Abstract
Primary biliary cirrhosis (PBC) is characterized by antimitochondrial antibodies (AMA), directed to the E2 component of the pyruvate dehydrogenase complex (PDC-E2). Notwithstanding the presence of mitochondria in virtually all nucleated cells, the destruction in PBC is limited to small intrahepatic bile ducts. The reasons for this tissue specificity remain unknown, although biliary epithelial cells (BEC) uniquely preserve the PDC-E2 epitope following apoptosis. Notably, PBC recurs in an allogeneic transplanted liver, suggesting generic rather than host-PBC-specific susceptibility of BEC. We used cultured human intrahepatic BEC (HIBEC) and other well-characterized cell lines, including, HeLa, CaCo-2 cells, and non transformed human keratinocytes and bronchial epithelial cells (BrEpC), to determine the integrity and specific localization of PDC-E2 during induced apoptosis. All cell lines, both before and after apoptosis, were tested with sera from patients with PBC (n=30), other autoimmune liver and rheumatic diseases (n=20), and healthy individuals (n=20), a mouse monoclonal antibody against PDC-E2, and AMA with an IgA isotype. PDC-E2 was found to localize unmodified within apoptotic blebs of HIBEC, but not within blebs of various other cell lineages studied. The fact that AMA- containing sera reacted with PDC-E2 on apoptotic BEC without a requirement for permeabilization suggests that the autoantigen is accessible to the immune system during apoptosis. In conclusion, our data indicate that the tissue (cholangiocyte) specificity of the autoimmune injury in PBC is a consequence of the unique characteristics of HIBEC during apoptosis and can be explained by exposure to the immune system of intact immunoreactive PDC-E2 within apoptotic blebs.
Keywords: autoimmunity, antimitochondrial antibodies, apoptosis, apoptotic bodies, cell clearance
Apoptotic cells are normally efficiently cleared after engulfment by ‘professional’ phagocytes followed by an anti-inflammatory response (1, 2). When such uptake is impaired, cell lysis can release intracellular components that are a potential source of autoantigenic stimulation (3-6) and autoimmunity onset (7-9). The presence of intact autoantigens within apoptotic blebs (10), their participation in the processes involved in autoantigen presentation (11), and the activation of innate immunity through macrophage cytokine secretion in concert (12) are likely links between apoptosis and autoimmunity. Of relevance to the autoimmune liver disease primary biliary cirrhosis (PBC), Odin and colleagues demonstrated that, following apoptosis of biliary epithelial cells (BEC), the autoantigenic E2 subunit of the pyruvate dehydrogenase complex (PDC-E2) remains immunologically intact and still recognizable as such by anti-mitochondrial autoantibodies (AMA) (13). It is reasoned that absence of glutathiolation (13) may contribute to this unique feature of the BEC.
We reasoned that immune-mediated BEC destruction would be accentuated if PDC-E2 were preserved in blebs during apoptosis. Indeed, this could lead to impaired clearance of such apoptotic cells, thus provoking an innate immune response and even the autoimmune destruction of bile ducts. This scenario would help explain the recurrence of PBC following orthotopic liver transplantation (14), as well as the therapeutic failure of immunosuppressive agents (15). We report herein the cellular topology of PDC-E2 during apoptosis of cultured human intrahepatic BEC (HIBEC) and other non-BEC cells. Our findings demonstrate that PDC-E2 is localized intact within blebs of apoptotic HIBEC and is thereby accessible to the immune system. We hypothesize that the unique HIBEC apoptotic features allow the exposure of a potent intracellular autoantigen to the PBC-associated multi-lineage autoimmune response that leads to the tissue-specific autoimmune injury.
Materials and Methods
Human sera and antibodies
Following informed consent, serum samples were obtained from patients diagnosed with PBC (n=30), systemic lupus erythematosus (n=5) (SLE), autoimmune hepatitis (n=5) (AIH), primary sclerosing cholangitis (n=5) (PSC), chronic hepatitis C (n=5) (CHC) and healthy individuals (n=10). PBC sera included 20 randomly chosen AMA-positive cases and 10 well-defined AMA-negative patients; thus the proportion of AMA-negative sera utilized in the present study is significantly higher than the normally expected frequency as these were specifically sought for this study. The diagnosis of all cases was based on established criteria for PBC (16), SLE (17), AIH (18), PSC (19) and by detection of serum HCV-RNA for CHC by PCR. As expected, 90% (27/30) of PBC cases were women and 70% were taking ursodeoxyxholic acid as the sole treatment at the time of enrolment. The mean age was 63±10 years and 17/30 patients had advanced PBC (histological stages III-IV). These clinical features did not differ between patients with AMA-positive or −negative PBC (data not shown).
Antibody reagents
Serum anti-PDC-E2 antibodies were tested using our well-defined assays with recombinant antigens, as explained below (20-22). We also utilized a previously described mouse monoclonal antibody (mAb) against PDC-E2, clone 2H-4C8 (23). Secondary antibodies Cy3-labeled anti-human-IgG, anti-human-IgA and anti-mouse-IgG were purchased from Jackson ImmunoResearch (West Grove, PA). Normal mouse IgG was obtained from Invitrogen (Carlsbad, CA) and FITC-labeled Annexin-V from BD Pharmingen (San Jose, CA). Monoclonal anti-human caspase-3 antibody was purchased from R&D systems (Minneapolis, MN). Negative controls were used throughout and included sera from healthy individuals. Additionally, we purified IgA-AMA from a patient diagnosed with PBC and a monoclonal gammopathy with high levels of IgA-AMA using Jacalin-agarose beads (Pierce, Rockford, IL) following the manufacturer's instructions. Serum IgA from a healthy subject were used as a control.
Detection of AMA
An established optimal amount (16 μg) of purified recombinant PDC-E2 was loaded onto a 10% mini protein gel (Bio-Rad Laboratories, Hercules, CA), fractionated at 170 V for 1 hour, and transferred overnight onto nitrocellulose membranes that were then cut into strips. AMA detection was performed as described (24). The samples to be analyzed included sera from PBC patients at an optimal dilution of 1:2000, monoclonal AMA (10-4 dilution), pooled AMA of IgA isotype (dilution 1:2000), and control IgA (1:2000). Blots were exposed on photographic membranes and images digitized with a FluorTech 8900 gel doc system (Alpha Innotech, San Leandro, CA) equipped with a chemiluminescent filter. The absence of AMA was further confirmed in the sera of the AMA negative patients using our standardized ELISA and pMIT-3 antigens (25).
Cell lines and culture conditions
The cells studied were cultured HIBEC, two human epithelial non-transformed primary cell cultures (human keratinocytes and bronchial epithelial cells) purchased from ScienCell (San Diego, CA) and two human tumor-derived laboratory cell lines, HeLa cells and CaCo-2 cells purchased from American Type Culture Collection (Manassas, VA). HIBEC were cultured in sterile medium supplemented with 2% fetal bovine serum (FBS), epithelial cell growth supplement (ScienCell, San Diego, CA, USA) and 1% penicillin in cell culture flasks coated with poly-L-lysine (Sigma-Aldrich, St Louis, MO). The other two epithelial cells were cultured under the same conditions in absence of FBS, as recommended by the manufacturer. HeLa and CaCo-2 cells were cultured using low glucose DMEM, supplemented with FBS 10% for HeLa cells and 20% for CaCo-2 cells, gentamicin (6 μg/ml), sodium pyruvate (110 mg/l), and L-glutamine (2mM). Cells were cultured at 37 °C in a humidified 5% CO2 incubator.
HIBEC were isolated from human liver tissue by the supplier and cryopreserved immediately after purification. This primary cell culture was characterized by an immunofluorescence method with antibodies to cytokeratin 18, 19 and vimentin which stained over 90% of the cells. All experiments on HIBEC were performed between cell passage 2 and 5.
Induction of apoptosis
We used biliary salts to induce apoptosis (26). To establish optimal conditions for the induction of apoptosis, we incubated all the cells types at 37 °C for 1,2,3 and 4 h using different concentrations (100 μM, 500 μM, 1 mM and 2 mM) of sodium glycochenodeoxycholate (GCDC) (Sigma-Aldrich) added to normal culture medium, and in absence of serum and growth factors (26). BS failed to induce any apoptotic effect in HeLa and Caco-2 cells so, in theise transformed cell lines, apoptosis induction was performed by ultraviolet B light (UV-B) irradiation, 1650 J/m2 for HeLa cells and 2200 J/m2 for CaCo-2, followed by incubation in fresh media for 8 (HeLa) or 16 (CaCo-2) hours (13). Apoptosis was also induced in HIBEC by UV-B irradiation (1650 J/m2) followed by incubation in fresh medium for 6 hours.
Quantification of apoptosis by flow cytometry
The cells to be analyzed for apoptosis were suspended in 200 μl of buffer containing 10 mM Hepes/NaOH (pH 7.4), 140 mM NaCl and 2.5 mM CaCl2. A total of 1×106 cells were stained for 15 min at room temperature in the dark with FITC-labeled Annexin-V and with propidium iodide (BD Pharmingen, San Jose, CA) to discriminate apoptotic from necrotic cells. The samples were immediately analyzed by flow cytometry and at least 10,000 events were counted. Stained cells were assessed on a FACScan flow cytometer (BD Immunocytometry Systems, San Jose, CA) upgraded by Cytec Development (Fremont, CA). Acquired data were analyzed with CELLQUEST Pro (BD Immunocytometry Systems) and FlowJo (Tree Star, Inc., Ashland, OR) software packages.
Immunostaining and confocal microscopy
Cells were washed twice with phosphate buffered saline (PBS) and incubated with FITC-labeled Annexin-V 1:10 for 15 min at RT in the dark. The samples were then fixed in 3.7% formaldehyde (5 min, RT), permeabilized with 0.2% Triton X-100 (5 min, RT) and blocked for 30 min at RT with 1× Universal blocking solution (Bio-Genex). Immunofluorecence staining was performed with human sera diluted 1:40 or monoclonal antibody diluted 1:80 (overnight, 4 °C), followed by Cy3-labeled secondary antibody diluted 1:500 for 1 hour at RT. The cells were co-stained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA) to visualize nuclear degeneration. All samples were stained when at 80% confluence; samples with less than 100 cells were excluded. Identical settings were used for all samples. Controls consisting of incubation of cells with developing secondary antibody alone did not demonstrate any detectable staining under the same conditions.
Staining of non-apoptotic and apoptotic HIBEC was also performed in absence of membrane perforating agent. The same protocol previously described was followed but Tryton X-100 omitted. Immunofluorescence-labeled samples were examined using a Pascal Zeiss confocal laser scanning microscope with a 100X oil-immersion objective.
Isolation and immunoblot analysis of apoptotic bodies
After induction of apoptosis, subcellular fragments were isolated by filtration and ultracentrifugation as described elsewhere (10). Briefly, the cell culture supernatant fluid was collected after apoptosis induction. Two additional centrifugation steps (500 × g, 5 min) were performed in order to remove the remaining cells. The supernatant fluid was then passed through a 1.2 μm non-pyrogenic, hydrophilic syringe filter. After centrifugation at 100,000 g for 30 min, the pellet containing apoptotic bodies (AB) was resuspended in lysis buffer containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Lysis was performed for 30 min on ice. Protein content of the samples was determined by the BCA (bicinconic acid) assay using a Nanodrop ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Each sample (20 μg) was diluted in loading buffer and subjected to a standard SDS-PAGE. After transfer to PVDF membranes, PDC-E2 was detected using the monoclonal antibody previously described (clone 2H-4C8) (23), and AMA-positive sera with two different AMA isotypes.
Results
Apoptosis in HIBEC
Apoptosis rates were evaluated in all cell lines by FACS (Annexin V and PI double staining) and immunofluorescence. Apoptotic cells were identified with confocal microscopy by morphological criteria: high nuclear density, chromatin condensation and nuclear fragmentation revealed with DAPI, and characteristic blebbing of the cell membrane revealed with Annexin-V; based on these characteristics an apoptotic index was established as (DAPI-apoptotic nuclei/total nuclei) × 100.
Bile salts (BS) accumulating in the human liver in the course of cholestatic conditions trigger liver injury and subsequent fibrosis, and it has been demonstrated that a constituent of the hydrophobic BS, glycochenodeoxycholate (GCDC), induces apoptosis of hepatocytes starting at a concentration of 50 μM (27). GCDC has also an apoptotic effect in cholangiocytes (26). The use of 1 mM GCDC in the absence of serum, or growth factors, induced apoptosis in 39% of HIBEC whereas UV-B irradiation (1650 J/m2 followed by incubation in fresh medium at 37 °C for 6 hours) induced apoptosis in just 11% of HIBEC; moreover, in our experience, a higher level of double positive PI/AnnexinV cells, most probably referable to necrotic cells, was generated following UVB irradiation compared to BS in HIBEC (Fig 1A). Under both sets of conditions, membrane blebbing and apoptotic fragments were observed in HIBEC using confocal microscopy (Figure 1C). The levels of apoptosis in HeLa and CaCo-2 after UVB irradiation were similar to those reported in the literature, i.e. 45%, and 60 % respectively (28). GCDC induced apoptosis in 49% of BrEpC and 52% of kerationocytes. On the other hand no substantial level of apoptosis was observed in HeLa and CaCo-2 cells after incubation with BS.
Figure 1.
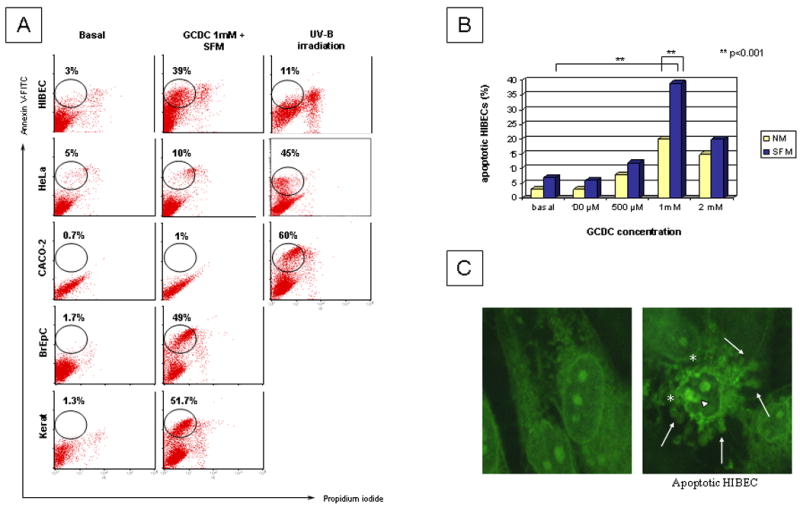
Human intrahepatic biliary epithelial cells (HIBEC) and four other cell types were cultured in normal media (NM) or serum free media (SFM) under optimal conditions to induce apoptosis, either by exposure to bile salts (glycochenodeoxycholate, GCDC) or ultraviolet B (UV-B) irradiation (see Methods). A. Induction of apoptosis was investigated by flow cytometry after double staining with FITC-labeled Annexin V and propidium iodide. The use of 1 mM GCDC in the absence of serum, or growth factors, induced apoptosis in 39% of HIBEC, 49% of BrEpC and 51.7% of keratinocytes whereas no significant apoptotic response was observed in transformed cell lines. UV-B irradiation induced apoptosis in just 11% of HIBEC and a higher level of double positive PI/AnnexinV cells, most probably referable to necrotic cells. B. Different concentrations of GCDC in NM or SFM were tested to establish pro-apoptotic concentrations for HIBECs, with 1mM proving optimal. C. HIBEC after exposure to GCDC 1mM in SFM were assessed by confocal microscopy, revealing typical apoptotic morphology with blebbing of the membrane (*), nucleolar degeneration (short arrow) and apoptotic fragments (long arrow). 100 × oil immersion objective was used.
In conclusion, GCDC 1mM in SFM was chosen to induce apoptosis in HIBEC, keratinocytes and BrEpC whereas apoptotic HeLa and CaCo-2 cells were generated by UVB irradiation. To exclude that a different method of apoptosis was the reason for different expression of PDC-E2, staining with mAb against PDC-E2 using UVB irradiated HIBEC was performed and the same results were observed.
PDC-E2 is not altered in apoptotic HIBEC and localizes within blebs
The localization of PDC-E2 was studied in apoptotic HIBEC by indirect immunofluorecence and compared with other human cell lines. Apoptotic cells were identified by morphological criteria; DAPI staining was preferred over the use of TUNEL to detect apoptotic cells because of its greater specificity, since TUNEL identifies fragmented DNA in both apoptotic and necrotic cells and hence may overestimate the number of apoptotic cells. First, HIBEC were stained with PBC sera (n=20) before and after induction of apoptosis, using both IgG and IgA AMA isotypes. As expected, non-apoptotic cells presented the typical punctuate cytoplasmic mitochondrial immunofluorecence pattern (Figure 2a), negative staining for Annexin-V, and normal nuclear morphology. After staining performed under the same conditions following induction of apoptosis with 1 mM GCDC in the absence of serum and growth factors, apoptotic HIBEC expressed positive PDC-E2 staining that could be localized with Annexin V within apoptotic blebs and fragments (Figure 2b). No detectable staining was noted on cells treated with either the secondary antibody alone or with normal mouse IgG instead of the mAb (data not shown). These findings were reproduced also in HIBEC following UV-B irradition and incubated in fresh media at 37 °C for 6 hours (data not shown).
Figure 2.
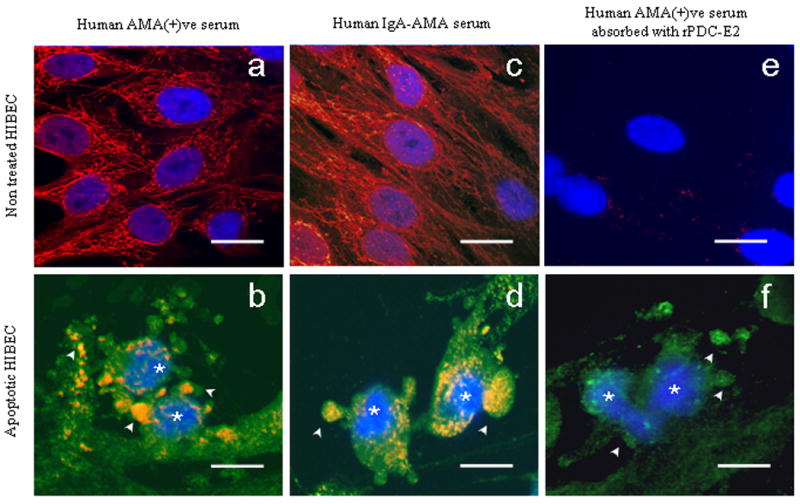
Staining of non-apoptotic or apoptotic HIBEC using an AMA-positive PBC serum (panels a, b), serum from a PBC patient with monoclonal production of IgA-AMA (panels c, d), and using the same AMA-positive serum after absorbtion with recombinant PDC-E2 (panels e, f). The immunofluorescence staining was performed with three fluorocromes: Cy3-conjugated secondary antibody (red, yellow-orange when co-stained with green), FITC-labeled Annexin-V (green), and DAPI (blue) for apoptosis detection. Apoptotic cells (*) were identified by morphological criteria: high nuclear density, chromatin condensation and nuclear fragmentation revealed with DAPI (blue), and characteristic blebbing of the cell membrane revealed with Annexin-V (green). Positive staining of blebs and apoptotic fragments (arrows) was observed using unabsorbed PBC sera and was virtually absent after absorption. Apoptosis was confirmed in all experiments (*). Scale bar represents 20 μm.
Immunofluorescence with murine mAb confirmed the results obtained with the human sera and apoptotic HIBEC retained the PDC-E2-specific staining within the apoptotic blebs. There were no differences in staining using each of the AMA-positive sera from the PBC patients according to age, disease stage, or therapy with ursodeoxycholic acid and the staining patterns were similar to those obtained with the AMA-IgA (Figures 2c,2d). Pre-incubation overnight with human recombinant PDC-E2 completely removed the capacity of the sera to stain normal and apoptotic cells (Figure 2). Serum samples from patients with AMA-negative PBC (n=10), AIH (n=5), SLE (n=5), PSC (n=5), CHC (n=5) and healthy controls (n=10) failed to produce any staining of HIBEC (Table 1).
Table 1.
Prevalence of staining for PDC-E2 in blebs of various cell types undergoing apoptosis using PBC and control sera. Of note, specific bleb staining was observed in virtually all HIBEC when AMA-positive PBC sera were used. This staining was not observed in non-apoptotic cells. Fisher's Exact Test was used to calculate statistical significance between HIBEC and the other cell types.
Control cells include in equal distribution, transformed cells (HeLa and CaCo-2 cells) and non-transformed human epithelial cells (keratinocytes and bronchial epithelial cells).
p value < 0.0001 compared to HIBEC
One third of the control sera were from healthy subjects, and two thirds were from patients with systemic lupus erythematosus, autoimmune hepatitis, primary sclerosing cholangitis or chronic hepatitis C.
Localization of PDC-E2 within apoptotic blebs is specific for HIBEC
PDC-E2 localization was investigated using the clone 2H-4C8 mAb and sera from patients with PBC and controls using a variety of different human cell lines (Table 1, Figure 3). All experiments were performed before and after induction of apoptosis under the same conditions as specified above for HIBEC. Prior to apoptosis induction, each of the cell lines manifested the typical cytoplasmic staining of mitochondria when the clone 2H-4C8 mAb was used (Figure 3, upper row). Following apoptosis induction none of the control cell lines had PDC-E2 staining (Figure 3, lower row). None of the cell lines used demonstrated detectable cytoplasmic staining when sera from patients with AMA-negative PBC, AIH, SLE, PSC, CHC or healthy controls were used (data not shown).
Figure 3.
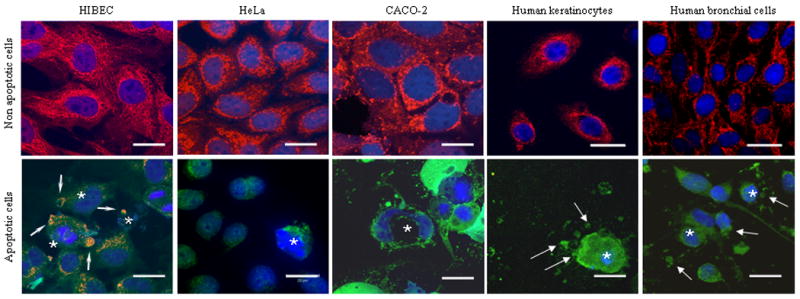
Apoptosis-dependent anti-PDC-E2 staining in various human cell types, with non-apoptotic cells shown in upper row and apoptotic cells in lower row. Immunofluorescence staining was performed with three fluorochromes, mAb against PDC-E2 and Cy3-conjugated secondary antibody (red, yellow-orange when co-stained with green); FITC-labeled Annexin-V (green); and DAPI (blue) for apoptosis detection. Apoptotic cells (*) were identified as described in Figure 2. Non- apoptotic cells (upper row) have a normal AMA pattern of immunofluorescence staining (red). Apoptotic cells (lower row) show differences between HIBEC that retained mitochondrial staining within blebs and fragments (arrow) after apoptosis, and other cell types (CACO-2 cells, HeLa cells, human keratinocytes and human bronchial epithelial cells) that lost mitochondrial staining after apoptosis. 100× oil immersion objective. Scale bar represents 20 μm.
These morphological observations were corroborated by Western Blot analysis wherein PDC-E2 was readily detected in lysates of isolated apoptotic bodies obtained from HIBEC, whereas no such reactivity was observed in lysates from HeLa and BrEpC (Figure 4).
Figure 4.
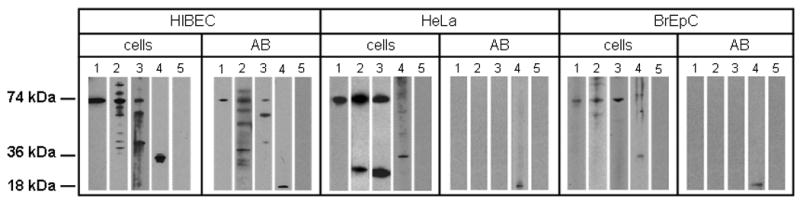
Western blots showing that PDC-E2 is localized unmodified within apoptotic bodies (AB) from human intrahepatic biliary cells (HIBEC) but not from other cell lineages. ABs were isolated by filtration and ultracentrifugation after induction of apoptosis (see Methods). PDC-E2 was detected by Western blot in lysates obtained from non-apoptotic cells and AB using (1) mAb against PDC-E2, (2) AMA positive serum, (3) AMA-IgA isotype, (4) mAb against caspase 3, and (5) serum from a healthy control. PDC-E2 was detected in ABs from HIBEC when tested with monoclonal Ab and with PBC sera of two different Ig isotypes. ABs from HeLa cells and BrEpC did not show the presence of PDC-E2 whereas, as expected, lysates from non-apoptotic cells did so. The 18 kDa subunit of caspase-3 generated during apoptosis is localized within apoptotic blebs, and hence exposure to mAb to caspase-3 was used to validate formation of blebs.
PDC-E2 in apoptotic blebs is accessible to antibody recognition
AMA staining in normal cells requires the use of a perforating agent due to the conservation of membrane integrity, we hypothesize that during apoptosis the mitochondrial antigen became accessible to the immune system. To determine whether the permeabilization agents were responsible for the access of autoantibodies to PDC-E2 within blebs, we stained HIBEC with the clone 2H-4C8 mAb in the absence of Triton X-100. Results obtained showed that while the absence of Triton X-100 did not modify the positive staining of blebs in apoptotic HIBEC (Figure 5); no differences were observed in the other cell lines since all of them lose AMA staining during apoptosis. These results were obtained with 12/20 AMA-positive PBC samples while sera from patients with AMA-negative PBC (n=5), AIH (n=5), SLE (n=5), PSC (n=5), CHC (n=5) or healthy controls (n=5), or the use of secondary antibody alone failed to demonstrate any staining (Table 3).
Figure 5.
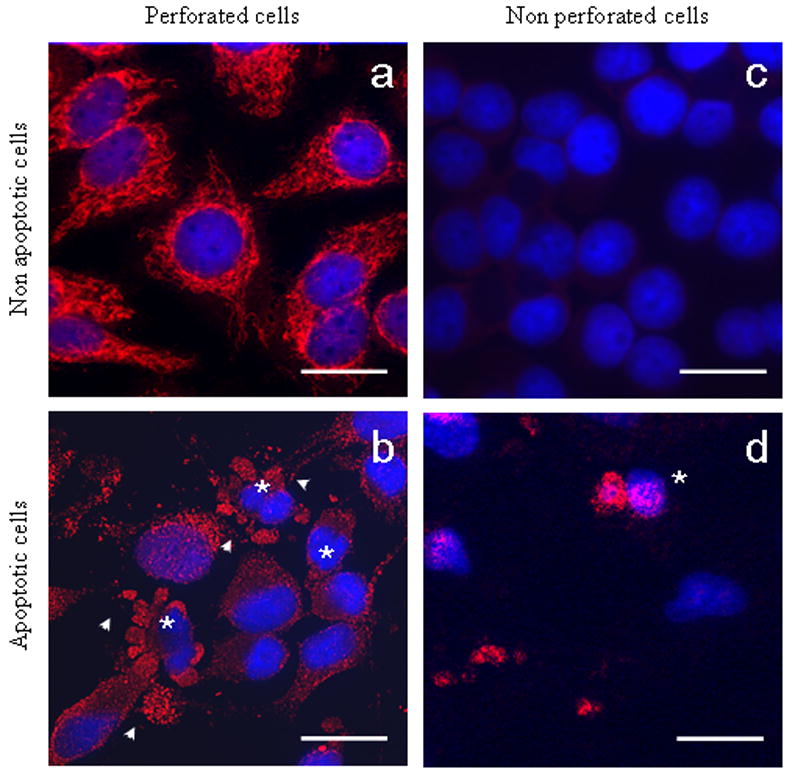
Staining of non-apoptotic (upper row) and apoptotic (lower row) HIBEC in absence or presence of a membrane permeabilizing agent, Triton X-100. AMA staining in normal cells requires pemeabilization of the cell (a); lacking this, no staining is observed due to retention of membrane integrity (c); apoptotic HIBEC (*) stained with a mAb against PDC-E2, with positive staining demonstrable within the blebs (arrow) (b); apoptotic HIBEC retained the bleb staining even without permeabilization (d). No staining was observed with serum from patients with AMA negative PBC, PSC, AIH, SLE, CHC or healthy subjects (not shown). Scale bar represents 20 μm.
Table 3.
Percentage of apoptotic HIBEC with positive staining for PDC-E2 in the presence or absence of a permeabilizating agent during the staining as described in the materials and methods. None of the control sera showed any positive staining. Values are expressed as mean ± SD. Student's t-test was used to calculate p values.
| Proliferating HIBEC | Apoptotic HIBEC | ||
|---|---|---|---|
| Sera (n) | Permeabilized | Non permeabilized | Permeabilized |
| mAb 2H-4C8 | 93 ± 7 | 37 ± 12 | 91 ± 5 * |
| PBC AMA + ve (20) | 88 ± 6 | 33 ± 7 | 88 ± 0.7 * |
| PBC AMA −ve (10) | 0 | 0 | 0 |
| Control (30) | 0 | 0 | 0 |
P value < 0.0001 compared to non permeabilized apoptotic HIBEC
Discussion
We herein demonstrate that PDC-E2, the major AMA autoantigen, is detectable in its antigenically reactive form within apoptotic blebs, specifically in cultured human BEC and not in other cell types, thus providing one explanation for the organ specific pathology noted in human PBC. We thus suggest that the unique characteristics of BEC during apoptosis might constitute the pathogenic link between the ubiquitous distribution and high degree of conservation across species of the AMA autoantigen and the organ specificity of PBC pathology.
It has been previously reported that PDC-E2 remains intact and retains its immunogenicity during BEC apoptosis due to a cell lineage specific lack of glutathiolation (13, 29). This ‘apoptotic exposure’ of PDC-E2 appears to be limited to BEC and it may ultimately have a critical pathogenic relevance to both inductive and effector stages of PBC. The formation of apoptotic bodies and fragments is essential during apoptosis to limit the escape of intracellular content and preclude any ensuing immunological responses against intracellular autoantigens with inflammatory reactions (30, 31). Nevertheless, apoptotic blebs and fragments can under some circumstances constitute a major source of immunogens in autoimmune diseases that involve the targeting of ubiquitous autoantigens (32). Thus dysregulation of apoptosis or the ineffective removal of apoptotic cells has been documented in patients with SLE (4, 7, 33) and the development of antibody-mediated myocarditis of infants born to mothers with anti-SSA/Ro-SSB/La (7). Also Kupffer cell engulfment of apoptotic bodies from hepatocytes promotes inflammation and fibrogenesis (34, 35).
Our observations may help seal several remaining gaps in the understanding of induction and perpetuation of PBC albeit raising new questions. First, intact PDC-E2 in apoptotic fragments from BEC could be taken up by intrahepatic dendritic cells and transferred to regional lymph nodes for priming of cognate T cells thus initiating PBC, but this attractive scenario still requires an explanation for the primum movens in the first place for apoptosis and this is likely not PBC-specific. Second, the accessibility of PDC-E2 within apoptotic blebs to autoantibodies appears to support the pathogenic role of AMA as well as T cells in the perpetuation of BEC injury even though antibody titers do not correlate with the clinical features or stages of PBC, and AMA-negative patients are clinically indistinguishable from their AMA-positive counterparts (36).
Nevertheless, the appearance of serum AMA does often herald disease onset sometimes by several years (16). Third, we can propose that PDC-E2 within apoptotic blebs will also be recognized by MHC class I-restricted CD8+ T cells; this point helps explain the BEC pathology in AMA negative PBC. Interestingly, our lab has recently demonstrated the presence of autoreactive T cells to PDC-E2 in AMA negative PBC patients (37). These data are also of particular relevance in view of the major pathogenic role of these cells in producing PBC-like liver lesions in animal models (38). Fourth, our findings are consistent with the likelihood that PBC cholangiocyte does not manifest any unique features that make it the target of autoimmunity (37), noting the frequent recurrence of PBC following allogeneic liver transplantation (14). The latter two issues may ultimately be combined with the fact that the donor and recipient MHC class I alleles are major determinants of the allograft outcome (39). Fifth and ultimately, the ensuing B and T cell autoreactive response may account for the perpetuation of the immune-mediated damage to BEC with a major role also played by elements of innate immunity which appears to be enhanced in PBC (40, 41).
Our data imply that the postapoptotic release of intact mitochondrial autoepitopes in small bile ducts is one contributor to this specificity. Indeed, we should note that, as previously reported, the overexpression of Bcl-2, specifically in apoptotic small BEC, inhibits PDC-E2 glutathiolation and prevents the loss of antigenicity (13, 42). However, other factors have also been incriminated in playing a role in the selective destruction of small BEC. In particular, there are dramatic differences in expression of trefoils in small versus large bile ducts, suggesting not only an imbalance of homeostasis, but also a differential ability to repair or restitute cell damage (43).
Our data also demonstrate that we are able to detect PDC-E2 without cell permeabilization. There are three explanations for this observation. First, PDC-E2 may leak out to the cell surface and is thus being detected on the cell membrane. Second, the cells undergoing apoptosis have holes in their cell membrane created by cellular proteases which allow passage into and localization of Ig in the bleb. Third, there may be a role for FcR mediated uptake in the apoptotic cell. Future experiments will address these possibilities.
In conclusion, the evidence provided herein leads to new scenarios in the pathogenesis of PBC and may constitute a credible link between the several convenient and inconvenient truths available thus far (37). However, it does not overcome all of the major challenges in PBC etiology, nor the need to ascertain the genetic basis of disease susceptibility and environmental triggers for cholangiocyte injury and apoptosis as an initial step in tolerance breakdown. treatments that could modulate apoptosis (44) should not be overlooked, and their assessment is warranted in recently established murine models for PBC (45-47).
Table 2.
Number of apoptotic cells in which blebs contain PDC-E2. The apoptotic cells were stained as described with sera from PBC AMA positive patients (n=20). Values are expressed as mean ± SD. Apoptotic index was derived from DAPI-apoptotic nuclei/total nuclei × 100. An individual apoptotic cell was considered positive when PDC-E2 staining was observed in at least one of its blebs. Student's t-test was used to calculate p values.
| Cell type | Apoptotic index | Cells with positive staining for PDC-E2 within blebs |
|---|---|---|
| HIBEC | 38 ± 2.2 | 88 ± 0.7 |
| Keratinocytes | 51 ± 0.7 | 0 ± 1.3 * |
| BrEpC | 48 ± 1.7 | 0 ± 0.4 * |
| HeLa | 42 ± 1.2 | 0 ± 0.7 * |
| CaCO-2 | 61 ± 3.5 | 0 ± 0.3 * |
p value < 0.0001 compared to HIBEC
Acknowledgments
We thank Professor Terence M Murphy, at UC Davis, for the use of the UV-B irradiator and Thomas P. Kenny for his help and technical assistance.
Financial support provided by National Institutes of Health grants DK70004 and DK39588.
Abbreviations
- PBC
Primary biliary cirrhosis
- AMA
antimitochondrial antibodies
- PDC-E2
E2 component of the pyruvate dehydrogenase complex
- BEC
biliary epithelial cells
- HIBEC
human intrahepatic biliary epithelial cells
- BrEpC
bronchial epithelial cells
- SLE
systemic lupus erythematosus
- AIH
autoimmune hepatitis
- PSC
primary sclerosing cholangitis
- CHC
chronic hepatitis C
- mAb
monoclonal antibody
- GCDC
sodium glycochenodeoxycholate
- UV-B
ultraviolet B light
- PBS
phosphate buffered saline
- PBS-T
PBS with 0.5% Tween 20
- FBS
fetal bovine serum
- DAPI
4′,6-diamidino-2-phenylindole
- AB
apoptotic bodies
- M
mitochondria
Contributor Information
Ana Lleo, Email: alleo@ucdavis.edu.
Carlo Selmi, Email: cfselmi@ucdavis.edu.
Pietro Invernizzi, Email: pietro.invernizzi@unimi.it.
Mauro Podda, Email: mauro.podda@unimi.it.
Ross L. Coppel, Email: ross.coppel@med.monash.edu.au.
Ian R. Mackay, Email: Ian.MacKay@med.monash.edu.au.
Gregory J. Gores, Email: gores.gregory@mayo.edu.
Aftab A. Ansari, Email: pathaaa@emory.edu.
Judy Van de Water, Email: javandewater@ucdavis.edu.
M. Eric Gershwin, Email: megershwin@ucdavis.edu.
References
- 1.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 2.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 3.Torok NJ. Apoptotic cell death takes its toll. Hepatology. 2007;46:1323–1325. doi: 10.1002/hep.21968. [DOI] [PubMed] [Google Scholar]
- 4.Perniok A, Wedekind F, Herrmann M, Specker C, Schneider M. High levels of circulating early apoptic peripheral blood mononuclear cells in systemic lupus erythematosus. Lupus. 1998;7:113–118. doi: 10.1191/096120398678919804. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Arguelles A, Brito GJ, Reyes-Izquierdo P, Perez-Romano B, Sanchez-Sosa S. Apoptosis of melanocytes in vitiligo results from antibody penetration. J Autoimmun. 2007;29:281–286. doi: 10.1016/j.jaut.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Salunga TL, Cui ZG, Shimoda S, Zheng HC, Nomoto K, Kondo T, Takano Y, et al. Oxidative stress-induced apoptosis of bile duct cells in primary biliary cirrhosis. J Autoimmun. 2007;29:78–86. doi: 10.1016/j.jaut.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Clancy RM, Neufing PJ, Zheng P, O'Mahony M, Nimmerjahn F, Gordon TP, Buyon JP. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allina J, Hu B, Sullivan DM, Fiel MI, Thung SN, Bronk SF, Huebert RC, et al. T cell targeting and phagocytosis of apoptotic biliary epithelial cells in primary biliary cirrhosis. J Autoimmun. 2006;27:232–241. doi: 10.1016/j.jaut.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lleo A, Invernizzi P, Selmi C, Coppel RL, Alpini G, Podda M, Mackay IR, et al. Autophagy: highlighting a novel player in the autoimmunity scenario. J Autoimmun. 2007;29:61–68. doi: 10.1016/j.jaut.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiller M, Bekeredjian-Ding I, Heyder P, Blank N, Ho AD, Lorenz HM. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ. 2008;15:183–191. doi: 10.1038/sj.cdd.4402239. [DOI] [PubMed] [Google Scholar]
- 11.Mandron M, Martin H, Bonjean B, Lule J, Tartour E, Davrinche C. Dendritic cell-induced apoptosis of human cytomegalovirus-infected fibroblasts promotes cross-presentation of pp65 to CD8+ T cells. J Gen Virol. 2008;89:78–86. doi: 10.1099/vir.0.83278-0. [DOI] [PubMed] [Google Scholar]
- 12.Lucas M, Stuart LM, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171:2610–2615. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 13.Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108:223–232. doi: 10.1172/JCI10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van de Water J, Gerson LB, Ferrell LD, Lake JR, Coppel RL, Batts KP, Wiesner RH, et al. Immunohistochemical evidence of disease recurrence after liver transplantation for primary biliary cirrhosis. Hepatology. 1996;24:1079–1084. doi: 10.1002/hep.510240517. [DOI] [PubMed] [Google Scholar]
- 15.Combes B, Emerson SS, Flye NL, Munoz SJ, Luketic VA, Mayo MJ, McCashland TM, et al. Methotrexate (MTX) plus ursodeoxycholic acid (UDCA) in the treatment of primary biliary cirrhosis. Hepatology. 2005;42:1184–1193. doi: 10.1002/hep.20897. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 19.Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21:870–877. doi: 10.1136/gut.21.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 21.Van de Water J, Gershwin ME, Leung P, Ansari A, Coppel RL. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988;167:1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van de Water J, Surh CD, Leung PS, Krams SM, Fregeau D, Davis P, Coppel R, et al. Molecular definitions, autoepitopes, and enzymatic activities of the mitochondrial autoantigens of primary biliary cirrhosis. Semin Liver Dis. 1989;9:132–137. doi: 10.1055/s-2008-1040504. [DOI] [PubMed] [Google Scholar]
- 23.Migliaccio C, Nishio A, Van de Water J, Ansari AA, Leung PS, Nakanuma Y, Coppel RL, et al. Monoclonal antibodies to mitochondrial E2 components define autoepitopes in primary biliary cirrhosis. J Immunol. 1998;161:5157–5163. [PubMed] [Google Scholar]
- 24.Miyakawa H, Tanaka A, Kikuchi K, Matsushita M, Kitazawa E, Kawaguchi N, Fujikawa H, et al. Detection of antimitochondrial autoantibodies in immunofluorescent AMA-negative patients with primary biliary cirrhosis using recombinant autoantigens. Hepatology. 2001;34:243–248. doi: 10.1053/jhep.2001.26514. [DOI] [PubMed] [Google Scholar]
- 25.Moteki S, Leung PS, Coppel RL, Dickson ER, Kaplan MM, Munoz S, Gershwin ME. Use of a designer triple expression hybrid clone for three different lipoyl domain for the detection of antimitochondrial autoantibodies. Hepatology. 1996;24:97–103. doi: 10.1002/hep.510240117. [DOI] [PubMed] [Google Scholar]
- 26.Drudi Metalli V, Mancino MG, Mancino A, Torrice A, Gatto M, Attili AF, Alpini G, et al. Bile salts regulate proliferation and apoptosis of liver cells by modulating the IGF1 system. Dig Liver Dis. 2007;39:654–662. doi: 10.1016/j.dld.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Guicciardi ME, Gores GJ. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig Liver Dis. 2002;34:387–392. doi: 10.1016/s1590-8658(02)80033-0. [DOI] [PubMed] [Google Scholar]
- 28.Vantieghem K, Overbergh L, Carmeliet G, De Haes P, Bouillon R, Segaert S. UVB-induced 1,25(OH)2D3 production and vitamin D activity in intestinal CaCo-2 cells and in THP-1 macrophages pretreated with a sterol Delta7-reductase inhibitor. J Cell Biochem. 2006;99:229–240. doi: 10.1002/jcb.20910. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura S, Van De Water J, Leung P, Odin JA, Yamamoto K, Gores GJ, Mostov K, et al. Caspase induction by IgA antimitochondrial antibody: IgA-mediated biliary injury in primary biliary cirrhosis. Hepatology. 2004;39:1415–1422. doi: 10.1002/hep.20175. [DOI] [PubMed] [Google Scholar]
- 30.Henson PM. Dampening inflammation. Nat Immunol. 2005;6:1179–1181. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- 31.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 34.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 35.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 36.Invernizzi P, Crosignani A, Battezzati PM, Covini G, De Valle G, Larghi A, Zuin M, et al. Comparison of the clinical features and clinical course of antimitochondrial antibody-positive and -negative primary biliary cirrhosis. Hepatology. 1997;25:1090–1095. doi: 10.1002/hep.510250507. [DOI] [PubMed] [Google Scholar]
- 37.Shimoda S, Harada K, Niiro H, Yoshizumi T, Soejima Y, Taketomi A, Maehara Y, et al. Biliary epithelial cells and primary biliary cirrhosis: The role of liver-infiltrating mononuclear cells. Hepatology. 2008;47(3):958–965. doi: 10.1002/hep.22102. [DOI] [PubMed] [Google Scholar]
- 38.Yang GX, L ZX, Chuang YH, Moritoki Y, Lan RY, Wakabayashi K, Ansari AA, Flavell RA, Ridgway WM, Coppel RL, Tsuneyama K, Mackay IR, Gershwin ME. Adoptive transfer of CD8+ T cells from dnTGFbetaRII mice induces autoimmune cholangitis in Rag1-/- mice. Hepatology. 2008;47(2):571–580. doi: 10.1002/hep.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinsmoen NL, Cornett KM, Kloehn R, Burnette AD, McHugh L, Flewellen BK, Matas A, et al. Pretransplant donor-specific and non-specific immune parameters associated with early acute rejection. Transplantation. 2008;85:462–470. doi: 10.1097/TP.0b013e3181612ead. [DOI] [PubMed] [Google Scholar]
- 40.Mao TK, Lian ZX, Selmi C, Ichiki Y, Ashwood P, Ansari AA, Coppel RL, et al. Altered monocyte responses to defined TLR ligands in patients with primary biliary cirrhosis. Hepatology. 2005;42:802–808. doi: 10.1002/hep.20859. [DOI] [PubMed] [Google Scholar]
- 41.Kikuchi K, Lian ZX, Yang GX, Ansari AA, Ikehara S, Kaplan M, Miyakawa H, et al. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304–312. doi: 10.1053/j.gastro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Charlotte F, L'Hermine A, Martin N, Geleyn Y, Nollet M, Gaulard P, Zafrani ES. Immunohistochemical detection of bcl-2 protein in normal and pathological human liver. Am J Pathol. 1994;144:460–465. [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura Y, Leung PS, Kenny TP, Van De Water J, Nishioka M, Giraud AS, Neuberger J, et al. Differential expression of intestinal trefoil factor in biliary epithelial cells of primary biliary cirrhosis. Hepatology. 2002;36:1227–1235. doi: 10.1053/jhep.2002.36157. [DOI] [PubMed] [Google Scholar]
- 44.Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 45.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 46.Irie J, Wu Y, Wicker LS, Rainbow D, Nalesnik MA, Hirsch R, Peterson LB, et al. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209–1219. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakabayashi K, Lian ZX, Moritoki Y, Lan RY, Tsuneyama K, Chuang YH, Yang GX, et al. IL-2 receptor alpha(-/-) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240–1249. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]


