Abstract
Although age is a major risk factor for stroke, physicians are often reluctant to use thrombolytic agents in those who are very old. No published study provides detailed information on the use of intravenous tissue plasminogen activator (tPA) in patients aged 90 years or older. We retrospectively reviewed the use of intravenous tPA for patients 90 years or older who were admitted with acute ischemic stroke to the hospital at 4 academic centers from March 1, 1999, to March 1, 2008. We reviewed the clinical features of the patients at presentation, complications, and outcomes. Twenty-two patients (11 women; median age, 93 years; range, 90-101 years) were identified who had received intravenous tPA for symptoms of acute ischemic stroke (average time to treatment, 143 minutes; range, 90-180 minutes; no tPA protocol violations; mean National Institutes of Health Stroke Scale score, 15; range, 5-28). Nearly all patients were highly functional at baseline (median modified Rankin Scale [mRS] score, 1; median Barthel Index score, 95), and all but one performed the activities of daily living with little or no assistance before stroke. By the 30-day follow-up, 2 patients (9%) had a favorable outcome (mRS score, 0-2) and 2 (9%) had moderate disability (mRS score, 3). Most patients died (n=10) or experienced severe disability, defined as an mRS score of 4 or 5 (n=5). Asymptomatic intracerebral hemorrhage occurred in 3 patients (14%) and was nonfatal. Most patients aged 90 years or older who received intravenous tPA for acute ischemic stroke had poor 30-day functional outcomes or died. Intravenous tPA treatment in this age group does not improve the outcome of ischemic stroke.
BI = Barthel Index; ICH = intracerebral hemorrhage; mRS = modified Rankin Scale; NIHSS = National Institutes of Health Stroke Scale; NINDS = National Institute of Neurological Disorders and Stroke; tPA = tissue plasminogen activator
People aged 85 years or older are the fastest growing segment of the older American population.1 One in every 9 baby boomers will live to at least the age of 90 years,2 and by 2050, it is estimated that there will be more than 55 million nonagenarians worldwide.3 Although age is a known risk factor for stroke, clinical trials have traditionally excluded4-9 or underrepresented10 patients older than 80 years. Intravenous tissue plasminogen activator (tPA) is the most beneficial therapy for emergent treatment of acute ischemic stroke.11 Detailed outcomes of intravenous tPA administration in patients aged 90 years or older have not been published.
PATIENTS AND METHODS
We reviewed the medical records of patients aged 90 years or older who were treated with intravenous tPA for an acute ischemic stroke from March 1, 1999, to March 1, 2008. Patients were admitted to 1 of 3 Mayo Clinic sites (Rochester, MN; Scottsdale, AZ; or Jacksonville, FL) or the University of Alberta (Edmonton, AB). Databases including patients who received tPA were searched by date of birth. Patients were also identified through admission logs from the neurology and neurosurgery intensive care units. All patients given tPA were admitted to the intensive care unit for at least 24 hours after thrombolysis, according to the protocols followed at each institution. International Classification of Diseases, Ninth Revision coding for ischemic stroke was also used to search and confirm that all patients who had received tPA had been identified. Baseline functioning and comorbidities were determined by the clinical assessments on record before stroke presentation and the history provided at admission. Stroke severity was measured using the National Institutes of Health Stroke Scale (NIHSS). Intravenous tPA protocol violations were identified using criteria from the National Institute of Neurological Disorders and Stroke (NINDS)4 study protocol and ECASS (European Cooperative Acute Stroke Study).5 Outcomes after intravenous tPA were quantitated by the Barthel Index (BI) and modified Rankin Scale (mRS) at 30- and 90-day follow-up. A favorable outcome was defined as an mRS score of2 or less. Additional outcome measures after administration of intravenous tPA were the presence of intracerebral hemorrhage (ICH) and discharge site (nursing home, assisted living facility, or independent living facility). When death occurred, cause and circumstances were noted.
RESULTS
A total of 22 patients (11 women; median age, 93 years; range, 90-101 years) received intravenous tPA for symptoms of acute ischemic stroke. Nearly all patients were highly functional at baseline (median mRS score, 1; BI, 95). Most patients (16 [73%]) performed their activities of daily living with little or no assistance before stroke. Comorbidities included hypertension (15 patients [68%]), atrial fibrillation (10 [45%]), symptomatic coronary artery disease (9 [41%]), congestive heart failure (6 [27%]), dyslipidemia (6 [27%]), Alzheimer dementia (1 [5%]), Lewy body dementia (1 [5%]), and mild cognitive impairment (1 [5%]).
The median NIHSS score on presentation was 16 (interquartile range, 11-19). More than half of the patients (14 [64%]) were severely affected at initial presentation (NIHSS score ≥15). The average time to treatment was 143 minutes (range, 90-180 minutes). All strokes were in the anterior circulation and deemed large vessel (17 [77%]), cardioembolic (3 [14%]), or indeterminate/cryptogenic (2 [9%]) in origin. There were no intravenous tPA protocol violations, and no differences in treatment protocols were identified among the 4 centers.
Asymptomatic ICH occurred in 3 patients (14%) and was detected incidentally on follow-up computed tomography of the head (2 patients) or magnetic resonance imaging of the brain (1 patient) within 36 hours of intravenous tPA administration.
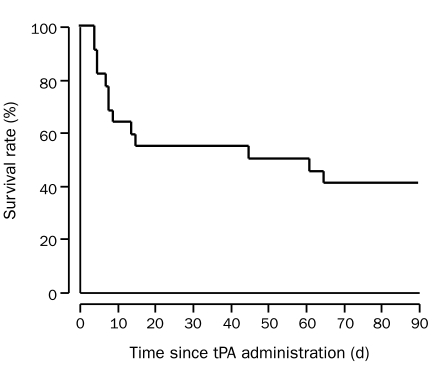
After 30 days, 2 patients (9%) had a favorable outcome, 2 (9%) experienced moderate disability (mRS score, 3), 6 (27%) experienced severe disability (mRS score, 4-5), and 10 (45%) died (mean mRS score increase, 3.5; Figure 1). The mRS score at 30 days was unavailable in 2 patients (9%), who were discharged from the hospital with an mRS score of 5. Although their mRS scores were unknown, both were alive 30 days after tPA administration. The median BI of surviving patients at 30-day follow-up was 20 (range, 0-90). All 6 patients with severe disability were discharged to a nursing home. The 2 patients with the highest prestroke mRS scores (mRS, 4) both died within 30 days. At 90 days, 9 patients (41%) were alive (Figure 2).
FIGURE 1.

Modified Rankin Scale (mRS) scores of patients aged 90 years or older before stroke (top bar) and 30 days after intravenous tissue plasminogen activator administration (bottom bar).
FIGURE 2.
Survival of patients aged 90 years or older after administration of intravenous tissue plasminogen activator (tPA) for acute ischemic stroke (n=22).
Poststroke survival in our cohort ranged from 4 days to 33 months. Information on the cause of death was available for all patients in the study. At the end of the study, 4 patients were alive, surviving 10, 24, 31, and 32 months after tPA administration.
Of the 14 patients (64%) with stroke evaluated using carotid artery ultrasonography, only 1 showed symptomatic stenosis. Of the 9 patients (41%) who underwent echocardiography (transesophageal, 6 [67%]; transthoracic: 3 [33%]), none experienced an intracardiac thrombus. Eight patients (36%) were in atrial fibrillation. Computed tomography of the head revealed evidence of infarction in all except one patient (21 [95%]). The 2 patients (9%) who underwent diffusion-weighted magnetic resonance imaging both had evidence of acute cerebral ischemia.
After tPA administration and hospitalization, 5 patients (23%) were given palliative care. For the 17 patients who died, the primary cause of death was the initial ischemic stroke (9 [53%]), aspiration pneumonia (3 [18%]), myocardial infarction (1 [6%]), or a second ischemic stroke more than 1 year later (1 [6%]). The cause of death was unknown or uncertain in 3 patients (17%), all of whom died after 90 days.
DISCUSSION
Good functional outcomes should not be expected when intravenous tPA is administered to nonagenarians with acute ischemic stroke. Our study cohort had higher 30- and 90-day mortality rates (55% and 59%, respectively) than those found in the available data for octogenarians11,12 and a similar mortality rate to that found in elderly patients with stroke who did not receive intravenous tPA.13 In contrast, a 90-day mortality rate of 32% to 40% is reported in the literature for intravenous tPA treatment of octogenarians after acute ischemic stroke.11,14
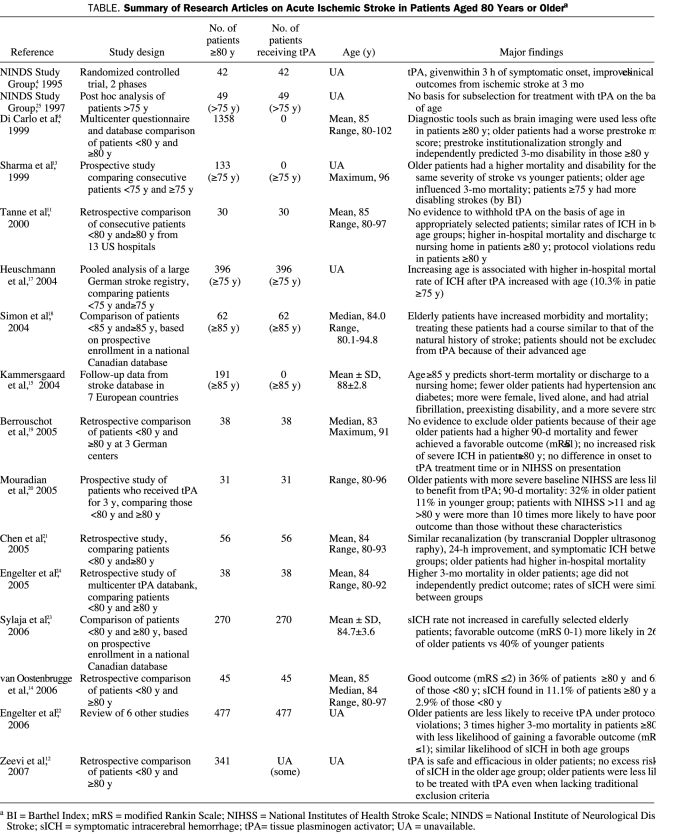
Class I data are absent for tPA administration in the elderly population4,11-25 (Table). In the NINDS trial,25 post hoc analysis of 49 patients older than 75 years found no support for subselection of patients for thrombolysis because of age. Nonetheless, tPA is not usually given to patients older than 80 years for reasons outside the traditional exclusion criteria.4 In some countries, intravenous tPA treatment is not recommended for elderly patients.
TABLE.
Summary of Research Articles on Acute Ischemic Stroke in Patients Aged 80 Years or Oldera
Previous studies have reported contradictory results on whether the rate of ICH is increased in carefully selected elderly patients. Notably, the high mortality rate in our study was not due to ICH, and tPA may be safer in this older age group than previously thought.
Withdrawal of support leading to respiratory failure was a major cause of death in our patients. Most of the patients who died did so as a direct result of the ischemic stroke. Treatment with tPA was not more likely than any other treatment to cause hemorrhage in the oldest old in our cohort. The rate of symptomatic ICH was reported to be 6.4% in the NINDS study,4 a cohort that included much younger patients. The lack of symptomatic ICH in our cohort may be due to the careful selection of patients aged 90 years or older for tPA administration.
Stroke severity is increased in the elderly population and is known to independently affect stroke outcomes.15 The patients in this study had more severe strokes (as defined by NIHSS scores) than those reported in other studies of younger cohorts, which suggests that the older group may a priori have worse outcomes with or without tPA.
This study has several limitations. First, it reports the results of a small cohort of elderly patients; however, currently it is the largest study of its kind. Other studies have included nonagenarians but have not focused on the oldest of the elderly patients. Second, because our analysis was retrospective, we were limited to the nonuniform decisions, investigations, and follow-up of clinicians at 4 different study centers. Finally, no comparison group was used. Age-matched patients with similar stroke severity who were not treated with thrombolytic agents were unavailable for comparison. Such a comparison would not have been ethical given the presumed benefits of tPA treatment in nonagenarians. Thus, our study did not allow us to arrive at conclusions about the efficacy of tPA treatment of stroke in nonagenarians. A randomized controlled trial of tPA treatment in elderly patients would be required.
The poor patient outcomes observed in this study were disconcerting because some recovery was expected and no intravenous tPA protocol violations occurred. Selection bias was likely because the final decision to administer tPA was made by the treating neurologist. However, the study patients presumably had the highest chance for improvement at the time of intravenous tPA administration.
CONCLUSION
Aggressive thrombolytic treatment with intravenous tPA is not futile in patients aged 90 years or older and is safe in carefully selected patients. However, functional outcomes are poor in most patients. In our study patients treated with intravenous tPA without protocol violations, 30-day survival was 55% and 90-day survival was 41%. Poor functional outcomes may also be related to the severity of the stroke on presentation.
REFERENCES
- 1.Hobbs FB. The elderly population. US Census Bureau Population Profile of the United States Web site http://www.census.gov/population/www/pop-profile/elderpop.html Accessed January 27, 2009
- 2.Takamura JC. US Department of Health and Human Services. Testimony on “The Graying of Nations.” Senate Special Committee on Aging Web site June8, 1998. http://www.hhs.gov/asl/testify/t980608b.html Accessed January 27, 2009
- 3.International Year of Older Persons: Demographics of Older Persons. United Nations Department of Public Information Web site September1999. http://www.un.org/NewLinks/older/99/older.htm Accessed January 27, 2009
- 4.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587 [DOI] [PubMed] [Google Scholar]
- 5.Hacke W, Kaste M, Fieschi C, et al. ECASS Study Group Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274(13):1017-1025 [PubMed] [Google Scholar]
- 6.Clark WM, Wissman S, Albers GW, et al. Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset: the ATLANTIS study: a randomized controlled trial. JAMA 1999;282(21):2019-2026 [DOI] [PubMed] [Google Scholar]
- 7.Clark WM, Albers GW, Madden KP, Hamilton S, Thrombolytic Therapy in Acute Ischemic Stroke Study Investigators The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study. Stroke 2000;31(4):811-816 [DOI] [PubMed] [Google Scholar]
- 8.Hacke W, Kaste M, Fieschi C, et al. Second European-Australasian Acute Stroke Study Investigators Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet 1998;352(9136):1245-1251 [DOI] [PubMed] [Google Scholar]
- 9.Hacke W, Kaste M, Bluhmki E, et al. ECASS Investigators Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329 [DOI] [PubMed] [Google Scholar]
- 10.Wardlaw JM, Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2003;3:CD000213 [DOI] [PubMed] [Google Scholar]
- 11.Tanne D, Gorman MJ, Bates VE, et al. Intravenous tissue plasminogen activator for acute ischemic stroke in patients aged 80 years and older: the tPA stroke survey experience. Stroke 2000;31(2):370-375 [DOI] [PubMed] [Google Scholar]
- 12.Zeevi N, Chhabra J, Silverman IE, Lee NS, McCullough LD. Acute stroke management in the elderly. Cerebrovasc Dis. 2007;23(4):304-308 Epub 2006 Dec 29 [DOI] [PubMed] [Google Scholar]
- 13.Sharma JC, Fletcher S, Vassallo M. Strokes in the elderly-higher acute and 3-month mortality—an explanation. Cerebrovasc Dis. 1999;9(1):2-9 [DOI] [PubMed] [Google Scholar]
- 14.van Oostenbrugge RJ, Hupperts RM, Lodder J. Thrombolysis for acute stroke with special emphasis on the very old: experience from a single Dutch centre. J Neurol Neurosurg Psychiatry 2006;77(3):375-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kammersgaard LP, Jørgensen HS, Reith J, Nakayama H, Pedersen PM, Olsen TS. Short- and long-term prognosis for very old stroke patients: Copenhagen stroke study. Age Ageing 2004;33(2):149-154 [DOI] [PubMed] [Google Scholar]
- 16.Di Carlo A, Lamassa M, Pracucci G, et al. European BIOMED Study of Stroke Care Group Stroke in the very old: clinical presentation and determinants of 3-month functional outcome: a European perspective. Stroke 1999;30(11):2313-2319 [DOI] [PubMed] [Google Scholar]
- 17.Heuschmann PU, Kolominsky-Rabas PL, Roether J, Misselwitz B, Lowitzsch K, German Stroke Registers Study Group Predictors of in-hospital mortality in patients with acute ischemic stroke treated with thrombolytic therapy. JAMA 2004;292(15):1831-1838 [DOI] [PubMed] [Google Scholar]
- 18.Simon JE, Sandler DL, Warwick Pexman JH, Hill MD, Buchan AM, Calgary Stroke Programme Is intravenous recombinant tissue plasminogen activator (rt-PA) safe for use in patients over 80 years old with acute ischaemic stroke?—the Calgary experience. Age Ageing 2004;33(2):143-149 [DOI] [PubMed] [Google Scholar]
- 19.Berrouschot J, Röther J, Glahn J, Kucinski T, Fiehler J, Thomalla G. Outcome and severe hemorrhagic complications of intravenous thrombolysis with tissue plasminogen activator in very old (≥80 years) stroke patients. Stroke 2005November;36(11):2421-2425 Epub 2005 Oct 6 [DOI] [PubMed] [Google Scholar]
- 20.Mouradian MS, Senthilselvan A, Jickling G, et al. Intravenous rt-PA for acute stroke: comparing its effectiveness in younger and older patients. J Neurol Neurosurg Psychiatry 2005;76(9):1234-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CI, Iguchi Y, Grotta JC, et al. Intravenous TPA for very old stroke patients. Eur Neurol. 2005;54(3):140-144 Epub 2005 Oct 18 [DOI] [PubMed] [Google Scholar]
- 22.Engelter ST, Bonati LH, Lyrer PA. Intravenous thrombolysis in stroke patients of ≥80 versus <80 years of age—a systematic review across cohort studies. Age Ageing 2006;35(6):572-580 [DOI] [PubMed] [Google Scholar]
- 23.Sylaja PN, Cote R, Buchan AM, Hills MD, Canadian Alteplase for Stroke Effectiveness Study (CASES) Investigators Thrombolysis in patients older than 80 years with acute ischaemic stroke: Canadian Alteplase for Stroke Effectiveness Study. J Neurol Neurosurg Psychiatry 2006July;77(7):826-829 Epub 2006 Feb 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelter ST, Reichhart M, Sekoranja L, et al. Thrombolysis in stroke patients 80 years and older: Swiss survey of IV thrombolysis. Neurology 2005December13;65(11):1795-1798 Epub 2005 Oct 12 [DOI] [PubMed] [Google Scholar]
- 25.NINDS t-PA Stroke Study Group Generalized efficacy of t-PA for acute stroke: subgroup analysis of the NINDS t-PA Stroke Trial. Stroke 1997;28(11):2119-2125 [DOI] [PubMed] [Google Scholar]