Abstract
OBJECTIVE: To determine whether sirolimus therapy is associated with neurologic complications, including stroke, among heart transplant recipients.
PATIENTS AND METHODS: We retrospectively studied patients who underwent heart transplant at Mayo Clinic's site in Rochester, MN, from January 1, 1988, through June 30, 2006.
RESULTS: Of 313 patients in the cohort, the medical regimen in 116 patients (37%) was switched from cyclosporine-based therapy to sirolimus. The hazard ratio of sirolimus for any neurologic or psychiatric event was 1.94 (95% confidence interval, 0.67-4.29). This hazard ratio was driven mainly by the association between sirolimus and the development of tremor and depression. Cerebrovascular events occurred with a cumulative incidence of 14% but did not occur in any of the patients who received sirolimus therapy. There were no cases of posterior reversible encephalopathy syndrome with sirolimus use.
CONCLUSION: No early or late episodes of major neurotoxicity occurred in heart transplant recipients using sirolimus immunosuppression. The absence of stroke and transient ischemic attacks in these high-risk transplant recipients treated with sirolimus is notable but needs confirmation in future studies.
No early or late episodes of major neurotoxicity occurred in this retrospective study of heart transplant recipients; the absence of stroke and transient ischemic attacks in these high-risk patients treated with sirolimus is notable but needs to be confirmed in future studies.
CI = confidence interval; HR = hazard ratio; IQR = interquartile range
Sirolimus is a recently introduced potent immunosuppressive agent that is used primarily after organ transplant because it inhibits a kinase (mammalian target of rapamycin) involved with proliferation of T and B lymphocytes.1 Sirolimus therapy is of considerable interest to neurologists who consult in transplant units. First, although a large study found no evidence of neurotoxicity with sirolimus therapy in kidney and liver transplant recipients,2 3 recent single case reports of heart, lung, or bone marrow transplant tentatively linked sirolimus therapy with posterior reversible encephalopathy syndrome.3-5 Second, heart transplant recipients are especially at risk of cerebrovascular events, having a 10-fold higher incidence compared with the risk in an age-matched healthy population.6 The effect of sirolimus on the development of stroke among heart transplant recipients is unknown. This is particularly relevant because sirolimus has also been reported to cause thrombotic microangiopathy and is known for its antiatherogenic properties.1 We investigated whether sirolimus therapy resulted in neurotoxicity in heart transplant recipients.
PATIENTS AND METHODS
We reviewed patient data from the cardiac transplant program at Mayo Clinic's site in Rochester, MN, from January 1, 1988, through June 30, 2006. The following sources of information were used: Mayo Clinic Transplant Database, Diagnostic Index Database, paper and electronic medical records, records of Mayo Clinic clinical laboratories, and results of imaging. Data were extracted regarding patients' histories and baseline characteristics, the early postoperative period (defined as the period between heart transplant and discharge from the hospital), and the occurrence of neurologic diseases. Patients' characteristics and neurologic events were noted by the treating health care professional.6 Immunosuppressive regimens were recorded at 2 and 6 months and at 2 and 6 years after transplant. Our study was approved by the Mayo Clinic Institutional Review Board. Two patients refused authorization for medical record research studies, and their data were excluded.
Data were summarized using means ± SDs or medians with interquartile ranges (IQRs) for continuous variables, as appropriate, and numbers and percentages for categorical variables. Parametric (t test or analysis of variance) and nonparametric tests (Mann-Whitney or Kruskal-Wallis) were used to identify differences between groups in continuous outcomes, and χ2 tests were used to compare categorical outcomes. Predictors that changed after baseline (eg, sirolimus use) were included in a Cox model as time-dependent predictors. Dosages and drug levels were not specifically evaluated. The Kaplan-Meier method adapted to account for the competing risk of death was used to describe cumulative incidences for neurologic complications. If a patient had recurrent similar neurologic complications, only the date of the first episode was included in the time-dependent analysis. If a patient had more than 1 different neurologic complications, all complications were included in the time-dependent analysis. Tests were performed at the 5% level and confidence intervals (CIs) calculated at the 95% level.
RESULTS
Baseline characteristics of the 313 study patients (median age, 52 years; IQR, 38-59 years), including 24 children (median age, 9 years; IQR, 4-14 years) and 289 adults (median age, 53 years; IQR, 44-59 years), have been described previously.3 Of the 313 patients, 198 (63%) developed heart failure that could be traced to its 2 most common causes, idiopathic dilated cardiomyopathy and ischemic heart failure. Multiorgan transplant was performed in 25 (8%) of the 313 patients (heart-liver-kidney in 14 patients [4%], heart-liver in 9 [3%], and heart-kidney in 2 [1%]) and was common in patients with amyloidosis (10/33 [30%]). Follow-up was complete for 306 (98%) of the 313 patients; 9 patients were lost to follow-up (4 patients after 1 year, 1 after 2 years, 1 after 5 years, and 3 after 6 years). During the study period, 95 (30%) of the 313 patients died, and the median (IQR) clinical follow-up was 5.5 (2.2-9.9) years.
The most frequently prescribed immunosuppressive treatments were corticosteroids (100%), cyclosporine (98%), and azathioprine (89%). Sirolimus for the treatment of heart transplant recipients was introduced at Mayo Clinic in 2002. Its use increased substantially over time. In 116 (37%) of the 313 patients in the cohort, the medical regimen was switched from cyclosporine-based therapy to sirolimus. In almost all cases, the medication switch was prompted by allograft vasculopathy or decrease of renal function. The number of patients taking sirolimus was 5 (2%) at 2 months, 21 (7%) at 6 months, 30 (10%) at 2 years, and 14 (5%) at 6 years after transplant.
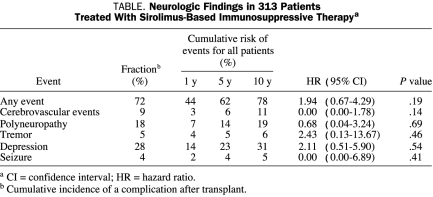
Common neurologic complications at follow-up were polyneuropathy and cerebrovascular events, stroke, and transient ischemic attacks (Table). The hazard ratio (HR) of sirolimus for any neurologic event was 1.94 (95% CI, 0.67-4.29). This HR was driven mainly by the association between sirolimus and the development of tremor and depression. Tremor was positional and rarely interfered with writing. Again, these associations were not significant with wide CIs.
TABLE.
Neurologic Findings in 313 Patients Treated With Sirolimus-Based Immunosuppressive Therapya
Stroke or transient ischemic attacks occurred in 29 (9%) of the 313 patients. The cumulative incidence of cerebrovascular events was 6%, 11%, and 14% at 5, 10, and 15 years, respectively. Cerebral infarction occurred in 19 patients, transient ischemic attack in 8 patients, and hemorrhage in 5 patients. Interestingly, none of the patients had a stroke or transient ischemic attack while taking sirolimus (95% CI for HR of sirolimus for cerebrovascular events, 0.00-1.78; P=.14).
Polyneuropathy was the most common peripheral nerve disease after transplant; it occurred in 40 (13%) of the 313 study patients. The HR of sirolimus for development of polyneuropathy was 0.68 (95% CI, 0.09-5.06). Occurrence of polyneuropathy was greatest in the 33 patients with amyloidosis (HR, 7.78; 95% CI, 4.30-14.0; P<.001); 18 patients (55%) with amyloidosis developed polyneuropathy. Of the 273 patients without amyloidosis, 35 (13%) developed polyneuropathy.
Seizures occurred in 12 patients (4%). None of these 12 patients developed seizures while taking sirolimus (95% CI for the HR of sirolimus for development of seizures, 0.00-6.89; P=.41). Three patients developed cyclosporine-based posterior reversible encephalopathy syndrome. None of these patients were taking sirolimus at the time of development of complaints compatible with this disease.
DISCUSSION
Sirolimus use does not cause major neurotoxicity in patients who undergo a single heart transplant or transplant combined with other viscera. This finding in 116 patients adds to an earlier experience involving 202 kidney and liver transplant recipients treated with sirolimus from 2001 to 2004.2 However, the follow-up in kidney and liver transplant recipients was brief (range, 15 days to 3 years), and the rate of neurologic complications was much lower compared with the current heart transplant cohort (6% vs 81%). The HR of sirolimus for occurrence of any neurologic event was approximately 2 but was not significant. This HR was driven mainly by the association between sirolimus therapy and the development of tremor and depression. Tremor can be regarded as a minor adverse neurologic effect and was reported during initial evaluation of the drug.7 Depression occurred frequently after heart transplant and was predictive of mortality (HR, 1.70; 95% CI, 0.99-2.91; P=.06).6 An association between sirolimus therapy and depression has not been described previously and is not likely to be causal. In contrast, treatment with the mammalian target of rapamycin inhibitor had antidepressant-like effects in 2 animal models of depression.8
We found 3 cases of posterior reversible encephalopathy syndrome, 1 of the major central nervous system complications after any type of transplant, but none of the patients were treated with sirolimus. Therefore, we doubt that the prior reported cases of patients who underwent heart, lung, or bone marrow transplant can be attributed to everolimus or sirolimus therapy.3-5 In those 3 patients, 1 patient was also taking cyclosporine,3 1 presented with hypertension,4 and 1 had atypical magnetic resonance imaging abnormalities of posterior reversible leukoencephalopathy syndrome.5,9 Although sirolimus appears to be less neurotoxic than calcineurin-inhibiting agents, neurologic adverse effects do occur. Progressive multifocal leukoencephalopathy, sympathetic dystrophy, and calcineurin-inhibitor-induced pain syndrome have all been associated with sirolimus use.1,5,10-12
Most interestingly, we observed no cerebrovascular event during treatment with sirolimus. Sirolimus has strong antiatherogenic properties.1,13 It inhibits vascular smooth muscle proliferation, and studies in animals have found an association between sirolimus dose and regression of cardiac allograft vasculopathy.13 A recent study involving 29 cardiac transplant recipients showed that substituting a calcineurin inhibitor with sirolimus as primary immunosuppression attenuates cardiac allograft vasculopathy.14 Because heart transplant recipients are at high risk of cerebrovascular events, this strategy could have important implications.6 Sirolimus-based immunosuppression may well prevent or retard development of cerebrovascular complications after heart transplant. This preliminary observation needs to be confirmed in another dataset of heart transplant recipients and may require objective evidence of reduced atherosclerotic disease.
Our study has several limitations. First, our dataset included patients who underwent transplant from 1988 through 2006. Sirolimus therapy was first used in the Mayo Clinic heart transplant program in 2002. Cardiac transplant management has evolved and improved considerably over the years. However, occurrence of major neurologic complications has not decreased during that time frame.6 Second, we did not evaluate sirolimus dosing ranges or therapeutic drug levels. We found no instances of major sirolimus-related neurotoxicity precluding evaluation of dose-relationship adverse effects with sirolimus drug levels.
Despite linkage to the same binding protein as tacrolimus, the similarly sounding drugs sirolimus and everolimus do not inhibit calcineurin, and their structure and effects are considerably different.1,2 Sirolimus has been used as a main immunosuppressant drug and has been combined with calcineurin inhibitors to avoid calcineurin-associated nephrotoxicity and neurotoxicity.1 Nevertheless, major adverse events associated with sirolimus have been reported, such as interstitial pneumonitis, alveolar hemorrhage, and wound infection, making it a far from ideal immunosuppressive drug.1 Because the Mayo Clinic experience with use of sirolimus in heart, kidney, liver, and combined transplants in more than 300 patients showed no major instances of neurotoxicity, sirolimus should be considered a useful replacement drug for patients with severe calcineurin neurotoxicity.
CONCLUSION
No early or late episodes of major neurotoxicity occurred among 116 heart transplant recipients using sirolimus immunosuppression. The absence of stroke and transient ischemic attacks in these high-risk transplant recipients treated with sirolimus is notable but needs confirmation in future studies.
Footnotes
Dr van de Beek is supported by grants from the Meerwaldt Foundation and the Netherlands Organization for Health Research and Development (ZonMw); NWO-Rubicon grant 2006 (019.2006.1.310.001).
REFERENCES
- 1.Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs 2007;67(3):369-391 [DOI] [PubMed] [Google Scholar]
- 2.Maramattom BV, Wijdicks EF. Sirolimus may not cause neurotoxicity in kidney and liver transplant recipients. Neurology 2004;63(10):1958-1959 [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz A, Nolan C, Lis E, Castro-Malaspina H, Perales MA. Posterior reversible encephalopathy syndrome due to sirolimus. Bone Marrow Transplant 2007May;39(10):653-654 Epub 2007 Mar 26 [DOI] [PubMed] [Google Scholar]
- 4.Tsagalou EP, Anastasiou-Nana MI, Margari ZJ, Vassilopoulos D. Possible everolimus-induced, severe, reversible encephalopathy after cardiac transplantation. J Heart Lung Transplant 2007;26(6):661-664 [DOI] [PubMed] [Google Scholar]
- 5.Bodkin CL, Eidelman BH. Sirolimus-induced posterior reversible encephalopathy. Neurology 2007;68(23):2039-2040 [DOI] [PubMed] [Google Scholar]
- 6.van de Beek D, Kremers W, Daly RC, et al. Effect of neurologic complications on outcome after heart transplant. Arch Neurol. 2008;65(2):226-231 [DOI] [PubMed] [Google Scholar]
- 7.Rapamune (sirolimus) [package insert] Philadelphia, PA: Wyeth Laboratories; 2003. http://www.fda.gov/cder/foi/label/2004/21110scf018_rapamune_lbl.pdf Accessed February 6, 2009 [Google Scholar]
- 8.Cleary C, Linde JA, Hiscock KM, et al. Antidepressive-like effects of rapamycin in animal models: implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Res Bull 2008July;76(5):469-473 Epub 2008 Apr 3 [DOI] [PubMed] [Google Scholar]
- 9.Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210 [DOI] [PubMed] [Google Scholar]
- 10.McCalmont V, Bennett K. Progressive multifocal leukoencephalopathy: a case study. Prog Transplant 2007;17(2):157-160 [DOI] [PubMed] [Google Scholar]
- 11.Molina MG, Diekmann F, Burgos D, et al. Sympathetic dystrophy associated with sirolimus therapy. Transplantation 2008;85(2):290-292 [DOI] [PubMed] [Google Scholar]
- 12.Collini A, De Bartolomeis C, Barni R, Ruggieri G, Bernini M, Carmellini M. Calcineurin-inhibitor induced pain syndrome after organ transplantation. Kidney Int 2006October;70(7):1367-1370 Epub 2006 Sep 6 [DOI] [PubMed] [Google Scholar]
- 13.Poon M, Badimon JJ, Fuster V. Overcoming restenosis with sirolimus: from alphabet soup to clinical reality. Lancet 2002;359(9306):619-622 [DOI] [PubMed] [Google Scholar]
- 14.Raichlin E, Bae JH, Khalpey Z, et al. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation 2007December4;116(23):2726-2733 Epub 2007 Nov 19 [DOI] [PubMed] [Google Scholar]