Abstract
Adiponectin is an adipocyte-derived cytokine that has attracted much attention because of its insulin-sensitizing effects in liver and skeletal muscle. Two adiponectin receptors, AdipoR1 and AdipoR2, have been cloned, but relatively little is known about their intracellular signaling mechanisms. We found that full-length adiponectin rapidly and robustly activates the ERK1/2 mitogen-activated protein kinase pathway in primary vascular smooth muscle, vascular endothelial cells, and hepatocytes. In a HEK293 cell model, we found that downregulating AdipoR1 and AdipoR2 simultaneously, but not individually, by RNA interference attenuated adiponectin-induced ERK1/2 activation, suggesting that either receptor was sufficient to mediate the response. Downregulation of T-cadherin, another adiponectin binding protein, enhanced the response. Downregulation of APPL1, an adapter protein and putative mediator of AdipoR1/R2 signaling, impaired adiponectin-stimulated ERK1/2 activation. Inhibiting PKA modestly attenutated ERK1/2 activation, while inhibition of Src family tyrosine kinases with PP2 abolished the response. The small GTPase inhibitor Clostridium difficile toxin B also produced complete inhibition. Adiponectin caused rapid, PP2-sensitive activation of Ras, but not the cAMP-regulated small GTPase, Rap1, suggesting that Src-dependent Ras activation is the dominant mechanism of adiponectin-stimulated ERK1/2 activation. To test whether Ras-ERK1/2 signaling by adiponectin was physiologically-relevant, we determined the effects of overexpressing AdipoR1, adiponectin, or both, on the rate of HEK293 cell growth. Overexpression of adiponectin alone, but not AdipoR1 alone, supported growth under serum-free conditions, while simultaneous expression of both led to further enhancement. Additional results suggest that adiponectin can exert proliferative effects by activating Ras signaling pathways.
Keywords: Adiponectin, Mitogen-Activated Protein Kinase, Mitogenesis, Ras, Tyrosine Kinase
Adiponectin is an abundant adipocyte-derived cytokine that plays an important role in regulating insulin sensitivity in peripheral tissues (1). It is uniquely expressed in adipose tissue and its high circulating levels (typically average 10µg/ml in men and 15µg/ml in women) are significantly decreased in the settings of obesity and diabetes mellitus, both in humans and murine models (1). Epidemiologically, low adiponectin levels correlate with the presence of cardiovascular disease, hypertension and the metabolic syndrome, clinical states associated with insulin resistance (2–5), as well as with increased breast cancer risk (5, 6). Experimentally, administration of recombinant adiponectin to adiponectin-deficient lipoatrophic diabetic mice and diabetic mice fed a high fat diet is sufficient to improve insulin sensitivity and suppress gluconeogenesis (7–9). Because of its potential to treat obesity-related insulin resistance, the structure and function of adiponectin has become the subject of considerable research interest.
Structurally, adiponectin belongs to the complement 1q family of proteins. The monomer contains three distinct structural domains: a signal peptide, a collagen-like stalk near the N-terminus, and a globular domain at the C-terminal end of the molecule. Circulating adiponectin is found primarily as disulfide-linked oligomers, composed of trimers, hexamers, and high molecular weight multimers containing up to 18 monomers (1, 10–14). It can also exist as a smaller molecular weight globular fragment that possesses biological activity when administered in vitro and in vivo (15). Eight naturally-occurring adiponectin polymorphisms have been described, some of which correlate with the presence of diabetes and hypoadiponectinemia. Two of these, G84R and G90S, fail to form high molecular weight multimers, suggesting that the oligomeric form of adiponectin is responsible for the biological function of adiponectin under normal physiological conditions (16).
Two distinct adiponectin receptors, AdipoR1 and R2, have been cloned (17). While both are expressed at detectable levels in most tissues and cell types, AdipoR1 is the major receptor expressed in skeletal muscle and heart, while AdipoR2 is most abundant in liver. Knockout mice lacking both AdipoR1 and R2 lose the metabolic actions effects adiponectin, exhibiting increased tissue triglyceride content, inflammation and oxidative stress, insulin resistance, and glucose intolerance, indicating that they are the predominant mediators of the metabolic effects of adiponectin in vivo (18). AdipoR1 and R2 are structurally similar, each consisting of seven predicted transmembrane domains, but they are structurally, topologically, and functionally distinct from heptahelical G protein-coupled receptors (GPCRs). The N-terminus of adiponectin receptors is cytoplasmic, while the C-terminus is extracellular, an orientation opposite to all reported GPCRs. Moreover, they do not appear to activate heterotrimeric G proteins. The ligand binding characteristics of AdipoR1 and R2 are distinguishable, in that only AdipoR1 appears to bind the cleaved globular form of adiponectin. AdipoR1 and R2 form homo- and heterodimers, although the biological significance of dimerization is unknown (17, 19). The glycosylphosphatidylinositol-anchored extracellular protein, T-cadherin, has also been identified as a binding protein for the hexameric and high molecular weight forms of adiponectin (20). Although T-cadherin lacks known biological function, it has been proposed to serve as a co-receptor for adiponectin.
Adiponectin elicits biological responses in most cell types. Its insulin-sensitizing actions are mediated primarily through activation of AMP-activated protein kinase (AMPK) and peroxisome proliferator activator receptor α (PPARα) signaling pathways, which facilitate fatty acid oxidation, glucose uptake and lactate production (2, 7, 8, 11). AdipoR1 and R2 appear to be functionally specialized with respect to these actions, since adenovirus-mediated expression of AdipoR1 in the liver of Leptin-receptor null mice preferentially enhances AMPK activation, while expression of AdipoR2 increases PPARα signaling. AdipoR1 and R2 knockout mice exhibit reciprocal loss of these functions (18). In endothelial cells, activation of the AMPK and Akt pathways by adiponectin stimulates angiogenesis and nitric oxide production (21, 22). In addition, adiponectin has been shown to activate the p38 and c-Jun-N-terminal kinase (JNK) pathways in myocytes and liver cells (23).
Despite intense interest, relatively little is currently known about the mechanisms by which the structurally unique adiponectin receptors elicit these pleiotropic cellular responses. In this study, we employed HEK293 cells, which express endogenous AdipoR1 and R2, to investigate adiponectin signaling. In these cells, adiponectin elicts rapid and robust activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) through a mechanism that involves Src-dependent Ras-activation. Furthermore, continuous exposure to adiponectin increases the rate of HEK293 cell growth. Since stimulation of Ras pathways is not a currently recognized element of adiponectin signaling, understanding how adiponectin affects mitogen-activated protein (MAP) kinase pathways may be relevant to efforts to target adiponectin receptors in the treatment of insulin resistance.
MATERIALS AND METHODS
Materials
Full-length adiponectin was from Axxora LLC (San Diego, CA). Clostridium difficile toxin B, H89, AG1478, PP2, Ro-31-8425, EGF, and PMA were from CalBiochem-EMD Biosciences Inc. (San Diego, CA). Isoproterenol was from Sigma Chemical Co. (St. Louis, MO). Eagle’s Minimal Essential Medium (MEM), fetal bovine serum and Penicillin/Streptomycin were from GIBCO (Grand Island, NY). Fugene 6 was from Roche Diagnostics (Indianapolis, IN). GeneSilencer was from Gene Therapy Systems Inc. (San Diego, CA). QuikChangeRII Site-Directed Mutagenesis kits were from Stratagene (Cedar Creek, TX). High Pure RNA Isolation kits were from Roche (Mannheim, Germany). iScript™ cDNA Synthesis kits and iQ SYBR Green Supermix were from BioRad Laboratories (Hercules, CA). Kits for assaying Ras and Rap1 activity were from Upstate Biotechnology Inc. (Lake Placid, NY) and Stressgen (Victoria, Alberta, Canada), respectively. Cell Titer-GloR cell viability assay kits were from Promega (Madison, WI). Mouse monoclonal anti-Myc IgG was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-APPL was from Novus Biological Inc (Littleton, CO) and rabbit polyclonal anti-T-cadherin was from ψProSci Inc (Poway, CA). Rabbit polyclonal anti-phospho-p44/42 ERK1/2 (pThr202 /pTyr204), anti-ERK1/2 (Thr202/Tyr204), anti-phosphop38α/β, anti-phospho-JNK1/2, anti-phospho-AMP kinase and anti-phospho-p90RSK, were from Cell Signaling Technology Inc (Beverly, MA). HA-11 affinity matrix was from Covance Inc. (Berkeley, CA). Rabbit polyclonal anti-FLAG IgG and anti-HA IgG were from Sigma Chemical Company (St. Louis, MO). Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse IgG were from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA).
cDNA constructs
Full-length cDNA clones of adiponectin (NM_004797.2), AdipoR1 (NM_015999.2) and AdipoR2 (NM_024551.2) were purchased from Origene (Rockville, MD). An N-terminal HA-epitope tag was introduced into the AdipoR1 clone and an N-terminal FLAG-epitope tag was introduced into the AdipoR2 clone immediately following the start codon. EcoRI sites were introduced into the 5’ and 3’ flanking regions of full-length adiponectin, which was subcloned into the pCXN2 expression plasmid (kindly provided by Dr. Jun-Ichi Miyazaki, Osaka University, Japan). An N-terminal FLAG-epitope was introduced into the adiponectin sequence following the predicted signal peptide sequence (amino acids 1–17). Sequence manipulations were performed using the QuikChangeRII Site-Directed Mutagenesis kit according to the manufacturer’s protocol.
Cell culture
HEK293 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in Eagle’s MEM supplemented with 10% (v/v) fetal bovine serum and penicillin-streptomycin. Monolayers were incubated for 5–6 h in serum-free growth medium supplemented with 10 mM HEPES (pH 7.4), 0.1% bovine serum albumin, and penicillin/streptomycin prior to stimulation.
Immunoprecipitation and immunoblotting
For HA-AdipoR1 and FL-AdipoR2 immunoprecipitation, 10 cm plates of appropriately transfected cells were solubilized in 1.0 ml lysis buffer [50 mM Tris-Base (pH 7.5), 0.5% deoxycholic acid, 1% Triton X-100, 10 mM EDTA, 0.5 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 5 µg/ml leupeptin, 5 µg/ml aprotinin, 1 µg/ml peps tatin A, 100 µM benzaminidine] and lysates clarified by centrifugation. HA-tagged AdipoR1 and FLAG-tagged AdipoR2 were immunoprecipited using 20 µl of 50% slurry of HA11 anti-HA or M2 anti-FLAG affinity matrix, respectively, in each case with constant agitation overnight at 4°C. Immune complexes were washed three times with lysis buffer, eluted with sample buffer [25 mM Tris pH6.8, 20% (w/v) SDS, 10% glycerol, 50 mM DTT, 0.01% (w/v) bromophenol blue] and resolved by SDS-Tris-Glycine polyacrylamide gel electrophoresis on precast SDS-free 4–20% gradient gels. Immunoprecipitated HA-AdipoR1, FL-AdipoR2 and Myc-tagged APPL1 were detected by immunoblotting with polyclonal IgG against the HA, FLAG or Myc epitopes.
For whole cell lysate immunoblots, monolayers were stimulated as described in the figure legends, and lysed directly in sample buffer. Samples containing 20 µg of cell protein were resolved by polyacrylamide gel electrophoresis and transferred to nitrocellulose. Proteins on nitrocellulose were detected with the appropriate monocloncal or polyclonal IgG (1:1000 dilution; overnight at 4 °C) using horseradish peroxidase-conjugated polyclonal donkey anti-mouse or anti-rabbit IgG (1:10,000 dilution; 1–2 h at room temperature) as secondary antibody. Immune complexes were visualized by enzyme-linked chemiluminescence recorded on X-Omat AR film and quantified using a Fluor-S MultiImager.
siRNA downregulation of adiponectin receptors, T-cadherin and APPL1 expression
Double-stranded siRNA composed of 21-nt duplex RNA and 2-nt 3’dTdT overhangs targeting AdipoR1 (‘5-AAGGACAACGACUAUCUGCUACA-3’) and AdipoR2 (5’-AAGGAGUUUCGUUUCAUGAUC GG-3’), corresponding to positions 313–335 and 1110–1130 from the start codon, respectively, were synthesized by QIAGEN (Valencia, CA). These siRNA sequences are identical to ones reported previously (17). A pool of three siRNAs from Ambion, Inc. (Austin, TX) (5’-CCGACCGA UCUUUCGGGAA-3’; 5’-GCGUGUACACUGCUCUCUU-3’; and, 5’-CGCUGUUUGUUUCAUAUA U-3’) were used to target T-cadherin. Two pooled siRNAs (5’-CACACCUGACCUCAAAACUUU-3’; and, 5’-UUAGGAUCUGAGUCUACAA-3’) were used to target APPL1. RNA duplexes with irrelevant sequences were used as negative controls. HEK293 cells at 50–70% confluence were plated in 6-well plates at least 24 h before siRNA transfection. GeneSilencer transfection reagent (6 µl) was diluted with serum free MEM (37 µl) and RNA mixtures containing 2.4 µg siRNA, 29 µl siRNA diluent, and 15 µl serum -free MEM were prepared. Both solutions were allowed to stand for 5 min at room temperature, then combined and mixed by inversion. The mixture was allowed to stand an additional 20 min at room temperature and then was added to cultures containing 1 ml of fresh serum-free MEM. Cells were incubated for 4 h at 37°C and then an additional 1 ml of MEM containing 20% FBS and 2% penicillin/streptomycin was added to each well. Twenty-four hours after transfection, cells were plated on Collagen I-coated 12-well plates and cultured an additional 24–48 h. The efficiency of mRNA downregulation was determined by quantitative real time PCR and protein immunoblotting performed 72 h after transfection.
Quantitative real time PCR
AdipoR1 and AdipoR2 mRNA abundance was assayed by quantitative real-time PCR using the iCycler iQ™ Multicolor Real-time Detection System (Bio-Rad, Hercules, CA). Total RNA was isolated from cells using the High Pure RNA Isolation Kit (Roche Diagnostics GmbH, Indianapolis, IN). cDNA was synthesized from each RNA sample (1 µg of total RNA per reaction) using the iScript™ cDNA Synthesis Kit (Bio-Rad) by amplifying the reaction one cycle in a GENIUS thermocycler (25 °C for 5 min, 42 °C for 3 0 min, 80 °C for 5 min). The amplified cDNA was used as a template for quantitative real-time PCR using iQ SYBR Green Supermix (Bio-Rad) according to the manufacturer’s instructions. Primer sets were designed to span intron-exon borders to distinguish amplified cDNA from genomic DNA. The conditions for the PCR reactions were denaturation at 95°C for 10 sec, annealing at 50°C for 45 sec, for 40 cycles. Data were analyzed using the iCycler software (Bio-Rad). Expression of AdipoR1, AdipoR2, T-cadherin and APPL1 mRNA in each sample was normalized to the mRNA expression level of the housekeeping gene Cyclophilin A. Specific primer pairs used for amplification were:
AdipoR1 sense 5’-ATATGATGTGCTCCCTGA-3’
AdipoR1 anti-sense 5’- ATGTTGCCAGTTTCTGTA-3’
AdipoR2 sense 5’-AAGAGCATTTTCAGAATACA-3’
AdipoR2 anti-sense 5’-TATTTGGGCGAAACATATAA-3’
T-cadherin sense 5’-AGGACCAGTCAATTCTAAAC-3’
T-cadherin anti-sense 5’-GTTATGTTTCTCAGAGCAAC-3’
APPL1 sense 5’-CAG GGTGGAAATTT AATGAG-3’
APPL1 anti-sense 5’-AGGTGATCTGAAAACAATATC-3’
Cyclophilin A (Peptidylprolyl Isomerase A) sense 5’-TGTTGATGTAGGCTTTATTT-3’
Cyclophilin A anti-sense 5’-CATTTTCAAT AT-3’.
Small GTPase activity
The activation state of Ras and Rap1 was determined by measuring binding of the GTP-bound form of Ras and Rap1 to Raf-1 RBD agarose or GST-ralGDS-RBD, respectively. HEK293 cells were grown to 85–90% confluence in 100 mm dishes, stimulated as described in the figure legends and lysed using the buffers supplied by the kit manufacturer. Lysates were clarified by centrifugation and incubated with Raf-1 RBD agarose or GST-ralGDS-RBD overnight at 4 C°. After washing, precipitated GTP-bound Ras and Rap1 were resolved by 4–20% Tris-glycine SDS-polyacrylamide gel electrophoresis and quantified by immunoblotting using anti-Ras or anti-Rap1 IgG.
Cell Growth
Transient transfection of HEK293 cells with plasmids encoding HA-AdipoR1, FLAG-adiponectin, or both, was performed on 50–70 percent confluent monolayers in 100mm dishes using FuGene 6 according to the manufacturer’s instructions. Each plate received 10 µg of total plasmid DNA per dish and 3 µl Fugene per µg DNA. Twenty-four hours after transfection, cells were split into Collagen I-coated 96 well plates at a density of 1–3 × 103 cells per well. The following day, growth medium was replaced with serum-free medium and cell number was assayed every 24 hours for three days using the Cell Titer-GloR Luminescent Kit (Promega, Madison, WI) according to manufacturer’s instructions. Luminescent signals were measured using a SPECTRAmax M2 instrument (Molecular Devices, Sunnyvale, CA). Data were analyzed using ANOVA with post hoc analysis (SigmaStat v3.0, SYSTAT, San Jose, CA) to determine the statistical significance of transfection with individual genes compared to cells transfected with empty vector at each time interval.
RESULTS
Full-length adiponectin activates the ERK1/2 pathway in vascular smooth muscle, vascular endothelial cells, hepatocytes and HEK293 cells
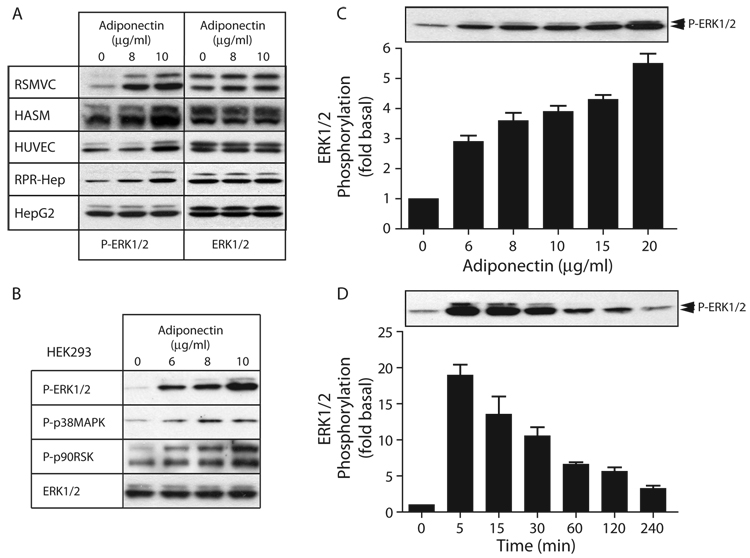
In addition to its effects on AMPK and PPARα, adiponectin stimulates Akt and nitric oxide production in endothelial cells (21, 22) and activates the JNK and p38 MAP kinase pathways in muscle and liver (23). Its reported effects on ERK1/2 activity have been variable. While the globular form of adiponectin activates ERK1/2 in cardiac fibroblast cells (24), previous studies failed to detect adiponectin-stimulated ERK1/2 phosphorylation in C2C12 myocytes (17). Figure 1A depicts the effect of full-length adiponectin on ERK1/2 phosphorylation in several primary cell types. Stimulation for 5 min increased ERK1/2 phosphorylation in vascular smooth muscle cells derived from rat and human aorta, human umbilical vein endothelial cells and primary rat hepatocytes. In contrast, immortalized HepG2 cells failed to respond, probably due to high basal levels of ERK1/2 phosphorylation. Figure 1B depicts the effects of adiponectin on MAP kinase pathway activity in serum-deprived HEK293 cells. Full-length adiponectin robustly activated the ERK1/2 and p38α/β pathways, and increased phosphorylation of the ERK1/2 substrate, p90RSK. We were unable to detect adiponectin-stimulated phosphorylation of JNK1/2 (data not shown). As shown in Figure 1C, adiponectin-stimulated ERK1/2 activation was dose dependent over concentrations in the 5–20 µg/ml range, consistent with its biological actions in other cells. As shown in Figure 1D, ERK1/2 activation was rapid in onset, with maximal phosphorylation occurring within 5 min of stimulation and with detectable increases persisting for at least 4 h.
Figure 1. Adiponectin activates the ERK1/2 pathway in primary vascular cells, hepatocytes and HEK293 cells.
A) Serum-deprived rat aortic vascular smooth muscle cells (RVSMC), human aortic smooth muscle cells (HASM), human umbilical vein endothelial cells (HUVEC), primary rat hepatocytes (RPR-Hep), and HepG2 cells were exposed to the indicated concentrations of full-length adiponectin for 5 min, after which phosphorylation of ERK1/2 was determined by immunoblotting whole cell lysates. Total ERK1/2 was blotted to confirm equal protein loading. B) Serum-deprived HEK293 cells were exposed to the indicated concentrations of full-length adiponectin for 5 min, after which phosphorylation of ERK1/2, p38α/β and p90RSK was determined by immunoblotting whole cell lysates. Total ERK1/2 was blotted to confirm equal protein loading. C) Concentration dependence of adiponectin-stimulated ERK1/2 phosphorylation. Cells were stimulated for 5 min with adiponectin (6 to 20µg/ml) and ERK1/2 phosphorylation determined. D) Time course of adiponectin-stimulated ERK1/2 phosphorylation. Cells were treated with 8 µg/ml adiponectin for the indicated time (5 min to 4 h) and ERK1/2 phosphorylation determined. In panels C–D, a representative immunoblot is shown above a bar graph depicting the Mean ± SEM determined in three separate experiments. ERK1/2 phosphorylation is expressed as the fold increase above the basal level in unstimulated cells.
Both AdipoR1 and AdipoR2 contribute to adiponectin-stimulated ERK1/2 activation
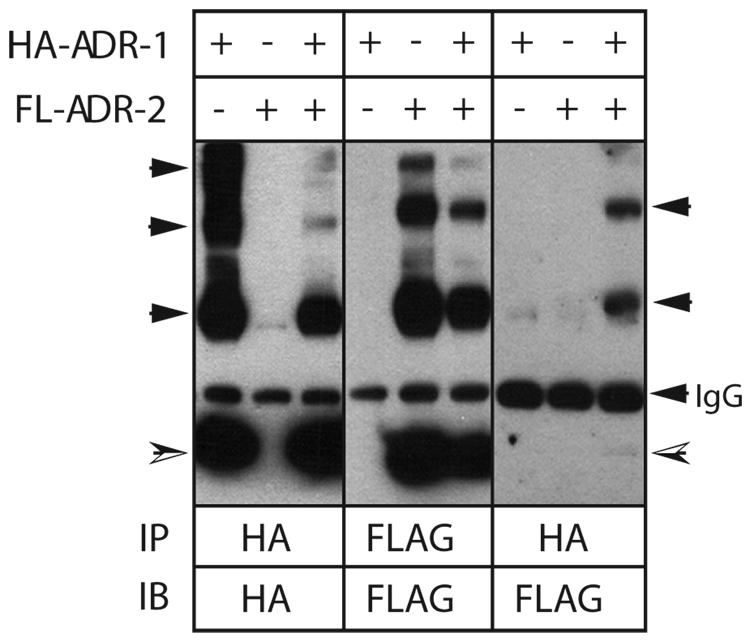
Previous studies have shown that AdipoR1 and AdipoR2 form homo- and heterodimers (17). This phenomenon is shown in Figure 2 using HEK293 cells transiently expressing HA epitope-tagged AdipoR1 (HA-AdipoR1) and FLAG epitope-tagged AdipoR2 (FL-AdipoR2). Under non-denaturing conditions, most immunoprecipitated HA-AdipoR1 migrated at its predicted monomeric molecular weight of approximately 42 kDa (left panel). However, a significant fraction (approximately 30%) exhibited slower electrophoretic mobility, consistent with either post-translational modification or the formation of receptor multimers. That these higher molecular weight species represented AdipoR1 was confirmed by probing identical blots with anti-AdipoR1 IgG (data not shown). A similar complex band pattern was observed with immunoprecipitated FL-AdipoR2 (center panel). When HA-AdipoR1 immunoprecipitates from cells expressing both receptors were probed for the presence of co-precipitated FL-AdipoR2, we detected FLAG-epitope in bands corresponding to the higher molecular weight species of FL-AdipoR2, suggesting that a fraction of the overexpressed receptors existed as relatively stable dimers or higher-order multimers (right panel).
Figure 2. Co-immunoprecipitation of overexpressed AdipoR1 and AdipoR2.
Serum-starved HEK293 cells transiently expressing HA epitope-tagged AdipoR1 (HA-ADR-1), FLAG epitope-tagged AdipoR2 (FL-ADR-2), or both, were lysed in detergent buffer and tagged receptors immunoprecipitated using anti-HA or anti-FLAG affinity resin. After resolution on SDS-free polyacrylamide gels, the presence of HA-AdipoR1 or FL-AdipoR2 was determined by immunoblotting for the HA or FLAG epitope. The bottom arrows indicate HA-AdipoR1 (42 kDa) and FL-AdipoR2 (35 kDa) bands migrating at their predicted monomeric molecular weight. The identity of the higher molecular weight species was confirmed by immunoblotting with anti-AdipoR1 and anti-AdipoR2 IgG (not shown). Data shown are from one of three experiments that gave similar results.
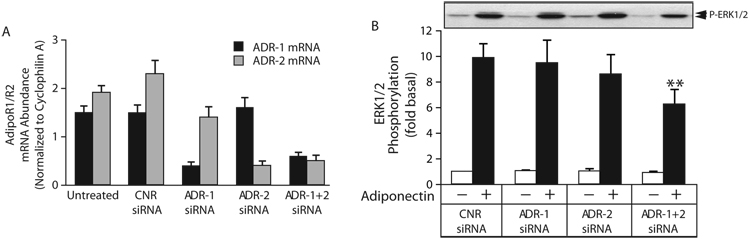
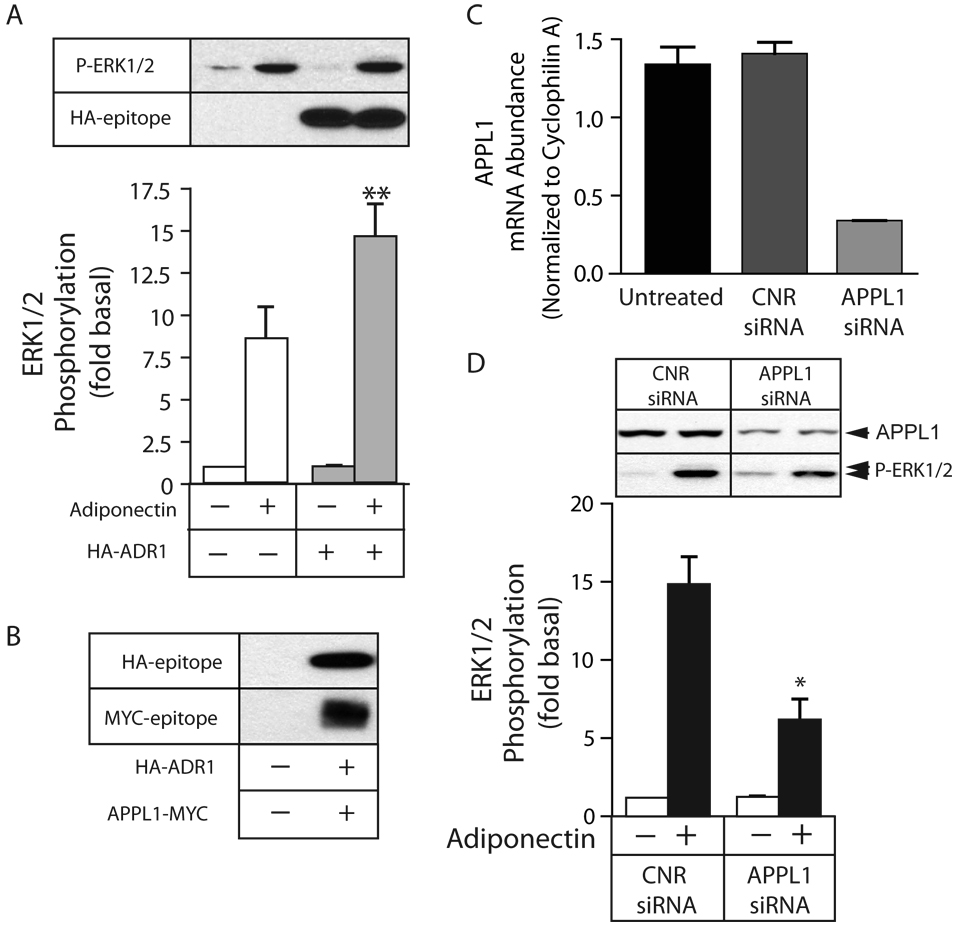
Data from AdipoR1 and AdipoR2 knockout mice suggest that the two receptors mediate different signaling events in liver, with AdipoR1 primarily acting on AMPK and AdipoR2 primarily affecting PPARα (18). To determine the role of AdipoR1 and AdipoR2 in ERK1/2 activation, we performed transcriptional downregulation of endogenous AdipoR1 and AdipoR2 using small interfering RNAs (siRNA). As shown Figure 3A, mRNA abundance of the two adiponectin receptors was similar in untreated HEK293 cells. Transfection with individual receptor-specific siRNA selectively reduced AdipoR1 or AdipoR2 mRNA levels by 75–80% compared to cells treated with scrambled siRNA. Simultaneous transfection with the two active siRNA sequences produced 60–75% reduction of each receptor mRNA. Figure 3B shows the effect of AdipoR1, AdipoR2 and AdipoR1/R2 mRNA downregulation on adiponectin-stimulated ERK1/2 activation. While neither siRNA alone was sufficient to attenuate the signal, in combination they reduced ERK1/2 activation by about 50% relative to scrambled siRNA treated cells.
Figure 3. Transcriptional down regulation of AdipoR1 and AdipoR2 attenuates adiponectin-mediated ERK1/2 activation.
A) Quantification of AdipoR1 and AdipoR2 mRNA level by real time PCR. HEK293 cells were transfected with scrambled control siRNA (CNR) or siRNA targeting AdipoR1 (ADR-1), AdipoR2 (ADR-2) or both (ADR-1+2) as described and the efficiency of transcriptional down regulation was assessed by quantitative real time PCR. The abundance of AdipoR1 and AdipoR2 mRNA in each sample was normalized to the mRNA expression levels of the housekeeping gene, Cyclophilin A. B) Effect of adiponectin receptor downregulation on adiponectin-stimulated ERK1/2 activation. Serum deprived cells were stimulated for 5 min with vehicle or full-length adiponectin (8 µg/ml) and ERK1/2 activity was determined by immunoblotting with anti-phospho-ERK1/2. ERK1/2 phosphorylation is expressed as the fold increase above the basal level in unstimulated, CNR-transfected cells. A representative phospho-ERK1/2 immunoblot is shown above a bar graph representing the Mean ± SEM from six independent experiments. ** less than CNR treated, p<0.005.
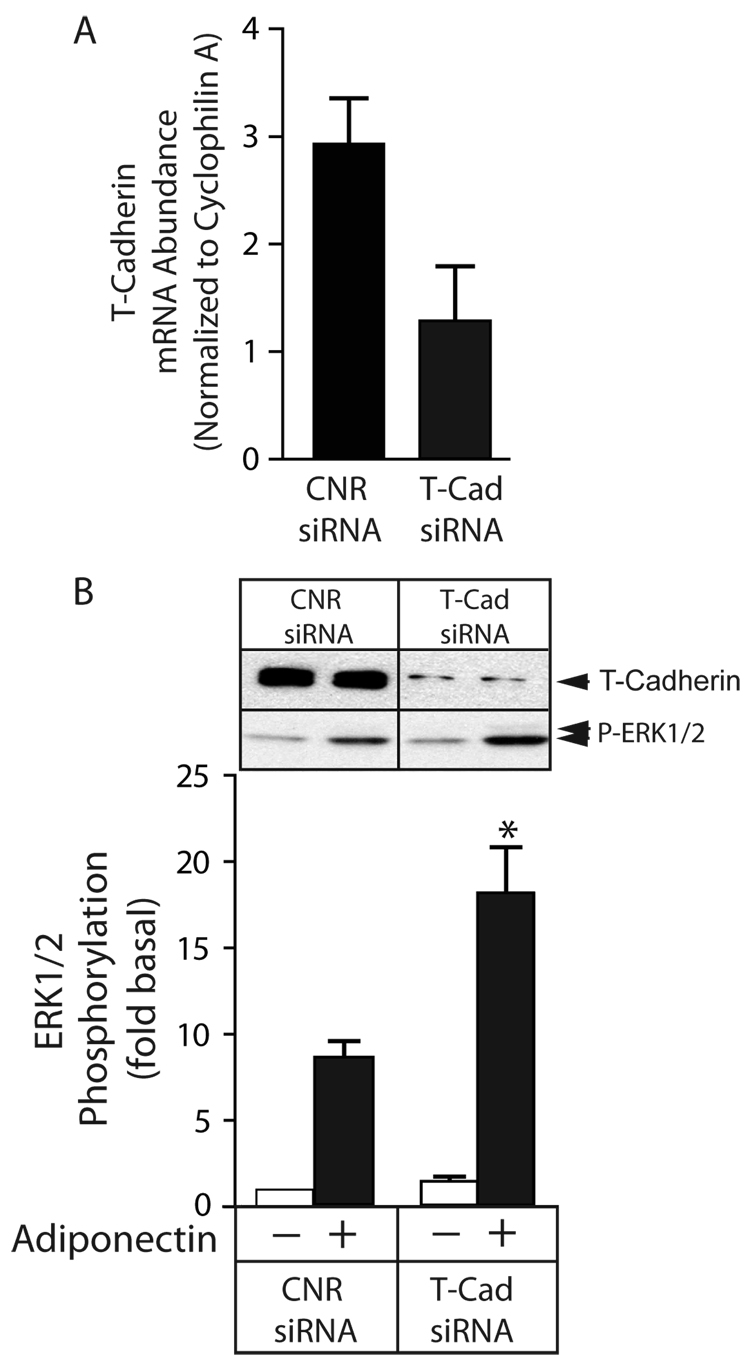
The opposite effect was observed when cells were treated with siRNA targeting T-cadherin. As shown Figure 4A, T-cadherin mRNA abundance was reduced by approximately 60% by T-cadherin targeted siRNA compared to the scrambled siRNA sequence. As shown in Figure 4B, this reduction was associated with a doubling of adiponectin-stimulated ERK1/2 phosphorylation. Collectively, these results suggest that adiponectin-stimulated ERK1/2 activation, like most of its reported biological effects, is mediated via AdipoR1 and AdipoR2. In contrast, T-cadherin, which has been proposed to act as an adiponectin co-receptor (20), either competes with AdipoR1/R2 for adiponectin binding or interferes with the coupling of adiponectin-bound AdipoR1/R2 to downstream effectors. The failure to achieve complete inhibition in AdipoR1/R2 siRNA treated cells probably reflects incomplete downregulation at the protein level rather than the existence of another, as yet unidentified adiponectin receptor.
Figure 4. Transcriptional down regulation of T-cadherin enhances adiponectin-mediated ERK1/2 activation.
A) Quantification of T-cadherin mRNA level by real time PCR. HEK293 cells were transfected with scrambled control siRNA (CNR) or siRNA targeting T-cadherin (T-Cad) as described and the efficiency of transcriptional down regulation was assessed by quantitative real time PCR. The abundance of T-cadherin mRNA in each sample was normalized to the mRNA expression levels of the housekeeping gene, Cyclophilin A. B) Effect of T-cadherin downregulation on adiponectin-stimulated ERK1/2 activation. Serum deprived cells were stimulated for 5 min with vehicle or full length adiponectin (8 µg/ml) and ERK1/2 activity was determined by immunoblotting with anti-phospho-ERK1/2. ERK1/2 phosphorylation is expressed as the fold increase above the basal level in unstimulated SCR transfected cells. Representative T-cadherin and phospho-ERK1/2 immunoblots are shown above a bar graph representing the Mean ± SEM of ERK1/2 phosphorylation from three independent experiments. * greater than CNR transfected, p<0.05.
The APPL1 adapter protein mediates AdipoR1/R2 stimulated ERK1/2 activation
The adapter protein, APPL1, has been reported to interact with the intracellular N-terminus of AdipoR1 (25). This interaction is stimulated by adiponectin and is involved in mediating adiponetic effects on lipid oxidation and glucose transport. Thus, we tested whether APPL1 plays a role in ERK1/2 activation by AdipoR1/R2 in HEK293 cells. As shown in Figure 5A, transient overexpression of HA epitope-tagged AdipoR1 significantly increased adiponectin-stimulated ERK1/2 phosphorylation with no effect on basal ERK1/2 activity. As shown in Figure 5B, HA-AdipoR1 and Myc-tagged APPL1 robustly coprecipitated under basal conditions. We did not detect an effect of adiponectin on APPL binding in this overexpression system (data not shown). To assess whether endogenous APPL1 played a role in ERK1/2 activation, we employed siRNA targeting APPL1. As shown in Figure 5C, transfection with active siRNA led to a 75% reduction in APPL1 mRNA levels compared to scrambled siRNA controls. As shown in Figure 5D, downregulation of APPL1 reduced adiponectin-stimulated ERK1/2 activation by about 60%, consistent with a signal transmitted via an AdipoR1/R2-APPL1 complex.
Figure 5. Downregulation of the adapter protein APPL1 inhibits adiponectin-mediated ERK1/2 activation.
A) Overexpression of AdipoR1 enhances adiponectin-stimulated ERK1/2 activation. HEK293 cells transfected with empty vector or an expression plasmid encoding HA-epitope-tagged AdipoR1 (HA-ADR1) were stimulated with full length adiponectin (AD; 8 µg/ml) for 5 min, after which phosphorylation of ERK1/2 was determined by immunoblotting. A representative anti-HA epitope immunoblot demonstrating expression of HA-ADR1 and a phospho-ERK1/2 immunoblot showing the resultant effects on adiponectin-stimulated ERK1/2 phosphorylation is shown above a a bar graph representing the Mean ± SEM for phospho -ERK1/2 from three independent experiments. ** greater than vector transfected, p<0.005. B) Coprecipitation of APPL1 with AdipoR1. HA-ADR1 was immunoprecipitated from HEK293 cells transfected with empty vector or plasmids encoding HA-ADR1 and Myc-tagged APPL1 using anti-HA agarose. Coprecipitating HA-ADR1 and Myc-APPL1 were detected by blotting the immunoprecipitates for the HA and Myc epitopes, respectively. C) Quantification of APPL1 mRNA level by real time PCR. HEK293 cells were transfected with scrambled control siRNA (CNR) or siRNA targeting APPL1 and the efficiency of transcriptional down regulation was assessed by quantitative real time PCR. D) Effect of APPL1 downregulation on adiponectin-stimulated ERK1/2 activation. Serum deprived cells were stimulated for 5 min with vehicle or full length adiponectin (8 µg/ml) and ERK1/2 activity was determined by immunoblotting with anti-phospho-ERK1/2. ERK1/2 phosphorylation is expressed as the fold increase above the basal level in unstimulated CNR-transfected cells. Representative APPL1 and phospho-ERK1/2 immunoblots are shown above a bar graph representing the Mean ± SEM from three independent experiments. ** greater than untransfected, p<0.005; * less than untransfected, p<0.05.
Adiponectin activates ERK1/2 in HEK293 cells primarily through Src-dependent Ras activation
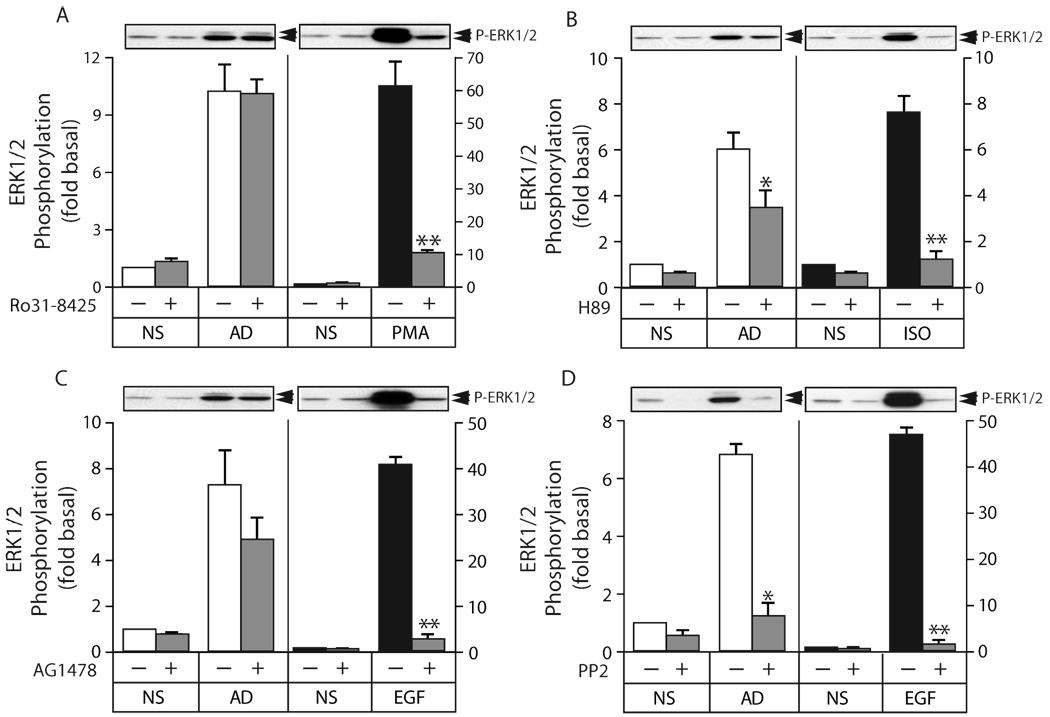
The ERK1/2 cascade is composed of three kinases that phosphorylate one another in succession; c-Raf-1 or B-Raf, MEK1/2 and ERK1/2 (26). Activity of the most proximal kinase, a Raf isoform, is commonly regulated by small GTPases, including Ras isoforms and Rap1. Ras classically functions in tyrosine kinase-mediated signaling and positively regulates c-Raf-1, while Rap-1 functions in cAMP signaling cascades, and activates the B-Raf isoform (27, 28). In addition, c-Raf-1 can undergo Ras-independent activation, notably as a consequence of phosphorylation by protein kinase C (PKC) (29). All three pathways exist in HEK293 cells (30, 31). To determine the mechanism by which AdipoR1/R2 stimulate ERK1/2, we initially employed a panel of selective inhibitors targeting protein kinase A (PKA), PKC, and Src family nonreceptor tyrosine kinases. We also tested an inhibitor of the epidermal growth factor (EGF) receptor tyrosine kinase, since transactivation of EGF receptors resulting from regulated shedding of membrane-associated EGF family peptide growth factors, accounts for Ras activation by diverse extracellular stimuli (32).
As shown in Figure 6A, treatment of HEK293 cells with the PKC inhibitor, R031-8425, at a concentration sufficient to block >90% of phorbol ester-stimulated ERK1/2 activation had no effect on the response to adiponectin. Figure 6B demonstrates that treatment with the PKA inhibitor, H89, produced partial, but significant, attenuation of the adiponectin signal under conditions where cAMP-dependent ERK1/2 activation via the β2-adrenergic receptor was abolished, indicating that cAMP-PKA signaling alone cannot account for adiponectin action. As shown in Figure 6C, the EGF receptor inhibitor, tyrphostin AG1478, at a concentration sufficient to block >95% of the EGF response did not significantly inhibit the adiponectin response. As shown in Figure 6D, the Src family kinase inhibitor, PP2, completely blocked both EGF and adiponectin-induced ERK1/2 phosphorylation. These data suggest that activation of the ERK1/2 cascade in HEK293 cells requires Src activity, but is independent of EGF receptor transactivation.
Figure 6. Effect of PKC, PKA, EGF receptor and Src inhibitors on adiponectin-mediated ERK1/2 activation.
A) Serum-deprived HEK293 cells were pretreated with the PKC inhibitor R031-8425 (1 µM) for 30 min prior to stimulation with full length adiponectin (8 µg/ml) or PMA (100 nM) for 5 min, after which phosphorylation of ERK1/2 was determined by immunoblotting. B) Cells were pretreated with the PKA inhibitor H89 (10 µM) for 30 min prior to stimulation with full length adiponectin (8 µg/ml) or isoproterenol (ISO; 1 µM) for 5 min, after which phosphorylation of ERK1/2 was determined. C) Cells were pretreated with the EGF receptor inhibitor AG1478 (200 nM) for 30 min prior to stimulation with full length adiponectin (8 µg/ml) or EGF (10 ng/ml) for 5 min, after which phosphorylation of ERK1/2 was determined. D) Cells were pretreated with the Src family kinase inhibitor PP2 (10 µM) for 30 min prior to stimulation with full length adiponectin (8 µg/ml) or EGF (10 ng/ml) for 5 min, after which phosphorylation of ERK1/2 was determined. In panels A–D, a representative phospho-ERK1/2 immunoblot is shown above a bar graph representing the Mean ± SEM from four independent experiments. ERK1/2 phosphorylation is expressed as the fold increase above the basal level in unstimulated cells not exposed to the inhibitor. * less than untreated, p<0.05; ** less than untreated, p<0.005.
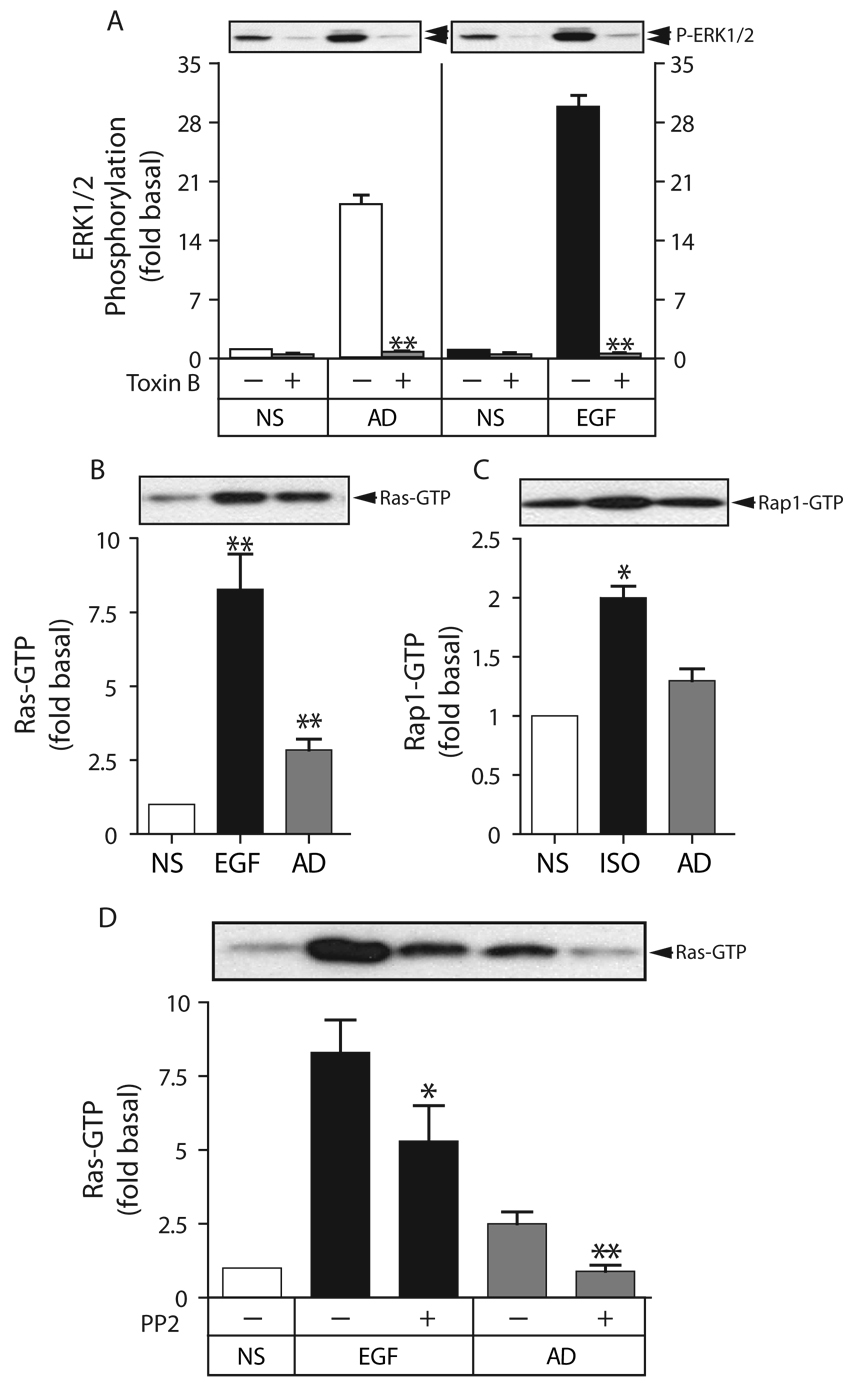
To initially determine whether small GTPases were involved in AdipoR1/R2 signaling to ERK1/2, we employed Clostridium difficile toxin B, a high molecular weight glucosyltransferase that non-selectively inhibits small GTPase activity. As shown in Figure 7A, toxin B treatment abrogated both adiponectin- and EGF-stimulated ERK1/2 activation. Phorbol ester (PMA)-stimulated ERK1/2 activation, which in HEK293 cells does not require Ras (33), was not significantly inhibited (data not shown). Because adiponectin-stimulated ERK1/2 activation was affected by PP2 and H89, suggesting involvement Src and cAMP-PKA signaling, respectively, we directly measured whether adiponectin treatment activated Ras and Rap1. As shown in Figure 7B and Figure 7C, adiponectin significantly increased GTP loading of Ras, but not Rap1. To determine whether Src activity was required for adiponectin to activate Ras, we tested whether PP2 affected adiponectin-stimulated Ras activation. As shown in Figure 7D, PP2, which partially inhibits EGF receptor-mediated Ras activation, completely blocked Ras-GTP loading stimulated by adiponectin. Collectively, these data suggest that the dominant mechanism by which AdipoR1/R2 stimulates ERK1/2 in HEK293 cells is by eliciting Src-dependent activation of Ras. A minor contribution from the PKA-Rap1 pathway cannot be excluded, since H89 produced partial inhibition of the ERK1/2 signal, but we were unable to detect significant adiponectin-stimulation of Rap1.
Figure 7. Adiponectin stimulates Src-dependent activation of Ras, but not Rap1, and small GTPase-dependent ERK1/2 activation.
A) Effect of the small GTPase inhibitor Clostridium difficile toxin B on adiponectin-mediated ERK1/2 activation. Serum-deprived HEK293 cells were pretreated with Toxin B (200 ng/ml) for 1 h prior to stimulation with vehicle (NS), full length adiponectin (AD; 8 µg/ml) or EGF (10 ng/ml) for 5 min, after which phosphorylation of ERK1/2 was determined by immunoblotting. A representative phospho-ERK1/2 immunoblot is shown above a bar graph representing the Mean ± SEM from four independent experiments. ERK1/2 phosphorylation is expressed as the fold increase above the basal level in unstimulated cells not exposed to Toxin B. ** less than untreated, p<0.005. B) Effect of adiponectin on Ras activation state. Cells were stimulated with adiponectin (8 µg/ml) or EGF (10 ng/ml) for 5 min, after which GTP-bound Ras was purified by precipitation with Raf-1 RBD agarose and quantified by immunoblotting as described. C) Effect of adiponectin on Rap1 activation state. Cells were stimulated with adiponectin (8 µg/ml) or isoproterenol (ISO; 1 µM) for 5 min, after which GTP-bound Rap1 was purified by precipitation with GST-RalGDS-RBD agarose and quantified by immunoblotting as described. In panels B–C, a representative immunoblot is shown above a bar graph representing the Mean ± SEM from three independent experiments. Ras and Rap1 activity are expressed as the fold increase above the level of GTP-bound GTPase detected in unstimulated cells (NS). * greater than NS, p<0.05; ** greater than NS, p<0.005. D, Effect of the Src inhibitor PP2 on adiponectin-stimulated Ras activation. Cells were pretreated with PP2 (10 µM) for 30 min prior to stimulation with full length adiponectin (8 µg/ml) or EGF (10 ng/ml) for 5 min, after which GTP-bound Ras was assayed as described. A representative immunoblot is shown above a bar graph representing the Mean ± SEM from three independent experiments. * less than untreated, p<0.05; ** less than untreated, p<0.005.
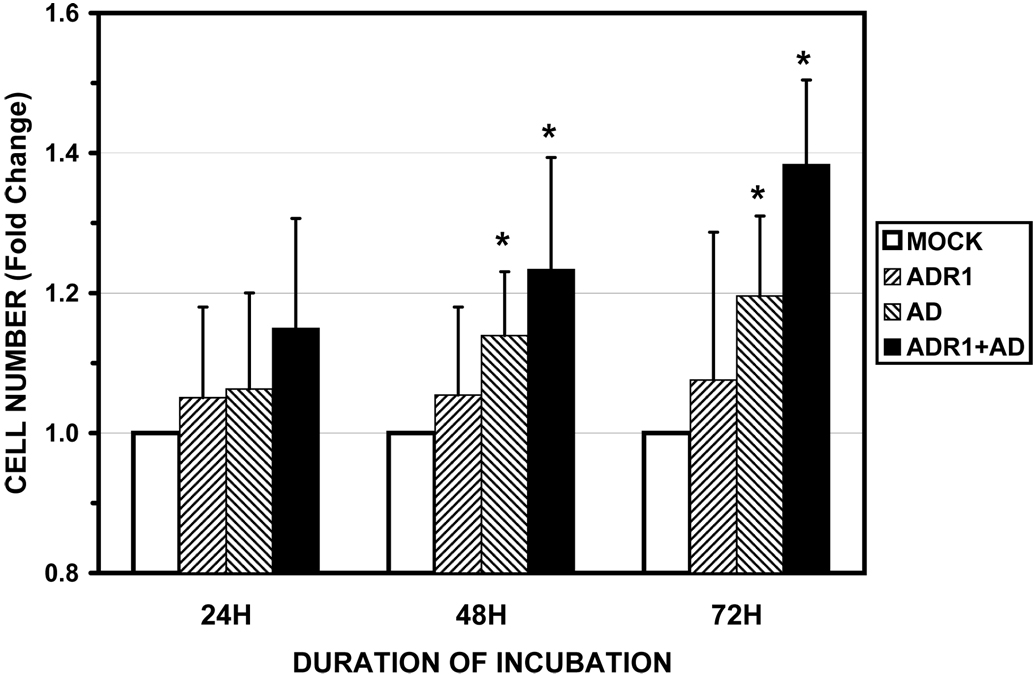
Adiponectin promotes serum-independent growth of HEK293 cells
ERK1/2 activity is essential for G0-G1 cell cycle transition and the passage of cells through mitosis or meiosis (26). We therefore hypothesized that continuous exposure to adiponectin might promote growth of HEK293 cells. To test this, cells were transiently transfected with expression plasmids encoding either HA-tagged AdipoR1, FLAG-tagged full-length adiponectin, or both, and cell growth rates were determined after 24–72 hours exposure to serum-free conditions. As shown in Figure 8, overexpression of HA-AdipoR1 alone, which does not affect basal ERK1/2 activity (Figure 5A), did not produce significant changes in cell number at any time point, indicating that the receptor alone, in the absence of adiponectin, had no effect on growth rate. In contrast, cells expressing FLAG-adiponectin alone showed significant increases in cell number at 48 and 72 h, and cells expressing both HA-AdipoR1 and FLAG-adiponectin showed a further increase. These data suggest that adiponectin, acting via AdipoR1/R2 is sufficient to promote cell growth.
Figure 8. Constitutive expression of adiponectin and AdipoR1 enhances the growth rate of HEK293 cells under serum-free conditions.
HEK293 cells transfected with empty vector or expression plasmids encoding HA-epitope tagged AdipoR1, FLAG-tagged adiponectin, or both, and viable cell number was determined every 24 h for 72 hr after serum withdrawal using the Cell Titer-GloR assay as described. Representative anti-HA and anti-adiponectin immunoblots demonstrating expression of HA-ADR1 and FLAG-adiponectin, respectively, are shown above a bar graph representing the Mean ± SEM from six independent experiments. Data are presented as the percent change in cell number compared to vector transfected cells (Mock). Viable cell number remained stable in vector transfected cells over the period of serum deprivation. * greater than vector transfected, p<0.05
DISCUSSION
Our results suggest that, beyond its known effects on AMPK and PPARα activity, activation of Ras pathways may be an important contributor to the biological actions of adiponectin. While adiponectin clearly plays an important role in regulating insulin-stimulated glucose disposal in major insulin-sensitive tissues like liver and skeletal muscle, AdipoR1/R2 are widely expressed. Moreover, adiponectin has been shown to stimulate additional signaling pathways in a variety of cell types (1, 2, 7, 8, 11). In human endothelial cells, adiponectin activates endothelial nitric oxide synthase (eNOS) (33). Adiponectin stimulates p38 MAP kinase activation in C2Cl2 myocytes and HEK293T cells and JNK1/2 activation in C2C12 myocytes and the HepG2 hepatocellular carcinoma line (17, 23). The globular form of adiponectin reportedly activates ERK1/2 in cardiac fibroblast cells (24). In primary vascular smooth muscle and endothelial cells, as well as HEK293 cells, we find that full-length adiponectin at physiologic concentrations activates the ERK1/2 MAP kinase pathways. While it is clear that adiponectin elicits pleiotropic responses, the signal transduction pathways by which these effects are generated are incompletely characterized. Here, we have focused on the receptors and signaling intermediates responsible for activation of ERK1/2 using HEK293 cells as a model system.
Two distinct types of membrane protein have been proposed to acts as receptors for adiponectin, the seven membrane spanning proteins AdipoR1 and AdipoR2 (17), and T-cadherin (20), an extracellular glycosylphosphatidylinositol-anchored protein, that like AdipoR1/R2, lacks intrinsic catalytic activity. Based on results from knockout mice, it is clear that AdipoR1/R2 mediate the major metabolic effects on adiponectin on glucose and lipid metabolism (18, 36). There appears to be a degree of functional specialization between AdipoR1 and AdipoR2, with the former being more tightly coupled to AMPK activation and the latter to PPARα. However only AdipoR1/R2 double knockout animals exhibit severe insulin resistance, suggesting at least partial redundancy (18). Similarly, both adiponectin receptors appear to regulate eNOS, since nitric oxide production is significantly attenuated only when both isoforms are downregulated (34). Our results are similar with respect to ERK1/2 activation, since siRNA-mediated downregulation reduced adiponectin-stimulated ERK1/2 activation only when AdipoR1 and AdipoR2 were targeted simultaneously. In contrast adiponectin binding to T-cadherin appears to dampen AdipoR1/R2 signaling, since its downregulation leads to a significant enhancement of ERK1/2 activation.
Because of their unique membrane topology and lack of intrinsic catalytic activity, it remains unclear how AdipoR1/R2 transmit signals intracellularly. Recently, the adapter protein APPL1 has emerged as an important element in AdipoR1/R2 signaling (25, 34). APPL1, which contains a pleckstrin homology domain, a phosphotyrosine-binding domain, and a leucine zipper motif, was initially identified in a yeast two-hybrid screen using the AdipoR1 N-terminus as bait, but it appears to bind to the N-terminus of both AdipoR1 and AdipoR2. It is reportedly involved in mediating adiponectin effects on glucose and lipid metabolism (25) and in stimulating phosphorylation of eNOS at Ser1177 to promote nitric oxide synthesis (34). Our results implicate APPL1 in AdipoR1/R2 mediated Ras signaling as well. We find that APPL1 co-precipitates with AdipoR1 and that downregulating APPL1 by RNA interference significantly reduces adiponectin stimulated ERK1/2 activation. Thus, APPL1 appears to be a key intracellular mediator of most of the adiponectin responses reported to date.
Activation of the ERK1/2 MAP kinase cascade is essential for cell cycle initiation and plays a key role in the control of cell proliferation, differentiation and survival (26). ERK1/2 serves as a point of convergence for diverse signal inputs. Classical receptor tyrosine kinases, such as the EGF receptor, primarily act via Ras and c-Raf-1, while GPCRs can alternatively activate c-Raf-1 directly via PKC or promote B-Raf-dependent activation of Rap1 via PKA and the cAMP-regulated guanine nucleotide exchange factor, Epac (36, 37). In addition, many receptors access Ras signaling pathways by stimulating matrix metalloprotease-dependent release of preformed EGF receptor ligands leading to transactivation of EGF receptors (32, 38, 39). While we find that pharmacologic inhibition of PKA attenuates adiponectin-stimulated ERK1/2 activation, we are unable to detect a significant increase in Rap1 GTP-loading. This is consistent with previous work that failed to show an increase in intracellular cAMP in response to adiponectin (17) and suggests that modulation of PKA signaling is at most a minor component of adiponectin regulation of ERK1/2. In contrast, inhibiting Src family nonreceptor tyrosine kinases abolishes the adiponectin response. While Src family kinases are involved in EGF receptor signaling, adiponectin-stimlated ERK1/2 activation does not appear to involve transactivated EGF receptors, since it is not significantly affected by pharmacologic inhibition of the EGF receptor. Consistent with a tyrosine kinase-dependent pathway, adiponectin significantly stimulates Ras GTP-loading in HEK293 cells and the response is blocked by Src inhibition, placing the nonreceptor tyrosine kinase upstream of Ras in the pathway.
Given the central role that ERK1/2 plays in controlling cell proliferation and our finding that AdipoR1/R2 mediates Ras-dependent ERK1/2 activation, we hypothesized that adiponectin might act as a growth factor. To test this we determined whether continuous exposure to adiponectin would permit HEK293 cells to grow under serum free conditions that normally cause growth arrest. Since only adipocytes endogenously express adiponectin, we introduced an expression plasmid encoding full-length adiponectin to provide a continuous source in culture. Overexpressing AdipoR1 alone, while sufficient to enhance adiponectin-stimulated ERK1/2 activation, predictably had no effect on either basal ERK1/2 activity or viable cell number under serum free conditions. In contrast, expression of full-length adiponectin was sufficient to promote growth and the effect was enhanced in AdipoR1 overexpressing cells.
Because of its ability to enhance insulin action in whole animal models and the apparent link between adiponectin deficiency, diabetes mellitus and vascular disease in humans, adiponectin has become the subject of intense interest as a possible therapeutic target in the treatment of type 2 diabetes mellitus. Clearly, the ability to regulate glucose and lipid metabolism by modulating AMPK and PPARα signaling in muscle and liver accounts for most of its potentially beneficial metabolic effects. Nonetheless, AdipoR1/R2 are widely expressed receptors and growing evidence suggests that they have additional actions in non-insulin-sensitive tissues. Our results clearly demonstrate that adiponectin regulates Ras-dependent signaling and that continuous exposure to adiponectin is sufficient to support serum-independent cell growth. Whether these effects are, on balance, beneficial or detrimental in vivo will have to be determined experimentally. Nonetheless our findings underscore the need for additional study of the molecular mechanisms of signaling employed by this unique class of transmembrane receptor.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the expert technical assistance of Ms. Andrea Semler.
Abbreviations
- AdipoR1/2
adiponectin receptor types 1 and 2
- AMPK
AMP-activated protein kinase
- APPL1
adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif
- EGF
Epidermal Growth Factor
- eNOS
endothelial nitric oxide synthase
- Epac
exchange protein directly activated by cAMP
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- G protein
guanine nucleotide-binding protein
- GPCR
G protein-coupled receptor
- JNK1/2
c-jun N-terminal kinase 1 and 2
- MAP
mitogen-activated protein
- MEM
Minimal Essential Medium
- PCR
polymerase chaim reaction
- PPARα
peroxisome proliferator activator receptor α
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol myristate acetate
- siRNA
small interfering RNA.
Footnotes
This work was supported by National Institutes of Health Grant DK58283 (L.M.L.) and Department of Veterans Affairs Merit (LML, RLK) and Research Enhancement Award Program (LML) awards.
REFERENCES
- 1.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 2.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 3.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 4.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 5.Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, Papadiamantis Y, Markopoulos C, Spanos E, Chrousos G, Trichopoulos D. Adiponectin and breast cancer risk. J. Clin. Endocrinol. Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 6.Miyoshi Y, Taguchi T, Tamaki Y, Noguchi S. Current status of endocrine therapy for breast cancer. Breast Cancer. 2003;10:105–111. doi: 10.1007/BF02967634. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 8.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 9.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc. Natl. Acad. Sci., U. S. A. 2004;101:10302–10307. doi: 10.1073/pnas.0403760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J. Biol. Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 12.Kishida K, Nagaretani H, Kondo H, Kobayashi H, Tanaka S, Maeda N, Nagasawa A, Hibuse T, Ohashi K, Kumada M. Disturbed secretion of mutant adiponectin associated with the metabolic syndrome. Biochem. Biophys. Res. Commun. 2003;306:286–292. doi: 10.1016/s0006-291x(03)00940-9. [DOI] [PubMed] [Google Scholar]
- 13.Tsao T-S, Murray HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J. Biol. Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 14.Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, Uchida S, Tsuchida A, Takekawa S, Kadowaki T. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–796. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- 15.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci., U. S. A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 19.Touyz RM. Endothelial cell IL-8, a new target for adiponectin: implications in vascular protection. Circ. Res. 2005;97:1216–1219. doi: 10.1161/01.RES.0000196745.09234.36. [DOI] [PubMed] [Google Scholar]
- 20.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci., U. S. A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 22.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazaki T, Bub JD, Uzuki M, Iwamoto Y. Adiponectin activates c-Jun NH2-terminal kinase and inhibits signal transducer and activator of transcription 3. Biochem. Biophys. Res. Commun. 2005;333:79–87. doi: 10.1016/j.bbrc.2005.05.076. [DOI] [PubMed] [Google Scholar]
- 24.Hattori Y, Hattori S, Akimoto K, Nishikimi T, Suzuki K, Matsuoka H, Kasai K. Globular adiponectin activates nuclear factor-κB and activating protein-1 and enhances angiotensin II-induced proliferation in cardiac fibroblasts. Diabetes. 2007;56:804–808. doi: 10.2337/db06-1405. [DOI] [PubMed] [Google Scholar]
- 25.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signaling and function. Nat. Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 26.Pearson G, Robinson F, Beers–Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 27.Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 28.DeRooij J, Zwartkruis FL, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 29.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp U. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–255. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 30.van Biesen T, Hawes BE, Luttrell DK, Krueger KM, Touhara K, Porfiri E, Sakaue M, Luttrell LM, Lefkowitz RJ. Receptor-tyrosine-kinase- and G beta gamma-mediated MAP kinase activation by a common signaling pathway. Nature. 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- 31.Schmitt JM, Stork PJ. beta-2-adrenergic receptor activates extracellular signal-regulated kinases (ERKs) via the small G protein rap1 and the serine/threonine kinase B-Raf. J. Biol. Chem. 2000;275:25342–25350. doi: 10.1074/jbc.M003213200. [DOI] [PubMed] [Google Scholar]
- 32.Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 2001;20:1594–1600. doi: 10.1038/sj.onc.1204192. [DOI] [PubMed] [Google Scholar]
- 33.Della Rocca GJ, van Biesen T, Daaka Y, Luttrell DK, Luttrell LM, Lefkowitz RJ. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J. Biol. Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 34.Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 35.Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, Ploj K, Gerdin AK, Arnerup G, Elmgren A, Berg AL, Oscarsson J, Linden D. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–593. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- 36.Gutkind JS. Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors. Sci. STKE. 2000 doi: 10.1126/stke.2000.40.re1. RE1 ( http://stke.sciencemag.org/cgi/content/full/-sigtrans;2000/40/pRE1) [DOI] [PubMed]
- 37.Luttrell LM. 'Location, location, location': activation and targeting of MAP kinases by G protein-coupled receptors. J. Mol. Endo. 2003;30:117–126. doi: 10.1677/jme.0.0300117. [DOI] [PubMed] [Google Scholar]
- 38.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr. Opin. Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 39.Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat. Rev. Mol. Cell Biol. 2003;4:651–657. doi: 10.1038/nrm1173. [DOI] [PubMed] [Google Scholar]