Abstract
Objective
Although erectile dysfunction (ED) has been associated with heart disease risk factors and large-vessel lower extremity arterial disease (LEAD), no community-based studies have reported the association between ED and small-vessel LEAD, despite the similar size of the arteries affected. We examined whether small-vessel LEAD is associated with ED, and whether this association is independent of cardiovascular risk factors and medications.
Methods and Results
Community-dwelling men, average age 71, completed the International Index of Erectile Function-5 questionnaire and had measurements recorded of toe-brachial index (TBI), a measure of small-vessel LEAD. TBI, 12 cardiovascular risk factors, and medications were used as categorical predictors in age-adjusted bivariate analyses, and as continuous covariates in multivariable linear regression analyses, to determine their independent association with severity of ED.
In the age-adjusted categorical model, the level of TBI (low, medium, high) was associated with the severity of ED (β = 0.364; 95% CI: 0.102, 0.625). In the final multivariable linear regression model, which controlled for age and systolic blood pressure, lower TBI (i.e., more severe small-vessel LEAD) was significantly and independently associated with more severe ED (β = 0.422; 95% CI: 0.019, 0.826).
Conclusions
The severity of small-vessel LEAD is significantly and independently associated with the severity of ED. The mechanism for this association remains to be determined, but these data are compatible with the hypothesis that concurrent ED and small-vessel LEAD signify a diffuse microvascular process involving multiple small-vessel arterial beds.
Keywords: erectile dysfunction, lower extremity arterial disease, cardiovascular risk factors, epidemiology
INTRODUCTION
Until recently, erectile dysfunction (ED) has been an under-reported and poorly understood source of morbidity in men. Today it is recognized that ED is an arterial disease associated with coronary artery disease risk factors [1] and angiographic evidence of coronary artery disease [2]. Lower extremity arterial disease (LEAD) includes small- and large-vessel disease, and is known to be associated with cardiovascular disease [3]. Little attention has been paid to the association between ED and LEAD, although they share common cardiovascular risk factors including age, diabetes, hypertension, dyslipidemia, sedentary lifestyle, obesity, alcohol use, and smoking [4–6], as well as endothelial dysfunction [7] and inflammation [8–10]. None of the three studies that have reported the association between ED and LEAD have specifically addressed the independent ED association with small-vessel LEAD [11–13].
LEAD is traditionally defined as an ankle-brachial index of ≤0.9 [14]. Measurement of ankle-brachial index alone does not isolate small-vessel LEAD. Recent research suggests that small- and large-vessel arterial disease, both usually grouped under LEAD, might have different risk factors and pathophysiological mechanisms [8, 15]. The toe-brachial index (TBI) is a measure of the toe pressure divided by the brachial pressure; it has been validated in multiple studies to be a sensitive and accurate measure for small-vessel LEAD in diabetics [14] and non-diabetics [16] by evaluating healing potential in surgeries dependent on small-vessel blood supply. TBI has been shown to be reproducible and precise [17].
Growing evidence indicates that ED in older men is a predominantly vascular disease [18]. Arterial vessel size has been suggested as a link between arterial disease in different arterial beds [19]. Because penile arteries range from 1.0 to 1.5mm in diameter and toe arteries range similarly from 1.0 to 2.0mm in diameter, ED might be closely linked to small-vessel LEAD. Based on shared risk factors and similar arterial size, we hypothesized that ED is significantly associated with small-vessel LEAD. We report here the first study of community-dwelling older men to examine the association between the severity of ED and the severity of small-vessel LEAD. ED was measured by the validated International Index of Erectile Function-5 (IIEF-5) questionnaire, the abbreviated international standard for sexual function assessment [20], and small-vessel LEAD was measured by using TBI. To isolate small-vessel LEAD, we excluded the 20 men who had proximal occlusion in the larger lower-extremity arteries (ankle-brachial index ≤0.9), which would be expected to reduce distal toe pressures [8]. We also evaluated whether any observed association was independent of validated medication use and 12 established cardiovascular risk factors.
METHODS
From 1972 through 1974, 82% of adult community-dwelling residents of Rancho Bernardo, California, predominantly middle- to upper-middle-class Caucasians, participated in a survey of cardiovascular risk factors. Noninstitutionalized survivors have been followed annually by mail and periodically by research clinic visits as part of the Rancho Bernardo Study. Participants who attended a voluntary research clinic visit between 1997 and 1999 and returned a mailed ED survey in 1998 are included in this report. The study was approved by the University of California, San Diego institutional review board; all participants gave written informed consent.
At the 1997–1999 clinic visit, we obtained a medical and medication history, and we measured seated resting blood pressure, fasting plasma glucose, total cholesterol, high- and low-density (i.e., HDL and LDL) cholesterol, and triglycerides. Lipids and lipoproteins were measured in a Lipid Research Clinic laboratory certified by the Centers for Disease Control using an Auto Analyzer I (Technicon Instruments, Tarrytown, New York); fasting plasma glucose was measured by a hexokinase method in a clinical laboratory. Height, weight, and waist and hip girth were measured using a standard protocol; body-mass index (kg/cm2) and waist-hip ratio (waist girth/hip girth) were calculated.
For LEAD testing, participants were rested recumbent with extremities at the level of the heart. Blankets were used for warmth to maintain peripheral circulation. Blood pressure measurements were taken twice in each arm using manual pneumatic cuff inflation-deflation and auscultation. Ankle blood pressure, measured twice at each ankle using a manual pneumatic cuff, was based on the return of pulse in the posterior tibialis artery during cuff deflation using an Elite Hand-held Doppler signal device (Imex Medical Systems, Inc., Golden, CO). Ankle-brachial index was calculated as a measure of large-vessel LEAD using the lower of the two blood pressures at each ankle divided by the highest brachial blood pressure. Toe pressure, measured twice using a manual pneumatic cuff inflated at the base of each great toe, was based upon the return of blood flow to the distal ventral toe pad using a photoplethysmograph (Imex Lab 3000, Imex Medical Systems, Inc., Golden, CO). TBI, which has been shown to have good reproducibility in the same person over time [17], was calculated as a measure of small-vessel LEAD using the lower of the two blood pressure measurements at each toe divided by the highest brachial blood pressure. To isolate small-vessel LEAD, we excluded men who had proximal occlusion in the larger lower-extremity arteries (ankle-brachial index ≤0.9), which would reduce distal toe pressures [8].
The 1998 mailed questionnaire included the IIEF-5, the standard short version of the original 15-item International Index of Erectile Function questionnaire, the international standard for the assessment of sexual function [21]. The IIEF-5 contains four items selected from the erectile domain portion of the full questionnaire and one item addressing sexual satisfaction [20]. Each item is answered on a scale of 0 to 5, with 0 denoting that the respondent was not sexually active, 1 being the most severe symptom, and 5 being no symptoms. ED was classified by the summed variable Erectile Dysfunction score (ED score) as no ED (22–25), mild ED (17–21), mild-moderate ED (12–16), moderate ED (8–11), and severe ED (5–7). According to the IIEF-5 protocol, men who fail to provide complete responses or report “no sexual activity” as a response to any of the questions cannot be classified. The IIEF-5 has been validated against the combination of patient-reported ED, physician diagnoses, and objective testing [20, 22].
The present analysis includes all sexually active respondents without evidence of large-vessel LEAD based on ankle-brachial index who had complete responses to IIEF-5 questions and toe pressure data. Statistical analyses were performed using Statistical Analysis System v9.1 software (SAS Institute, Cary, North Carolina). We analyzed ED score and each continuous independent variable graphically. To correct for a non-normal distribution, we log-transformed ED score, fasting plasma glucose, high-density cholesterol, triglycerides, number of grams of alcohol per week, and the number of pack-years of cigarettes.
Cardiovascular risk factors and medications were entered as categorical variables versus ED score in bivariate analyses. Included risk factors and medications were TBI level, diabetes (defined as any of the following: fasting plasma glucose ≥126mg/dl, use of diabetes medications, history of diabetes), hypertension (any of the following: systolic blood pressure ≥140mmHg, diastolic blood pressure ≥90mmHg, use of blood pressure medications, history of high blood pressure), body-mass index (normal: ≤24kg/cm2, overweight: >24 but <30kg/cm2, obese: ≥30kg/cm2), hyperlipidemia by diabetes status (for non-diabetics: fasting total cholesterol ≥200mg/dl, triglycerides ≥200mg/dl, or LDL cholesterol ≥130mg/dl; for diabetics: fasting LDL cholesterol ≥100mg/dl; for any person: fasting HDL cholesterol <40mg/dl), waist-hip ratio (normal: ≤0.95, elevated: >0.95), exercise ≥3 times per week, cigarette smoking (ever), alcohol use (none, moderate: >0 but <140g/week, heavy: ≥140g/week), and use of beta-blockers, calcium-channel blockers, diuretics, angiotensin-converting enzyme inhibitors, anti-angina medications, or “other heart medications”. Because no consensus exists to classify TBI levels, we defined TBI levels based on the data from this study, with low indicating participants with TBI >1 standard deviation (SD = 0.17) below the cohort TBI mean, medium indicating TBI ≤1 SD below the mean and ≤1 SD above the mean, and high indicating TBI >1 SD above the mean. Because age is strongly associated with the prevalence of ED [4], we examined the age-adjusted association between ED score and each risk factor using ANCOVA, and reported parameter estimate β as a measure of magnitude of effect.
Cardiovascular risk factors and medications with two-sided P <0.20 from the bivariate analyses were entered into a multivariable linear regression model to examine the independent effect of cardiovascular risk factors and medications on ED score. In addition to age, clinical measures were entered as continuous variables where possible and stepwise selection was used to create a final parsimonious model. Interactions between variables were modeled and included if statistically significant. Magnitude of effect and statistical significance were evaluated by the parameter estimate β and 95% confidence intervals of each risk factor. The F-statistic and adjusted R2 statistic were reported for the overall strength of the model.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
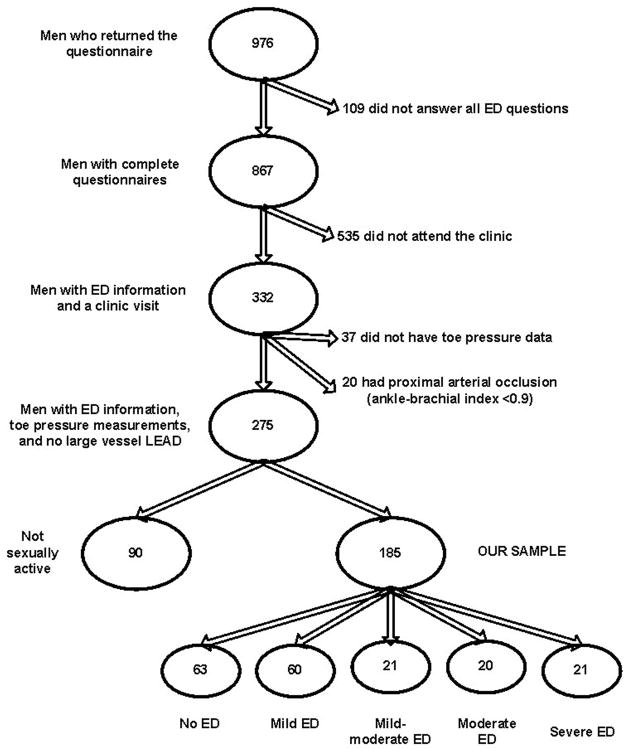
Of the original cohort of 1810 men (enrolled 1972–1974), 976 (54%) of the surviving community dwelling participants aged 44 to 94 years returned the 1998 mailed ED survey. The main reason for non-response was death before the 1998 survey. As shown in Figure 1, among the respondents, 109 were excluded for incomplete questionnaire responses and 535 men who completed the questionnaire were excluded because they did not attend the research clinic for TBI measurement. Other exclusions were 37 participants who did not have toe pressure data, 20 who had ankle-brachial index ≤0.9 (indicating significant large-vessel arterial occlusion), and 90 who returned the mailed IIEF-5 questionnaire but said they were not sexually active, leaving 185 participants for this report.
Figure 1.
Participant inclusion and exclusion.
As shown in Table 1, their average age was 71 ±10 years and their average ED score was 18±6, which corresponds to the category of mild-ED (17–21). One standard deviation above the ED score mean corresponds to no-ED (22–25) and one standard deviation below corresponds to mild-moderate-ED (12–16). The average TBI was 0.83 ± 0.17 with lower TBI indicating worse small-vessel LEAD.
Table 1.
Cohort characteristics, continuous variables, follow-up clinic visit 1997–1999 (n=185).
| Characteristic | Mean ± standard deviation |
|---|---|
| Age | 70.7 ± 9.6 |
| Weight (kg) | 81.2 ± 11.7 |
| Height (cm) | 175 ± 6.4 |
| Erectile dysfunction-score | 17.8 ± 6.5 |
| Toe-brachial index | 0.83 ± 0.17 |
| Ankle-brachial index* | 1.12 ± 0.06 |
| Systolic blood pressure (mmHg) | 133 ± 19 |
| Diastolic blood pressure (mmHg) | 76.4 ± 8.9 |
| Body-mass index (kg/cm2) | 26.6 ± 3.2 |
| Waist-hip ratio | 0.93 ± 0.06 |
| Fasting plasma glucose (mg/dl) | 106 ± 15.3 |
| Total cholesterol (mg/dl) | 190.8 ± 31.1 |
| High-density lipoprotein cholesterol (mg/dl) | 50.6 ± 12.6 |
| Low-density lipoprotein cholesterol (mg/dl) | 116.5 ± 28.4 |
| Triglycerides (mg/dl) | 123.0 ± 101.1 |
| Alcohol (grams/week) | 86.6 ± 87.8 |
| Tobacco (pack-years) | 16.8 ± 23.0 |
Ankle-brachial index of included cohort, >0.9 by definition
As shown in Table 2, the number of men in each age decade ranged from 26 to 36, and the number in each of the no-ED (63) and mild-ED categories (60) equaled the total number of participants in the mild-moderate-ED or worse categories (62). Only 6.5% of these elderly survivors had diabetes, but a majority had hypercholesterolemia (69.6%), were overweight (57.8%), and had ever smoked cigarettes (61.1%). In 1997–1999, less than 15 percent of these men were taking a heart or blood pressure medication.
Table 2.
Cohort characteristics, prevalence of categorical variables and medications, follow-up clinic visit 1997–1999 (n=185).
| Risk Factor | Category | Number (% total) |
|---|---|---|
| Age | <60 | 27 (10.3) |
| 60–65 | 26 (14.1) | |
| 65–70 | 36 (19.5) | |
| 70–75 | 31 (16.8) | |
| 75–80 | 34 (18.4) | |
| >80 | 31 (16.8) | |
| Erectile dysfunction | No ED | 63 (34.1) |
| Mild ED | 60 (32.4) | |
| Mild-moderate ED | 21 (11.4) | |
| Moderate ED | 20 (10.8) | |
| Severe ED | 21 (11.4) | |
| Toe-brachial index | 0.2–0.4 | 4 (2.2) |
| 0.4–0.6 | 18 (9.7) | |
| 0.6–0.8 | 48 (25.9) | |
| 0.8–1.0 | 91 (49.2) | |
| 1.0–1.2 | 24 (13.0) | |
| Body-mass index | Normal | 54 (29.2) |
| Overweight | 107 (57.8) | |
| Obese | 24 (13.0) | |
| Elevated waist-hip ratio (>0.95) | 64 (34.8) | |
| Hypertension | 83 (44.9) | |
| Diabetes | 12 (6.5) | |
| Hyperlipidemia | 128 (69.6) | |
| Beta-blocker use | 25 (13.5) | |
| Calcium channel blocker use | 29 (15.7) | |
| Diuretic use | 28 (15.1) | |
| Angiotensin converting enzyme inhibitor use | 27 (14.6) | |
| Anti-angina medication use | 4 (2.2) | |
| Other heart medication use | 19 (10.3) | |
| Exercise ≥3 times per week | 36 (19.5) | |
| Tobacco use ever | 113 (61.1) | |
| Alcohol use | None | 38 (20.5) |
| Moderate | 106 (57.3) | |
| Heavy | 41 (22.2) |
Participants with ankle-brachial index ≤0.9 were excluded from analysis
Because age on a continuous scale showed a very strong association with ED score in a bivariate model (F-statistic = 5.31, p <0.001), all subsequent analyses were adjusted for age. As shown in Table 3, categorical TBI level (low, medium, high), body-mass index (normal, overweight, obese), alcohol use (none, moderate, heavy), and diuretic use were each significantly associated with ED. Three additional factors, hypertension, beta-blocker use, calcium-channel blocker use, and smoking each showed a marginally significant (p ≤0.15) association with ED. Neither diabetes nor hyperlipidemia was a significant predictor of ED score in these age-adjusted analyses.
Table 3.
Age-adjusted bivariate analysis — categorical cardiovascular risk factors and medications on the logarithm of ED score.
| Categorical risk factor | Parameter estimate β [95% CI] |
P-value | |
|---|---|---|---|
| Toe-brachial index level* | 0.02 | ||
| Medium vs. low | 0.129 [−0.051, 0.309] | ||
| High vs. low | 0.364 [0.102, 0.625] | ||
| Diabetes | −0.046 [−0.318, 0.225] | 0.74 | |
| Hypertension | −0.131 [−0.267, 0.004] | 0.06 | |
| Smoker | 0.099 [−0.037, 0.235] | 0.15 | |
| Body-mass index | 0.01 | ||
| Overweight vs. normal | −0.185 [−0.336, −0.034] | ||
| Obese vs. normal | −0.317 [−0.536, −0.097] | ||
| Hyperlipidemia | −0.057 [−0.191, 0.077] | 0.40 | |
| Elevated waist-hip ratio | −0.071 [−0.212, 0.069] | 0.32 | |
| Exercise ≥3 times per week | −0.001 [−0.170, 0.167] | 0.99 | |
| Alcohol use† | 0.04 | ||
| Moderate vs. none | −0.102 [−0.271, 0.067] | ||
| Heavy vs. none | −0.257 [−0.458, −0.056] | ||
| Beta-blocker use | −0.170 [−0.366, 0.025] | 0.09 | |
| Calcium channel blocker use | −0.179 [−0.365, 0.008] | 0.06 | |
| Diuretic use | −0.206 [−0.391, −0.020] | 0.03 | |
| Angiotensin-converting enzyme inhibitor use | −0.093 [−0.283, 0.097] | 0.33 | |
| Anti-angina medication use | −0.080 [−0.547, 0.386] | 0.73 | |
| Other heart medication use | −0.122 [−0.346, 0.102] | 0.28 |
Toe-brachial index level — low <0.66, medium ≥0.66 but ≤1.00, high >1.00
Alcohol use — moderate >0 but <140g/week, heavy ≥140g/week
Based upon the bivariate analyses, age, TBI, body-mass index, systolic and diastolic blood pressure (which were not correlated with each other), alcohol (grams/week), cigarettes (pack-years), and use of beta-blockers, diuretics, and calcium-channel blockers, were regressed on the logarithm of ED score in a multivariable linear regression model. Stepwise selection produced a model that fit the data well (F = 15.4, p < 0.001) and accounted for 19% of the variation in ED score (adjusted R2 = 0.19) (Table 4). The three risk factors that were significantly associated with ED score were TBI (β = 0.422, 95% CI: 0.019, 0.826), age (β = −0.008, 95% CI: −0.0003, −0.016), and systolic blood pressure (β = −0.008, 95% CI −0.004, −0.012).
Table 4.
Final multivariable linear regression model of significant continuous risk factors on logarithm of ED score — resulting changes in ED score from variation in risk factors.
| Risk factor | Parameter estimate β [95% CI]* |
Variation in risk factor | Resulting ΔED score (no-ED)† |
Resulting ΔED score (mild- ED)† |
Resulting ΔED score (mild-moderate-ED)† |
Resulting ΔED score (moderate-ED)† |
Resulting ΔED score (severe-ED)† |
|---|---|---|---|---|---|---|---|
| Age | −0.008 [−0.0003, −0.016] | Increased 10 years | −1.8 | −1.5 | −1.1 | −0.7 | −0.5 |
| Toe-brachial index | 0.422 [0.019, 0.826] | Decreased 1 SD‡ (0.17) | −1.6 | −1.3 | −1.0 | −0.7 | −0.4 |
| Systolic blood pressure | −0.008 [−0.004, −0.012] | Increased 10mmHg | −1.8 | −1.5 | −1.1 | −0.7 | −0.5 |
Overall model adjusted R2 = 0.19, F-statistic = 15.4, p < 0.001
No-ED (range 22–25) represented by ED score = 23.5, mild-ED (range 17–21) = 19, mild-moderate-ED (range 12–16) = 14, moderate-ED (range 8–11) = 9.5, severe-ED (range 5–7) = 6
SD = standard deviation
Lower TBI, increasing age, and increasing systolic blood pressure were independently associated with more severe ED (i.e., a lower ED score). A decrease in TBI from high to medium (1 standard deviation, 0.17) resulted in a decrease of ED score, ranging from 1.6 in participants with no ED, to 0.4 in participants with severe ED (Table 4). An increase of 10 years of age or an increase in 10mmHg of systolic blood pressure resulted in a decrease of ED score, ranging from 1.8 (no ED) to 0.5 (severe ED).
DISCUSSION
To our knowledge, this is the first community-based study reporting an independent association between isolated small-vessel LEAD and ED. After adjusting for age and examining 12 classical cardiovascular disease risk factors and several medications, we found that small-vessel LEAD assessed by low TBI was significantly associated with ED severity. In the age-adjusted categorical model, TBI level (low, medium, high) was associated with the severity of ED (p = 0.02). In the multivariable linear regression model, which controlled for age and systolic blood pressure, lower TBI (i.e., more severe small-vessel LEAD) remained significantly and independently associated with more severe ED (β = 0.422, 95% CI: 0.019, 0.826).
As expected, age was a strong risk factor for ED, stronger than TBI, probably in part because age is better measured (less inter-individual variation) than TBI. The strong association of blood pressure with small-vessel LEAD is a novel finding, and somewhat surprising in that in a previous report from this cohort [15], the authors were unable to show association between small-vessel LEAD and blood pressure. That study differs from this report in that participants were on average younger, less obese, and had lower fasting glucose levels. No data on ED were available from the earlier study.
The three published studies suggesting a common pathologic process for ED and LEAD predominantly examined large-vessel LEAD, not small-vessel LEAD, and considered few covariates. One was a retrospective study of an insurance database [11] in which men with reported ED were more likely than those without ED to develop LEAD. The authors did not describe clinical methods for LEAD diagnosis, and did not control for blood pressure. In the other two studies [12, 13], the authors reported that narrowing of the larger lower extremity arteries (based on Doppler ultrasound) was more common in men with ED, and that men with lower extremity arterial narrowing and ED had significantly worse penile arterial insufficiency; they did not report small-vessel LEAD or control for any covariates.
One study of hypertensive patients using an entirely different methodology also supports a small-vessel association with ED. Prisant and colleagues [23] reported a marginal association of ED with small-vessel elasticity in the radial artery. They did not measure LEAD. Because all patients had hypertension, they were unable to examine the radial artery-ED association independent of hypertension. Nevertheless their report is important because small-vessel elasticity is likely one of the important vascular causes of ED [24].
Our larger study of community-dwelling older men and measurement of multiple risk factors and TBI, an indicator of end-organ small-vessel dysfunction, allowed us to demonstrate that small-vessel LEAD in men without large-vessel LEAD was significantly and independently associated with ED. By excluding large-vessel LEAD, we eliminated the contribution of proximal vessel occlusion on distal perfusion and isolated arterial disease in the blood vessel size most similar to penile arteries. The distinction between large-vessel LEAD and small-vessel LEAD is important because evidence is increasing that large-vessel LEAD and small-vessel LEAD might have different risk factors. In a prospective study, Aboyans and colleagues [8] reported that smoking, cholesterol, and certain inflammatory markers were risk factors for progression of large-vessel LEAD, but only diabetes was a risk factor for small-vessel LEAD progression. In the early cross-sectional report by Criqui and colleagues [15], multiple cardiovascular risk factors were significantly associated with large-vessel LEAD in men and women, but no cardiovascular risk factors were associated with small-vessel LEAD. These results are compatible with our finding that small-vessel LEAD is significantly associated with ED independent of 12 cardiovascular risk factors and medication use. Although the current results suggest that small-vessel LEAD, not large-vessel LEAD, is associated with severity of ED, we could not compare large-vessel LEAD with ED separately because only 20/295 (6.8%) men in this Rancho Bernardo cohort had large-vessel LEAD (Figure 1).
There are several possible mechanisms for the small-vessel LEAD-ED association. Inflammation may be a common underlying pathophysiological process. Aso and colleagues [9] reported a significant association of high serum high sensitivity CRP and fibrinogen levels with worse TBI but not ankle-brachial index in patients with diabetes. In a randomized clinical trial of physical activity, ED was also reported to have a close link with serum CRP levels [10]. However, Aboyans and colleagues reported that CRP was associated with large-vessel LEAD (p= 0.051) but not with small-vessel LEAD [8].
Although no studies of the association between small-vessel LEAD and endothelial dysfunction have been reported, endothelial dysfunction has been linked to both carotid [25] and brachial artery disease [7]. Because penile erection requires multiple intermediaries including smooth muscle dilatation and endothelium-derived nitric oxide, ED and peripheral vascular endothelial dysfunction may share a defect in this pathway [7].
Arterial diameter may at least partly explain the association between small-vessel LEAD and ED because both processes affect arteries approximately 1–2mm in diameter. ED symptoms often precede coronary artery symptoms [2]; the latter are thought to reflect ischemia in larger arteries (3–4mm in diameter). Montorsi and colleagues [19] have hypothesized that arterial size is the etiological link between ED and coronary artery disease based on the appearance of ED prior to coronary heart disease. These authors postulated that the same atherosclerotic process occurs in penile and coronary arterial beds, but ED symptoms appear prior to cardiac symptoms due to the smaller penile vessels. Other evidence that small-diameter arterial beds are closely associated with ED comes from a study by Kawanishi and colleagues [26], who reported that pathologic retinal arteries in ED patients were significantly and independently associated with peak systolic penile velocity, independent of age, hypertension, hyperlipidemia, and cigarette smoking.
Of the 12 cardiovascular risk factors we examined, only age and blood pressure had a significant independent association with ED in the final multivariable analysis. The age-ED association could be due to stroke, neuropathy, decreased testosterone, decreased vascular compliance, depression, or medication-related co-morbidity [24]. The age-specific prevalence of ED in Rancho Bernardo was similar to the prevalence reported by others [4]. The independent and significant association of higher systolic blood pressure with ED observed here is also consistent with some other reports [18]. Although it is well known that blood pressure medications can cause ED [4], the blood pressure-ED association reported here was independent of antihypertensive medication use once blood pressure was statistically controlled for in the models. Other studies have reported a similar lack of association between ED and beta-blocker and diuretic use when hypertension is considered [5].
Diabetes is a well known risk factor for ED [5, 6], but no association was observed here. The prevalence of diabetes was low in this cohort of elderly community-dwelling survivors, and was higher (by the same diagnostic criteria) in an earlier evaluation of this cohort [1]. We postulate that the low rate of diabetes reflects survival bias because men with diabetes died earlier. In addition, the IIEF-5 protocol excluded men who are not sexually active, who were more likely to have diabetes [1].
The present study is subject to other limitations. Although the multivariable model was able to account for a relatively large percentage of the ED (19% is large for an observational study), limitations of self-reported behaviors, residual confounding, and participation and survival bias — common to all prospective studies of the elderly — likely contributed to the unexplained variation. Because participants were mostly ambulatory, participation bias might explain the low prevalence of large-vessel LEAD (6.8%) in Rancho Bernardo compared to another community-based cohort of similar age (12.4%) [27]. These biases would be expected to underestimate the magnitude of associations, that is, to give a conservative bias.
This study has several strengths. Fifty-four percent of survivors from the original 1972–1974 cohort returned the questionnaire. None of the participants in this study reported claudication, consistent with the observation that clinical claudication represents only a fraction of persons who have subclinical or unrecognized LEAD [15]. Unlike previous reports, we used a validated ED questionnaire, controlled for multiple potential confounding factors, and measured small-vessel LEAD (while excluding large-vessel LEAD) to isolate the blood vessel size most similar to penile arteries. Most importantly, the Rancho Bernardo cohort is community-based, not patient-based or selected for symptoms of LEAD, which minimizes the biases inherent in these other sampling methods.
In conclusion, in this study of older, community-dwelling men, the severity of small-vessel LEAD was significantly associated with the severity of ED, independent of age, cardiovascular disease risk factors, and medication use. We hypothesize that erectile dysfunction might be a marker for a diffuse microvascular disease that occurs concurrently in similarly sized arterial beds in the presence of age and elevated blood pressure. Other studies of small-vessel LEAD are needed to confirm these findings and to further characterize the pathophysiological mechanisms underlying small-vessel disease and its link to erectile dysfunction.
Acknowledgments
Acknowledgments: none
Funding sources: This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK 31801 and National Institute on Aging Grant AG-07181.
Footnotes
Disclosures: none
Contributor Information
Shua J. Chai, Department of Internal Medicine, University of California, San Diego Medical Center.
Elizabeth Barrett-Connor, Division of Epidemiology, Department of Family and Preventive Medicine, School of Medicine, University of California, San Diego.
Anthony Gamst, Department of Biostatistics, School of Medicine, University of California, San Diego.
References
- 1.Fung MM, Bettencourt R, Barrett-Connor E. Heart disease risk factors predict erectile dysfunction 25 years later: the Rancho Bernardo Study. J Am Coll Cardiol. 2004;43:1405–1411. doi: 10.1016/j.jacc.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 2.Montorsi F, Briganti A, Salonia A, Rigatti P, Margonato A, Macchi A, Galli S, Ravagnani PM, Montorsi P. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003;44:360–364. doi: 10.1016/s0302-2838(03)00305-1. discussion 364–365. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. Jama. 1993;270:487–489. [PubMed] [Google Scholar]
- 4.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139:161–168. doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- 5.Blanker MH, Bohnen AM, Groeneveld FP, Bernsen RM, Prins A, Thomas S, Bosch JL. Correlates for erectile and ejaculatory dysfunction in older Dutch men: a community-based study. J Am Geriatr Soc. 2001;49:436–442. doi: 10.1046/j.1532-5415.2001.49088.x. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Morales A, Sanchez-Cruz JJ, Saenz de Tejada I, Rodriguez-Vela L, Jimenez-Cruz JF, Burgos-Rodriguez R. Prevalence and independent risk factors for erectile dysfunction in Spain: results of the Epidemiologia de la Disfuncion Erectil Masculina Study. J Urol. 2001;166:569–574. doi: 10.1016/s0022-5347(05)65986-1. discussion 574–575. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser DR, Billups K, Mason C, Wetterling R, Lundberg JL, Bank AJ. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol. 2004;43:179–184. doi: 10.1016/j.jacc.2003.07.042. [DOI] [PubMed] [Google Scholar]
- 8.Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, Fronek A. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623–2629. doi: 10.1161/CIRCULATIONAHA.105.608679. [DOI] [PubMed] [Google Scholar]
- 9.Aso Y, Okumura K, Inoue T, Matsutomo R, Yoshida N, Wakabayashi S, Takebayashi K, Inukai T. Results of blood inflammatory markers are associated more strongly with toe-brachial index than with ankle-brachial index in patients with type 2 diabetes. Diabetes Care. 2004;27:1381–1386. doi: 10.2337/diacare.27.6.1381. [DOI] [PubMed] [Google Scholar]
- 10.Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D’Andrea F, D’Armiento M, Giugliano D. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. Jama. 2004;291:2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 11.Blumentals WA, Gomez-Caminero A, Joo S, Vannappagari V. Is erectile dysfunction predictive of peripheral vascular disease? Aging Male. 2003;6:217–221. doi: 10.1080/13685530312331309752. [DOI] [PubMed] [Google Scholar]
- 12.Vicari E, Arcidiacono G, Di Pino L, Signorelli S, Arancio A, Sorrentino F, Battiato C, D’Agata R, Calogero AE. Incidence of extragenital vascular disease in patients with erectile dysfunction of arterial origin. Int J Impot Res. 2005;17:277–282. doi: 10.1038/sj.ijir.3901312. [DOI] [PubMed] [Google Scholar]
- 13.Vicari E, Di Pino L, La Vignera S, Fratantonio E, Signorelli S, Battiato C, Calogero AE. Peak systolic velocity in patients with arterial erectile dysfunction and peripheral arterial disease. Int J Impot Res. 2006;18:175–179. doi: 10.1038/sj.ijir.3901387. [DOI] [PubMed] [Google Scholar]
- 14.Orchard TJ, Strandness DE., Jr Assessment of peripheral vascular disease in diabetes Report and recommendations of an international workshop sponsored by the American Diabetes Association and the American Heart Association September 18–20, 1992 New Orleans, Louisiana. Circulation. 1993;88:819–828. doi: 10.1161/01.cir.88.2.819. [DOI] [PubMed] [Google Scholar]
- 15.Criqui MH, Browner D, Fronek A, Klauber MR, Coughlin SS, Barrett-Connor E, Gabriel S. Peripheral arterial disease in large vessels is epidemiologically distinct from small vessel disease. An analysis of risk factors. Am J Epidemiol. 1989;129:1110–1119. doi: 10.1093/oxfordjournals.aje.a115233. [DOI] [PubMed] [Google Scholar]
- 16.Ramsey DE, Manke DA, Sumner DS. Toe blood pressure. A valuable adjunct to ankle pressure measurement for assessing peripheral arterial disease. J Cardiovasc Surg (Torino) 1983;24:43–48. [PubMed] [Google Scholar]
- 17.Sahli D, Svensson M, Lidgren J, Ojbrandt K, Eriksson JW. Evaluation of simple non-invasive techniques for assessment of lower extremity arterial disease. Clin Physiol Funct Imaging. 2005;25:129–134. doi: 10.1111/j.1475-097X.2005.00597.x. [DOI] [PubMed] [Google Scholar]
- 18.Burchardt M, Burchardt T, Baer L, Kiss AJ, Pawar RV, Shabsigh A, de la Taille A, Hayek OR, Shabsigh R. Hypertension is associated with severe erectile dysfunction. J Urol. 2000;164:1188–1191. [PubMed] [Google Scholar]
- 19.Montorsi P, Ravagnani PM, Galli S, Rotatori F, Briganti A, Salonia A, Rigatti P, Montorsi F. The artery size hypothesis: a macrovascular link between erectile dysfunction and coronary artery disease. Am J Cardiol. 2005;96:19M–23M. doi: 10.1016/j.amjcard.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 21.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 22.Cappelleri JC, Siegel RL, Osterloh IH, Rosen RC. Relationship between patient self-assessment of erectile function and the erectile function domain of the international index of erectile function. Urology. 2000;56:477–481. doi: 10.1016/s0090-4295(00)00697-x. [DOI] [PubMed] [Google Scholar]
- 23.Prisant LM, Loebl DH, Jr, Waller JL. Arterial elasticity and erectile dysfunction in hypertensive men. J Clin Hypertens (Greenwich) 2006;8:768–774. doi: 10.1111/j.1524-6175.2006.05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 25.Corrado E, Rizzo M, Muratori I, Coppola G, Novo S. Association of elevated fibrinogen and C-reactive protein levels with carotid lesions in patients with newly diagnosed hypertension or type II diabetes. Arch Med Res. 2006;37:1004–1009. doi: 10.1016/j.arcmed.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Kawanishi Y, Kimura K, Nakanishi R, Numata A, Taguchi H. Retinal vascular findings and penile cavernosal artery blood flow. BJU Int. 2003;92:977–980. doi: 10.1111/j.1464-410x.2003.04504.x. [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]