Abstract
Male Syrian hamsters (Mesocricetus auratus) treated with moderately high doses (5.0mg/kg/day) of anabolic/androgenic steroids (AAS) during adolescence (P27–P56) display highly escalated offensive aggression. The current study examined whether adolescent AAS-exposure influenced the immunohistochemical localization of phosphate-activated glutaminase (PAG), the rate-limiting enzyme in the synthesis of glutamate, a fast-acting neurotransmitter implicated in the modulation of aggression in various species and models of aggression, as well as glutamate receptor 1 subunit (GluR1). Hamsters were administered AAS during adolescence, scored for offensive aggression using the resident-intruder paradigm, and then examined for changes in PAG and GluR1 immunoreactivity in areas of the brain implicated in aggression control. When compared with sesame oil-treated control animals, aggressive AAS-treated hamsters displayed a significant increase in the number of PAG- and area density of GluR1- containing neurons in several notable aggression regions, although the differential pattern of expression did not appear to overlap across brain regions. Together, these results suggest that altered glutamate synthesis and GluR1 receptor expression in specific aggression areas may be involved in adolescent AAS-induced offensive aggression.
INTRODUCTION
In a number of previous studies, we have used developmentally immature Syrian hamsters (Mesocricetus auratus) as an adolescent animal model to examine the link between developmental anabolic/androgenic steroid (AAS) exposure and the behavioral neurobiology of offensive aggression. Behavioral data from these studies indicated that animals repeatedly exposed AAS during adolescent development display highly elevated levels of offensive aggression when tested immediately following the exposure period on the first behavioral interaction [11,20–23,25,31,46,47,59,60]. The finding that adolescent AAS-treated hamsters demonstrated highly escalated offensive aggression in the absence of prior social interactions and dominance cues suggested that adolescent exposure to AAS stimulated aggression directly, perhaps by affecting the development and/or activity of neural circuits that regulate this behavior.
In hamsters, the anterior hypothalamus (AH) appears to be at the center of a neural network of reciprocal connections between the lateral septum (LS), medial amygdala (MeA) and ventrolateral hypothalamus (VLH) that regulates offensive aggression [12]. In a number of prior studies, we have shown that adolescent AAS exposure produces dramatic alterations in the development and activity of several neurochemical systems in these brain regions, suggesting that hypothalamic and amygdaloid nuclei may be an important point of convergence for developmental changes that underlie the generation of the AAS-induced aggressive phenotype [11,20–23,25,31,46,47,59,60]. While compelling, this data set is limited in that it only considers the role of very few and select neurotransmitter systems implicated in the aggressive response in adolescent AAS-induced aggression. It is probable that other neurotransmitter systems implicated in this behavioral response are influenced by chronic adolescent AAS exposure and contribute to the AAS-induced aggressive phenotype.
Glutamate has been firmly established as the predominant excitatory transmitter in the hypothalamus [4,45,70], however as expected glutamate is widely distributed through the CNS [7]; for review see [75]. Neuronal glutamate is produced in brain by the enzyme phosphate-activated glutaminase (PAG) [29] and the expression of this enzyme has been used in a number of previous studies to visualize and quantify glutamate-transmitting neurons in the CNS [1,33–35,44,71]. Glutamate activity has been localized to various brain regions in rat, including the hypothalamus, lateral septum, bed nucleus of the stria terminalis, and medial amygdala [8,15,28,32,36,68,69], i.e., areas of the brain implicated in aggressive behavior in hamsters [6,12–14,16,30,38,56,57,65]. Indeed, glutamate activity has been extensively linked to aggression in a range of animal models, where it appears to be positively associated with the aggressive behavioral phenotype. For instance, in rats, the “hypothalamic attack area” (HAA) has been shown to possess dense glutamatergic activity [32], and infusion of L-glutamate itself into a similar region of the cat hypothalamus activates an aggressive response [5]. Interestingly, genetically aggressive species, such as fighting bulls also show increased ratios of the excitatory amino acids (including glutamate and aspartate) to their inhibitory counterparts, such as GABA and glycine, in the fastigial nucleus of the cerebellum, the anterior and posterior collicui, and the pons, as compared to non-aggressive breeds [52,53].
The excitatory nature of the glutamate neural system on aggression has been linked to the action of glutamate at receptors representing each of the fast-acting ionotropic glutamate receptor families, i.e., N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), and kainate (KA) glutamate receptors. For instance, in rats co-infusion of a glutamate KA receptor agonist and a GABA A subtype receptor antagonist into the HAA has been shown to elicit attack behavior in experienced animals [27], while peripheral administration of NMDA receptor antagonists blocks morphine withdrawal-induced aggression in mice [66,67]. Of the AMPA family of glutamate receptors, knock-out mice lacking the glutamate receptor 1 subunit (GluR1) show vastly reduced aggressive behavior in a variety of agonistic paradigms, including the resident-intruder paradigm [72], indicating that signaling through glutamate receptors expressing this subunit may play an important role in aggression facilitation. GluR1 mRNA expression has been localized throughout hypothalamic and limbic brain areas, including the anterior, medial, and ventrolateral hypothalamic nuclei, lateral septum, bed nucleus of the terminal stria, and the amygdaloid nuclei [62,69], consistent with a putative role in aggressive behavior. In addition, GluR1-containing receptors co-localize within androgen receptor-expressing neurons in the hypothalamus and limbic system, and hypothalamic GluR1 protein levels are reduced castrated rats compared to intact and testosterone treated castrated rats [15]. Thus, of all the ionotropic glutamate receptors, those expressing the GluR1 subunit present as an ideal initial candidates for the investigation of the relationship between glutamate receptor expression and adolescent AAS-induced aggression. It is possible that AAS exposure during adolescent development escalates aggressive responding by altering glutamate and/or GluR1 activity in brain areas important for aggression control. To date however, it is unknown whether adolescent AAS exposure has any effects on the development and/or activity of the glutamate neural system.
Given this documented association between the glutaminergic neural system, aggression and androgens, the present study was conducted to establish a link between the expression of Glu- and GluR1- containing neurons and adolescent AAS-induced offensive aggression using the sub-adult Syrian hamster as an animal model. In Syrian hamsters the adolescent period of development can be identified as the time between postnatal days 27 and 60 (P27–P60). Weaning generally occurs between P23–P25 with the onset of puberty beginning around one week later [48]. During this developmental time period, hamsters wean from their dams, leave the nest, establish new solitary nest sites, participate in social relationships, and learn to defend their territory using offensive aggression [63,76]. To establish whether adolescent AAS exposure altered glutaminergic activity in various brain regions implicated in the control of offensive aggression, we employed immunohistochemistry utilizing an antibody specific against PAG and the GluR1 receptor subunit to visualize and quantify Glu- and GluR1- containing neurons.
METHODS
Animals
Prepubertal male Syrian hamsters (P21–P23) were obtained from Charles River Laboratories (Wilmington, MA), individually housed in polycarbonate cages, and maintained at ambient room temperature (22–24°C with 55% relative humidity) on a reverse light-dark cycle of (14L: 10D; lights off at 07:00). Food and water were provided ad libitium. For aggression testing, stimulus (intruder) males of equal size and weight to the experimental animals were obtained from Charles River one week prior to the behavioral test, group housed at five animals per cage in large polycarbonate cages, and maintained as above to acclimate to the animal facility. All intruders were prescreened for low-level social interest and avoidance (i.e., Disengage and Evade) and submission (i.e., Tail-up Freeze, Flee, and Fly-away) one day prior to the aggression test to control for behavioral differences between stimulus animals. Stimulus animals displaying no social interest and/or submissive postures were excluded from use in the behavioral assay. All studies using live animals were produced with approval by The Animal Care and Use Committee at Northeastern University and all methods used were consistent with guidelines provided by the National Institute of Health for the scientific treatment of animals.
Experimental Treatment
Adolescent Syrian hamsters (P27) were weighed and randomly distributed into two groups (n = 10 animals/group). One group of animals received daily subcutaneous (SC) injections (0.1ml – 0.2ml) of an AAS mixture consisting of 2mg/kg testosterone cypionate, 2mg/kg nortestosterone, and 1mg/kg dihydroxytestosterone undecylate (Steraloids Inc., Newport, R.I.), for 30 consecutive days (P27–P56) as previously described [31]. This treatment regime, designed to mimic a chronic use regimen [54,55], has been shown repeatedly to produce highly aggressive animals in greater than 90% of the treatment pool [11,20,21,25,31]. The second group of hamsters received SC injections of sesame oil alone. The day following the last injection (P58), animals were tested for offensive aggression using the resident-intruder paradigm, sacrificed 24 hours later (i.e., on P59), and the brains removed and processed for immunohistochemistry for PAG and GluR1 receptors as detailed below.
Aggression Testing
Experimental animals were tested for offensive aggression using the resident-intruder paradigm, a well-characterized and ethologically valid model of offensive aggression in Syrian hamsters [18,43]. Briefly, an intruder of similar size and weight was introduced into the home cage of the experimental animal (resident) and the resident was scored for specific and targeted aggressive responses including lateral attacks and flank bites as described [25]. An attack was scored each time the resident animal would pursue and then either: (1) lunge toward and/or (2) confine the intruder by upright and sideways threat; each generally followed by a direct attempt to bite the intruder’s dorsal and/or flank target area(s). The latency to attack and bite was defined as the period of time between the beginning of the behavioral test and the first attack and bite of the residents toward an intruder. In the case of no attacks and/or bites, latencies to attack and bite were assigned the maximum latency (i.e., 600s). In addition, residents were measured for social interest toward intruders (i.e., contact time between resident and intruder) to control for nonspecific effects of AAS treatment. Contact time was defined as the period of time during which the resident deliberately initiated contact with the intruder either through olfactory investigation (i.e., sniffing) or aggression. Each aggression test lasted for 10 min and was videotaped and coded by two observers unaware of the hamsters’ experimental treatment. No intruder was used for more than one behavioral test to control for the effects of repeated exposure to conspecifics on the behavior of intruders, and all animals were tested during the first four hours of the dark cycle under dim-red illumination to control for circadian influences on behavioral responding.
Immunohistochemistry
One day following the behavioral test for aggression, AAS and sesame oil-treated hamsters were anesthetized with 80mg/kg Ketamine and 12mg/kg Xylazine and the brains fixed by transcardial perfusion with 4% paraformaldehyde. Brains were removed, post-fixed for 90 mins in perfusion fixative, then cryoprotected in 30% sucrose in phosphate buffered saline (1xPBS; 0.001M KH2PO4, 0.01M Na2HPO4, 0.137M NaCl, 0.003M KCl, pH 7.4) overnight at 4°C. A consecutive series of 35 μm coronal brain sections from experimental and control animals were cut on a freezing rotary microtome, collected as free floating sections in 1x PBS, and then alternating sections were labeled for PAG or GluR1 in one standardized immunohistochemical run specific modifications of an existing protocol [10]. For PAG immunohistochemistry, sections were washed 3 × 5 mins in 0.1M PBS, then incubated in antibody buffer comprised of 10% normal goat serum (NGS) in 0.3% PBSTx (1xPBS/0.5% Triton X-100) for 90 mins. Primary antibody (a monoclonal mouse anti-PAG serum, courtesy of T. Kaneko, Kyoto University, Kyoto, Japan) was prepared in antibody buffer diluted to a final concentration of 1:5000 and incubation with free-floating brain sections was carried out overnight at 33°C. The specificity of this antibody has been well established [34] and the antiserum has been used to identify and study neuronal glutamate-containing neurons in the CNS in a number of studies by other laboratories [32,43,70]. Sections were then rinsed 3 × 5 minutes with 0.3% PBSTx, incubated for 90 minutes in biotinylated secondary horse anti-mouse IGg (Vector Laboratories, Burlingame, CA.) in 0.3% PBSTx then rinsed again 3 × 5 minutes in 0.3% PBSTx and incubated for 90 mins in Avidin-biotin-complex (Vectastain ABC kit; Vector Laboratories, Burlingame, CA.) in 0.3% PBSTx. The peroxidase reaction was revealed using 0.5% 3,3′-diaminobenzidine in distilled water as per manufacture’s recommendations (DAB Kit, Vectastain; Vector Laboratories, Burlingame, CA.). Omissions of primary and secondary antibodies were performed as specificity controls during the procedure. GluR1 immunohistochemistry was largely similar to that used for PAG staining. Briefly, sections were washed 3 × 5 minutes in 0.1M PBS followed by 5% hydrogen peroxide for 10 mins, rinsed 3 × 10 mins with 0.3% PBSTx, and then incubated in antibody buffer comprised of 10% NGS in PBS for 1 hr. Primary GluR1 antibody (Chemicon, Temecula, CA) was prepared in antibody buffer diluted to final concentration of 1:1000 and free-floating brain sections were incubated overnight at 21°C on a rotation wheel. Subsequently, sections were incubated for 2 hrs in biotinylated secondary goat anti-rabbit IGg (Vector Laboratories, Burlingame, CA.) in PBS with 10% NGS, rinsed again 3 × 5 minutes in PBS and incubated for 1 hr in Avidin-biotin-complex (Vectastain ABC kit; Vector Laboratories, Burlingame, CA.) in PBS. The peroxidase reaction was revealed as above. As with PAG immunochemistry, omissions of primary and secondary antibodies were performed during the procedure as controls.
Image Analysis
The number of PAG immunoreactive cell bodies was determined within specific brain areas using the BIOQUANT NOVA 5.0 computer-assisted microscopic image analysis software package as previously described [10,21,58]. The areas analyzed were selected based on data from previous studies implicating these regions as important for the regulation of offensive aggression, with the notable exception of the S1 somatosensory neocortex (S1) included in the assay as a non-aggression area used for control purposes. The specific aggression areas examined included the anterior hypothalamus (AH) with specific focus on a distinct cellular compartment within the AH, namely the latero-anterior hypothalamic (LAH) brain region (i.e., the brain area in the AH just ventro-lateral to the nucleus circularis and dorso-medial to the medial supraoptic nucleus), the medial and lateral divisions of the bed nucleus of the stria terminalis (BNST), the dorsal, intermediate, and ventral parts of the lateral septal nucleus (LS), the medial amygdaloid nucleus (MeA), and the ventrolateral hypothalamus (VLH). Slides from each animal were coded by an experimenter unaware of the experimental conditions and BIOQUANT NOVA 5.0 image analysis software running on a Pentium III CSI Open PC computer (R&M Biometrics, Nashville, TN, USA) was utilized to identify the brain Region Of Interest (ROI). Specifically, with the aid of The Hamster Atlas [51], a standard computer-generated parcel was drawn to outline the entire ROI at low power (4X) using a Nikon E600 microscope. Each brain region was assigned a separate and distinct ROI parcel, formatted in size specifically for that brain area, with the notable exception of the S1 cortex control region where placement of a size appropriate parcel was not feasible. Then, under 10X magnification images were assigned a threshold value at a standard RGB-scale level empirically determined by observers unaware of the treatment conditions, such as to allow detection of stained PAG-ir cells with moderate to high intensity, while suppressing lightly stained elements. This threshold value was then applied across subjects to control for changes in background staining and differences in foreground staining intensity between animals. The illumination was kept constant for all measurements. PAG-ir cells were identified in each field using a mouse driven cursor and then quantification of PAG-immunopositive cells was performed manually by the experimenter using the BIOQUANT software. Measurements at 10X continued until all PAG-positive cells throughout the entire ROI were quantified. Two to six independent measurements were taken from several consecutive sections of each animal (n=6) per treatment group depending upon the identification of the exact position of the nucleus within the region of interest, and the size of the nucleus in the rostral-caudal plane. Then, the number of PAG-ir cells was determined for each ROI, averaged for each brain region per animal and used for statistical analysis. The density of GluR1-ir was similarly determined. Briefly, under 40X magnification, images were assigned a threshold value as above such as to allow detection of stained GluR1-ir elements (neuropil and somata) showing moderate to high intensity, while suppressing lightly stained elements. and this threshold value was then applied across subjects. GluR1-ir elements were identified in each field using a mouse driven cursor and then GluR1-ir density measurements were performed automatically by the BIOQUANT software. Two to three independent measurements of GluR1 elements were taken from several consecutive sections of each animal per treatment group, and then the quantity (density) of GluR1-ir signal was determined for each region of interested and used for statistical analysis.
Statistical Analysis
Behavioral Studies
The results from the aggression tests were compared between AAS- and sesame oil-treatment groups. Data for all behavioral measures were compared using Student’s t-test (two-tailed).
PAG/GluR1 Immunoreactivity
The number of PAG-ir cells and the density of GluR1-ir was compared between treatment groups by Student’s t-Test (two-tailed) for each area analyzed. The α level for all comparisons was set at 0.05.
RESULTS
Aggressive Behavior
As seen previously in a number of published studies [11,20–23,25,31,59,60], hamsters treated with anabolic steroids during the majority of adolescent development showed significantly elevated levels of offensive aggression. Specifically, AAS animals demonstrated a greater than two-fold increase in the number of lateral attacks (t (16) = 2.97, p < 0.01) and more than three times the number of flank bites (t (16) = 3.32, p < 0.01) compared to their oil-treated counterparts (Fig. 1). Similarly, although the latency to first attack was not significantly different as a function of treatment (t (16) = −0.99, p > 0.05), latency to first bite was decreased in AAS-treated hamsters versus their controls (t (16) = −2.42, p < 0.05). Social contact time did not differ between groups, t (16) = 0.08, p > 0.05.
Figure 1.
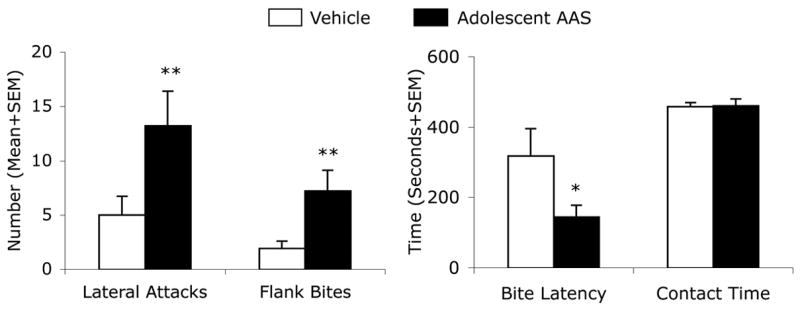
Adolescent AAS treatment increases offensive aggression. Number of Lateral Attacks and Flank Bites, as well as Latency to First Attack and Total Contact Time in Vehicle- (White Bars) and AAS- (Black Bars) treated residents. Bars denote SEM. *p<0.05; **p<0.01; Mann-Whitney, two-tailed (number of attacks and bites), Student’s t-test, two-tailed (attack latency and contact time).
PAG Immunohistochemistry
Adolescent AAS treatment altered the immunohistochemical expression of PAG-containing cells in three brain areas important for aggressive behavior, namely the hypothalamus, amygdala and the lateral septum, when compared with oil-treated controls. For example, in oil-treated controls, PAG immunostaining in the AH revealed relatively few PAG-containing neuronal somata indicative of the normal distribution of glutamate neurons in this brain region in hamsters ([17] and see Fig. 2A,C) and rats [35]. By comparison, aggressive, AAS-treated hamsters displayed an increased number of PAG immunopositive somata in the AH (Fig. 2B,D). Quantitative analysis of PAG-ir neurons in the AH showed that AAS-treated animals had a 2 fold increase in PAG immunopositive somata when compared to oil-treated controls (Fig. 3). This difference was statistically significant [AH, t(13) = 2.35, p < 0.05]. However, closer examination of this phenomenon revealed a visually detectable increase in the number of PAG-ir neurons in a select subregion of AH, namely the latero-anterior hypothalamic nucleus (LAH) – the ventro-lateral portion of the AH (Fig. 2C and B). Similar increases in the number of PAG-containing neurons were detected in subregions of the amygdala and septum. For instance, aggressive, AAS-treated hamsters exhibited a 2–3 fold increase in the number of PAG immunopositive somata in the MeA (t (11)=3.06, p < 0.01) and LS (t (12) = 3.49, p < 0.01) compared to sesame oil-treated controls (Fig. 3). Interestingly, aggressive, AAS-treated animals did not show an increase in PAG immunopositive somata compared to non-aggressive, oil-treated controls in several other brain regions implicated in the control of offensive aggression in hamster, most notably the BNST (t (10) = 0.82) and VLH (t (13) = 1.25) (p > 0.05 each comparison). Further, no significant differences were found between treatment groups in the S1 cortex (t (13) = 1.25, p > 0.05), i.e., a brain area not involved in regulation of aggressive behavior in the hamster.
Figure 2.
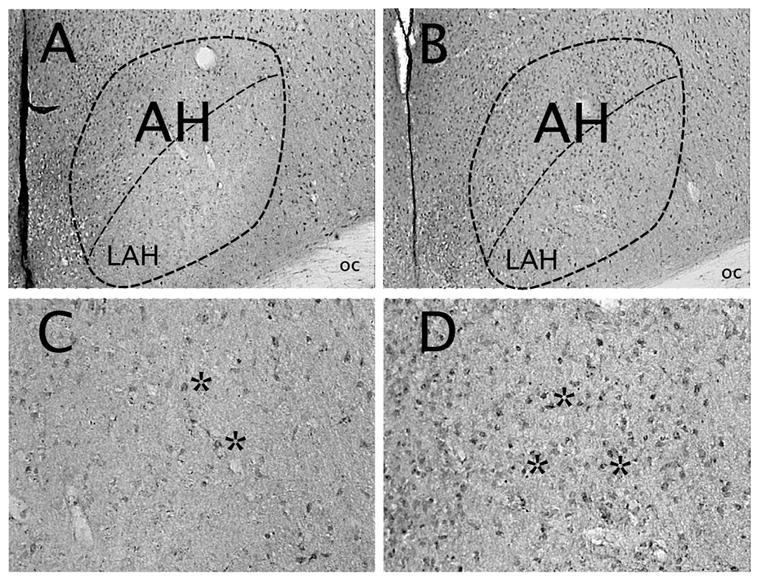
Brightfield photomicrographs of a coronal section through the Syrian hamster hypothalamus. Shown are PAG-containing cells (astericks) within the anterior hypothalamus (encircled) of Vehicle- (A, C) and AAS- (B, D) treated hamsters. High power photomicrographs in C and D represent PAG immunostaining in the ventro-lateral aspects of the anterior hypothalamus delineated below the center dashed line in A and B, i.e., the latero-anterior hypothalamic (LAH) brain region. oc, optic chiasm.
Figure 3.
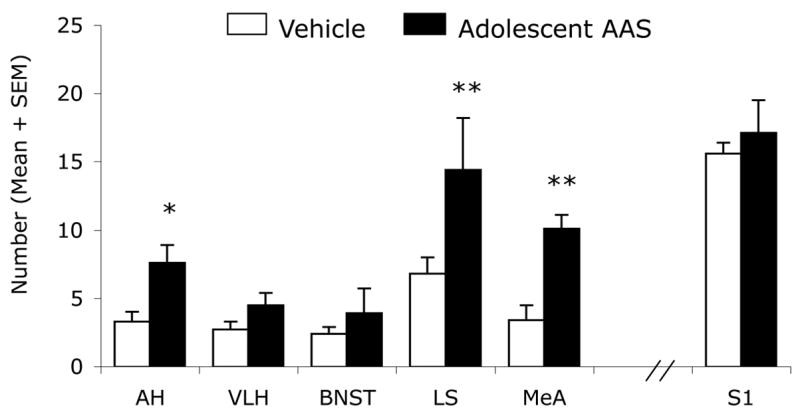
Number of PAG-immunoreactive cells in select brain regions of Vehicle (White Bars) and AAS (Black Bars) treated hamsters. * p<0.05, ** p< 0.01; Student’s t-test, two-tailed.
GluR1Immunohistochemistry
In aggressive, AAS-treated hamsters, the immunohistochemical staining pattern for GluR1 receptors was also altered in areas of the hamster brain implicated in offensive aggression, including those in the hypothalamus. For example, in oil-treated controls, the staining of GluR1 receptors displayed a moderately dense pattern of GluR1 receptor immunoreactive fibers and puncta in the VLH with very few GluR1 receptor-containing somata (astericks) indicative of the normal distribution of receptor localization in this brain region (Fig. 4A). By comparison, aggressive, AAS-treated hamsters displayed an increase in the density of GluR1 immunoreactive staining (fibers and neuronal somata (somata identified by astericks) in the VLH (Fig. 4B) compared to non-aggressive, oil-treated controls (Fig. 4A,B). Analysis of the density of GluR1-ir staining in the VLH showed that aggressive, AAS-treated hamsters had approximately 3-fold more GluR1 immunopositive signal (fibrous and somata staining) when compared to non-aggressive, oil-treated controls (Fig. 4, see Graph). This difference was statistically significant [t (10) = 3.68, p < 0.01]. Similar results were found in the BNST where aggressive, AAS-treated animals displayed significantly more GluR1-ir staining [t (12) = 3.10, p < 0.01] (i.e., approximately 2-fold) than non-aggressive, oil-treated controls (Fig. 4, see Graph). However, not all brain regions implicated in the aggressive response showed significant alterations in the density of GluR1 immunoreactive puncta/somata following adolescent AAS exposure (Fig. 4, see Graph). For instance, similar densities of GluR1 receptor-containing puncta and somata were found in the AH (t (11) = 1.05), MeA (t (11) = 0.34), and LS (t (10) = 1.21) (p > 0.05 each comparison) of both AAS- and oil-treated hamsters. Similarly, no significant differences were found in the density of GluR1 immunoreactive puncta/somata in the S1 cortex (t (13) = 0.08, p > 0.05), a brain area not involved in aggressive behavior in the hamster.
Figure 4.
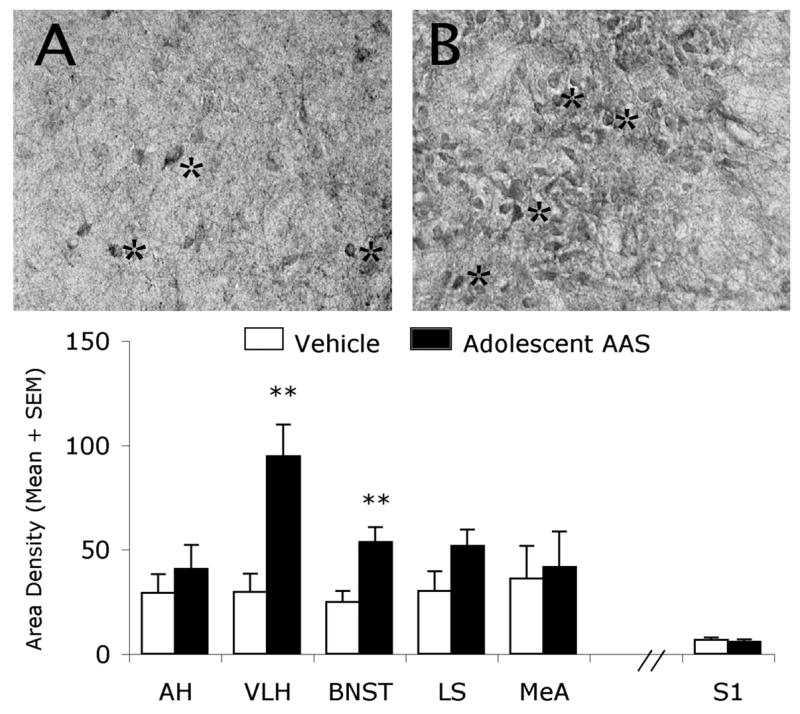
Brightfield photomicrographs of a coronal section through the Syrian hamster hypothalamus. Shown are GluR1-containing cells (astericks) within the ventrolateral hypothalamus of Vehicle- (A) and AAS- (B) treated hamsters. Graph represents density of GluR1-immunostaining (neuropil and somata) in select brain regions of Vehicle (White Bars) and AAS (Black Bars) treated hamsters. ** p< 0.01; Student’s t-test, two-tailed.
DISCUSSION
Aggressive, adolescent AAS treated animals had significant increases in the number and density of glutamate and GluR1-expressing neurons compared to non-aggressive, vehicle-treated controls, although the patterns of change observed across the neuraxis did not parallel one another. For instance, in the AAS-treated hamsters, there were greater than two-fold more PAG-ir neurons in the AH, MeA, and LS. Meanwhile, two- to three- fold increases in the density of GluR1-ir were observed in the BNST and VLH of aggressive, adolescent AAS treated animals. This disparate pattern of expression between transmitter and receptor across different brain sites implicated in aggression control is reminiscent, at least in part, of that observed for arginine vasopressin (AVP), i.e., another neurochemical signal shown previously to stimulate offensive aggression in a number of animal models and species. For instance, aggressive, adolescent AAS-treated hamsters show increased AVP in the AH, but not other brain sites associated with and/or implicated in aggression control, e.g., the BNST, LS, MeA, VLH [23,24,31]. Conversely, AVP V1A subtype receptor binding activity is increased in the BNST, LS, MeA, and VLH of aggressive, AAS-treated animals, while levels of receptor activity remain unchanged in the AH [11]. While most assuredly indicative of different molecular mechanisms, that aside, these data combined indicate that adolescent AAS exposure increases AVP tone across a number of brain regions implicated in aggression control – likely facilitating the development of the aggressive phenotype in drug treated animals. The findings presented in the current study are suggestive of a similar trend. Here aggressive, adolescent AAS-treated hamsters show increased PAG expression (indicative of increased glutamate production/activity) in the AH, MeA, and LS but not other brain sites associated with and/or implicated in aggression control, e.g., the BNST and VLH. However, increases in GluR1 glutamate receptors are noted in these latter brain sites, but not in the aforementioned brain regions. Together, these data strongly suggest that adolescent AAS exposure also increases glutaminergic tone across a number of the same brain regions implicated in aggression control – similarly suggestive of a facilitation the development of the aggressive phenotype in drug treated animals. Of particular relevance are the findings of an increase in the production/activity of neurotransmitters that facilitate aggression in the AH brain region of aggressive, AAS-treated animals, i.e., AVP and glutamate. Given this patterns of overlap, these data suggest that AVP and glutamate may interact in the AH to control adolescent, AAS-induced aggression in hamsters. Indeed, there is precedent that AVP and glutamate interact in the AH to control flank marking in hamsters, i.e., a stereotypic motor behavior that is part of the ethogram of offensive aggression in this species [3]. In these studies, the activation of flank marking by AVP delivered directly into the AH requires the activity of glutamate – linking the activity of these two neural systems in this particular brain region to the regulation of agonistic behavior.
Overlap between the altered production and/or activity of the aggression-stimulating neurotransmitter glutamate and that of other neural components implicated in aggression control is not limited to the AVP neural system in the AH. Indeed, in recent studies we have shown a dramatic reduction in afferent fibers containing serotonin (5HT – i.e., a neurochemical signal previously shown to inhibit aggression) to the AH of aggressive, adolescent AAS-treated hamsters [21], indicating a reduced 5HT tone in this brain region in AAS-exposed animals. Aggressive, adolescent AAS-treated hamsters also express fewer post-synaptic 5HT1A- [60] and pre-synaptic 5HT1B- [20] receptors in the AH, while the expression of post-synaptic 5HT1B receptors appears to be up-regulated in neurons located in a select subregion of AH [20] namely the hamster equivalent of the latero-anterior hypothalamic nucleus – the ventro-lateral portion of the AH located between the two AVP containing compartments of the AH (i.e., the mSON and NC). Interestingly, more detailed analysis of the data presented in this report indicates that the observed increases in PAG appear to be localized to a great extent within the LAH, suggesting that perhaps the 5HT1B-containing neurons in this brain region are glutaminergic – and that both PAG and 5HT1B receptors are up-regulated in these neurons in aggressive, adolescent AAS-treated hamsters. Preliminary studies support the former assertion as we have shown that PAG containing neurons in the LAH express 5HT1B receptors (data not shown). Interestingly, in a separate set of studies we also showed that aggressive, adolescent AAS-treated animals showed persistent activation of neurons within this same subregion of the AH [59], suggesting that 5HT1B receptor-containing glutamate neurons in the LAH brain region may become constitutively active following adolescent AAS exposure. Thus, together, in the case of aggressive, adolescent AAS-treated animals, the enhanced stimulatory AVP tone combined with the loss of inhibitory 5HT tone that exists in the LAH as a result of AAS exposure might result in the chronic activation of downstream glutamate neurons in this brain region, facilitating the development of a heightened aggressive response pattern. This hypothesis is currently under investigation in the laboratory.
As mentioned above, however, there is increased PAG and GluR1 receptor expression (indicative of increased glutaminergic tone) in several other parts of the forebrain implicated in the control of aggression that should not be overlooked. For instance, neurons in the MeA and BNST regulate aggressive response patterns in rats, mice, prairie voles, and hamsters [6,12,19,38,41,42,64,73,74], and increased neuronal activation has been observed in these brain regions in aggressive, experienced fighter hamsters after an aggressive encounter with other hamsters [12,38]. In aggressive, adolescent AAS treated animals increases in excitatory AVP V1a receptor binding [11], decreases in inhibitory 5HT afferent innervation [21,22], and alterations in 5HT1B receptor expression [22] have all been observed in the MeA and BNST. The enhanced tone of stimulatory AVP combined with the loss of inhibitory 5HT tone that occur in the MeA and BNST as a result of AAS exposure, along with the increase in PAG- and GluR1- expression (or glutaminergic tone) observed in these brain regions, reflect a neurobiologic state consistent with facilitating the development of a heightened aggressive response pattern in drug exposed animals. Similarly, the VLH has been implicated in the modulation of offensive aggression in hamsters [12–14], and projects extensively into other relevant regions, including the AH and BNST [61]. Alterations in the AVP and 5HT neural systems that are consistent with the development of the aggressive phenotype have been observed in the VLH of aggressive, adolescent AAS treated hamsters [11,21,22]. Similarly here the increased GluR1 expression in the VLH may reflect an increase in excitatory glutamate tone in this brain region. Combined with the aforementioned neural changes that also occur here, increased glutaminergic tone may function to activate a feed-forward circuit to facilitate the development of excessive offensive responding in animals exposed to AAS during adolescence.
These data notwithstanding, there are findings from the current study that are inconsistent with this general hypothesis. For example, in this study we show increases in PAG-ir neurons in the LS in aggressive, adolescent AAS-treated animals. However, in this brain region, no significant changes in 5HT afferent innervation and/or 5HT1A or 1B receptor expression have been observed in aggressive, AAS-treated hamsters [20,21,60], nor are there any alterations in neuronal activation in this brain site [20,21,60]. Further, aggressive responding has been shown to be inhibited by LS activity in hamsters [56,57], suggesting that an increase in the excitatory glutaminergic tone in this brain site may serve to suppress aggressive behavior. While it is true that the LS has previously been reported to be active following bouts of fighting in hamsters [38,40], this brain region has not been specifically characterized as one critical for the regulation of offensive aggression in hamsters. For example, in hamsters, activation of the LS has been implicated in defensive aggression, mating and scent marking [2,3,37–39]. Therefore, activity in these brain regions may reflect gross alterations in social behavior and responsiveness and have less of an affect on offensive aggression, remaining consistent with the central hypothesis regarding the relationship between site specific neural activation and the development of the offensive aggressive phenotype.
Speculation aside, currently the mechanisms by which adolescent exposure to AAS would up-regulate PAG expression is unclear. Certainly, there is strong evidence that glutamate affects steroid hormones – glutamatergic activity is critical for the onset of puberty, and appears to underlie secretion of lutenizing hormone, testosterone, and follicle stimulating hormone [4,9]. In fact the development of the glutamatergic system continues through the pubertal years, not reaching full maturation until adulthood [26,49,50]. Glutamate concentrations begin to rise during postnatal development and peak at or very shortly after, the onset of puberty [50]. Such ongoing pubertal development might leave glutamatergic systems particularly vulnerable to pharmacological insult by AAS. However, evidence is considerably sparse regarding an association in this direction – i.e., whereby glutamate levels are influenced by sex steroids. Our data clearly suggest such a developmental sensitivity, and are bolstered by previous studies that have reported a degree of hypothalamic and limbic GluR1 and GluR2/3 receptor dependence upon sex steroid manipulations [15]. Further research is necessary to determine the specific mechanisms underlying the increased expression in hypothalamic and limbic PAG and GluR1 observed in this study.
In summary, existing literature strongly implicate a role for the excitatory glutamatergic systems in the mediation of offensive aggression. In the current report, we explored the possibility that alterations in the expression of PAG and the ionotropic AMPA GluR1 subunit might be affected by chronic, high-dose steroid treatment in adolescent hamsters. Elevations in expression of both PAG and GluR1 were observed in key brain areas previously associated with offensive behavior, including the AH, VLH, MeA, BNST, and LS. The patterns of expression appeared to complement, rather than parallel one another, and overall, the hypothalamic-limbic aggression areas demonstrated an increase in general glutamatergic tone and activity in aggressive, AAS-treated animals.
Acknowledgments
This work was supported by a research grant (R01) DA10547 from NIDA to R.H.M.. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH or Northeastern University. R.H.M. would like to extend special thanks to Dr. K.A. Melloni for support and encouragement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aoki C, Kaneko T, Starr A, Pickel VM. Identification of mitochondrial and non-mitochondrial glutaminase within select neurons and glia of rat forebrain by electron microscopic immunocytochemistry. J Neurosci Res. 1991;28:531–48. doi: 10.1002/jnr.490280410. [DOI] [PubMed] [Google Scholar]
- 2.Bamshad M, Albers HE. Neural circuitry controlling vasopressin-stimulated scent marking in Syrian hamsters (Mesocricetus auratus) J Comp Neurol. 1996;369:252–63. doi: 10.1002/(SICI)1096-9861(19960527)369:2<252::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Bamshad M, Cooper TT, Karom M, Albers HE. Glutamate and vasopressin interact to control scent marking in Syrian hamsters (Mesocricetus auratus) Brain Res. 1996;731:213–6. doi: 10.1016/0006-8993(96)00670-1. [DOI] [PubMed] [Google Scholar]
- 4.Brann DW, Mahesh VB. Glutamate: a major neuroendocrine excitatory signal mediating steroid effects on gonadotropin secretion. J Steroid Biochem Mol Biol. 1995;53:325–9. doi: 10.1016/0960-0760(95)00070-g. [DOI] [PubMed] [Google Scholar]
- 5.Brody JF, DeFeudis PA, DeFeudis FV. Effects of micro-injections of L-glutamate into the hypothalamus on attack and flight behaviour in cats. Nature. 1969;224:1330. doi: 10.1038/2241330a0. [DOI] [PubMed] [Google Scholar]
- 6.Bunnell BN, Sodetz FJ, Jr, Shalloway DI. Amygdaloid lesions and social behavior in the golden hamster. Physiol Behav. 1970;5:153–61. doi: 10.1016/0031-9384(70)90059-4. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JR, Bloom FE, Roth RH. Amino Acid Transmitters. In: Cooper JR, Bloom FE, Roth RH, editors. The Biochemical Basis of Neuropharmacology. Oxford University Press; New York, NY: 2003. pp. 105–150. [Google Scholar]
- 8.Csaki A, Kocsis K, Kiss J, Halasz B. Localization of putative glutamatergic/aspartatergic neurons projecting to the supraoptic nucleus area of the rat hypothalamus. European Journal of Neuroscience. 2002;16:55–68. doi: 10.1046/j.1460-9568.2002.02059.x. [DOI] [PubMed] [Google Scholar]
- 9.DeCavel C, Van den Pol AN. Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. The Journal of Comparative Neurology. 1992;316:104–116. doi: 10.1002/cne.903160109. [DOI] [PubMed] [Google Scholar]
- 10.DeLeon KR, Grimes JM, Connor DF, Melloni RH., Jr Adolescent cocaine exposure and offensive aggression: involvement of serotonin neural signaling and innervation in male Syrian hamsters. Behav Brain Res. 2002;133:211–20. doi: 10.1016/s0166-4328(02)00004-9. [DOI] [PubMed] [Google Scholar]
- 11.DeLeon KR, Grimes JM, Melloni RH., Jr Repeated anabolic-androgenic steroid treatment during adolescence increases vasopressin V(1A) receptor binding in Syrian hamsters: correlation with offensive aggression. Horm Behav. 2002;42:182–91. doi: 10.1006/hbeh.2002.1802. [DOI] [PubMed] [Google Scholar]
- 12.Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- 13.Delville Y, Mansour KM, Ferris CF. Serotonin blocks vasopressin-facilitated offensive aggression: interactions within the ventrolateral hypothalamus of golden hamsters. Physiol Behav. 1996;59:813–6. doi: 10.1016/0031-9384(95)02166-3. [DOI] [PubMed] [Google Scholar]
- 14.Delville Y, Mansour KM, Ferris CF. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol Behav. 1996;60:25–9. doi: 10.1016/0031-9384(95)02246-5. [DOI] [PubMed] [Google Scholar]
- 15.Diano S, Naftolin F, Horvath TL. Gonadal steroids target AMPA glutamate receptor-containing neurons in the rat hypothalamus, septum, and amygdala: a morphological and biochemical study. Endocrinology. 1997;138:778–789. doi: 10.1210/endo.138.2.4937. [DOI] [PubMed] [Google Scholar]
- 16.Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–40. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer SG, Melloni RH., Jr Increased phosphate activated glutaminase expression in hypothalamic and limbic nuclei of anabolic/androgenic steroid treated hamsters. Journal of Neurosci Abst. 2006;32:816–19. [Google Scholar]
- 18.Floody OR, Pfaff DW. Aggressive behavior in female hamsters: the hormonal basis for fluctuations in female aggressiveness correlated with estrous state. J Comp Physiol Psychol. 1977;91:443–64. doi: 10.1037/h0077341. [DOI] [PubMed] [Google Scholar]
- 19.Gammie SC, Nelson RJ. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 2001;898:232–41. doi: 10.1016/s0006-8993(01)02189-8. [DOI] [PubMed] [Google Scholar]
- 20.Grimes JM, Melloni RH. Serotonin 1B receptor activity and expression modulate the aggression-stimulating effects of adolescent anabolic steroid exposure in hamsters. Behavioral Neuroscience. 2005;119:1184–94. doi: 10.1037/0735-7044.119.5.1184. [DOI] [PubMed] [Google Scholar]
- 21.Grimes JM, Melloni RH., Jr Serotonin modulates offensive attack in adolescent anabolic steroid-treated hamsters. Pharmacol Biochem Behav. 2002;73:713–21. doi: 10.1016/s0091-3057(02)00880-8. [DOI] [PubMed] [Google Scholar]
- 22.Grimes JM, Melloni RHJ. Prolonged alterations in the serotonin neural system following the cessation of adolescent anabolic-androgenic steroid exposure in hamsters (Mesocricetus auratus) Behavioral Neuroscience. 2006;120:1242–51. doi: 10.1037/0735-7044.120.6.1242. [DOI] [PubMed] [Google Scholar]
- 23.Grimes JM, Ricci LA, Melloni RH. Plasticity in anterior hypothalamic vasopressin correlates with aggression during anabolic/androgenic steroid withdrawal. Behav Neurosci. 2006;120:115–24. doi: 10.1037/0735-7044.120.1.115. [DOI] [PubMed] [Google Scholar]
- 24.Grimes JM, Ricci LA, Melloni RH. Alterations in anterior hypothalamic vasopressin, but not serotonin, correlate with teh temporal onset of aggressive behavior during adolescent anabolic-steroid exposure in hamsters. Behav Neurosci. 2007 doi: 10.1037/0735-7044.121.5.941. Under Review. [DOI] [PubMed] [Google Scholar]
- 25.Grimes JM, Ricci LA, Melloni RH., Jr Glutamic acid decarboxylase (GAD65) immunoreactivity in brains of aggressive, adolescent anabolic steroid-treated hamsters. Horm Behav. 2003;44:271–80. doi: 10.1016/s0018-506x(03)00138-7. [DOI] [PubMed] [Google Scholar]
- 26.Hall RA, Bahr BA. AMPA receptor development in rat telencephalon: [3H]AMPA binding and western blot studies. Journal of Neurochemistry. 1994;63:1658–1665. doi: 10.1046/j.1471-4159.1994.63051658.x. [DOI] [PubMed] [Google Scholar]
- 27.Haller J, Abraham I, Zelena D, Juhasz G, Makara GB, Kruk MR. Aggressive experience affects the sensitivity of neurons towards pharmacological treatment in the hypothalamic attack area. Behav Pharmacol. 1998;9:469–75. doi: 10.1097/00008877-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Halpain S, Wiecsorek CM, Rainbow TC. Localization of L-glutamate receptors in rat brain by quantitative autoradiography. Journal of Neuroscience. 1984;4:2247–2259. doi: 10.1523/JNEUROSCI.04-09-02247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamberger AC, Chiang GH, Sandoval E, Cotman CW. Glutamate as a CNS transmitter. II. Regulation of synthesis in the releasable pool. Brain Research. 1979;168:531–541. doi: 10.1016/0006-8993(79)90307-x. [DOI] [PubMed] [Google Scholar]
- 30.Hammond MA, Rowe FA. Medial preoptic and anterior hypothalamic lesions: influences on aggressive behavior in female hamsters. Physiol Behav. 1976;17:507–13. doi: 10.1016/0031-9384(76)90115-3. [DOI] [PubMed] [Google Scholar]
- 31.Harrison RJ, Connor DF, Nowak C, Nash K, Melloni RH., Jr Chronic anabolic-androgenic steroid treatment during adolescence increases anterior hypothalamic vasopressin and aggression in intact hamsters. Psychoneuroendocrinology. 2000;25:317–38. doi: 10.1016/s0306-4530(99)00057-8. [DOI] [PubMed] [Google Scholar]
- 32.Hrabovszky E, Halasz J, Meelis W, Kruk MR, Liposits Z, Haller J. Neurochemical characterization of hypothalamic neurons involved in attack behavior: glutamatergic dominance and co-expression of thyrotropin-releasing hormone in a subset of glutamatergic neurons. Neuroscience. 2005;133:657–66. doi: 10.1016/j.neuroscience.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 33.Kaneko T, Itoh K, Shigemoto R, Mizuno N. Glutaminase-like immunoreactivity in the lower brainstem and cerebellum of the adult rat. Neuroscience. 1989;32:79–98. doi: 10.1016/0306-4522(89)90109-7. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko T, Mizuno N. Immunohistochemical study of glutaminase-containing neurons in the cerebral cortex and thalamus of the rat. J Comp Neurol. 1988;267:590–602. doi: 10.1002/cne.902670411. [DOI] [PubMed] [Google Scholar]
- 35.Kaneko T, Urade Y, Watanabe Y, Mizuno N. Production, characterization, and immunohistochemical application of monoclonal antibodies to glutaminase purified from rat brain. J Neurosci. 1987;7:302–9. doi: 10.1523/JNEUROSCI.07-01-00302.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kocsis K, Kiss J, Goercs T, Halasz B. Metabotropic glutamate receptor in vasopressin, CRF and VIP hypothalamic neurones. Neuroendocrinology. 1998;9:4029–4033. doi: 10.1097/00001756-199812210-00008. [DOI] [PubMed] [Google Scholar]
- 37.Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J Neuroendocrinol. 1999;11:547–59. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- 38.Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–36. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- 39.Kollack-Walker S, Newman SW. Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J Neurobiol. 1997;32:481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 40.Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–55. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koolhaas JM, Moor E, Hiemstra Y, Bohus B. The testosterone-dependent vasopressinergic neurons in the medial amygdala and lateral septum: involvement in social behavior of male rats. In: Jard S, Jamison R, editors. Vasopressin. 1991. pp. 213–219. [Google Scholar]
- 42.Koolhaas JM, Van den Brink THC, Roozendal B, Boorsma F. Medial amygdala and aggressive behavior: Interaction between testosterone and vasopressin. Aggressive Behavior. 1990;16:223–229. [Google Scholar]
- 43.Lerwill CJ, Makings P. The agonistic behavior of the golden hamster. Animal Behavior. 1971;19:714–721. [Google Scholar]
- 44.Manns ID, Mainville L, Jones BE. Evidence for glutamate, in addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain neurons projecting to the entorhinal cortex. Neuroscience. 2001;107:249–63. doi: 10.1016/s0306-4522(01)00302-5. [DOI] [PubMed] [Google Scholar]
- 45.Meeker RB, Swanson DJ, Greenwood RS, Hayward JN. Quantitative mapping of glutamate presynaptic terminals in the supraoptic nucleus and surrounding hypothalamus. Brain Res. 1993;600:112–22. doi: 10.1016/0006-8993(93)90408-f. [DOI] [PubMed] [Google Scholar]
- 46.Melloni RH, Jr, Connor DF, Hang PT, Harrison RJ, Ferris CF. Anabolic-androgenic steroid exposure during adolescence and aggressive behavior in golden hamsters. Physiol Behav. 1997;61:359–64. doi: 10.1016/s0031-9384(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 47.Melloni RH, Jr, Ferris CF. Adolescent anabolic steroid use and aggressive behavior in golden hamsters. Ann N Y Acad Sci. 1996;794:372–5. doi: 10.1111/j.1749-6632.1996.tb32546.x. [DOI] [PubMed] [Google Scholar]
- 48.Miller LL, Whitsett JM, Vandenbergh JG, Colby DR. Physical and behavioral aspects of sexual maturation in male golden hamsters. J Comp Physiol Psychol. 1977;91:245–59. doi: 10.1037/h0077315. [DOI] [PubMed] [Google Scholar]
- 49.Miranda-Contreras L, Benitez-Diaz PR, Mendoza-Briceno RV, Delgado-Saez MC, Palacios-Pru EL. Levels of amino acid neurotransmitters during mouse cerebellar neurogenesis and in histotypic cerebellar cultures. Developmental Neuroscience. 1999;21:147–158. doi: 10.1159/000017377. [DOI] [PubMed] [Google Scholar]
- 50.Miranda-Contreras L, Mendoza-Briceno RV, Palacios-Pru EL. Levels of monoamine and amino acid neurotransmitters in the developing male mouse hypothalamus and in histotypic hypothalamic cultures. International Journal of Developmental Neuroscience. 1998;16:403–412. doi: 10.1016/s0736-5748(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 51.Morin LPaWRI. A Stereotaxic Atlas of The Golden Hamster Brain. Academic Press; 2001. [Google Scholar]
- 52.Munoz-Blanco J, Porras Castillo A. Changes in neurotransmitter amino acids content in several CNS areas from aggressive and non-aggressive bull strains. Physiol Behav. 1987;39:453–7. doi: 10.1016/0031-9384(87)90372-6. [DOI] [PubMed] [Google Scholar]
- 53.Munoz-Blanco J, Yusta B, Cordoba F. Differential distribution of neurotransmitter amino acids from the limbic system of aggressive and non-aggressive bull strains. Pharmacol Biochem Behav. 1986;25:71–5. doi: 10.1016/0091-3057(86)90232-7. [DOI] [PubMed] [Google Scholar]
- 54.Pope HG, Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiatry. 1994;51:375–82. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- 55.Pope HG, Katz DL, Champoux R. Anabolic-androgenic steroid use among 1010 college men. Physician Sports Med. 1988;16:75–81. doi: 10.1080/00913847.1988.11709554. [DOI] [PubMed] [Google Scholar]
- 56.Potegal M, Blau A, Glusman M. Effects of anteroventral septal lesions on intraspecific aggression in male hamsters. Physiol Behav. 1981;26:407–12. doi: 10.1016/0031-9384(81)90167-0. [DOI] [PubMed] [Google Scholar]
- 57.Potegal M, Blau A, Glusman M. Inhibition of intraspecific aggression in male hamsters by septal stimulation. Physiol Psychol. 1981;9:213–218. doi: 10.1016/0031-9384(81)90167-0. [DOI] [PubMed] [Google Scholar]
- 58.Ricci LA, Grimes JM, Melloni RH., Jr Serotonin type-3 receptors modulate the aggression-stimulating effects of adolescent cocaine exposure. Behav Neurosci. 2004;118:1097–1110. doi: 10.1037/0735-7044.118.5.1097. [DOI] [PubMed] [Google Scholar]
- 59.Ricci LA, Grimes JM, Melloni RH., Jr Lasting changes in neuronal activation patterns in select forebrain regions of aggressive, adolescent anabolic/androgenic steroid-treated hamsters. Behav Brain Res. 2007;176:344–352. doi: 10.1016/j.bbr.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ricci LA, Rasakham S, Grimes JM, Melloni RH. Serotonin 1A receptor activity and expression modulate adolescent anabolic/androgenic steroid induced aggression in hamsters. Pharmacol Biochem Behav. 2006;85:1–11. doi: 10.1016/j.pbb.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 61.Ricciardi KH, Turcotte JC, De Vries GJ, Blaustein JD. Efferent projections from the ovarian steroid receptor-containing area of the ventrolateral hypothalamus in female guinea pigs. J Neuroendocrinol. 1996;8:673–85. [PubMed] [Google Scholar]
- 62.Rogers SW, Hughes TE, Hollmann M, Gasic GP, Deneris ES, Heinemann S. The characterization and localization of the glutamate receptor subunit GluR1 in the rat brain. J Neurosci. 1991;11:2713–24. doi: 10.1523/JNEUROSCI.11-09-02713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schoenfeld TA, Leonard CM. Behavioral Development in the Syrian Golden Hamster. In: Siegel HI, editor. The Hamster: Reproduction and Behavior. Plenum Press; New York: 1985. [Google Scholar]
- 64.Shibata S, Yamamoto TY, Ueki S. Differential effects of medial, central and basolateral amygdaloid lesions on four models of experimentally-induced aggression in rats. Physiol Behav. 1982;28:289–94. doi: 10.1016/0031-9384(82)90077-4. [DOI] [PubMed] [Google Scholar]
- 65.Sodetz FJ, Bunnell BN. Septal ablation and the social behavior of the golden hamster. Physiol Behav. 1970;5:79–88. doi: 10.1016/0031-9384(70)90017-x. [DOI] [PubMed] [Google Scholar]
- 66.Sukhotina IA. Morphine withdrawal-facilitated aggression is attenuated by morphine-conditioned stimuli. Pharmacol Biochem Behav. 2001;68:93–8. doi: 10.1016/s0091-3057(00)00429-9. [DOI] [PubMed] [Google Scholar]
- 67.Sukhotina IA, Bespalov AY. Effects of the NMDA receptor channel blockers memantine and MRZ 2/579 on morphine withdrawal-facilitated aggression in mice. Psychopharmacology (Berl) 2000;149:345–50. doi: 10.1007/s002130000386. [DOI] [PubMed] [Google Scholar]
- 68.Van den Pol AN. Glutamate and aspartate immunoreactivity in hypothalamic presynaptic axons. The Journal of Neuroscience. 1991;11:2087–2101. doi: 10.1523/JNEUROSCI.11-07-02087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van den Pol AN, Hermans-Borgmeyer I, Hofer M, Ghosh P, Heinemann S. Ionotropic glutamate-receptor gene expression in hypothalamus: localization of AMPA, kainate, and NMDA receptor RNA with in situ hybridization. The Journal of Comparative Neurology. 1994;343:428–444. doi: 10.1002/cne.903430307. [DOI] [PubMed] [Google Scholar]
- 70.van den Pol AN, Trombley PQ. Glutamate neurons in hypothalamus regulate excitatory transmission. J Neurosci. 1993;13:2829–36. doi: 10.1523/JNEUROSCI.13-07-02829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van der Gucht E, Jacobs S, Kaneko T, Vandesande F, Arckens L. Distribution and morphological characterization of phosphate-activated glutaminase-immunoreactive neurons in cat visual cortex. Brain Res. 2003;988:29–42. doi: 10.1016/s0006-8993(03)03332-8. [DOI] [PubMed] [Google Scholar]
- 72.Vekovischeva OY, Aitta-Aho T, Echenko O, Kankaanpaa A, Seppala T, Honkanen A, Sprengel R, Korpi ER. Reduced aggression in AMPA-type glutamate receptor GluR-A subunit-deficient mice. Genes Brain Behav. 2004;3:253–65. doi: 10.1111/j.1601-1848.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 73.Vochteloo JD, Koolhaas JM. Medial amygdala lesions in male rats reduce aggressive behavior: interference with experience. Physiol Behav. 1987;41:99–102. doi: 10.1016/0031-9384(87)90137-5. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767:321–32. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- 75.Watkins JC. L-Glutamate as a central neurotransmitter: looking back. Biochemical Society Transactions. 2000;28:297–310. [PubMed] [Google Scholar]
- 76.Whitsett JM. The development of aggressive and marking behavior in intact and castrated male hamsters. Horm Behav. 1975;6:47–57. doi: 10.1016/0018-506x(75)90022-7. [DOI] [PubMed] [Google Scholar]


