Abstract
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is activated by Ca2+ entry into neurons. Autophosphorylation of T286 is of special importance because it makes the enzyme active in the absence of Ca2+, providing a biochemical memory that is critical for plasticity. To understand the factors controlling the duration of this state of CaMKII, we studied dephosphorylation of CaMKII in the post-synaptic density (PSD), a structure that defines a neuronal subcompartment critical for plasticity. We found that PSD-resident PP1 can dephosphorylate many sites on CaMKII, but not the T286 site that produces Ca2+-independent activity. This, together with previous work showing that soluble PP2A cannot dephosphorylate PSD CaMKII, provides a novel explanation for the in vivo persistence of T286 phosphorylation: after activated CaMKII translocates from the cytoplasm to the PSD, structural constraints prevent phosphatases from dephosphorylating T286. These results also suggest that the PSD is more than a simple scaffold for synaptic proteins; it may act to regulate the activity of proteins by positioning them in orientations that either prevent or favor specific biochemical reactions.
Keywords: learning and memory, long-term potentiation, phosphatase, PP1, protein kinase
Short-term and long-term synaptic modifications are triggered by brief periods of synaptic activity during which Ca2+ elevation in the cytoplasm is critical. Such Ca2+ elevations trigger biochemical networks that result in structural and functional changes at synapses. For instance, learning events produce long-term potentiation (LTP) of synapses within the hippocampus (Whitlock et al. 2006). Ca2+/calmodulin-dependent protein kinase II (CaMKII; EC number 2.7.11.17) has been implicated in both short-term and longterm synaptic plasticity (reviewed in Lisman et al. 2002; Elgersma et al. 2004; Colbran and Brown 2004). Importantly, the molecule itself is persistently altered by brief Ca2+ elevation. Ca2+-dependent autophosphorylation at T286 (Lai et al. 1986; Miller and Kennedy 1986; Miller et al. 1988) makes the enzyme active even in the absence of sustained high levels of Ca2+. Such activation occurs during LTP induction and can persist for hours (Fukunaga et al. 1993). When this persistence is blocked by a mutation that prevents T286 phosphorylation, both learning and LTP are greatly reduced (Giese et al. 1998). It is thus crucial to understand the mechanisms that control persistent activation of synaptic CaMKII.
This persistence is not straightforward to understand. An important pool of CaMKII is contained within the postsynaptic density (PSD), which also contains a high concentration of the phosphatase, PP1, an enzyme that can dephosphorylate CaMKII. Various theoretical models involving the balance of kinase autophosphorylation and phosphatase activities have been proposed to explain persistence (Miller et al. 2005); however, a recent study using reconstituted soluble CaMKII and PP1 was unable to demonstrate a persistent ‘on-state’ (Bradshaw et al. 2003). One possible explanation is that persistence requires special conditions that occur in the PSD. To test this possibility, we examined the stability of switching within purified PSDs. Our results show that CaMKII in the PSD can be switched into a persistent ‘on-state’ and reveal a surprising underlying mechanism: while PSD PP1 can indeed dephosphorylate sites on CaMKII, as previously shown (Huang et al. 2001; Colbran 2004), it cannot dephosphorylate the critical pT286 site. This finding takes on special importance because previous work showed that PP2A, the major phosphatase that dephosphorylates soluble CaMKII, is ineffective at dephosphorylating CaMKII once it has translocated into the PSD (Strack et al. 1997a). The PSD is thus a zone where pT286 is unlikely to be dephosphorylated and this may help to explain how the CaMKII switch stays in the ‘on-state.’ We further show that the pT286 site is not buried within the CaMKII protein; thus the inability of PSD PP1 to dephosphorylate this site arises because of precise positioning of CaMKII and PP1 by scaffolding proteins. These results point to a novel mechanism for persistence of a memory switch and suggest that the structure of the PSD controls the biochemical reactions that occur within it. The PSD may be considered a ‘signaling machine’ (Kennedy 2000) that modifies biochemical reactions by controlling the physical relationships between enzymes and substrates.
Experimental procedures
PSD preparation
Post-synaptic densities were prepared from the cerebral cortex of either adult Sprague Dawley rats or frozen rat brains (collected and frozen within 2 min of decapitation by Pel-Freeze Biologicals, Rogers, AR, USA) by the method of Carlin (Carlin et al. 1980) as described in Dosemeci et al. (2000). Brains were thawed in isotonic sucrose and homogenized in the same medium. The synaptosome fraction was obtained by sucrose density gradient centrifugation at 200 000 g and treated with 0.5% Triton X-100. The detergent insoluble pellet was further fractionated by sucrose density gradient centrifugation at 200 000 g and treated again with 0.5% Triton X-100 and 75 mmol/L KCl. The purified PSD preparation was stored in 33% glycerol at −20°C. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and western blot analysis were performed on the extracted fraction to determine the integrity of the PSD preparation. Protein concentration in the PSD was estimated using the modified Lowry protein estimation kit (Sigma, St Louis, MO, USA). Immediately prior to use, the PSD sample was pre-incubated with 0.1 mol/L dithiothreitol (DTT) for 1–2 h on ice with occasional mixing to reverse the artifactual formation of disulfide bonds in the PSD that are known to occur during isolation (Sui et al. 2000).
CaMKII autophosphorylation
The standard phosphorylation medium contained 20 mmol/L HE-PES, pH 7.4, 20 mmol/L DTT, 5 mmol/L MgCl2, 100 µmol/L ATP, 50 µg/mL leupeptin, 40 µg/mL CaM, and 1 mmol/L CaCl2. In some reactions γ-32P ATP with specific activity 170–1700 cpm/pmol was used. The phosphatase inhibitor microcystin-LR (0.5 µmol/L), when used, was added after pre-incubation. An aliquot from this was used as the zero time point sample to measure basal autophosphorylation on the kinase. The phosphorylation reaction was carried out at 30°C or 37°C and aliquots were removed at different time intervals. The reaction was continued for a maximum of 20–30 min. In each case, the reaction was arrested by mixing the samples with either SDS sample buffer for immunoblots or by addition of ELISA buffer (10 mmol/L Tris, pH 7.5 and 150 mmol/L NaCl; TBS) containing 10 mmol/L EDTA pH 8.0, 1.0 mmol/L EGTA pH 8.0, protease inhibitors (Roche, Indianapolis, IN, USA), and Ser/Thr phosphatase inhibitor cocktail (Sigma).
CaMKII activity assay
Assays were performed in a final volume of 50 µL containing 50 mmol/L PIPES pH 7.0, 15 mmol/L MgCl2, 1 mmol/L CaCl2 (for + samples), 5 mmol/L EGTA (for ) samples), 1 µg/mL bovine serum albumin (BSA), 10 µmol/L autocamtide 3, 10 µg/mL calmodulin, and 50 µmol/L ATP (1 Ci/mmol). Reactions were started by addition of autophosphorylated PSD samples (made as described above) with approximately 2.5 µg protein per reaction. Reactions were run for 30 s and stopped by addition of 50 µL of 10% trichloro acetic acid. The samples were then microfuged for 3 min. Supernatant of about 25 µL was spotted onto a strip of phosphocellulose paper and the paper strips were washed with water for 15–30 min. Strips were dried and counted on a tritium channel (Cherenkov radiation). n = 3 for each experiment and data are presented as mean ± SEM.
Immunoblotting
Proteins were separated by SDS–PAGE and transferred to nitrocellulose electrophoretically. pT286 levels were measured using phospho-pT286 specific antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1 : 1000. The specificity of the antibody was tested using autophosphorylated wild type and T286A CaMKII. The antibody failed to recognize T286A CaMKII on immunoblots (data not shown). CaMKII alpha levels were measured using the monoclonal antibody (Santa Cruz) at 1 : 1000. horseradish peroxidase-conjugated secondary antibody at 1 : 5000 was used and the blot developed using electro chemiluminescence substrate. Immunoblots, blots, and autoradiograms were quantitated using Quantity-One software (Biorad, Hercules, CA, USA). Statistical analysis was performed using Excel (Microsoft Corp., Redmond, WA, USA) or JMP (SAS Institute, Cary, NC, USA). The data shown are averages from at least three separate experiments.
pT286 ELISA
Samples were bound to the wells (0.5 µg of protein per well) of 96-well assay plate by overnight incubation at 4°C. Each sample was assayed in triplicate. The plates were washed with TBS and blocked with TBS containing 1% BSA (TBS-BSA) by incubating at 25°C for 1 h. The samples were then incubated overnight at 4°C with the primary antibodies, rabbit anti-pT286 CaMKII, and mouse anti-CaMKII alpha, at 1 : 500 dilutions in TBS-BSA. Plates were then washed thrice with TBS-BSA and incubated with alkaline phosphatase-conjugated anti-rabbit and anti-mouse IgG, respectively (1 : 5000 in TBS-BSA) for 1 h at 25°C. After a final set of washes, the amount of phosphorylated CaMKII and the corresponding amounts of total kinase present at each time point was determined by treating the wells with 4 methyl umbelliferyl phosphate substrate system (Sigma) and measuring the fluorescence with excitation at 360 nm and emission at 460 nm (Cytofluor; Applied Biosystem, Foster City, CA, USA). Assays were performed at a protein concentration in the linear range for the antibody used. The fluorescence measured at each time point with the phospho-specific antibody was normalized to amount of total protein to generate the normalized data. n = 3 for each experiment and data are presented as mean ± SEM.
Dephosphorylation by exogenous phosphatase
The effect of exogenous soluble phosphatase on dephosphorylating maximally autophosphorylated CaMKII in the PSD was studied using λ phosphatase (New England Biolabs, Ipswich, MA, USA) 400 U and PP1 (New England Biolabs) 0.025, 0.25, and 2.5 U. Phospho CaMKII was made in the manner described above. The phosphorylated PSD kinase was then treated with the soluble phosphatase under conditions prescribed by the manufacturer. The reactions were carried out at 30°C for 10 min and the phosphorylation at T286 on CaMKII was quantitated using ELISA. n = 3 and data are presented as mean ± SEM.
Phosphatase assay
Phosphatase activity in the PSD was measured using the Enzchek phosphatase assay kit (Molecular Probes; Invitrogen Corp., Carlsbad, CA, USA). The PSD sample was diluted in 20 mmol/L HEPES, 1 mmol/L DTT, and 50 µg/mL leupeptin to a final concentration of 0.01 µg/µL. The phosphatase reaction was carried out in a microtiter plate, with 0.5 µg of protein per well and the substrate 6,8-difluoro4-methylumbelliferyl phosphate at a final concentration of 100 µmol/L. The samples were incubated at 30°C for about 30 min and the fluorescence was measured in a fluorometer (Cytofiuor) using excitation at 360 nm and emission detection at 460 nm. The moles of product formed in the 6,8-difluoro4-methylumbelliferyl phosphate reaction was calculated from a standard curve generated using the reference standard 6,8-difiuoro-7-hydroxy-methylcoumarin provided in the kit. PP1 activity was measured as the activity inhibited by high (0.25 or 2.5 µmol/L), but not low (2.5 nmol/L) okadaic acid (Strack et al. 1997a) or by microcystin-LR (1 nmol/L or 0.4 µmol/L). n = 3 for activity determination.
Results
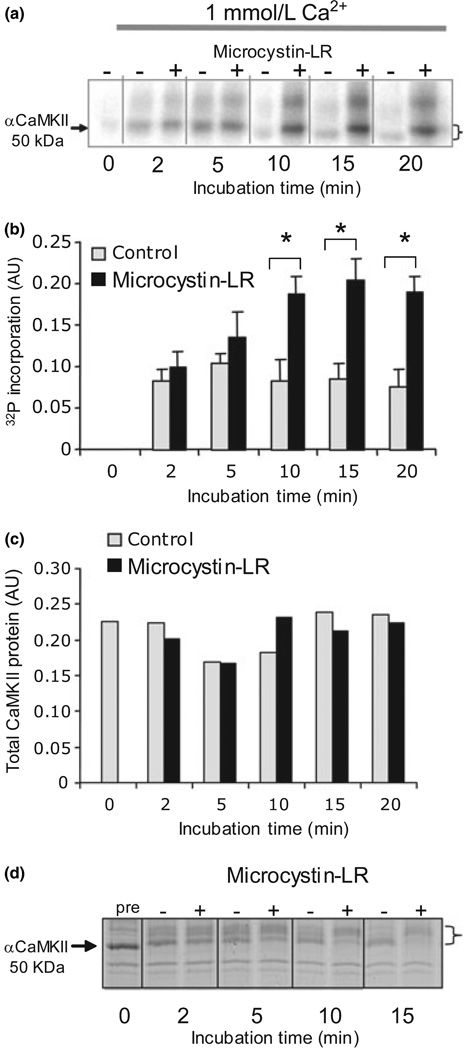
We first attempted to replicate previous work showing that the PSD contains PP1 capable of dephosphorylating CaMKII within the PSD. Using established protocols (Dosemeci and Reese 1993), we found that after addition of Ca2+/calmodulin, the CaMKII endogenous to purified PSDs becomes phosphorylated over time (Fig. 1a–c), as judged by incorporation of 32P. Inhibition of phosphatase activity by 0.5 µmol/L microcystin-LR (which does not affect CaMKII activity Dosemeci and Reese 1993) leads to higher levels of phosphorylation, indicating that the extent of phosphate incorporation depends on the balance of kinase and phosphatase activities within the PSD. Phosphorylation is accompanied by a decrease in mobility on SDS–PAGE that can be visualized by Coomassie staining and is reversed by phosphatase (Fig. 1d). The shift in phosphate and protein signal indicates that all the phosphate we are seeing is associated with CaMKII. These findings are consistent with previous reports indicating that PSD CaMKII, in the sustained presence of Ca2+, can be rapidly phosphorylated and then undergo slower dephosphorylation by PSD PP1 (Dosemeci and Reese 1993; Strack et al. 1997a).
Fig. 1.
Phosphorylation of post-synaptic density (PSD) -bound Ca2+/calmodulin-dependent protein kinase II (CaMKII) is controlled by the balance of kinase and phosphatase activities. (a) 32P incorporation into PSD CaMKII under standard conditions in 1 mmol/L Ca2+ increases in Ca2+/CaM but plateaus at a lower level in reactions without microcystin-LR. Phosphorylation is accompanied by a shift in apparent molecular weight (indicated by a bracket). The higher molecular weight phospho-band that undergoes a similar shift and dephosphorylation is βCaMKII. (b) Phospho-CaMKII band intensity on the autoradiogram was quantified from three separate experiments using a phosphor-imager. After 10 min, there was a significant (p < 0.05, anova) difference between (+) and (−) microcystin-LR values. (c) Total kinase, quantified from parallel blots probed with the anti-αCaMKII (1 : 1000), did not change significantly. (d) Coomassie stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis profile of PSD proteins phosphorylated under standard conditions with 1 mmol/L Ca2+ showing that Ca2+/CaM initially shifts the apparent molecular weight of aCaMKII (indicated by arrow) to a higher value, followed by a return towards the unshifted value. The presence of microcystin-LR (+) blocked downward shift compared with sample in which there was no microcystin-LR (−). The magnitude of the shift is indicated by a bracket.
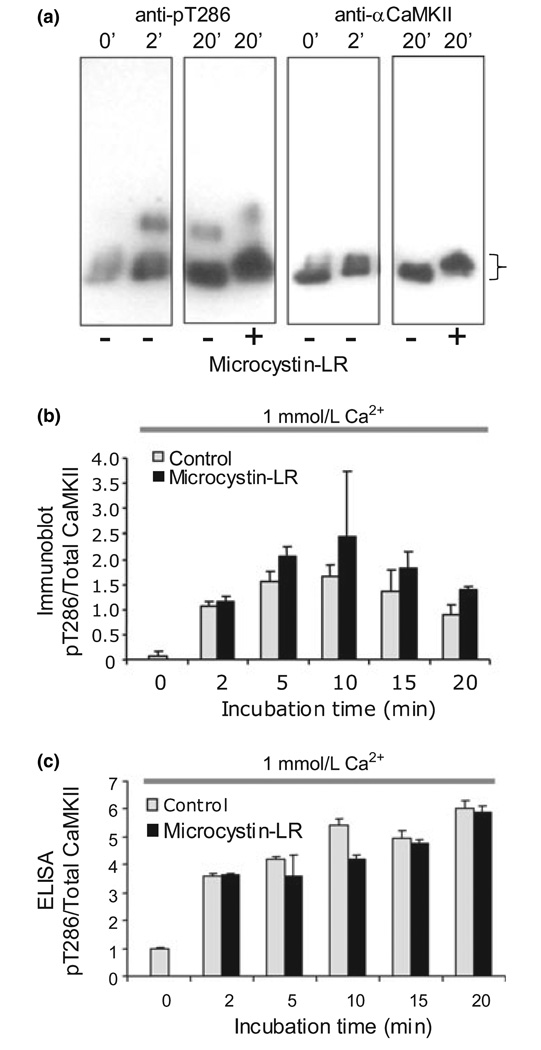
Because CaMKII has many autophosphorylation sites (Hanson et al. 1989), neither relative mobility nor total 32P content address the state of T286, the autophosphorylation site associated with constitutive activity of CaMKII and its role in plasticity. It is known that many other sites (Miller et al. 1988; Hanson et al. 1989), notably T253 in PSD CaMKII (Dosemeci et al. 1994; Migues et al. 2006), can be phosphorylated in a Ca2+/CaM-dependent fashion. To determine if pT286 is one of the sites dephosphorylated by endogenous phosphatase, we used a phospho-specific antibody directed against pT286 to probe immunoblots of samples treated in the same manner as in the radioactive phosphate experiments. Parallel blots were probed with an antibody that recognized total αCaMKII to normalize for protein levels. Figure 2a shows that addition of Ca2+/CaM results in a fast and significant increase in pT286 that accompanies the shift in apparent molecular weight. However, whereas, phosphatase activity was evident when incorporation into all sites was measured (see effect of microcystin-LR in Fig. 1), phosphatase inhibitor had little consequence for pT286 levels (Fig. 2a and b). When quantified and normalized to total kinase, there was no significant effect of phosphatase inhibitor on pT286 levels (Fig. 2b, p > 0.05, anova). A noticeable reversal of the upward mobility shift in the same gels indicated that dephosphorylation of the other CaMKII sites was occurring under these conditions. These data were unexpected and seemed to indicate that dephosphorylation visualized by reversal of the activation-dependent molecular weight shift or removal of radioactive phosphate is not due to dephosphorylation of pT286, but rather due to dephosphorylation of other sites on the kinase.
Fig. 2.
pT286 on Ca2+/calmodulin-dependent protein kinase II (αCaMKII) is not dephosphorylated by post-synaptic density (PSD) phosphatase during sustained Ca2+ elevation. (a) PSDs were phosphorylated under standard conditions with 1 mmol/L Ca2+ for the indicated times, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose and probed with antipT286 (1 : 1000, left panels) and anti-αCaMKII (1 : 1000, right panels). The higher molecular weight band seen in the anti-pT286 blots is βCaMKII. Phosphorylation of αCaMKII is accompanied by a shift in apparent molecular weight (indicated by a bracket). (b) Quantification of the pT286 levels on αCaMKII normalized to total kinase from immunoblots. pT286 increases significantly with initial phosphorylation, but does not show any significant microcystin-LR-dependent difference on further incubation (p > 0.05, anova). (c) PSDs phosphorylated under the same conditions as in panel (a) were subjected to ELISA for pT286. No dephosphorylation of pT286 was detected (p > 0.05, anova).
Given the surprising inability of endogenous phosphatase to dephosphorylate pT286, we sought to test the integrity and levels of phosphatase in our PSD preparation. PP1 protein has previously been shown to be enriched in PSDs by immunoblotting (Vinade and Dosemeci 2000). Using an exogenous fluorescent substrate and a solid phase assay system, we found that the endogenous PSD phosphatase activity was inhibited by okadaic acid and microcystin at concentrations that inhibit PP1 (Sheppeck et al. 1997). Low concentrations of okadaic acid that would inhibit PP2A but not PP1 were ineffective, consistent with previous work indicating that PSD phosphatase is primarily PP1 (Shields et al. 1985; Strack et al. 1997a). The microcystin-sensitive PP1 activity in our preps (67.5 ± 1.2 nmoles/min/mg) was comparable with that reported in PSDs (Strack et al. 1997b) or in a crude particulate fraction (Shields et al. 1985; Sim et al. 1994).
We also wanted to verify the lack of pT286 dephosphorylation by a second method. To quantify pT286 dephosphorylation more accurately, we developed an ELISA in which total PSD pT286 could be measured. Using the ELISA, we again found no evidence of pT286 dephosphorylation in PSDs when they were maintained in high Ca2+ (Fig. 2c p > 0.05). These data were obtained from the same batch of PSDs used in the bulk dephosphorylation assays in Fig 1 and Fig 2 in which we saw robust dephosphorylation of CaMKII. Similar results were also obtained with other preparations and were not dependent on whether the starting material was fresh or frozen brain.
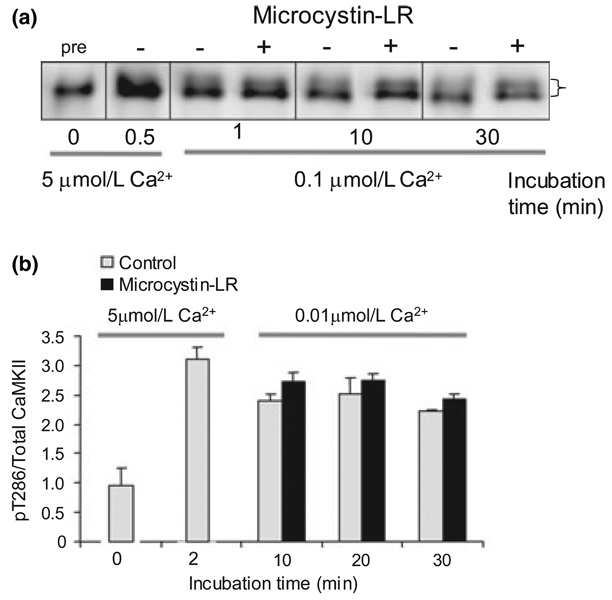
The stability of pT286 under conditions of high Ca2+ could be attributed to the continued Ca2+-dependent activation of CaMKII. A question of importance to switch function under physiological conditions is whether the switch, once turned on by Ca2+ elevation, will stay on after Ca2+ is reduced to the low levels characteristic of the resting physiological state. We found that reducing Ca2+ to 0.1 µmol/L (the physiological resting level, Fig. 3a) or even lower, to 0.01 µmol/L (Fig. 3b) after initial autophosphorylation, did not reduce pT286 stability. These data indicate that once T286 becomes phosphorylated, it is stable to the activity of the endogenous PSD phosphatase even when Ca2+ returns to resting levels. This is the first biochemical evidence that the PSD CaMKII can function as a switch, retaining information about a transient elevation of Ca2+.
Fig. 3.
Maintenance of Ca2+-stimulated T286 phosphorylation after lowering Ca2+. (a) Post-synaptic density proteins were phosphorylated under standard conditions with 5 µmol/L Ca2+. After initial phosphorylation, Ca2+ was lowered to 0.1 µmol/L (physiological resting level) and pT286 phosphorylation monitored ± microcystin-LR by immunoblotting with anti-pT286. (b) Post-synaptic density proteins were phosphorylated under standard conditions with 5 µmol/L Ca2+. After initial phosphorylation, Ca2+ was lowered to 0.01 µmol/L and phosphorylation monitored by ELISA. No significant effect of microcystin-LR was detected (p > 0.05, multifactoral anova).
Persistence of T286 phosphorylation may be important in maintaining the Ca2+-independent activity of this enzyme. Testing this at physiological temperature in vitro is complicated by the fact that the autophosphorylated kinase exhibits substantial thermal instability (Kuret and Schulman 1985; Lai et al. 1986). Even in the face of decreasing total activity, however, the phosphatase sensitivity of Ca2+-independent activity can still be assessed by comparison of activity in autophosphorylated PSDs maintained with or without microcystin-LR. We find that initial autophosphorylation produces a substantial increase in Ca2+-independent activity (measured in 5 mmol/L EGTA; at 2 min, 865 ± 109% of basal Ca2+-independent activity, n = 6). Over the course of 20 min, total activity declines (data not shown), but there is no significant difference in autonomous activity ± microcystin-LR at any time point. At 10 min, autonomous activity in the presence of microcystin is 98.4 ± 5.4% of autonomous activity in the absence of the inhibitor, and at 20 min it was 88.7 ± 20.6% (p > 0.05, anova for the whole time course). These results are consistent with our measurements of pT286 and indicate that the enzyme is persistently activated by a brief elevation of Ca2+.
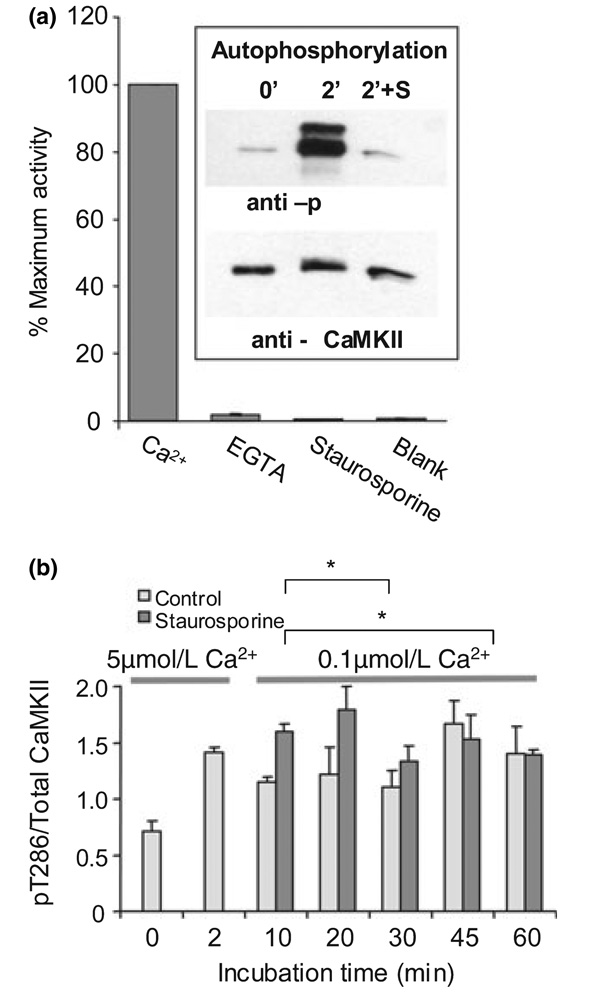
The stability of activity and phosphorylation at resting Ca2+ argues that maintained Ca2+-dependent activation of CaMKII (or other intrinsic PSD enzymes) is not required for maintained phosphorylation of T286. However, as phosphorylation of T286 allows CaMKII to become active in the absence of Ca2+/CaM, it was possible that the stability of pT286 was due to rephosphorylation by autonomously active CaMKII subunits. To determine if autonomous kinase activity was required, we looked at the effects of kinase inhibitor on the persistence of T286 phosphorylation. We used staurosporine under conditions of demonstrated efficacy for blocking Ca2+-dependent pT286 phosphorylation (Fig. 4a) to test this. If stability required rephosphorylation, then adding staurosporine after initial phosphorylation would promote net dephosphorylation of pT286. Figure 4b shows that blocking CaMKII activity after the first 2 min did not affect pT286 stability. Thus, once turned on by a Ca2+-dependent reaction, the PSD CaMKII switch stays on simply because there is no effective phosphatase activity.
Fig. 4.
Rephosphorylation is not responsible for persistent pT286. (a) Staurosporine inhibits post-synaptic density (PSD) Ca2+/calmodulin-dependent protein kinase II (CaMKII) activity and T286 autophosphorylation. Total CaMKII activity was measured in the presence of 1 mmol/L CaCl2, and Ca2+-independent activity assayed in 5 mmol/L EGTA. Reactions were run for 30 s. Activity in blank (no PSDs) and staurosporine (1 mmol/L Ca2+ + 2 µmol/L drug) reactions was significantly different from activity in the presence EGTA (p < 0.05, anova, indicated by *) but not from each other (p > 0.05, anova), indicating that staurosporine inhibits both the Ca2+-dependent and Ca2+-independent components of CaMKII activity. Autophosphorylation of T286 (inset) was probed by immunoblotting of unphosphorylated (0′) and phosphorylated (2′ reaction in 1 mmol/L CaCl2) PSD preparation. Addition of 2 µmol/L staurosporine to the autophosphorylation reaction blocked T286 phosphorylation. Total µCaMKII immunoreactivity was equivalent between samples. The higher molecular weight band on the anti-pT286 blot is βCaMKII. (b) Phosphorylation of PSDs was performed in 5 µmol/L Ca2+. At 10 min, Ca2+ was lowered to 0.1 µmol/L and staurosporine (2 µmol/L) added to half the sample. pT286 levels were measured by ELISA. No significant effect of staurosporine was detected (p > 0.05, multifactoral anova).
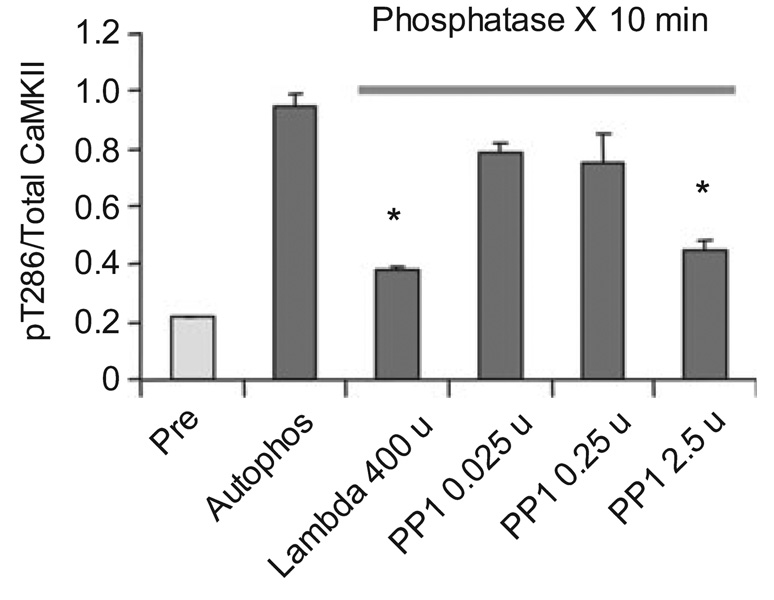
Stability of the LTP-induced increase in T286 phosphorylation over the course of hours (Fukunaga et al. 1993; Heynen et al. 1996; Barria et al. 1997; Lengyel et al. 2004; Ahmed and Frey 2005) may be important for maintenance of memory, but plasticity also requires mechanisms for resetting the switch. When synapses undergo activity-dependent depotentiation, pT286 is dephosphorylated by PP1 (Huang et al. 2001). In the cell, the catalytic subunit of PP1 is not freely diffusible, but is bound to other subunits that target it to specific sites (Colbran et al. 1997; Watanabe et al. 2001). Indeed, there are at least three PP1 binding subunits in the PSD (Colbran 2004). It is thus important to understand why PP1 cannot dephosphorylate CaMKII under basal conditions. One possibility is that pT286 is occluded by other proteins or is buried within the protein (this occurs in protein kinase C Sweatt et al. 1998). Alternatively, rigid positioning of PP1 may keep its active site away from exposed pT286 residues. To distinguish between these possibilities we applied λ phosphatase or the free catalytic subunit of PP1 to autophosphorylated PSDs. We found that both dephosphorylated pT286 (Fig. 5). These results clearly indicate that T286 is not buried and suggest that precise positioning of PP1 in the PSD matrix makes it unable to dephosphorylate pT286, while allowing it to dephosphorylate other sites on CaMKII.
Fig. 5.
Dephosphorylation of T286 by exogenous catalytic subunits of phosphatases (PP1 or λ phosphatase). Phosphorylation was carried out for 2 min in the presence of 1 mmol/L Ca2+. The indicated amount of phosphatase was added for 10 min at 30°C and pT286 levels measured by ELISA. A total of 400 U of k phosphatase and 2.5 U of PP1 significantly decreased pT286 levels (p < 0.01, anova).
Discussion
Our results show that CaMKII in the PSD can act as a stable switch, even in the presence of substantial phosphatase activity. The reason for the stability is surprising: even though the PP1 in the PSD can dephosphorylate some sites on CaMKII, it cannot dephosphorylate the T286 site that is critical for constitutive activation of CaMKII. We show that persistent Ca2+-independent kinase activity is present, but not necessary for the maintenance of T286 phosphorylation, at least on the time scale we have measured. Thus, the critical factor important for persistence appears to be structural; CaMKII and PP1, both of which are held in the PSD by a number of protein-protein interactions are held in such a position that PP1 simply cannot reach pT286.
These conclusions were made possible through the use of a phospho-specific antibody to pT286 that allowed us to look at this site in isolation and differentiate it from other CaMKII sites. Dephosphorylation of synaptic CaMKII has previously been addressed only in terms of total phosphate, although some studies interpreted this as reflecting the dynamics of T286 phosphorylation, extrapolating from studies on soluble CaMKII that showed this is a preferred autophosphorylation site when the reaction is performed at 0–4°C (Schworer et al. 1988). Autophosphorylation of T286, however, is not the principal reaction for PSD-bound CaMKII under physiological conditions; the highest level of phosphorylation at 37°C appears to be on T253 (Dosemeci et al. 1994), and even at 4°C, only 30% of the phosphate is in T286 (Rich et al. 1989). The large number of non-T286 sites that can be potentially dephosphorylated by PSD phosphatase make it important to use a method that specifically monitors pT286.
These results have implications for the mechanism by which pT286 persists after LTP or learning. Taken together with previous work on CaMKII translocation (Shen and Meyer 1999; Otmakhov et al. 2004), our data suggest a simple model. Soluble cytoplasmic CaMKII translocates to the synapse in response to the Ca2+ elevation triggered by the opening of NMDA channels during LTP induction. At the synapse, CaMKII binds to proteins within the PSD, including the NMDA channel (Strack and Colbran 1998; Leonard et al. 1999; Bayer et al. 2001; Barria and Malinow 2005). Once in the PSD, pT286 is protected from dephosphorylation. Previous work showed that PSD CaMKII was protected from dephosphorylation by PP2A (Strack et al. 1997a), but left open the possibility that it could be dephosphorylated by PSD PP1. Our results show that T286 cannot be attacked by this phosphatase and therefore make it clear how T286 can remain phosphorylated and thereby serve as a biochemical memory important in LTP maintenance (Sanhueza et al. 2007).
How is pT286 protected from dephosphorylation by PSD-resident PP1? One hypothesis is that the site becomes buried within the kinase, as can occur with certain phosphorylation sites of protein kinase C (Sweatt et al. 1998). However, this is not the case for CaMKII because pT286 can be dephosphorylated by exogenously applied soluble PP1 catalytic subunit or λ phosphatase. The differential dephosphorylation of sites on CaMKII by PSD PP1 must thus arise from different accessibility of the bound PP1 to these sites. PP1 is held in the PSD by scaffolding proteins. Apparently these proteins position PP1 so rigidly and so precisely that it has access to some sites on CaMKII but not to T286. To our knowledge, this is the first evidence that scaffold-dependent positioning can be so precise that it determines which site on a substrate is targeted. How this finding translates to the intact cell where the PSD is bathed in cytosol is unknown, but the fact that protection of pT286 is so robust suggests that the relative relationship between PP1 and CaMKII molecules within the PSD is very stereotyped. This type of organization could also be used to block or enhance other biochemical reactions within the PSD, implying that our understanding of reactions between other synaptic molecules, much of which is based on soluble phase experimentation, needs to be reevaluated in the context of PSD structure.
Acknowledgements
This work was supported by R01 NS50944 as part of the NSF/NIH Collaborative Research in Computational Neuroscience program (to JEL), a grant from the Packard Foundation Interdisciplinary Science Program (to I. Epstein), R01 GM54408 (to LCG), R01 NS 27337 (to JEL), and NINDS intramural funds. We would like to thank Rebecca Mongeon for help with initial studies and Anatol Zhabotinsky for suggesting the examination of switching in the PSD and early help in the design of experiments. We also thank Roger Colbran for useful discussions and comments on the manuscript.
Abbreviations used
- BSA
bovine serum albumin
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- DTT
dithiothreitol
- LTP
long-term potentiation
- PSD
post-synaptic density
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
References
- Ahmed T, Frey JU. Plasticity-specific phosphorylation of CaMKII, MAP-kinases and CREB during late-LTP in rat hippocampal slices in vitro. Neuropharmacology. 2005;49:477–492. doi: 10.1016/j.neuropharm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Bradshaw JM, Kubota Y, Meyer T, Schulman H. An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling. Proc. Natl. Acad. Sci USA. 2003;100:10512–10517. doi: 10.1073/pnas.1932759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J. Cell. Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ. Protein phosphatases and calcium/calmodulin-dependent protein kinase II-dependent synaptic plasticity. J. Neurosci. 2004;24:8404–8409. doi: 10.1523/JNEUROSCI.3602-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr. Opin. Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Colbran RJ, Bass MA, McNeill RB, Bollen M, Zhao S, Wadzinski BE, Strack S. Association of brain protein phosphatase 1 with cytoskeletal targeting/regulatory subunits. J. Neurochem. 1997;69:920–929. doi: 10.1046/j.1471-4159.1997.69030920.x. [DOI] [PubMed] [Google Scholar]
- Dosemeci A, Reese TS. Inhibition of endogenous phosphatase in a postsynaptic density fraction allows extensive phosphorylation of the major postsynaptic density protein. J. Neurochem. 1993;61:550–555. doi: 10.1111/j.1471-4159.1993.tb02158.x. [DOI] [PubMed] [Google Scholar]
- Dosemeci A, Gollop N, Jaffe H. Identification of a major autophosphorylation site on postsynaptic density-associated Ca2+/calmodulin-dependent protein kinase. J. Biol. Chem. 1994;269:31330–31333. [PubMed] [Google Scholar]
- Dosemeci A, Reese TS, Petersen J, Tao-Cheng JH. A novel particulate form of Ca2+/calmodulin-dependent protein kinase II in neurons. J. Neurosci. 2000;20:3076–3084. doi: 10.1523/JNEUROSCI.20-09-03076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma Y, Sweatt JD, Giese KP. Mouse genetic approaches to investigating calcium/calmodulin-dependent protein kinase II function in plasticity and cognition. J. Neurosci. 2004;24:8410–8415. doi: 10.1523/JNEUROSCI.3622-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, Stoppini L, Miyamoto E, Muller D. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 1993;268:7863–7867. [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Kapiloff MS, Lou LL, Rosenfeld MG, Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its auto-regulation. Neuron. 1989;3:59–70. doi: 10.1016/0896-6273(89)90115-3. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Abraham WC, Bear MF. Bidirectional modification of CA1 synapses in the adult hippocampus in vivo. Nature. 1996;381:163–166. doi: 10.1038/381163a0. [DOI] [PubMed] [Google Scholar]
- Huang CC, Liang YC, Hsu KS. Characterization of the mechanism underlying the reversal of long term potentiation by low frequency stimulation at hippocampal CA1 synapses. J. Biol. Chem. 2001;276:48108–48117. doi: 10.1074/jbc.M106388200. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Kuret J, Schulman H. Mechanism of autophosphorylation of the multifunctional Ca2+/calmodulin-dependent protein kinase. J. Biol. Chem. 1985;260:6427–6433. [PubMed] [Google Scholar]
- Lai Y, Nairn AC, Greengard P. Autophosphorylation reversibility regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc. Natl. Acad. Sci. USA. 1986;83:4253–4257. doi: 10.1073/pnas.83.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel I, Voss K, Cammarota M, Bradshaw K, Brent V, Murphy KP, Giese KP, Rostas JA, Bliss TV. Autonomous activity of CaMKII is only transiently increased following the induction of long-term potentiation in the rat hippocampus. Eur. J. Neurosci. 2004;20:3063–3072. doi: 10.1111/j.1460-9568.2004.03748.x. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc. Natl Acad. Sci. USA. 1999;96:3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Migues PV, Lehmann IT, Fluechter L, Cammarota M, Gurd JW, Sim AT, Dickson PW, Rostas JA. Phosphorylation of CaMKII at Thr253 occurs in vivo and enhances binding to isolated postsynaptic densities. J. Neurochem. 2006;98:289–299. doi: 10.1111/j.1471-4159.2006.03876.x. [DOI] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Miller SG, Patton BL, Kennedy MB. Sequences of autophosphorylation sites in neuronal type II CaM kinase that control Ca2+-independent activity. Neuron. 1988;1:593–604. doi: 10.1016/0896-6273(88)90109-2. [DOI] [PubMed] [Google Scholar]
- Miller P, Zhabotinsky AM, Lisman JE, Wang XJ. The stability of a stochastic CaMKII switch: dependence on the number of enzyme molecules and protein turnover. PLoS. Biol. 2005;3:e107. doi: 10.1371/journal.pbio.0030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J. Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DP, Colbran RJ, Schworer CM, Soderling TR. Regulatory properties of calcium/calmodulin-dependent protein kinase II in rat brain postsynaptic densities. J. Neurochem. 1989;53:807–816. doi: 10.1111/j.1471-4159.1989.tb11777.x. [DOI] [PubMed] [Google Scholar]
- Sanhueza M, McIntyre CC, Lisman JE. Reversal of synaptic memory by CaMKII inhibitor. J. Neurosci. 2007;27:5190–5199. doi: 10.1523/JNEUROSCI.5049-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schworer CM, Colbran RJ, Keefer JR, Soderling TR. Ca2+/calmodulin-dependent protein kinase II: identification of a regulatory autophosphorylation site adjacent to the inhibitory and calmodulin-binding domains. J. Biol. Chem. 1988;263:13486–13489. [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Sheppeck JE, 2nd, Gauss CM, Chamberlin AR. Inhibition of the Ser-Thr phosphatases PP1 and PP2A by naturally occurring toxins. Bioorg. Med. Chem. 1997;5:1739–1750. doi: 10.1016/s0968-0896(97)00146-6. [DOI] [PubMed] [Google Scholar]
- Shields SM, Ingebritsen TS, Kelly PT. Identification of protein phosphatase 1 in synaptic junctions: dephosphorylation of endogenous calmodulin-dependent kinase II and synapse-enriched phosphoproteins. J. Neurosci. 1985;5:3414–3422. doi: 10.1523/JNEUROSCI.05-12-03414.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim AT, Ratcliffe E, Mumby MC, Villa-Moruzzi E, Rostas JA. Differential activities of protein phosphatase types 1 and 2A in cytosolic and particulate fractions from rat forebrain. J. Neurochem. 1994;62:1552–1559. doi: 10.1046/j.1471-4159.1994.62041552.x. [DOI] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 1998;273:20698–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J. Neurochem. 1997a;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- Strack S, Choi S, Lovinger DM, Colbran RJ. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J. Biol. Chem. 1997b;272:13467–13470. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- Sui CW, Chow WY, Chang YC. Effects of disulfide bonds formed during isolation process on the structure of the postsynaptic density. Brain. Res. 2000;873:268–273. doi: 10.1016/s0006-8993(00)02544-0. [DOI] [PubMed] [Google Scholar]
- Sweatt JD, Atkins CM, Johnson J, English JD, Roberson ED, Chen SJ, Newton A, Klann E. Protected-site phosphorylation of protein kinase C in hippocampal long-term potentiation. J. Neurochem. 1998;71:1075–1085. doi: 10.1046/j.1471-4159.1998.71031075.x. [DOI] [PubMed] [Google Scholar]
- Vinade L, Dosemeci A. Regulation of the phosphorylation state of the AMPA receptor GluR1 subunit in the postsynaptic density. Cell. Mol. Neurobiol. 2000;20:451–463. doi: 10.1023/A:1007019030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Huang HB, Horiuchi A, da Cruze Silva EF, Hsieh-Wilson L, Allen PB, Shenolikar S, Greengard P, Nairn AC. Protein phosphatase 1 regulation by inhibitors and targeting subunits. Proc. Natl. Acad. Sci. USA. 2001;98:3080–3085. doi: 10.1073/pnas.051003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]