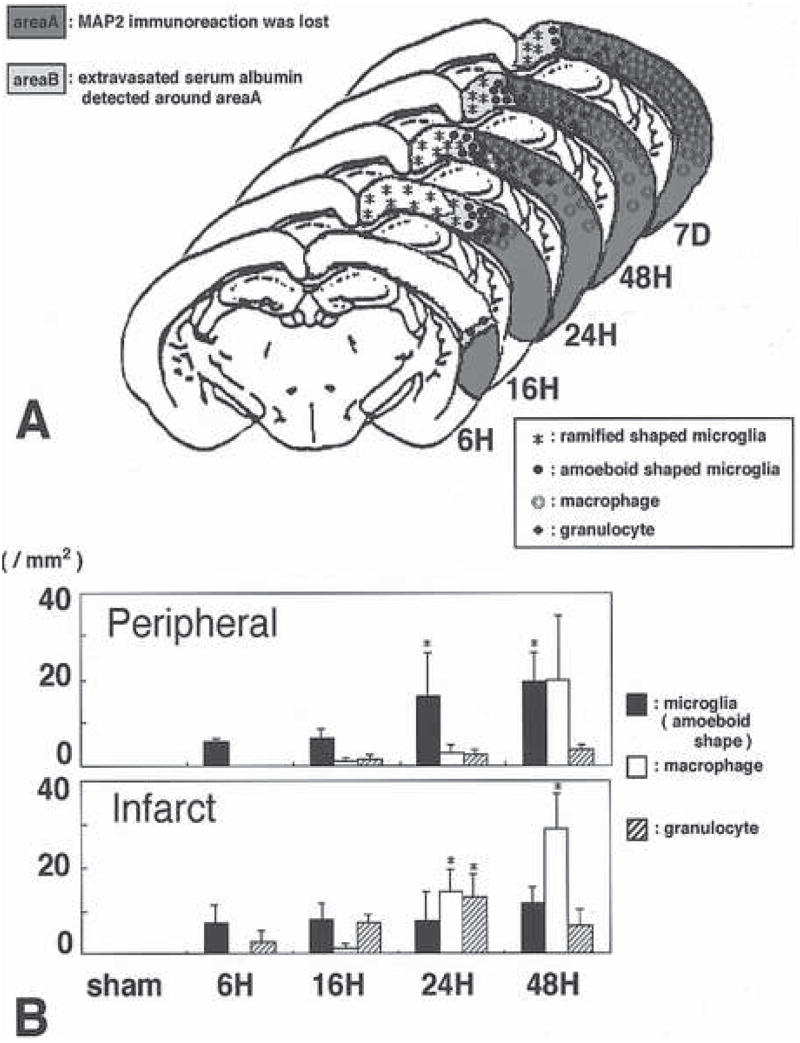
Within the first 24 hours following the onset of focal ischemia, peripheral blood cells of the monocyte/macrophage lineage enter the neuropil. At the same time microglia become activated, undergo shape change, and express markers of inflammation. Mabuchi et al have detailed the relative appearance and kinetics of these two related cell types in the ischemic brain parenchyma (Figure 5).(Mabuchi et al., 2000)
Figure 5.
Distribution of activated microglial cells compared with peripheral blood macrophages in the evolution of focal cerebral ischemia in the rat. A, topographical distribution of activated microglia in the peripheral region of the ischemic lesion and the incursion of macrophages within the region of ischemic injury identified by MAP2 immunoreactivity at various times after MCA:O. B, relative distribution of microglia, macrophages, and PMN leukocytes identified in the peripheral region and the central region of the evolving infarction. Figure 4 from Mabuchi et al used with the permission of the principal author.(Mabuchi et al., 2000)
6.6.2 Mast cells
Several recent reports have highlighted the potential roles of mast cell degranulation in microvascular and tissue responses to ischemia in the CNS.(Strbian et al., 2006; Strbian et al., 2007a; Strbian et al., 2007b) Located in association with astroglial processes in the vicinity of microvessels at their branch points, and on their luminal aspect,(Goldschmidt et al., 1984; Dimitriadou et al., 1990) these cells associate with astrocytes and matrix substrates (e.g. laminin, fibronectin).(Thompson et al., 1993) Mast cells can release TNF-α and IL-1β.
Individual cells and structural elements of the neurovascular unit participate and facilitate the contributions of cellular inflammation and can respond to the humoral stimuli. How the individual cells interact with each other is not so clear. A unifying element in these processes appears to be represented by the ECM and its fate.
7.0 Effects of ischemia on vascular elements within the neurovascular unit
Occlusion of a brain-supplying artery produces an abrupt decrease in regional CBF, although residual flow through the ischemic region exists even despite no flow through the brain-supplying artery. While a small number of experimental studies have failed to show persistent decreases in residual flow, the level of resolution of these techniques has not been sufficient to detect “no-reflow.” Nonetheless, significant alterations in both microvessel integrity and in the blood occur within the first minutes of flow cessation. These lead to obstruction of microvessels within the “territory-at-risk,” focal loss of permeability barriers, and changes in endothelium-astrocyte relationships.(del Zoppo and Mabuchi, 2003) An untested hypothesis is that the microvascular occlusions directly cause neuron injury.
Within hours of proximal MCA:O (in the striatum of the non-human primate), obstruction within the microvasculature occurs.(del Zoppo et al., 1991; Mori et al., 1992) Focal “no-reflow” results when the endothelium is activated, thereby leading to expression of the leukocyte adhesion receptors P-selectin and ICAM-1, the activation of PMN leukocytes, and their lodgment in the microvessel bed.(Granger et al., 1989; Bevilacqua et al., 1989; del Zoppo et al., 1991; McEver, 1991; Okada et al., 1994b; Haring et al., 1996b) These resulting microvessel defects of focal “no-reflow” can be prevented by the acute inhibition of PMN leukocyte β2-integrin-ICAM-1 interactions.(Mori et al., 1992) The microvessel obstructions also contain activated platelets and fibrin, caused by the generation of thrombin.(Okada et al., 1994a; Abumiya et al., 2000) The deposition of fibrin within the microvasculature originates from the exposure of perivascular TF to the plasma column and activation of prothrombin (factor II) to thrombin. Fibrin formation can be decreased significantly by blocking TF-factor VIIa interactions and preventing thrombin generation.(Okada et al., 1994a) Platelet activation entails interaction of the platelet surface integrin receptor αIIBβ3 with fibrin(ogen). Inhibition of these interactions via organic inhibitors or RGD-containing peptides can inhibit focal “no-reflow” in a dose-dependent manner in rodent or non-human primate focal ischemia models.(Abumiya et al., 2000) With increased concentrations of the inhibitor, significant symptomatic hemorrhage results. Those findings indicate that normal platelet function is required to suppress the risk of hemorrhage during focal ischemia, that the risk of hemorrhage can be manipulated by altering platelet function, and that the range of the risk-to-benefit is relatively narrow when platelet-fibrinogen interactions are disrupted.(Choudhri et al., 1998; Abumiya et al., 2000)
Whether inhibiting PMN leukocyte adherence within the microvasculature or preventing fibrin deposition can lead to a reduction in neuron injury has not yet been studied. But, separate experiments that have examined the impact of the acute use of low molecular weight heparins (LMWHs, e.g. enoxaparin) in rodent models of MCA occlusion, have shown significantly decreased in residual volumes of infarction and improvement in behavioral outcome.(Stutzmann et al., 2002) Early studies in the non-human primate had already shown that heparin (and ticlopidine in combination) could significantly reduce the density of microvessel occlusions in the ischemic territory. Those studies suggest that by limiting coagulation system activation, and blocking the effects of endothelial cell activation, the effects of Virchow’s triad can be reduced. It should be remembered that the vascular consequences of inflammation and coagulation system activation are interconnected.
Those observations indicate that within the target regions of ischemic injury, in addition to endothelial cell activation products of coagulation system activation (e.g. thrombin, fibrin) and platelet activation contribute to focal “no-reflow.”(Mori et al., 1992; Okada et al., 1994a; Abumiya et al., 2000) These events generate intravascular occlusions that would be expected to reduce local flow. Furthermore, in the post-capillary venules ischemia initiates endothelial adhesion receptor expression that promotes PMN leukocyte rolling, firm adherence, and the conditions for transmigration into the abluminal tissue. The endothelial cell-matrix-astrocyte interfaces are the staging structures for this transmigration. The impact of free radical generation at this interface,(Kontos et al., 1992) and of protease release during leukocyte transit have been speculated upon. Because the generation of some of the members of the MMP family, for instance, derive from the glial compartment it has been difficult to be certain of the contribution of transitting leukocytes in this process. But, the very early (~2 hours post-ischemia) appearance of pro-MMP-2, u-PA, and other proteases suggests that the parenchymal responses to ischemia could precede those of leukocyte courses.(Heo et al., 1999; Chang et al., 2003; Fukuda et al., 2004)
Transudation of fluid as edema occurs within the period of very early endothelial cell activation, and may have a number of sources, including increased endothelial cell permeability, the expression of water transit channels (e.g. of the SUR-1 type),(Simard et al., 2006) alterations in aquaporin-4 expression (co-expressed with dystroglycan),(Neely et al., 2001; Milner et al., 2008b) loss of endothelial cell-matrix adherence, alterations in astrocyte end-foot adherence, and others.
It is certain that focal ischemia initiates major alterations in the integrity of microvessels in the same territory-at-risk. But, sorting out the contributions of inflammatory cells and their mediators to these very early events is still quite incomplete.
8.0 Summary
A growing database indicates that injury to neurons within the ischemic territory and dynamic changes in the supply microvasculature occur simultaneously in consequence of occlusion of a brain-supplying artery. The neurovascular unit is a hypothetical construct that links structurally and functionally the components of the cerebral microvasculature with their interdependent neurons via intervening astrocytes that are a component of individual microvessels. The microvascular blood-endothelial cell interface is the staging area for cellular inflammation that is stimulated by the effects of focal ischemia on the territory-at-risk. The cerebral extravascular tissue generates inflammatory mediators and serves (like the microvasculature) as a target for their action. However, recent data indicates that by 2 hours after the onset of focal ischemia it is neurons (within the striatum) more distant from their neighboring microvessels that are significantly more likely to display injury acutely, negating a role for cellular inflammation within this early period. Within the neurovascular unit individual cell types have immune and/or inflammatory response properties. However, currently our knowledge of how the individual cell types in the CNS interact (e.g. endothelium and astrocyte end-feet, astrocytes and neurons) in response to an inflammatory stimulus is quite limited. This implies the need to understand the regional and whole tissue responses. Of more general consideration are the effects of inflammatory stimuli on the integrity of the ECM in the microvascular compartment of the neurovascular unit and the extravascular compartment, and the consequences for the entire unit. Ischemia causes the rapid processing of elements within the ECM, and some of these have mediator properties. These issues point to the “neurovascular unit” concept as a framework for identifying inter-relationships among cells that have heretofore been considered in isolation, for redirecting unsuccessful interventional work targeting ischemic neurons, and for understanding more incisively the responses of cerebral tissue to the inflammatory consequences of focal ischemia and directing potential treatments more successfully.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abumiya T, Fitridge R, Mazur C, Copeland BR, Koziol JA, Tschopp JF, Pierschbacher MD, del Zoppo GJ. Integrin alpha(IIb)beta(3) inhibitor preserves microvascular patency in experimental acute focal cerebral ischemia. Stroke. 2000;31:1402–1410. doi: 10.1161/01.str.31.6.1402. [DOI] [PubMed] [Google Scholar]
- Abumiya T, Lucero J, Heo JH, Tagaya M, Koziol JA, Copeland BR, del Zoppo GJ. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:1038–1050. doi: 10.1097/00004647-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Aloisi F. Immune function of microglia. GLIA. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Washington R, Wagnerova J, Dore-Duffy P. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res. 1996;52:127–142. doi: 10.1006/mvre.1996.0049. [DOI] [PubMed] [Google Scholar]
- Bär T. Morphometric evaluation of capillaries in different laminae of rat cerebral cortex by automatic image analysis: Changes during development and aging. In: Cervos-Navarro J, editor. Advances in Neurology. New York: Raven Press; 1978. pp. 1–9. [PubMed] [Google Scholar]
- Bär T. The vascular system of the cerebral cortex. Adv Anat Embryol Cell Biol. 1980;59(I–VI):1–62. doi: 10.1007/978-3-642-67432-7. [DOI] [PubMed] [Google Scholar]
- Bär T. Patterns of vascularization in the developing cerebral cortex. Ciba Foundation Symposium. 1983;100:20–36. doi: 10.1002/9780470720813.ch3. [DOI] [PubMed] [Google Scholar]
- Bernstein JJ, Getz R, Jefferson M, Kelemen M. Astrocytes secrete basal lamina after hemisection of rat spinal cord. Brain Res. 1985;327:135–141. doi: 10.1016/0006-8993(85)91507-0. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, Stengelin S, Gembrone MA, Jr, Seed B. Endothelial leukocyte adhesion molecule 1: An inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1162–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Carlson SS, Hockfield S. Central nervous system. In: Comper WD, editor. Extracellular Matrix. Vol. 1. Melbourne: Harwood Academic Publishers; 1996. pp. 1–23. [Google Scholar]
- Chang DI, Hosomi N, Lucero J, Heo JH, Abumiya T, Mazar AP, del Zoppo GJ. Activation systems for matrix metalloproteinase-2 are upregulated immediately following experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:1408–1419. doi: 10.1097/01.WCB.0000091765.61714.30. [DOI] [PubMed] [Google Scholar]
- Choudhri TF, Hoh BL, Zerwes HG, Prestigiacomo CJ, Kim SC, Connolly E, Sander E, Jr, Kottirsch G, Pinsky DJ. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J Clin Invest. 1998;102:1301–1310. doi: 10.1172/JCI3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Braun PE, Jackson DL, Kottis V, McKerracher L. Laminin overrides the inhibitory effects of peripheral nervous system and central nervous system myelin-derived inhibitors of neurite growth. J Neurosci Res. 1995;42:594–602. doi: 10.1002/jnr.490420417. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Silengo L, Tarone G. α6β1 integrin (laminin receptor) is down-regulated by tumor necrosis factor α and interleukin-1 beta in human endothelial cells. J Biol Chem. 1992;267:18303–18307. [PubMed] [Google Scholar]
- del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R. Integrin-Matrix Interactions in the Cerebral Microvasculature. Arterioscler Thromb Vasc Biol. 2006;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Schmid-Schönbein GW, Mori E, Copeland BR, Chang C-M. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–1284. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Lambracht-Hall M, Reichler J, Theoharides TC. Histochemical and ultrastructural characteristics of rat brain perivascular mast cells stimulated with compound 48/80 and carbachol. Neuroscience. 1990;39:209–224. doi: 10.1016/0306-4522(90)90234-u. [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. GLIA. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Isolation and characterization of cerebral microvascular pericytes. Meth Mol Med. 2003;89:375–382. doi: 10.1385/1-59259-419-0:375. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Pericytes: Pluripotent cells of the blood brain barrier. Curr Pharm Des. 2008;14:1581–1593. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- Engvall E, Davis GE, Dickerson K, Ruoslahti E, Varon S, Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: Localization of the neurite-promoting site. J Cell Biol. 1986;103:2457–2465. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenstermacher J, Nakata H, Tajima A, Lin S-Z, Otsuka T, Acuff V, Wei L, Bereczki D. Functional variations in parenchymal microvascular systems within the brain. Magn Reson Med. 1991;19:217–220. doi: 10.1002/mrm.1910190205. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt RC, Hough LB, Glick SD, Padawer J. Mast cells in rat thalamus: nuclear localization, sex difference and left-right asymmetry. Brain Res. 1984;323:209–217. doi: 10.1016/0006-8993(84)90291-9. [DOI] [PubMed] [Google Scholar]
- Gonzales C, Lin RCS, Chesselet MF. Relative sparing of GABAergic interneurons in the striatum of gerbils with ischemia-induced lesions. Neurosci Lett. 1992;135:53–58. doi: 10.1016/0304-3940(92)90134-s. [DOI] [PubMed] [Google Scholar]
- Granger DN, Benoit JN, Suzuki M, Grisham MB. Leukocyte adherence to venular endothelium during ischemia- reperfusion. Am J Physiol. 1989;257:G683–G688. doi: 10.1152/ajpgi.1989.257.5.G683. [DOI] [PubMed] [Google Scholar]
- Grant DS, Kleinman HK. Regulation of capillary formation by laminin and other components of the extracellular matrix. EXS. 1997;79:317–333. doi: 10.1007/978-3-0348-9006-9_13. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/reperfusion. J Cereb Blood Flow Metab. 1996;16:1373–1378. doi: 10.1097/00004647-199611000-00036. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26:2120–2126. doi: 10.1161/01.str.26.11.2120. [DOI] [PubMed] [Google Scholar]
- Haring H-P, Akamine P, Habermann R, Koziol JA, del Zoppo GJ. Distribution of integrin-like immunoreactivity on primate brain microvasculature. J Neuropathol Exp Neurol. 1996a;55:236–245. doi: 10.1097/00005072-199602000-00012. [DOI] [PubMed] [Google Scholar]
- Haring H-P, Berg EL, Tsurushita N, Tagaya M, del Zoppo GJ. E-selectin appears in non-ischemic tissue during experimental focal cerebral ischemia. Stroke. 1996b;27:1386–1392. doi: 10.1161/01.str.27.8.1386. [DOI] [PubMed] [Google Scholar]
- Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39:51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Herken R, Gotz W, Thies M. Appearance of laminin, heparan sulphate proteoglycan and collagen type IV during initial stages of vascularisation of the neuroepithelium of the mouse embryo. J Anat. 1990;169:189–195. [PMC free article] [PubMed] [Google Scholar]
- Hoogerbrugge PM, Suzuki K, Poorthuis BJ, Kobayashi T, Wagemaker G, van Bekkum DW. Donor-derived cells in the central nervous system of twitcher mice after bone marrow transplantation. Science. 1988;239:1035–1038. doi: 10.1126/science.3278379. [DOI] [PubMed] [Google Scholar]
- Hosomi N, Lucero J, Heo JH, Koziol JA, Copeland BR, del Zoppo GJ. Rapid differential endogenous plasminogen activator expression after acute middle cerebral artery occlusion. Stroke. 2001;32:1341–1348. doi: 10.1161/01.str.32.6.1341. [DOI] [PubMed] [Google Scholar]
- Hurwitz AA, Berman JW, Rashbaum WK, Lyman WD. Human fetal astrocytes induce the expression of blood-brain barrier specific proteins by autologous endothelial cells. Brain Res. 1993;625:238–243. doi: 10.1016/0006-8993(93)91064-y. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos CD, Wei EP, Williams JI, Kontos HA, Povlishock JT. Cytochemical detection of superoxide in cerebral inflammation and ischemia in vivo. Am J Physiol. 1992;263:H1234–H1242. doi: 10.1152/ajpheart.1992.263.4.H1234. [DOI] [PubMed] [Google Scholar]
- Liesi P. Do neurons in the vertebrate CNS migrate on laminin? EMBO J. 1985;4:1163–1170. doi: 10.1002/j.1460-2075.1985.tb03755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Jackson P, Tester AM, Diaconu E, Overall CM, Blalock JE, Pearlman E. Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro-Gly-Pro. Am J Pathol. 2008;173:144–153. doi: 10.2353/ajpath.2008.080081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, Hori M, Matsumoto M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31:1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Lucero J, Feng A, Koziol JA, del Zoppo GJ. Focal cerebral ischemia preferentially affects neurons distant from their neighboring microvessels. J Cereb Blood Flow Metab. 2005;25:257–266. doi: 10.1038/sj.jcbfm.9600027. [DOI] [PubMed] [Google Scholar]
- McEver RP. Selectins: Novel receptors that mediate leukocyte adhesion during inflammation. Thromb Haemost. 1991;65:223–228. [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Berg GI, Spatz M, del Zoppo GJ. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008a;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Spatz M, del Zoppo GJ. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab. 2008b;28:812–823. doi: 10.1038/sj.jcbfm.9600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori E, del Zoppo GJ, Chambers JD, Copeland BR, Arfors KE. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke. 1992;23:712–718. doi: 10.1161/01.str.23.5.712. [DOI] [PubMed] [Google Scholar]
- Mydel P, Shipley JM, ir-Kirk TL, Kelley DG, Broekelmann TJ, Mecham RP, Senior RM. Neutrophil elastase cleaves laminin-332 (laminin-5) generating peptides that are chemotactic for neutrophils. J Biol Chem. 2008;283:9513–9522. doi: 10.1074/jbc.M706239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano N, Aoyagi M, Hirakawa K. Extracellular matrix modulates the proliferation of rat astrocytes in serum-free culture. GLIA. 1993;8:71–76. doi: 10.1002/glia.440080202. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom BR, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci USA. 2001;98:14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg P, Waller S. Age-dependent vulnerability of brain choline acetyltransferase activity to transient cerebral ischemia in rats. Stroke. 1989;20:495–500. doi: 10.1161/01.str.20.4.495. [DOI] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994a;25:1847–1853. doi: 10.1161/01.str.25.9.1847. [DOI] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Mori E, Tung M-M, Thomas WS, del Zoppo GJ. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994b;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- Reed RK, Rubin K, Wiig H, Rodt SÅ. Blockade of β1-integrins in skin causes edema through lowering of interstitial fluid pressure. Circ Res. 1992;71:978–983. doi: 10.1161/01.res.71.4.978. [DOI] [PubMed] [Google Scholar]
- Risau W, Hallmann R, Albrecht U, Henke-Fahle S. Brain astrocytes induce the expression of an early cell surface marker for blood-brain barrier specific endothelium. EMBO J. 1986;5:3179–3183. doi: 10.1002/j.1460-2075.1986.tb04627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SK, Owolabi SA, Bagley J, Morin N, Cheng E, LeBlanc BW, Kim M, Harty P, Waxman SG, Saab CY. Activated polymorphonuclear cells promote injury and excitability of dorsal root ganglia neurons. Exp Neurol. 2008;210:286–294. doi: 10.1016/j.expneurol.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirén AL, Heldman E, Doron D, Lysko PG, Yue TL, Liu Y, Feuerstein G, Hallenbeck JM. Release of proinflammatory and prothrombotic mediators in the brain and peripheral circulation in spontaneously hypertensive and normotensive Wistar-Kyoto rats. Stroke. 1992;23:1643–1651. doi: 10.1161/01.str.23.11.1643. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV. Inflammation and brain edema: new insights into the role of chemokines and their receptors. Acta Neurochir. 2006;Suppl 96:444–450. doi: 10.1007/3-211-30714-1_91. [DOI] [PubMed] [Google Scholar]
- Strbian D, Karjalainen-Lindsberg ML, Kovanen PT, Tatlisumak T, Lindsberg PJ. Mast cell stabilization reduces hemorrhage formation and mortality after administration of thrombolytics in experimental ischemic stroke. Circulation. 2007a;116:411–418. doi: 10.1161/CIRCULATIONAHA.106.655423. [DOI] [PubMed] [Google Scholar]
- Strbian D, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab. 2006;26:605–612. doi: 10.1038/sj.jcbfm.9600228. [DOI] [PubMed] [Google Scholar]
- Strbian D, Tatlisumak T, Ramadan UA, Lindsberg PJ. Mast cell blocking reduces brain edema and hematoma volume and improves outcome after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007b;27:795–802. doi: 10.1038/sj.jcbfm.9600387. [DOI] [PubMed] [Google Scholar]
- Stutzmann JM, Mary V, Wahl F, Grosjean-Plot O, Uzan A, Pratt J. Neuroprotective profile of enoxaparin, a low molecular weight heparin, in in vivo models of cerebral ischemia or traumatic brain injury in rats: a review. CNS Drug Review. 2002;8:1–30. doi: 10.1111/j.1527-3458.2002.tb00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaya M, Haring H-P, Stuiver I, Wagner S, Abumiya T, Lucero J, Lee P, Copeland BR, Seiffert D, del Zoppo GJ. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Liu K-F, Copeland B, Seiffert D, Engler R, Garcia JH, del Zoppo GJ. DNA scission after focal brain ischemia. Temporal differences in two species. Stroke. 1997;28:1245–1254. doi: 10.1161/01.str.28.6.1245. [DOI] [PubMed] [Google Scholar]
- Temporin K, Tanaka H, Kuroda Y, Okada K, Yachi K, Moritomo H, Murase T, Yoshikawa H. IL-1beta promotes neurite outgrowth by deactivating RhoA via p38 MAPK pathway. Biochem Biophys Res Commun. 2008;365:375–380. doi: 10.1016/j.bbrc.2007.10.198. [DOI] [PubMed] [Google Scholar]
- The National Institutes of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Thompson HL, Thomas L, Metcalfe DD. Murine mast cells attach to and migrate on laminin-, fibronectin-, and matrigel-coated surfaces in response to Fc epsilon RI-mediated signals. Clin Exp Allergy. 1993;23:270–275. doi: 10.1111/j.1365-2222.1993.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Thornton P, Pinteaux E, Allan SM, Rothwell NJ. Matrix metalloproteinase-9 and urokinase plasminogen activator mediate interleukin-1-induced neurotoxicity. Mol Cell Neurosci. 2008;37:135–142. doi: 10.1016/j.mcn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha 6 beta 4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- Wang X, Yue T-L, Barone F, White R, Gagnon R, Feuerstein G. Concommitant cortical expression of TNF-α and IL-1β mRNA following transient focal ischemia. Mol Chem Neuropathol. 1994;23:103–114. doi: 10.1007/BF02815404. [DOI] [PubMed] [Google Scholar]
- Warth A, Mittelbronn M, Wolburg H. Redistribution of the water channel protein aquaporin-4 and the K+ channel protein Kir4.1 differs in low- and high-grade human brain tumors. Acta Neuropathol (Berl) 2005;109:418–426. doi: 10.1007/s00401-005-0984-x. [DOI] [PubMed] [Google Scholar]
- Webersinke G, Bauer H, Amberger A, Zach O, Bauer HC. Comparison of gene expression of extracellular matrix molecules in brain microvascular endothelial cells and astrocytes. Biochem Biophys Res Commun. 1992;189:877–884. doi: 10.1016/0006-291x(92)92285-6. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Suzuki T, Yamaya H, Matsuura N, Onodera H, Kogure K. Possible involvement of interleukin-1 brain edema formation. Neurosci Lett. 1992;142:45–47. doi: 10.1016/0304-3940(92)90616-f. [DOI] [PubMed] [Google Scholar]
- Yang GY, Gong C, Quin Z, Ye W, Mao Y, Bertz AL. Inhibition of TNFα (attenuates infarct volume and ICAM-1 expression in ischemic mouse brain. Neuroreport. 1998;9:2131–2134. doi: 10.1097/00001756-199806220-00041. [DOI] [PubMed] [Google Scholar]
- Young AR, Touzani O, Derlon J-M, Sette G, MacKenzie ET, Baron J-C. Early reperfusion in the anesthetized baboon reduces brain damage following middle cerebral artery occlusion: a quantitative analysis of infarction volume. Stroke. 1997;28:632–637. doi: 10.1161/01.str.28.3.632. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo M, Gobbo S, Rosengarten B, Hossmann K, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]