Abstract
Bile acids and other steroids modify BK channel activity contributing to nongenomic modulation of myogenic tone. The protein regions that sense steroid action, however, remain unknown. Accessory BK β1 subunits are necessary for lithocholate to activate BK channels and vasodilate. Using recombinant channels in POPE/POPS bilayers we now demonstrate that complex proteolipid domains and cytoarchitecture are unnecessary for β1 to mediate lithocholate action; β1 and a simple phospholipid microenvironment suffice. Since β1 senses lithocholate but β4 does not, we made chimeras swapping regions between these subunits and, following channel heterologous expression, demonstrate that β1 TM2 is a bile acid-recognizing sensor.
Keywords: MaxiK channel, KCNMB1, lithocholic acid, bile acids, steroids
1.Introduction
In most vertebrates, including humans, bile acids are synthesized from cholesterol and facilitate digestion and absorption of dietary lipids. These processes involve bile acid interactions with membrane and cytosolic proteins, requiring specific bile acid-recognition domains in the proteins themselves [1-3].
Besides their well-established role in gastrointestinal physiology and disease, bile acids are increasingly recognized as multifunctional signaling molecules, and a variety of bile acid receptor proteins have emerged. Bile acids are natural ligands of the farnesoid-X receptor, a transcription factor that controls genes involved in bile acid and cholesterol homeostasis [4]. Bile acids are ligands of the G-protein-coupled receptor TGR5 [5], and activators of MAP kinase. Thus, these steroids possess endocrine functions, controlling energy use and body weight [6]. In addition, bile acids activate vitamin D receptors [7] and facilitate internal Ca2+ mobilization via ryanodine and IP3 receptors [8], which makes these steroids active players in Ca2+ homeostasis. In spite of the growing list of proteins that recognize bile acids as endogenous ligands, the identification of specific bile acid-recognition domains in these receptor proteins remains largely elusive.
It has been known for decades that lithocholate (LC) and other bile acids are effective vasodilators in human physiology and disease [9,10]. In a recent study, we demonstrated that LC-induced vasodilation is blunted after genetic ablation of KCNMB1, which codes for the β1 subunit of the large conductance, calcium- and voltage-gated potassium (BK) channel [11], a channel that critically controls vascular tone [12,13]. In most tissues, BK channels are heterooligomers consisting of pore-forming (α, encoded by Slo1), and accessory (β1-β4) subunits, with β1 being abundant in smooth muscle [13]. Studies in cell-free membrane patches demonstrate that β1 expression is necessary for LC to increase the activity of BK channels cloned from arterial myocytes [11].
Natural membranes, however, are complex organelles, with a myriad of molecular entities [14] that can mediate or, at least, modify, the bile acid-channel interaction. Whether LC-sensing by BK β1 requires the complex proteolipid environment of natural cell membranes or, rather, the BK channel and its immediate, simple lipid medium suffice, remains unknown. We addressed this question and identified the specific BK β1 protein domain that recognizes LC leading to channel activation.
2. Materials and Methods
2.1. Bilayer experiments
BK α cDNA (“cbv1”) was cloned from rat cerebral artery myocytes (AY330293, [15]). BK β1 cDNA was a generous gift from Maria Garcia (Merck, Whitehouse station, NJ). HEK293 cells transiently transfected with cbv1 or cbv1+BK β1 cDNAs were grown to confluence, pelleted, and resuspended on ice in 10 ml of buffer solution (mM): 30 KCl, 2 MgCl2, 10 HEPES, 5 EGTA; pH 7.2. A membrane preparation was obtained using a sucrose gradient as described elsewhere [16], and aliquots were stored at -80°C.
Channels were incorporated by adding 10-15 μl of membrane preparation onto bilayers cast of 1-palmitoyl-2-oleoyl-phosphatidylethanolamine (POPE) and 1-palmitoyl-2-oleoyl-phosphatidylserine (POPS), 3:1 (w/w). Lithocholate and cholesterol were introduced into the lipid mixture at final concentrations of 33 and 5-37 mol%, respectively. Lipids were dried under N2 gas and resuspended in 25 mg/ml decane. Vertical, 80-120 pF bilayers were formed by painting the lipid mix across a 200 μm hole in a deldrin cup (Warner, Hamden, CT). Vesicle fusion was promoted by osmosis, with the cis chamber (to which the membrane preparation was added) being hyperosmotic to the trans. Solutions consisted of (mM): cis, 300 KCl, 10 HEPES, 1.47 HEDTA, 1.05 CaCl2 (Ca2+free≅10 μM), pH 7.2; trans, 30 KCl, 10 HEPES, 0.1 HEDTA, pH 7.2. Currents were obtained at 0 mV using a bilayer amplifier (Warner BC-525D), low-pass filtered at 1 and sampled at 5 kHz using Digidata 1322A and pCLAMP 8.0 (Molecular Devices, Union City, CA).
2.2. Xenopus oocyte experiments
Cbv1 cDNA ligated to the PCR-XL-TOPO cloning vector (Invitrogen, Carlsbad, CA) was cleaved by BamHI (Invitrogen) and XhoI (Promega, Madison, WI), and inserted into pOX for expression in Xenopus laevis oocytes. pOX-cbv1 was linearized with NotI (Promega) and transcribed in vitro using T3 (Ambion, Austin, TX). pOX was a generous gift from Aguan Wei (Washington Univ., Saint Louis, MO).
Beta1/β4 chimeric cDNAs were constructed using overlap extension or custom synthesized (IDT, Coralville, IA), subcloned in pOx or pcDNA3, linearized, and transcribed in vitro using T3 or T7 RNA polymerase (Ambion). Sequences of cDNA constructs were determined at the Univ. Tennessee Molecular Research Center.
cRNA was dissolved in diethyl polycarbonate-treated water at 5 (cbv1) and 15 (β1, β4 or chimeras) ng/μl; 1 μl aliquots were stored at -70°C. Xenopus laevis (NASCO, Fort Atkinson, WI; Xenopus Xpress, Plant City, FL) oocytes were isolated as described [17]. Cbv1 cRNA was injected alone (2.5 ng/μl) or with chimeric β1/β4 (7.5 ng/μl) cRNAs, giving molar ratios ≥6:1 (β:α). cRNA injection (23 nl/oocyte) was conducted using a modified micropipette (Drummond, Broomall, PA) 48-72 h. before patch-clamp recordings. Oocytes were prepared for patch-clamping as described [17].
Currents were recorded from inside-out (I/O) or outside-out (O/O) patches using an EPC8 amplifier (HEKA, Lambrecht/Pfalz, Germany) at 1 kHz using a low-pass, eight-pole Bessel filter (902LPF, Frequency Devices, Haverhill, MA). Data were sampled at 5 kHz using Digidata 1320A and pCLAMP 8.0 (Molecular Devices). Bath and electrode solutions contained (mM): 135 K+ gluconate, 5 EGTA, 1 MgCl2, 0.01 CaCl2, 15 HEPES, 10 glucose, pH 7.35. Free Ca2+ was calculated with MaxChelator Sliders and determined using Ca2+-selective electrodes. Patch-recording and ground electrodes were made as described [17]. Bath solution containing either vehicle or 150 μM LC was applied onto excised patches using a pressurized DAD12 system (ALA, New York, NY). Experiments were carried out at room temperature.
2.3. Chemicals
5β-cholanic acid 3α-ol (LC) and iberiotoxin were purchased from Steraloids (Newport, RI) and Alomone (Jerusalem, Israel), respectively. All other chemicals were purchased from Sigma (St. Louis, MO). On the day of the experiment, an LC stock solution (333 mM) in dimethyl sulfoxide (DMSO) was freshly prepared by sonication for 5 min. For oocyte recordings, the LC stock solution was diluted 1/10 in 95% ethanol, and further diluted in bath solution to final concentration. The DMSO/ethanol vehicle in bath solution (≤0.05/≤0.45%) was used as control (before) and wash of (after) LC application.
2.4. Data Analysis
Data were analyzed with pCLAMP 8.0 (Molecular Devices). As index of channel activity we used the product of the number of channels in the patch/bilayer (N) and the channel open probability (Po). NPo was obtained from all-points amplitude histograms [17] from ≥30 sec of continuous recording under each experimental condition. Further analysis, plotting and fitting were conducted using Origin 7.0 (Originlab, Northampton, MA) and InStat 3.0 (GraphPad Software, San Diego, CA). Statistical analysis was conducted using one-way ANOVA and Bonferroni's multiple comparison test; significance was set at P<0.05. Data are expressed as mean±SEM; n=number of patches/bilayers.
3. Results
We first determined whether the complex proteolipid environment of natural cell membranes is necessary for BK β1 sensing of LC. Thus, we probed LC on homomeric cbv1 and heteromeric cbv1+β1 channels that were reconstituted into planar 3:1 POPE:POPS (w/w) bilayers. We chose this binary bilayer to validate basal channel behavior with our previous characterization of native skeletal muscle BK channels [18] and recombinant slo1 from human brain [16] reconstituted into this bilayer type. Upon cbv1 channel incorporation, we recorded unitary current events that displayed distinct features of BK channel openings: large current amplitude (e.g., 14 pA at 0 mV in 300/30 mM [K+]i/[K+]o; Fig.1A); increase in Po as voltage was made more positive (Suppl. Fig. 1A), and/or [Ca2+]i was increased (not shown). Within a wide voltage range (-60 to 0 mV), cbv1 channels required more Ca2+i to achieve a given (N)Po than that required by cbv1+β1 (Fig. 1A left vs. right panels). This demonstrates that β1 increases the channel apparent Ca2+-sensitivity, as previously shown for other slo1 channels [19]. The increase resulted in a parallel leftward shift in the V-Po plot (Suppl. Fig. 1A), and served to indicate the presence of functional BK β1.
Fig.1. Lithocholic acid activates BK channels in binary phospholipid bilayers.
A. Currents obtained after incorporation of cbv1+β1 (right) or cbv1 (left) channels into POPE:POPS 3:1 (w/w) bilayers in absence (top) or presence of lithocholate (middle) or cholesterol (bottom); arrows=baseline. B. Averaged responses of cbv1 (left) and cbv1+β1 (right) to the steroid presence. In A and B: [steroids]=33 mol%; V=0 mV; free Ca2+i =0.3 μM (for cbv1+β1) and 10 μM (for cbv1); LC, lithocholate; CHS, cholesterol. * P<0.05 vs. POPE/POPS bilayer; ** P<0.01 vs. POPE/POPS bilayer.
Lithocholate was introduced into the lipid mix at 20% (w/w), which corresponds to a molar fraction of 0.33. This is roughly equivalent to 300 μM LC in the aqueous phase, a concentration that is fully effective to activate native and recombinant BK channels [10,11]. Cbv1+β1 NPo in POPE/POPS bilayers containing LC were consistently higher than those in LC absence (Fig. 1A right). The increased NPo in LC far exceeds any possible maximum due to NPo spontaneous oscillations found in POPE/POPS alone (Suppl. Fig. 1B). Furthermore, the average increase in NPo (∼×3, n=3) induced by LC was identical to that observed when LC 150-300 μM was applied to native cerebral artery BK channels or to cbv1+β1 expressed in oocytes [11]. In contrast, cbv1 NPo remained insensitive to LC presence in the bilayer (Fig. 1A left; Fig. 1B) (n=5), mimicking the LC-refractoriness of cbv1 expressed in natural membranes [11]. Collectively, these results indicate that complex membrane cytoarchitecture and heterogeneous proteolipid domains are unnecessary for β1 to mediate LC action on BK channels. Rather, cbv1+β1 and a simple phospholipid microenvironment are sufficient.
On the other hand, as previously shown with brain BK channels [20] and hslo1 [16] reconstituted into binary phospholipid bilayers, 33 mol% cholesterol consistently inhibited cbv1 channels (Fig. 1A,B). The decrease in cbv1 steady-stated activity (Po) was a monotonic function of cholesterol concentration in the bilayer (IC50∼20 mol%). This reduction in Po was evident at cholesterol levels present in natural membranes (7.5-50 mol% of total lipid), with cbv1 Po reaching minimum values at 33-37 mol% cholesterol (Suppl. Fig. 2). Notably, cbv1+β1 channels displayed a cholesterol response identical to that of cbv1 (Fig. 1B). Thus, β1 are required for LC, but not cholesterol, modulation of BK channels in simple, binary bilayers. Conceivably, this specificity involves LC-recognition by specific protein domains, leading to the hypothesis that a region(s) in BK β1 behaves as a specific LC-sensor.
Based on the fact that β1, but not β4, confers LC sensitivity to the BK channel [11], we constructed chimeric β1/β4 (termed “B1L4” and “B4L1”) where the extracellular loops were swapped (Fig. 2A; Suppl. Fig. 3). After chimeric subunit coexpression with cbv1 in X. oocytes, we probed LC action on Po using conditions similar to those used to test LC on channels containing wild type (wt) β1. LC was applied to the intracellular side of inside-out (I/O) patches at a concentration of 150 μM, which is known to activate (EC90) both recombinant and native BK channels [10,11]. All experiments were performed at 10 μM free [Ca2+]i, which is detected in the vicinity of the BK channel in arterial myocytes [21]. Taking advantage that LC action on BK channels is voltage-independent [11], records were obtained with the membrane potential clamped within -40 and +20 mV to obtain similar basal (i.e., in absence of LC) NPo values across patches. Functional expression of a given chimeric β was determined by the channel response to iberiotoxin (Ibtx) block in O/O patches, which is blunted by extracellular loops of the β4-type [22] (Suppl. Fig. 4).
Fig. 2. The BK β1 extracellular loop is not involved in lithocholate sensing.
A. Cartoon showing the β1/β4 chimeras constructed by swapping their extracellular loops. L=extracellular loop; B=background region (i.e., transmembrane domains+cytosolic ends). Regions from β1 are given in black while those from β4 are in grey. B. Inside-out patch-clamp recordings demonstrate that lithocholate activates cbv1+B1L4 (top) but not cbv1+B4L1 (bottom) channels. V=-30 mV; symmetric Ca2+=10 μM. C. Averaged channel responses to LC. ** P<0.01 vs. vehicle-containing solution; n=6 (B1L4), n=4 (B4L1).
Characteristically, LC caused an increase in cbv1+B1L4 NPo >×2 times (n=6; Fig. 2B,C). In contrast, LC consistently failed to modify cbv1+B4L1 NPo (n=4; Fig. 2B,C). Thus, the β1 extracellular loop is not necessary for this subunit to provide LC sensitivity to the BK channel. Rather, any possible β1 subunit LC-sensor is located in transmembrane-intracellular domains.
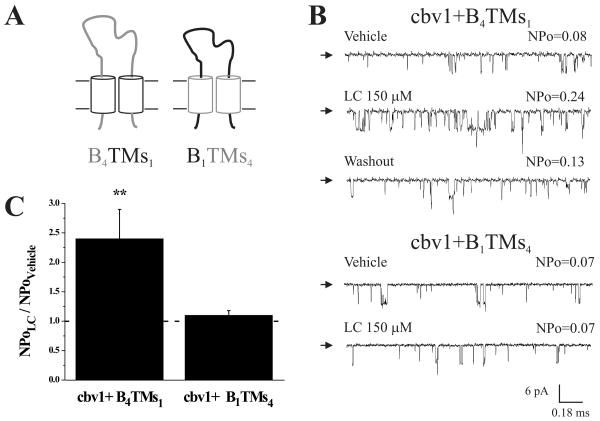
We next coexpressed cbv1 with chimeric β1/β4 that had their transmembrane domains swapped (“B4TMs1” and “B1TMs4”; Fig. 3A). Functional expression of a given chimera was determined by evaluating the Ibtx sensitivity of the channel (Suppl. Fig. 4). Under conditions identical to those described in the previous section, LC characteristically increased cbv1+B4TMs1 NPo ≈×2.4 times (n=4; Fig. 3B,C). In contrast, LC consistently failed to modify cbv1+B1TMs4 NPo (n=4; Fig. 3B,C). Therefore, the intracellular ends of β1 are not critical in LC-sensing, but the subunit transmembrane regions include the LC sensor.
Fig. 3. β1 transmembrane domains confer LC sensitivity to the BK channel.
A. Cartoon showing the β1/β4 chimeras with swapped transmembrane domains (TMs). B=background (in this case: extracellular loop+cytosolic ends). Regions from β1 are given in black; those from β4 are given in grey. B. Inside-out patch-clamp recordings demonstrate that lithocholate activates cbv1+B4TMs1 (top) but not cbv1+B1TMs4 (bottom). V=-30 mV; symmetric [Ca2+]=10 μM. C. Averaged channel responses to LC. ** P<0.01 vs. vehicle-containing solution; n=4 (B4TMs1), n=4 (B1TMs4).
To determine which, if any, transmembrane domain of the BK β1 is sufficient to provide LC-sensitivity to the channel, we next constructed chimeric β4 containing either TM1 (“B4TM11”) or TM2 (“B4TM21”) from β1 (Fig. 4A). Functional coexpression of these chimeras with cbv1 was confirmed by the resistance of the heteromeric channel-mediated currents to Ibtx (Suppl. Fig. 4). In 5 out of 5 patches, LC failed to increase cbv1+B4TM11 NPo, indicating that β1 TM1 is not sufficient to provide LC-sensitivity to the channel (Fig. 4B,C). In contrast, LC reversibly increased cbv1+B4TM21 NPo in every patch studied (n=6), with an average increase of ≈×2 times over control (Fig. 4B,C). Moreover, cbv1+B4TM21 responses to LC were similar to those of cbv1+wt β1 (Fig. 4D), which pinpoints TM2 as the β1 protein domain that senses LC presence rendering activation of BK channels.
Fig.4. β1 TM2 is the BK channel lithocholate sensor.
A. Cartoon showing chimeric subunits that contain either TM1 or TM2 from β1 on a β4 background (B=the rest of the protein). Regions from β1 are given in black, those from β4 are in grey. B. Inside-out patch-clamp recordings demonstrate that lithocholate activates cbv1+B4TM21 (bottom) but not cbv1+B4TM11 (top) channels. V=-30 mV; symmetric Ca2+=10 μM. C. Averaged channel responses to LC. ** P<0.01 vs. vehicle-containing solution; n=5 (B4TM11), n=6 (B4TM21). D. Lithocholate activation of cbv1+B4TM21 (n=6) and cbv1+wt β1 (n=7) are identical.
4. Discussion
Nongenomic effects of steroids are receiving increasing attention as they may lead to discovery of novel receptor protein sites, with possible development of steroid analogs that have therapeutic use. Most steroids that are critical in human physiology and disease, including bile acids, can modify smooth muscle tone independently of genomic actions [9,11]. Indeed, 17-beta estradiol, xenoestrogens, androgens, gluco- and mineralocorticoids, progestagens, bile acids and cholesterol can all directly (i.e., independently of cell integrity or metabolism) modify the activity of BK channels, an action that explains or, at least, contributes to, the myogenic effect of these steroids. In contrast to bile acid data [11], we show here that the activity of BK channels cloned from vascular smooth muscle is modulated by cholesterol independently of BK β1 (Fig. 1). This subunit is also unnecessary for tamoxifen [23] or 17β-estradiol direct activation of BK channels, with α+β2, α+β4 [24] or just α [25] subunits being sufficient for estrogen action. In turn, corticosterone activates more effectively β4- than β2-containing BK channels while dehydroepiandrosterone shows an opposite selectivity and testosterone appears not to discriminate among these two subunits [24]. In contrast, LC concentrations that are maximally effective in activating cbv1+β1 completely fail to modulate cbv1+β4 [11]. Therefore, β1 TM2 should be considered as a rather specific bile acid-recognizing, instead of a nonselective, steroid-sensing, protein domain.
Among physiological steroids, LC and analogs are distinctively planar amphiphiles, with a convex hydrophobic and a concave hydrophilic hemisphere in the bile acid ring structure. Remarkably, planar polarity is critical for bile acids to activate smooth muscle BK channels [10]. In LC, polarity is provided by the carboxylate in the side chain and the single hydroxyl attached to the steroid ring. Based on data with different bile acid analogs and electrophysiological determinations, we advanced a putative model in which LC inserted normally to the bilayer plane, with LC ionized carboxylate pointing away from the bilayer core, the convex hemisphere facing the bilayer leaflets and the concave hemisphere facing the channel subunit(s) [10,11]. In absence of structural data, we cannot determine the contribution of channel slo1 and beta1 subunits, or even yet-to-be identified accessory proteins, if any, to the LC actual binding site. However, pharmacological constraints, including stereospecificity in LC activation of native BK channels containing beta1 subunits (Dopico et al., 2002) are consistent with the idea that the beta1 TM2 domain, at least, contributes to the LC binding site. If so, and following the LC insertion model mentioned above, the BK β1 TM2 domain should provide distinct interacting amino acid residues for the hydroxyl and/or the concave hydrophobic ring area of LC. Notably, β1 contains several Thr/Ser residues in TM2 that could interact with the LC hydroxyl via hydrogen bonding. In contrast, BK β4, which fails to sense LC [11], largely lacks these polar residues in its TMs. The bile acid-sensing domain discovered here also contains a much larger proportion of hydrophobic amino acids (82%) than TM1. Remarkably, BK channel activation by bile acids strongly correlates with the hydrophobicity of the steroidal nucleus, LC being the most effective activator [10]. While identification of the several amino acids that interact with LC requires further study, present results clearly demonstrate that β1 TM2 is a selective LC docking domain.
The newly discovered bile acid sensor contains 23 amino acid residues (157-178aa; Suppl. Fig. 3) Remarkably, its protein sequence does not show major identity (<5%) with any of the few bile acid-binding domains identified in other proteins. In addition, BK β1 is highly expressed in smooth muscle, and LC fails to activate BK channels that include accessory βs other than β1 [26]. Therefore, BK β1 TM2 may serve as a “selective conduit” that allows bile acids (and eventual synthetic analogs) to modulate BK channels only in tissues where β1 is abundant, such as smooth muscle.
Supplementary Material
Acknowledgments
We deeply thank Aster Sigel (UCLA) and Maria T. Asuncion-Chin (UTHSC) for technical assistance, and Abby Parrill (UMemphis) for helpful discussion. Supported by NIH grants HL77424 (A.M.D.) and HL54970 (L.T.).
List of Abbreviations
- LC
lithocholate
- BK
large conductance, calcium- and voltage-gated potassium
- POPE
1-palmitoyl-2-oleoyl-phosphatidylethanolamine
- POPS
1-palmitoyl-2-oleoyl-phosphatidylserine
- DMSO
dimethyl sulfoxide (DMSO)
- N
number of channels present in the membrane patch/lipid bilayer
- Po
channel open probability
- Ibtx
iberiotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang EY, Phelps MA, Banerjee A, Khantwal CM, Chang C, Helsper F, Swaan PW. Topology scanning and putative three-dimensional structure of the extracellular binding domains of the apical sodium-dependent bile acid transporter (SLC10A2) Biochemistry. 2004;43(36):11380–92. doi: 10.1021/bi049270a. [DOI] [PubMed] [Google Scholar]
- 2.Tochtrop GP, Richter K, Tang C, Toner JJ, Covey DF, Cistola DP. Energetics by NMR: site-specific binding in a positively cooperative system. Proc Natl Acad Sci USA. 2002;99(4):1847–52. doi: 10.1073/pnas.012379199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ragona L, Catalano M, Luppi M, Cicero D, Eliseo T, Foote J, Fogolari F, Zetta L, Molinari H. NMR dynamic studies suggest that allosteric activation regulates ligand binding in chicken liver bile acid-binding protein. J Biol Chem. 2006;281(14):9697–709. doi: 10.1074/jbc.M513003200. [DOI] [PubMed] [Google Scholar]
- 4.Modica S, Moschetta A. Nuclear bile acid receptor FXR as pharmacological target: are we there yet? FEBS Lett. 2006;580(23):5492–9. doi: 10.1016/j.febslet.2006.07.082. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298(5):714–9. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 6.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25(7):1419–25. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296(5571):1313–6. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 8.Gerasimenko JV, Flowerdew SE, Voronina SG, Sukhomlin TK, Tepikin AV, Petersen OH, Gerasimenko OV. Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors. J Biol Chem. 2006;281(52):40154–63. doi: 10.1074/jbc.M606402200. [DOI] [PubMed] [Google Scholar]
- 9.Bomzon A, Ljubuncic P. Bile acids as endogenous vasodilators? Biochem Pharmacol. 1995;49:581–89. doi: 10.1016/0006-2952(94)00428-o. [DOI] [PubMed] [Google Scholar]
- 10.Dopico AM, Walsh JV, Jr, Singer JJ. Natural bile acids and synthetic analogues modulate large conductance Ca2+-activated K+ (BKCa) channel activity in smooth muscle cells. J Gen Physiol. 2002;119(3):251–73. doi: 10.1085/jgp.20028537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukiya AN, Liu J, Toro L, Dopico AM. Beta1 (KCNMB1) Subunits Mediate Lithocholate Activation of Large-Conductance Ca2+-Activated K+ Channels and Dilation in Small, Resistance-Size Arteries. Mol Pharmacol. 2007a;72(2):359–69. doi: 10.1124/mol.107.034330. [DOI] [PubMed] [Google Scholar]
- 12.Brenner R, Perez G, Bonev A, Eckman D, Kosek J, Wiler S, Patterson A, Nelson M, Aldrich R. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–6. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 13.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci. 2002;17:156–61. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 14.Dopico AM, Tigyi GJ. A glance at the structural and functional diversity of membrane lipids. Methods Mol Biol. 2007;400:1–13. doi: 10.1007/978-1-59745-519-0_1. [DOI] [PubMed] [Google Scholar]
- 15.Jaggar J, Li A, Parfenova H, Liu J, Umstot E, Dopico A, Leffler C. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97:805–12. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowley JJ, Treistman SN, Dopico AM. Cholesterol antagonizes ethanol potentiation of human brain BKCa channels reconstituted into phospholipid bilayers. Mol Pharmacol. 2003;64(2):365–72. doi: 10.1124/mol.64.2.365. [DOI] [PubMed] [Google Scholar]
- 17.Dopico A, Anantharam V, Treistman S. Ethanol increases the activity of Ca2+-dependent K+ (mslo) channels: Functional interaction with cytosolic Ca2+ J Pharmacol Exp Ther. 1998;284:258–68. [PubMed] [Google Scholar]
- 18.Chu B, Dopico AM, Lemos JR, Treistman SN. Ethanol potentiation of calcium-activated potassium channels reconstituted into planar lipid bilayers. Mol Pharmacol. 1998;54(2):397–406. doi: 10.1124/mol.54.2.397. [DOI] [PubMed] [Google Scholar]
- 19.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275(9):6453–61. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 20.Chang HM, Reitstetter R, Mason RP, Gruener R. Attenuation of channel kinetics and conductance by cholesterol: an interpretation using structural stress as a unifying concept. J Membr Biol. 1995;143(1):51–63. doi: 10.1007/BF00232523. [DOI] [PubMed] [Google Scholar]
- 21.Perez GJ, Bonev AD, Nelson MT. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol. 2001;281:C1769–75. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- 22.Meera P, Wallner M, Toro L. A neuronal β subunit (KCNMB4) makes the large conductance, voltage- and Ca2+- activated K+ channels resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA. 2000;97:5562–7. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan RK. Tamoxifen alters gating of the BK alpha subunit and mediates enhanced interactions with the avian beta subunit. Biochem Pharmacol. 2005;70(1):47–58. doi: 10.1016/j.bcp.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 24.King JT, Lovell PV, Rishniw M, Kotlikoff M, Zeeman M, McCobb D. Beta2 and beta4 subunits of BK channels confer differential sensitivity to acute modulation by steroid hormones. J Neurophysiol. 2006;95:2878–88. doi: 10.1152/jn.01352.2005. [DOI] [PubMed] [Google Scholar]
- 25.Korovkina VP, Brainard AM, Ismail P, Schmidt T, England S. Estradiol binding to maxi-K channels induces their down-regulation via proteasomal degradation. J Biol Chem. 2004;279:1217–23. doi: 10.1074/jbc.M309158200. [DOI] [PubMed] [Google Scholar]
- 26.Bukiya A, Toro L, Dopico AM. BK channel β1 subunits are specific effectors of cholane-induced channel activation. American Heart Association Scientific sessions; Orlando, FL: 2007. p. 1367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.