Abstract
Coronary artery disease (CAD) is the leading cause of morbidity and mortality in the United States, and a high concentration of low-density lipoprotein cholesterol (LDL-C) is a major risk factor for CAD. Current guidelines recommend the use of statins to lower LDL-C levels for the primary prevention of CAD based on an individual's risk factor profile and baseline LDL-C level. For moderaterisk individuals, those with 2 or more major risk factors for CAD and a Framingham risk score of 10% to 20%, the recommendation is to use a statin to lower LDL-C levels to less than 130 mg/dL. However, up to 40% of individuals who develop CAD have LDL-C levels lower than this cutoff. In 2004, the National Cholesterol Education Program Adult Treatment Panel III guidelines were updated to include an LDL-C goal of less than 100 mg/dL for individuals at moderately high risk of developing CAD. The guidelines identified several risk factors that when present would favor the use of pharmacological therapy to achieve this more aggressive LDL-C goal. This review evaluates the evidence supporting an LDL-C target of less than 100 mg/dL for moderately high-risk individuals and reviews those risk factors that when present help identify patients who would benefit from achieving this lower LDL-C goal. English-language publications in MEDLINE and references from relevant articles published between January 1, 1980, and November 30, 2008, were reviewed. Main keywords searched were coronary artery disease, hyperlipidemia, statins, cardiac risk factors, inflammatory markers, metabolic syndrome, and coronary artery calcium.
AFCAPS/TexCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study; AHA = American Heart Association; ASCOT-LLA = Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm; CAC = coronary artery calcium; CAD = coronary artery disease; CRP = C-reactive protein; HDL-C = high-density lipoprotein cholesterol; JUPITER = Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin; LDL-C = low-density lipoprotein cholesterol; MI = myocardial infarction; NCEP ATP III = National Cholesterol Education Program Adult Treatment Panel III; PROSPER = Prospective Study of Pravastatin in the Elderly at Risk
Cardiovascular disease, in particular coronary artery disease (CAD), is the leading cause of morbidity and mortality in both men and women of all racial groups in the United States.1 An elevated low-density lipoprotein cholesterol (LDL-C) level is a major risk factor for CAD, and several large, randomized, primary prevention trials have shown that lowering LDL-C levels with statins reduces the risk of major coronary events and coronary death.2-4 Furthermore, the reduction in CAD events is proportional to the reduction in LDL-C level achieved with statin therapy.5 The results of these trials support a strategy of “lower is better” and argue for more early treatment and more aggressive lowering of LDL-C levels for the primary prevention of CAD.
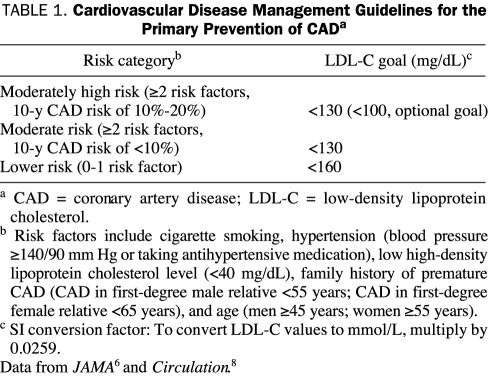
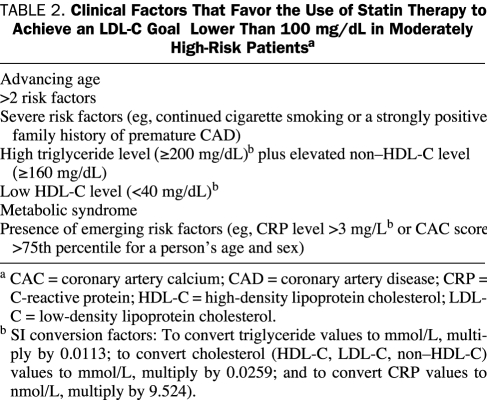
The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) and American Heart Association (AHA) guidelines recommend the use of statins for the primary prevention of CAD based on an individual's risk factor profile and LDL-C level.6,7 In 2004, the NCEP ATP III guidelines were updated and, for the primary prevention of CAD, individuals were stratified into 3 risk categories (Table 1).8 The major change in the updated guidelines for the primary prevention of CAD was the lowering of the LDL-C goal to an optional or more reasonable goal of less than 100 mg/dL (to convert to mmol/L, multiply by 0.0259) for individuals at moderately high risk of CAD (those with ≥2 major CAD risk factors and a 10-year risk of 10%-20%). The NCEP ATP III identified several clinical factors that would favor using a statin in this group to lower LDL-C levels to the more aggressive goal of less than 100 mg/dL (Table 2).8
TABLE 1.
Cardiovascular Disease Management Guidelines for the Primary Prevention of CADa
TABLE 2.
Clinical Factors That Favor the Use of Statin Therapy to Achieve an LDL-C Goal Lower Than 100 mg/dL in Moderately High-Risk Patientsa
For editorial comment, see page 307
These recommendations have important clinical implications because up to 40% of individuals who develop CAD have LDL-C levels that are lower than the more modest goal of less than 130 mg/dL that most physicians follow when initiating lipid-lowering therapy to prevent CAD.9 The rationale for the recommended LDL-C goal of less than 100 mg/dL is based on epidemiological and clinical outcome studies. The aim of this article is to review the clinical evidence and recent studies that support a more aggressive strategy of statin use to lower LDL-C levels to less than 100 mg/dL in patients at higher risk of developing CAD. English-language publications in MEDLINE and references from relevant articles published between January 1, 1980, and November 30, 2008, were reviewed. Main keywords searched were coronary artery disease, hyperlipidemia, statins, cardiac risk factors, inflammatory markers, metabolic syndrome, and coronary artery calcium. Articles were screened on the basis of importance, quality, and relevance to the aims of the review.
ADVANCING AGE AND SEVERE AND MULTIPLE RISK FACTORS
In older individuals, the LDL-C goal is lower because this population has a much greater burden of coronary atherosclerosis than younger individuals and therefore increased CAD morbidity and mortality. The prevalence of clinical CAD is almost 3-fold higher for individuals older than 60 years compared with younger individuals, and CAD is the leading cause of death among older individuals.1
Many healthy older individuals without clinical CAD have subclinical atherosclerotic disease and are at increased risk of a major cardiovascular event. In the Cardiovascular Health Study, a longitudinal, population-based study of persons 65 years and older, among 3584 individuals without clinical CAD, 61% of men and 49% of women were found to have subclinical cardiovascular disease.10 Those individuals had a high rate of cardiovascular events, with evidence of subclinical cardiovascular disease. In a follow-up period of 2.4 years, the cardiovascular event rate was 8.2% for men and 3.8% for women with subclinical cardiovascular disease, almost 3-fold higher than for individuals without subclinical cardiovascular disease. Therefore, older individuals have a high prevalence of subclinical cardiovascular disease, are at increased risk of CAD, and would be candidates for aggressive therapies, including lipid lowering, to prevent cardiovascular events.
PROSPER (Prospective Study of Pravastatin in the Elderly at Risk) was the first trial to examine the effects of statins in the treatment of elderly patients with or at high risk of developing CAD.11 In this study, 5804 men and women aged 70 to 82 years were randomized to pravastatin, 40 mg, or placebo. After a mean follow-up of 3.2 years, pravastatin was associated with a 15% relative reduction in the risk of the primary end point, coronary death, nonfatal myocardial infarction (MI), or fatal and nonfatal stroke, compared with placebo (P=.014). In addition, pravastatin was associated with substantial reductions in the risk of major coronary events and CAD mortality. The findings from PROSPER demonstrate the benefit of statin therapy in older individuals at risk of CAD.
The Framingham risk score is heavily weighted toward age, such that older men may have a calculated 10-year CAD risk score of 10% to 20% based solely on their age. Age should not be the only factor when deciding to initiate statin therapy to achieve an LDL-C goal of less than 100 mg/dL in older individuals. Intensive lowering of LDL-C levels should be reserved for older individuals who have additional CAD risk factors. In PROSPER, the greatest reduction in CAD events with statin therapy was seen in individuals in the lowest tertile for high-density lipoprotein cholesterol (HDL-C).11 In ASCOT-LLA (Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm), atorvastatin substantially reduced CAD risk in older patients with hypertension,3 who had on average 3 to 4 major risk factors for CAD. Emerging risk factors may also help to better assess CAD risk in an older individual. If an older patient has an elevated C-reactive protein (CRP) level or a high coronary artery calcium (CAC) score, the treating physician should consider initiation of intensive lipid-lowering therapy to aggressively reduce the level of LDL-C.
In addition to advancing age, the updated guidelines identified the presence of more than 2 risk factors or the presence of severe risk factors, such as continued cigarette smoking or a strongly positive family history of premature atherosclerotic cardiovascular disease, as factors that favor the use of a lipid-lowering drug to achieve the lower LDL-C goal of less than 100 mg/dL.8 Several prospective cohort studies have shown that most individuals who develop CAD have at least 1 major risk factor for CAD.12-14 Using data from the Framingham study and NHANES III (Third National Health and Nutrition Examination Survey), more than 90% of CAD events in the US population were predicted to occur in individuals with at least 1 risk factor for CAD. Furthermore, CAD rates increased for both men and women with the number of risk factors present.13 In the INTERHEART study, a large, international case-control study designed to assess the importance of coronary heart disease risk factors worldwide, the risk of clinical CAD also increased with the number of risk factors present. Cigarette smoking was an important clinical risk factor, accounting for 36% of the population's attributable risk of acute MI.14
It is clear from epidemiological studies that the risk of CAD increases with the number of risk factors present. The recommendation in the 2004 NCEP ATP III update for a lower LDL-C goal of less than 100 mg/dL in individuals with multiple or severe risk factors is based on data from the ASCOT-LLA trial.3 The ASCOT-LLA study included 10,305 patients with hypertension and at least 3 other risk factors but no prior CAD who were randomized to either placebo or atorvastatin, 10 mg/d. The study was planned for an average follow-up period of 5 years but was stopped early after a median follow-up of 3.3 years because of the markedly positive findings observed in the atorvastatin group. In both treatment groups, the mean LDL-C level at baseline was 131 mg/dL; however, at trial completion, atorvastatin lowered the LDL-C level by 29% to 88 mg/dL and reduced the risk of the primary end point of nonfatal MI and fatal CAD by 36% (P=.0005) compared with placebo.3 Atorvastatin also reduced the risk of fatal and nonfatal stroke by 27% (P=.024), total cardiovascular events by 21% (P=.0005), and total coronary events by 29% (P=.0005). The benefit of atorvastatin was independent of baseline total cholesterol levels. The results from ASCOT-LLA support treating persons with multiple cardiovascular risk factors and a 10-year CAD risk of 10% to 20% with a statin to achieve an LDL-C goal of less than 100 mg/dL.
FAMILY HISTORY OF PREMATURE CAD
The updated NCEP ATP III guidelines identified a strongly positive family history of premature atherosclerotic cardiovascular disease as a severe risk factor that, when present in moderately high-risk individuals, would favor the use of a lipid-lowering drug to achieve an LDL-C goal of less than 100 mg/dL. An individual with a first-degree male relative who had a CAD event before the age of 55 years or a first-degree female relative who had a CAD event before the age of 65 years is considered to have a strongly positive family history of premature atherosclerotic cardiovascular disease. Retrospective cohort studies have shown that a parental history of premature CAD increases the risk of CAD 2- to 3-fold,15,16 whereas a sibling history of CAD can increase CAD risk further.16
Several recent studies have observed that younger individuals with a family history of premature CAD have a high prevalence of subclinical coronary atherosclerosis. Michos et al17 studied 102 healthy young women who had a sibling history of premature CAD. Using electron beam computed tomography to assess for coronary calcium, 40% of these women had a high coronary calcium score. In SIRCA (Study of the Inherited Risk of Coronary Calcium), 52% of women and 78% of men with a family history of premature CAD, but without CAD or diabetes mellitus, had a high CAC score.18 We studied 89 younger men and women with a family history of premature CAD but a low Framingham risk score and found that 38% had a high CAC score.19 In MESA (Multi-Ethnic Study of Atherosclerosis), not only did individuals with a family history of premature CAD have a higher prevalence of CAC but also when present CAC was more advanced compared with individuals without a family history of premature CAD.20 Coronary artery calcifications have been shown to be an independent risk factor for cardiovascular events, and the higher the CAC score the greater the CAD risk.21
Although a family history of premature CAD is associated with a substantially increased CAD risk, LDL-C levels in individuals with a family history of premature CAD are usually not elevated. In MESA, the mean LDL-C level was 118 mg/dL among 1044 individuals with a reported family history of premature CAD, whereas in individuals without a family history of CAD, the mean LDL-C levels were similar at 117 mg/dL (P=.21).20 Using an LDL-C level of 130 mg/dL or greater as the threshold for initiating lipid-lowering therapy will deny many of these high-risk individuals the benefits of statin treatment.
METABOLIC SYNDROME, HIGH TRIGLYCERIDE LEVELS, AND LOW HDL-C LEVELS
The metabolic syndrome represents a constellation of lipid and nonlipid risk factors that together increase the risk of CAD independently of LDL-C levels.22,23 The NCEP ATP III defines the metabolic syndrome as a diagnosis of 3 or more of the following risk factors: waist circumference greater than 40 inches for men or greater than 35 inches for women, triglyceride levels of 150 mg/dL or higher (to convert to mmol/L, multiply by 0.0113), HDL-C levels of less than 40 mg/dL for men or less than 50 mg/dL for women (to convert to mmol/L, multiply by 0.0259), blood pressure of 130/85 mm Hg or higher, and fasting glucose level of 110 mg/dL or higher (to convert to mmol/L, multiply by 0.0555),7 although recent guidelines from the American Diabetes Association define impaired fasting glucose as a level of 100 mg/dL or higher.24 Retrospective analyses of primary prevention trials have shown that the metabolic syndrome is associated with an increased risk of developing CAD irrespective of LDL-C levels and that patients with the metabolic syndrome benefit from statin therapy. The AFCAPS/TexCAPS (Air Force/Texas Coronary Atherosclerosis Prevention Study) recruited 6605 men and women with dyslipidemia and no previous history of CAD who were randomized to lovastatin, 20 to 40 mg, or placebo. In placebo-treated patients, the metabolic syndrome was associated with a 40% increased risk of developing major coronary events. Furthermore, patients with the metabolic syndrome were at increased risk irrespective of their Framingham risk score.25 The WOSCOPS (West of Scotland Coronary Prevention Study) randomized 6595 men with hypercholesterolemia and no history of MI to pravastatin, 40 mg, or placebo. In a subanalysis of 6447 patients, the metabolic syndrome was associated with a 76% increased risk of CAD events, despite similar mean LDL-C levels in men with and without the metabolic syndrome. In addition, compared with placebo, pravastatin lowered the risk of CAD by 27% in men with the metabolic syndrome.26 These studies indicate that the metabolic syndrome predicts increased cardiovascular risk independently of the Framingham risk score and the level of LDL-C.
Individuals with the metabolic syndrome often have LDL-C levels in the normal range, and therefore their risk of CAD may be underestimated. This increased risk of CAD may be explained by their dyslipidemia, which is characterized by a high preponderance of small, dense LDL-C particles, along with high levels of triglycerides and low levels of HDL-C. The primary mechanism by which statins prevent cardiovascular disease is by lowering levels of LDL-C; however, statins also reduce triglyceride levels, have modest effects in increasing HDL-C levels, and are the most effective drug for lowering the concentration of small, dense LDL-C particles. In the NASDAC (New Atorvastatin Starting Doses: A Comparison) study, a post hoc analysis of almost 200 patients with hypertriglyceridemia (triglyceride level ≥200 mg/dL) showed that the LDL-C particle concentration decreased and the LDL-C particle size increased with each dose of atorvastatin. Higher doses more favorably changed particle size and concentration than did lower doses.27 Several statin trials have shown modest increases in HDL-C levels across a range of doses.2,4,28-31 A meta-analysis has revealed that for every 1-mg/dL increase in HDL-C, there is an estimated 2% to 4% decrease in cardiovascular risk, independent of other risk factors, including LDL-C level.32 Thus, the effects of lowering the concentration of small, dense LDL-C particles and triglycerides and increasing HDL-C levels may explain the benefit of statins in patients with the metabolic syndrome.
In AFCAPS/TexCAPS, which enrolled patients who had average levels of LDL-C but low HDL-C and elevated triglyceride levels, lovastatin reduced LDL-C levels by 25% and reduced the incidence of first acute major cardiovascular events by 37% compared with placebo (P<.001).2 Furthermore, similar benefit was observed in those individuals with lower compared to higher baseline LDL-C levels. This study adds further support to the finding that statin therapy can lower risk in individuals with the metabolic syndrome who have average levels of LDL-C. Thus, for individuals with the metabolic syndrome who are unable to achieve their lipid goals with lifestyle changes alone, use of lipid-lowering therapy to achieve an LDL-C goal of less than 100 mg/dL is an appropriate option to reduce their risk of CAD.
EMERGING RISK FACTORS
The updated NCEP ATP III guidelines identified 2 emerging risk factors that would favor an LDL-C goal of less than 100 mg/dL for the primary prevention of CAD in moderately high-risk individuals: elevated serum high-sensitivity CRP level greater than 3 mg/L (to convert to nmol/L, multiply by 9.524) and a coronary calcium level in the higher than 75th percentile for a person's age and sex.
Inflammation plays a key role in both the development and progression of atherosclerotic CAD. The most commonly used inflammatory marker in clinical practice is CRP, and large prospective studies among individuals with no history of cardiovascular disease have shown that an individual's baseline level of CRP is a strong and independent predictor of future cardiovascular events.33,34 The AHA has published guidelines identifying a CRP level less than 1 mg/L as low risk, a CRP level of 1 through 3 mg/L as moderate risk, and a CRP level of greater than 3 mg/L as high risk.35
In clinical practice, the most useful roles of CRP are to augment risk assessment and to identify individuals who would benefit from lipid-lowering therapy. Ridker et al33 measured CRP and LDL-C levels in 27,939 participants in the Women's Health Study. After adjusting for traditional risk factors, the risk of a first cardiovascular event increased 2.3-fold for women in the highest compared with the lowest quintile of CRP (P<.001). Furthermore, baseline CRP levels predicted future cardiovascular events more strongly than did baseline LDL-C levels. In another analysis of the Women's Health Study, CRP level and family history of CAD were added to traditional risk factors to assess cardiovascular risk. This new risk score (Reynolds Risk Score) was a more accurate predictor of cardiovascular risk, and it reclassified approximately 50% of low- and intermediate-risk women into either higher or lower risk categories.36
Individuals with elevated CRP levels but normal LDL-C levels are at increased risk of a cardiovascular event. In the Women's Health Study, almost half of all cardiovascular events occurred in women whose LDL-C level was less than 130 mg/dL. The cardiovascular risk of women with normal LDL-C levels but elevated levels of CRP was similar to that of women whose LDL-C levels were elevated.33 A post hoc analysis of the AFCAPS/TexCAPS trial showed that patients with LDL-C levels below the median (150 mg/dL) and CRP levels above the median (1.60 mg/L) were at increased risk of cardiovascular events and that lovastatin reduced the risk of a cardiovascular event by 42% compared with placebo in these patients.37 These studies suggest that individuals with normal LDL-C levels (<130 mg/dL) but elevated CRP levels are at increased risk of CAD and that statin therapy can reduce their high risk.
The recently published JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) was the first study to show that statin therapy could reduce cardiovascular morbidity and mortality among individuals with normal levels of LDL-C (<130 mg/dL) but elevated levels of CRP (>2.0 mg/L).38 JUPITER randomized 17,802 healthy men (≥50 years) and women (≥60 years) to rosuvastatin, 20 mg, or placebo. Among patients treated with rosuvastatin, LDL-C levels were reduced by 50% (from a median of 108 mg/dL at baseline to 55 mg/dL at 1 year), and CRP levels were reduced by 37% (from 4.2 mg/L at baseline to 2.2 mg/L at 1 year). JUPITER was designed as a 4-year trial but was stopped after 1.9 years because of a statistically significant 44% reduction in the primary end point: a composite of nonfatal MI, nonfatal stroke, hospitalization for unstable angina, revascularization, and cardiovascular death in the rosuvastatin-treated group (P<.00001). In addition, in patients treated with rosuvastatin, there was a statistically significant 55% reduction in nonfatal MI (P=.002), a 48% reduction in the risk of stroke (P=.002), and a 47% reduction in the combined end point of MI, stroke, or cardiovascular death (P<.00001). This benefit was seen across the subgroups of study patients. Among the 6801 women included in JUPITER, rosuvastatin reduced the risk of major cardiovascular events by 46%. The investigators also reported a benefit of rosuvastatin among lower-risk individuals, including nonsmokers, those without the metabolic syndrome, and those with a Framingham risk score of less than 10%.
In a meta-analysis of the primary prevention statin trials before JUPITER, the number of patients needed to be treated to prevent 1 major CAD event during an average of 4.3 years was 60.39 In JUPITER, the number needed to treat for 4 years was 31, and if the risk was projected for an average of 5 years as done in previous statin trials, the number needed to treat to prevent 1 major CAD event decreased to 25.38 The more intensive lowering of LDL-C levels that was achieved in JUPITER compared with most previous primary prevention statin trials may explain the greater benefit observed in that study.
The findings from JUPITER will expand the population of patients who would benefit from intensive LDL-C level lowering with statin therapy. JUPITER enrolled individuals not typically considered for LDL-C-lowering therapy: those whose LDL-C levels fall below the threshold to initiate LDL-C-lowering therapy for primary prevention. Although participants in JUPITER had levels of LDL-C in the normal range, they were at increased risk of CAD because of their high levels of CRP. The median CRP level was 4.2 mg/L in the rosuvastatin group, and most patients had CRP levels that would be classified as high risk according to the AHA guidelines (>3.0 mg/L).35 Whether individuals with lower levels of CRP will benefit from statin therapy remains unknown. The findings from JUPITER justify treating individuals (men >50 years and women >60 years) who have normal LDL-C levels with intensive statin therapy if they are at moderate risk and have elevated levels of CRP.
Coronary artery calcium can be measured by cardiac computed tomography, and the amount of coronary calcium measured with this modality mirrors the extent of coronary atherosclerosis. Multiple studies have shown that the amount of CAC, reported typically as a CAC score, predicts CAD events beyond standard risk factors and can predict CAD risk better than the Framingham risk score.21 In the St Francis Heart Study, CAC scoring was performed in 4903 healthy individuals who were then followed up for an average of 4.3 years for a combined outcome of all coronary events, nonhemorrhagic stroke, and peripheral vascular surgery.40 The CAC score predicted CAD events independently of standard risk factors and was superior to the Framingham risk score in the prediction of CAD events. Compared with individuals with CAC scores less than 100, individuals with CAC scores of 100 or higher had a 9.2- to 11.1-fold increased risk of all cardiovascular events, all CAD events, and the composite end point of coronary death and nonfatal MI. These relative risks were substantially higher than values typically reported for other cardiovascular risk factors.
Before publication of the updated NCEP ATP III guidelines in 2004, the Prevention Conference V and the American College of Cardiology and AHA reported that measuring CAC is of value in patients found to be at intermediate risk to improve the accuracy of predicting their CAD risk.41,42 The NCEP ATP III guidelines support these recommendations, stating that in persons at intermediate risk of CAD, a high CAC score (>75th percentile for age and sex) identifies advanced coronary atherosclerosis and provides a rationale for more intensive lipid-lowering therapy to achieve the more aggressive LDL-C target of less than 100 mg/dL. However, the availability and cost of CAC scoring currently limit its use in the primary prevention of CAD.
DISCUSSION
The current evidence supports a strategy of early and aggressive lowering of LDL-C levels for the primary prevention of CAD. Cohen et al43 studied a group of individuals with nonsense mutations in the PCSK9 gene that caused low levels of LDL-C. Although their LDL-C level was only 28% lower than the population without the PCSK9 mutation, their CAD risk was 88% lower. The implications from that study are that a low level of LDL-C throughout life is associated with a very low risk of CAD. Lowering LDL-C levels with a statin also reduces CAD risk. In a large meta-analysis of 14 randomized primary prevention trials, statin treatment was associated with an overall 28% reduction in risk of first major coronary events.5 Accumulating evidence from clinical trials, including the recently published JUPITER, suggests that, in primary prevention, the lower the achieved LDL-C level, the lower the cardiovascular risk. Because many individuals who develop CAD have normal or mildly elevated levels of LDL-C that are lower than current LDL-C targets, there is a strong argument to use statins to decrease LDL-C levels to even lower targets in individuals at increased CAD risk.
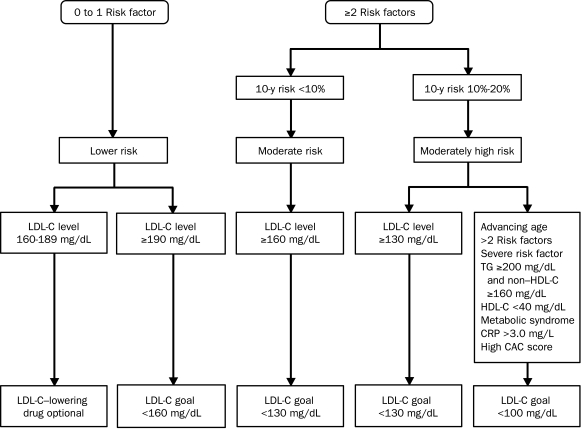
In 2004, the NCEP ATP III introduced a new optional LDL-C goal of less than 100 mg/dL for moderately high-risk individuals with 2 or more CAD risk factors and a 10-year risk of 10% to 20%.8 The presence of the following risk factors should favor the use of LDL-C-lowering drugs to decrease the level of LDL-C to well below 100 mg/dL (Figure): advancing age; multiple or severe risk factors, such as cigarette smoking or a strong family history of premature CAD; the metabolic syndrome; high triglyceride levels; low levels of HDL-C; high CRP levels; or an elevated CAC score (>75th percentile for a person's age and sex).
FIGURE.
Treatment algorithm for the primary prevention of coronary artery disease (CAD). The main changes in the updated National Cholesterol Education Program Adult Treatment Panel III guidelines are the lower low-density lipoprotein cholesterol (LDL-C) goal of less than 100 mg/dL for individuals with 2 or more CAD risk factors and a Framingham risk score of 10% to 20%. The presence of multiple or severe risk factors, an elevated level of C-reactive protein (CRP), or other risk factors identified in the updated guidelines justify more intensive LDL-C level lowering with drug therapy to achieve a lower LDL-C goal of less than 100 mg/dL. CAC = coronary artery calcium; HDL-C = high-density lipoprotein cholesterol; TG = triglycerides. SI conversion factors: To convert cholesterol (HDL-C, LDL-C) levels to mmol/L, multiply by 0.0259; to convert CRP values to nmol/L, multiply by 9.524; to convert TG values to mmol/L, multiply by 0.0113.
CONCLUSION
Physicians need to treat moderate-risk patients with lipid-lowering therapy to achieve a lower LDL-C goal of less than 100 mg/dL if the patients have multiple risk factors, an elevated CRP level, or other clinical risk factors identified in the updated NCEP ATP III guidelines. With increased adherence to these guidelines, opportunity exists to substantially reduce the incidence of a first cardiovascular event in higher-risk patients.
Acknowledgments
Editorial support was provided by Chris Cadman of Envision Pharma, a medical writer funded by Pfizer.
Footnotes
Dr Karalis has received research and grant support from Pfizer and Abbott Pharmaceuticals and is on the speaker's bureau for Pfizer, Abbott, Sanofi-Aventis, and Merck-Schering-Plough Pharmaceuticals.
REFERENCES
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008January;117(4):e25-e146 Epub 2007 Dec 17 [DOI] [PubMed] [Google Scholar]
- 2.Downs JR, Clearfield M, Weis S, et al. AFCAPS/TexCAPS Research Group Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA 1998;279(20):1615-1622 [DOI] [PubMed] [Google Scholar]
- 3.Sever PS, Dahlöf B, Poulter NR, et al. ASCOT Investigators Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361(9364):1149-1158 [DOI] [PubMed] [Google Scholar]
- 4.Shepherd J, Cobbe SM, Ford I, et al. West of Scotland Coronary Prevention Study Group Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333(20):1301-1307 [DOI] [PubMed] [Google Scholar]
- 5.Cholesterol Treatment Trialists' (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomized trials of statins [published corrections appear in Lancet. 2005;366(9494):1358 and 2008;371(9630):2084] Lancet 2005October8;366:(9493) 1267-1278 Epub 2005 Sep 27 [DOI] [PubMed] [Google Scholar]
- 6.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285(19):2486-2497 [DOI] [PubMed] [Google Scholar]
- 7.Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation 2002;106(3):388-391 [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Cleeman JI, Merz CN, et al. Coordinating Committee of the National Cholesterol Education Program Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines [published correction appears in Circulation. 2004;110(6):763] Circulation 2004;110(2):227-239 [DOI] [PubMed] [Google Scholar]
- 9.Rubins HB, Robins SJ, Collins D, et al. Department of Veterans Affairs HDL Intervention Trial Study Group Distribution of lipids in 8,500 men with coronary artery disease. Am J Cardiol. 1995;75(17):1196-1201 [DOI] [PubMed] [Google Scholar]
- 10.Kuller LH, Shemanski L, Psaty BM, et al. Subclinical disease as an independent risk factor for cardiovascular disease. Circulation 1995;92(4):720-726 [DOI] [PubMed] [Google Scholar]
- 11.Shepherd J, Blauw GJ, Murphy MB, et al. PROSPER Study Group Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360(9346):1623-1630 [DOI] [PubMed] [Google Scholar]
- 12.Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA 2003;290(7):891-897 [DOI] [PubMed] [Google Scholar]
- 13.Vasan RS, Sullivan LM, Wilson PW, et al. Relative importance of borderline and elevated levels of coronary heart disease risk factors [published correction appears in Ann Intern Med. 2005;142(8):681] Ann Intern Med. 2005;142(6):393-402 [DOI] [PubMed] [Google Scholar]
- 14.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364(9438):937-952 [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones DM, Nam BH, D'Agostino RB, Sr, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA 2004;291(18):2204-2211 [DOI] [PubMed] [Google Scholar]
- 16.Murabito JM, Pencina MJ, Nam BH, et al. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA 2005;294(24):3117-3123 [DOI] [PubMed] [Google Scholar]
- 17.Michos ED, Vasamreddy CR, Becker DM, et al. Women with a low Framingham risk score and a family history of premature coronary heart disease have a high prevalence of subclinical coronary atherosclerosis. Am Heart J. 2005;150(6):1276-1281 [DOI] [PubMed] [Google Scholar]
- 18.Valdes AM, Wolfe ML, Tate HC, Gefter W, Rut A, Rader DJ. Association of traditional risk factors with coronary calcification in persons with a family history of premature coronary heart disease: the study of the inherited risk of coronary atherosclerosis. J Investig Med. 2001;49(4):353-361 [DOI] [PubMed] [Google Scholar]
- 19.Sailam V, Karalis DG, Agarwal A, et al. Prevalence of emerging cardiovascular risk factors in younger individuals with a family history of premature coronary heart disease and low Framingham risk score. Clin Cardiol. 2008;31(11):542-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasir K, Budoff MJ, Wong ND, et al. Family history of premature coronary heart disease and coronary artery calcification: Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2007August7;116(6):619-626 Epub 2007 Jul 23 [DOI] [PubMed] [Google Scholar]
- 21.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006October17;114(16):1761-1791 Epub 2006 Oct 2 [DOI] [PubMed] [Google Scholar]
- 22.Sundström J, Risérus U, Byberg L, Zethelius B, Lithell H, Lind L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ 2006April15;332(7546):878-882 Epub 2006 Mar 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis 2004;173(2):309-314 [DOI] [PubMed] [Google Scholar]
- 24.Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance; implications for care. Diabetes Care 2007;30(3):753-759 [DOI] [PubMed] [Google Scholar]
- 25.Girman CJ, Rhodes T, Mercuri M, et al. 4S Group. AFCAPS/TexCAPS Research Group The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Am J Cardiol. 2004(2);93(2):136-141 [DOI] [PubMed] [Google Scholar]
- 26.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 2003July29;108(4):414-419 Epub 2003 Jul 14 [DOI] [PubMed] [Google Scholar]
- 27.Karalis DG, Ishisaka DY, Luo D, Ntanios F, Wun CC. Effects of increasing doses of atorvastatin on the atherogenic lipid subclasses commonly associated with hypertriglyceridemia. Am J Cardiol. 2007August1;100(3):445-449 Epub 2007 Jun 13 [DOI] [PubMed] [Google Scholar]
- 28.Pedersen TR, Faergeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial [published correction appears in JAMA. 2005;294(24):3092] JAMA 2005;294(19):2437-2445 [DOI] [PubMed] [Google Scholar]
- 29.Scandanavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344(8934):1383-1389 [PubMed] [Google Scholar]
- 30.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360(9326):7-22 [DOI] [PubMed] [Google Scholar]
- 31.Cannon CP, Braunwald E, McCabe CH, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators Intensive versus moderate lipid lowering with statins after acute coronary syndromes [published correction appears in N Engl J Med. 2006;354(7):778] N Engl J Med. 2004April8;350(15):1495-1504 Epub 2004 Mar 8 [DOI] [PubMed] [Google Scholar]
- 32.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation 1989;79(1):8-15 [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557-1565 [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men [published correction appears in N Engl J Med. 1997;337(5):356] N Engl J Med. 1997;336(14):973-979 [DOI] [PubMed] [Google Scholar]
- 35.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107(3):499-511 [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score [published correction appears in JAMA. 2007;297(13):1433] JAMA 2007;297(6):611-619 [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Rifai N, Clearfield M, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study Investigators Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344(26):1959-1965 [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008November20;359(21):2195-2207 Epub 2008 Nov 9 [DOI] [PubMed] [Google Scholar]
- 39.Thavendiranathan P, Bagai A, Brookhart MA, Choudhry NK. Primary prevention of cardiovascular diseases with statin therapy: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(21):2307-2313 [DOI] [PubMed] [Google Scholar]
- 40.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165 [DOI] [PubMed] [Google Scholar]
- 41.Smith SC, Jr, Greenland P, Grundy SM. Prevention Conference V. Beyond secondary prevention: identifying the high-risk patient for primary prevention: executive summary. Circulation 2000;101(1):111-116 [DOI] [PubMed] [Google Scholar]
- 42.O'Rourke RA, Brundage BH, Froelicher VF, et al. American College of Cardiology/American Heart Association expert consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation 2000;102(1):126-140 [DOI] [PubMed] [Google Scholar]
- 43.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264-1272 [DOI] [PubMed] [Google Scholar]