Abstract
Ezetimibe is a new lipid-lowering agent that inhibits intestinal absorption of dietary cholesterol. It substantially lowers low-density lipoprotein cholesterol levels when used alone or in combination with statins. However, its effect on cardiovascular mortality remains unknown. We reviewed peer-reviewed published literature on the effect of ezetimibe on different phases of atherosclerosis. MEDLINE, EMBASE, BIOSIS, and other Web of Knowledge databases were searched for relevant abstracts and articles published in the English language that compared ezetimibe and statins as modulators of atherosclerosis. On the basis of the available evidence, ezetimibe appears to reduce inflammation when used in combination with statins, but its effect on endothelial function is mixed and less clear. The effect of ezetimibe on coronary disease progression or prevention of cardiovascular events is currently unknown. Use of ezetimibe as a second- or third-line agent to achieve low-density lipoprotein cholesterol treatment goals seems appropriate on the basis of the available evidence.
CAD = coronary artery disease; CIMT = carotid artery intima-media thickness; CVD = cardiovascular disease; ENHANCE = Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression; FMD = flow mediated dilatation; FBF = forearm blood flow; HMG-CoA = 3-hydroxy-3-methylglutaryl coenzyme A; hs-CRP = high-sensitivity C-reactive protein; LDL = low-density lipoprotein; LDL-C = LDL cholesterol; NPC1L1 = Niemann-Pick C1-like 1 protein
Remarkable progress has been made in the understanding and treatment of blood lipid abnormalities in the past 3 decades. The development of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) in particular has led to substantial improvements in the treatment of lipid abnormalities. Statins improve cardiovascular disease (CVD) outcomes by lowering low-density lipoprotein cholesterol (LDL-C) levels1,2 and also by affecting the process of atherosclerosis through several possible nonlipid mechanisms, such as reduction of inflammation3 and reversal of endothelial dysfunction.4 In part because of concerns of the relatively common occurrence of adverse effects from statins, intense efforts are ongoing to develop effective and well-tolerated lipid-altering agents.
Ezetimibe is a new lipid-lowering agent that inhibits intestinal cholesterol absorption and substantially reduces LDL-C levels when used alone or in combination with statin therapy.5 However, the role of ezetimibe in lipid management has been debated in view of limited evidence defining the impact of ezetimibe on major adverse cardiac events. Proponents of the LDL-C level lowering position point out that ezetimibe would be expected to lower cardiovascular risk because it lowers LDL-C levels, and they cite many studies that have shown a reduction in cardiovascular events with any amount of reduction in LDL-C level, independent of the mechanism of that reduction.6 Others take a more conservative stance and suggest that ezetimibe should be used only as a second- or third-line agent until more evidence is available regarding the impact of ezetimibe on CVD events,7 particularly since evidence shows that an alternative therapy (statin therapy) has beneficial effects on CVD events. Until longer-term outcome studies of ezetimibe are available, these 2 viewpoints may not ever be reconciled, leaving physicians to judge the role of ezetimibe using currently available evidence. To help clarify the potential role of ezetimibe in lipid management and in risk reduction strategies, we reviewed published reports on ezetimibe and its impact on various steps in the process of atherosclerosis.
For editorial comment, see page 307
METHODS
We performed a computerized search to identify clinical trials that compared the effect of ezetimibe and statins as modulators of traditional CVD risk factors (lipid levels, blood pressure, glycemic control), novel risk factors (inflammation, thrombosis, lipid peroxidation), markers of subclinical atherosclerosis (coronary calcification, endothelial dysfunction, arterial intima-media thickness), and clinical events. MEDLINE (from 1966 to October 2008), EMBASE (from 1980 to October 2008), BIOSIS, Cochrane Collaborative databases, and Web of Knowledge databases (including www.clinicaltrials.gov and www.scopus.com) were searched for relevant journal articles. We also manually searched the references of cited articles for pertinent material. The following search terms were used: ezetimibe, statins or HMG-CoA reductase inhibitors, inflammation, high-sensitivity CRP (C-reactive protein), endothelial function/dysfunction or flow mediated dilatation (FMD), intimamedia thickness or IMT, lipid peroxidation, platelet aggregation, coronary calcification, blood pressure, and hyperglycemia or hypoglycemia. The search was restricted to articles and abstracts published in the English language. The abstracts of the cited articles were reviewed and summarized by 1 of the authors (F.J.A.) to determine relevance. All studies that were found by the authors to meet the criteria of our review were retrieved for further consideration. Studies were included in this review if they were from prospective trials, compared ezetimibe in one arm (alone or in combination with statins) with statins in the other arm (irrespective of the agent or dose used), and reported adequate data to allow comparison of both study arms on the end point in question. In addition, studies were included if they assessed the impact of ezetimibe on at least 1 of the pathway steps in the process of atherosclerosis. When an abstract from a meeting and a full article referred to the same trial, only the full article was included in the analysis. When there were multiple reports from the same trial, we used the most complete and/or most recently reported data. The quality of each study included in our review was individually evaluated by 1 of the authors (F.J.A.) using the criteria outlined by Jadad et al.8
MECHANISMS OF ACTION FOR EZETIMIBE
Ezetimibe is the first of a new class of highly selective cholesterol absorption inhibitors. Through a mechanism that is not yet fully elucidated, ezetimibe appears to block a protein transporter called Niemann-Pick C1-like 1 protein (NPC1L1)9 that is located at the apical membrane of the small intestine enterocytes. The result of ezetimibe's action on NPC1L1 in the small intestine is to decrease the absorption of dietary and biliary cholesterol in the small intestine and subsequently decrease the delivery of LDL to the liver.10 Increased clearance of plasma LDL through the liver ensues through up-regulation of LDL receptors on the surface of hepatocytes.11 Statins lower serum LDL levels through up-regulation of LDL receptors in the liver, albeit through a different mechanism (inhibition of HMG-CoA reductase). In addition, statins reduce serum LDL levels by reducing hepatic cholesterol production. Furthermore, NPC1L1 is expressed in human hepatocytes and is similarly blocked by ezetimibe. However, the clinical effects and possible off-target effects of the interaction between ezetimibe and hepatic NPC1L1 are unclear.
EVIDENCE OF EZETIMIBE'S IMPACT ON ATHEROSCLEROSIS
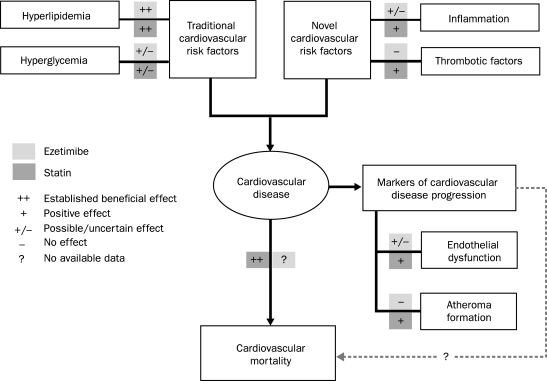
To help clarify the potential clinical role of ezetimibe based on currently available evidence, particularly its role in CVD risk reduction, one needs to consider the published evidence regarding ezetimibe's impact on the major risk factors and pathophysiologic steps in the process of atherosclerosis (Figure).
FIGURE.
Impact of ezetimibe and statin therapy on various steps in the process of atherosclerosis.
TRADITIONAL CVD RISK FACTORS
Effects on Blood Lipids
Ezetimibe substantially reduces LDL-C levels (-17.2% to -22.3%) when used alone compared with placebo.12,13 When used in combination with different statins, an additive reduction in LDL-C levels14,15 is observed (-6% to -20%), along with favorable changes in high-density lipoprotein cholesterol, triglycerides, and apolipoprotein B100 levels in hyperlipidemic patients.11
Effects on Other Traditional CVD Risk Factors
Limited studies have suggested a potential beneficial effect of ezetimibe on glucose metabolism in both animal16 and human17 models, although the results are somewhat mixed18 and preliminary. Whether ezetimibe therapy is associated with a reduced incidence of type 2 diabetes mellitus is unclear. No published reports could be identified that assessed the potential impact of ezetimibe therapy on other traditional CVD risk factors, including blood pressure and obesity.
The limited data on ezetimibe's effects on glucose and blood pressure mirror a similarly unclear picture of the effect that statins have on these factors. Some limited reports suggest that pravastatin is associated with a lower incidence of diabetes19 and a neutral effect on glycemic indices.20 In contrast, atorvastatin use led to worse blood glucose control compared with pravastatin.21
NOVEL CVD RISK FACTORS
Several investigators have studied the potential impact of ezetimibe on novel CVD risk factors, including its effect on inflammatory markers, thrombotic factors, and lipid peroxidation.
Inflammation
Inflammation is recognized as a major component of the process of atherogenesis and a contributor to plaque rupture.22,23 Higher levels of circulating markers of systemic inflammation, mainly high-sensitivity (hs) CRP, are associated with an increased risk of myocardial infarction and ischemic stroke in asymptomatic patients24 and a heightened risk of major adverse cardiac events in those with established disease.25 Measurement of hs-CRP levels in patients with an intermediate 10-year risk of CVD appears to be helpful in further risk stratification of such patients.26 A recently published study,27 JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin), found that statin therapy lowers cardiovascular risk in persons with elevated hs-CRP levels. However, debate continues regarding the most appropriate role for hs-CRP as a risk indicator, particularly in its potential role as a target of preventive therapies.28
Key findings from published reports of ezetimibe therapy and inflammation are as follows. First, several studies have shown that ezetimibe monotherapy produced overall a modest, nonsignificant reduction in hs-CRP levels compared with placebo29 (7.4% vs -2.8%). Pearson et al30 pooled data from 3 randomized controlled trials5,31,32 that compared the efficacy and safety of ezetimibe-simvastatin combination therapy relative to simvastatin monotherapy. In 2541 hyperlipidemic men and women, ezetimibe-simvastatin combination therapy was more effective in lowering hs-CRP levels than simvastatin alone (-31% vs -14.3%; P<.001). This effect was noticed across all available simvastatin dosages, with an additional 14.1% to 19.4% reduction in hs-CRP levels with 10 mg of ezetimibe combined with any simvastatin dose. In contrast, in a small study of 40 hyperlipidemic patients, Efrati et al33 reported that adding ezetimibe to ongoing therapy with 40 mg of simvastatin was less effective in reducing the hs-CRP level than doubling the statin dose. The dissimilar results in these 2 studies may be due to the differences in study design, particularly the smaller number of participants in the latter study.
Second, Ballantyne et al34 compared atorvastatin-ezetimibe combination therapy with atorvastatin alone and reported an overall larger reduction in hs-CRP levels with the combination therapy compared with atorvastatin monotherapy (-41% vs -31%; P<.01). Unlike the findings in the aforementioned simvastatin studies, addition of ezetimibe produced an incremental reduction in the hs-CRP level with only the higher 80-mg atorvastatin dose (-62% vs -43%; P<.01) but not with the lower 10-mg dose (-25% vs -27%), despite the consistent benefit observed with LDL-C level lowering across the whole dosing range of atorvastatin.
Third, ezetimibe-simvastatin combination therapy was also compared with atorvastatin monotherapy.35 Investigators observed no further reduction in hs-CRP levels with the addition of ezetimibe to simvastatin compared with the corresponding dose of atorvastatin in 1902 patients with above-target LDL-C values. They concluded that the reduction in hs-CRP levels was similar in the 2 treatment groups (24.8% vs 25.1%). This conclusion should be interpreted with caution because the degree of reduction in LDL-C levels differed between the treatment groups.36 When comparing dosages that achieved equivalent reductions in LDL-C levels, atorvastatin alone produced greater reductions in hs-CRP levels than ezetimibe-simvastatin combination therapy.
Fourth, Catapano et al37 reported a greater reduction in LDL-C levels with ezetimibe-simvastatin combination therapy relative to rosuvastatin but a similar change in hs-CRP levels in both groups. A study by Ballantyne et al38 compared rosuvastatin monotherapy to rosuvastatin-ezetimibe combination therapy and found a significant reduction in hs-CRP levels with the combination therapy compared with statin monotherapy (-46% vs 29%; P<.001).
In summary, currently available data suggest that ezetimibe may have a synergistic effect on hs-CRP levels when combined with statins, a finding that is consistent with the results of a recent meta-analysis.39 However, the mechanism of this effect and the interaction of ezetimibe with different statins are still in need of clarification. In addition, the high correlation between the change in LDL-C levels and that in hs-CRP levels suggests that most of the anti-inflammatory effect of LDL-C-lowering therapies is related to the magnitude of change in LDL-C, rather than an LDL-independent effect of statins or other lipid-lowering therapies. The clinical importance of pleiotropic benefits of statins and ezetimibe in prevention of vascular disease has not been firmly elucidated.
Thrombotic Factors
Patients with hyperlipidemia have increased platelet aggregation,40 which may contribute to CVD risk in these patients. Although therapy with statins has been associated with reduced platelet aggregation through a mechanism that is LDL independent,41 data from 2 studies suggest no such effect with ezetimibe therapy. Piorkowski et al42 showed that atorvastatin (40 mg) produced greater reduction in markers of platelet activation than atorvastatin (10 mg) combined with ezetimibe, 10 mg/d, in patients with stable coronary artery disease (CAD) despite achieving a similar LDL-C reduction in both groups. Similar findings were also reported by Hussein et al43 despite considerable methodological variation in the 2 studies.
Lipid Peroxidation
Oxidation of LDL particles has been identified as an early step in the process of atherosclerosis. Oxidized LDL is less likely to be taken up by hepatic LDL receptors and more likely to be taken up by monocytes in the arterial wall. This latter phenomenon initiates a cascade of events that results ultimately in endothelial injury and dysfunction.44 Statins have been reported to have a possible positive effect on LDL oxidation.45 In one report,43 ezetimibe lowered the peroxidation tendency of LDL in 22 hyperlipidemic patients. In addition, ezetimibe therapy was shown to reduce the serum level of oxidized cholesterol significantly (>50%) in 7 healthy volunteers fed an oxidized cholesterolrich diet.46 The clinical importance of this observation is unknown.
SUBCLINICAL MARKERS OF ATHEROSCLEROSIS
Various markers of atherosclerosis have been developed to help identify otherwise healthy individuals who show evidence of early atherosclerosis and who are at risk of future CVD events. Such markers include noninvasive measurement of carotid artery intima-media thickness (CIMT), endothelial function, arterial stiffness, coronary calcification, and ankle-brachial index. Limited available data on the impact of ezetimibe on measures of subclinical atherosclerosis are described subsequently.
Carotid Artery Intima-Media Thickness
Numerous studies have reported that CIMT is associated with the risk of CVD,47 and CIMT has been used in several studies as a subclinical marker of atherosclerosis48 and as a surrogate end point of CVD.49 In addition, serial CIMT measurements, as a marker of CVD progression and/or regression, were used to study the effect of various interventions on CVD.50-61 The advent of more advanced imaging techniques and software has made this imaging modality even more attractive in accurately detecting and quantifying changes in CIMT.
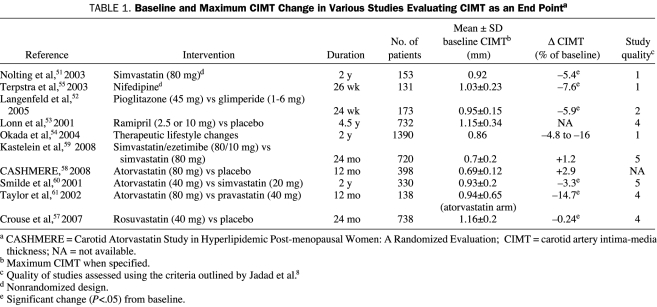
Data are limited on ezetimibe and changes in CIMT. One recent study that used CIMT measurements to assess the impact of ezetimibe therapy on atherosclerosis produced as many questions as answers. In the ENHANCE (Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression) study,59 the effect of ezetimibe-simvastatin on CIMT was investigated by Kastelein et al in persons with familial hypercholesterolemia who were randomized to receive simvastatin, 80 mg, and either ezetimibe, 10 mg, or placebo. The primary end point was a change in CIMT after 24 months of treatment. At the conclusion of the study, the group receiving simvastatin-ezetimibe combination therapy had significantly reduced LDL-C (-39.1% vs 55.6%; P<.01) and hs-CRP (-49.2% vs -23.5%; P<.01) levels compared with the simvastatin group. However, despite the difference in LDL-C level lowering, the primary outcome, CIMT change, did not differ between the treatment groups (+0.0033 mm for simvastatin vs +0.0182 mm for simvastatin-ezetimibe; P=.15). Although these results suggest that ezetimibe did not promote additional CIMT improvement, 81% of the study participants were already receiving statin therapy before the start of the study. This fact may have affected CIMT stabilization and/or regression for study participants even before the study began, thus dampening the potential impact of ezetimibe on CIMT change during the study. In support of this concern, the mean maximum CIMT at baseline in participants in the ENHANCE study was only 0.70 mm, a value significantly smaller than the corresponding CIMT values from other studies in which a treatment benefit on CIMT was reported (Table 1).
TABLE 1.
Baseline and Maximum CIMT Change in Various Studies Evaluating CIMT as an End Pointa
Some commentators have questioned the use of CIMT as an end point in the ENHANCE study,62 using the results of the previously reported CASHMERE (Carotid Atorvastatin Study in Hyperlipidemic Post-menopausal Women: A Randomized Evaluation)58 to support this point. In CASHMERE, 398 postmenopausal women (average age, 56 years) were randomized to receive either atorvastatin, 80 mg, or placebo and were followed up for 12 months using CIMT as an outcome measure. Reportedly, no changes in atherosclerosis burden could be detected at the end of the study, even with the use of atorvastatin therapy. This study has several limitations: (1) it involved a small number of postmenopausal women with moderate hyperlipidemia, relatively high levels of high-density lipoprotein cholesterol, and low CIMT measures at baseline; (2) it had a high dropout rate in both the treatment and the placebo groups; (3) study duration was too short to detect changes in CIMT, especially in a population at low risk of progression of atherosclerosis; and (4) it has not yet been published in a peer-reviewed journal. Recently, a secondary analysis from SANDS (Stop Atherosclerosis in Native Diabetics Study63) was released.64 One-third of patients (Native Americans without prior CVD who had diabetes) randomized to an aggressive LDL-C goal of less than 70 mg/dL required ezetimibe to reach the treatment goal. Among patients in the aggressive arm, 62% of those who received ezetimibe plus a statin and 61% of those who received statin therapy only showed either no change or a reduction in CIMT at 36 months of follow-up compared with 39% of patients in the arm of a standard LDL-C goal of less than 100 mg/dL (P<.001). In this nonprespecified secondary analysis, the magnitude of LDL-C level lowering was similar in both groups of the aggressive arm: -32.3 in patients who received statin monotherapy and -31.1 in the group that received the statin-ezetimibe combination therapy. In addition, multivariate analysis showed that change in LDL-C level independently affected CIMT change, whereas ezetimibe use did not.
Endothelial Function and Arterial Stiffness
Since endothelial dysfunction is considered an early step in the current understanding of atherogenesis65 and a key player in plaque progression and rupture,66 detection of endothelial function impairment that predates the presence of clinically important plaque burden may help identify a subgroup of patients at higher risk of future development of cardiovascular events.67 Similarly, increased arterial stiffness is predictive of coronary disease and stroke even after adjustment for other CVD risk factors.68 Statin therapy has been reported to improve measures of arterial function.69-71 Several studies have also explored the potential impact of ezetimibe on arterial function.
Settergren et al72 demonstrated a comparable reduction in LDL-C and hs-CRP levels in patients with stable CAD and dysglycemia who were treated with either simvastatin (80 mg) or simvastatin (10 mg) and ezetimibe (10 mg) combination therapy. In addition, both groups had similar improvement in FMD, a measure of endothelial function, after 6 weeks of therapy (+0.9% vs +1.5%, P=.39). In another report,73 60 patients from a similar population were randomized according to their statin status. Statin-naive patients were randomized to receive either ezetimibe alone or atorvastatin, 40 mg, alone. Patients who were receiving a long-term dosage of simvastatin at 20 mg/d had 10 mg of ezetimibe added to their ongoing simvastatin therapy and those receiving 10 mg of atorvastatin were switched to 40 mg/d of atorvastatin. All patients received study medication for 4 weeks. Forearm blood flow (FBF) was measured by the venous occlusion plethysmography technique to assess endothelial function. Study investigators found that patients in the ezetimibe or simvastatin (20 mg)-ezetimibe combination groups had no improvement in their FBF after 4 weeks of therapy, whereas a statistically significant increase in FBF was noted among participants in the 2 atorvastatin groups (statin naive and statin exposed). They concluded that ezetimibe use in patients with stable CAD was not associated with improvement in endothelial function, whereas use of atorvastatin was associated with improved endothelial function.
Landmesser et al74 evaluated the role of ezetimibe on endothelial function in patients with heart failure by using FMD measurements, expressed as the percentage of dilatation of radial artery after relief of wrist arterial occlusion. Patients were randomized to receive either 10 mg of ezetimibe or 10 mg of simvastatin. At the end of 4 weeks, both groups had a similar reduction in LDL-C level (15.6% vs 15.4%), whereas only the patients receiving simvastatin had an improvement in endothelial function based on FMD improvements.
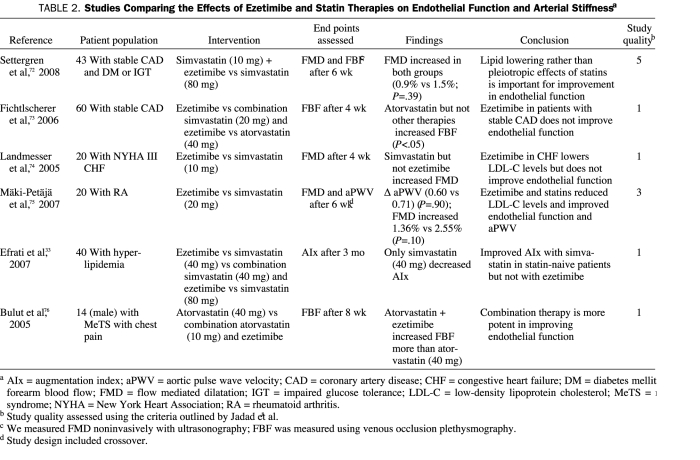
Mäki-Petäjä et al75 assessed changes in arterial function, using FMD and arterial stiffness, as measured by arterial pulse wave velocity in a cohort of patients with rheumatoid arthritis but no concomitant CVD, renal disease, or diabetes. Patients were randomized to receive either 10 mg of ezetimibe or 20 mg of simvastatin in a double-blind, crossover manner. Despite a larger reduction in LDL-C levels in the simvastatin group (-38.7% vs -17.9%; P=.001), patients in both groups had substantial improvement in arterial pulse wave velocity (-7.23% vs -7.40%) and FMD (37.2% vs 64.9%; P=.10). In contrast, Efrati et al33 found no improvement in augmentation index with ezetimibe use, either singly or in combination with simvastatin therapy. Table 2 summarizes the clinical studies that evaluated the effect of ezetimibe on arterial health, including an assessment of the quality of these studies using the criteria outlined by Jadad et al.8
TABLE 2.
Studies Comparing the Effects of Ezetimibe and Statin Therapies on Endothelial Function and Arterial Stiffnessa
In summary, studies with more rigorous methods72,75 showed comparable improvement in endothelial function and arterial stiffness with both ezetimibe monotherapy and combination therapy, whereas the studies with less rigorous methods did not.33,73,74 However, the latter group of studies has multiple methodological concerns that limit the strength of their conclusions. The results of the study by Mäki-Petäjä et al75 may not be applicable to the general population because it was exclusively performed in persons with rheumatoid arthritis, a condition that is in itself highly associated with inflammation and endothelial dysfunction.77
On the basis of currently available evidence, ezetimibe appears to have a positive, protective effect on endothelial function, although this association needs to be further confirmed by additional long-term clinical trials.
Other Measures of Atherosclerosis
Several other methods to assess subclinical CVD have been developed, including coronary calcification scanning and measurement of the ankle-brachial blood pressure index. In addition, quantifying changes in atherosclerotic burden over time is now feasible with intravascular ultrasonography, which has been used to assess CAD progression or regression. We identified no published studies that have addressed the effect of ezetimibe therapy on these markers of atherosclerosis.
Cardiovascular Events
To date, the long-term effect of ezetimibe on cardiovascular events is largely unknown. The SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) study,78 a placebo-controlled study designed primarily to assess the possible effect of intensive lipid lowering with simvastatin-ezetimibe combination therapy on aortic valve stenosis, showed a trend toward reduction in ischemic events (a secondary end point of the study) in the treatment group relative to placebo (15.7% vs 20.1%; P=.02) during a median follow-up of 52 months. However, it is possible that this reduction was due to the effects of simvastatin, not ezetimibe. IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a randomized, prospective, placebo-controlled clinical trial comparing the impact of simvastatin monotherapy with simvastatin-ezetimibe combination therapy on cardiovascular outcomes in patients with acute coronary syndromes, is currently under way and will shed more light on the role of ezetimibe in hyperlipidemia management when the results become available in 2012.
Adverse Reactions
Clinical efficacy trials of ezetimibe, which were not powered to detect differences in adverse events, have shown no increased incidence of ezetimibe-induced muscle or liver injury relative to placebo or statin monotherapy.79 Reports of serious ezetimibe-related myopathy80 and liver toxic effects81 exist in the literature, but to date it does not appear that ezetimibe exacerbates statin-induced myopathy.82 Recent reports have raised concerns about an association between ezetimibe and an increased incidence of cancer.78 However, analysis of pooled cancer data from 3 large ezetimibe trials found no sufficient evidence of an association between ezetimibe and cancer.83
CONCLUSION
Ezetimibe, a new lipid-lowering agent, can substantially lower LDL-C levels either alone or in combination with statin therapy. However, data are limited regarding the impact of ezetimibe on CVD morbidity and mortality. Until those data become available, the decision to use ezetimibe in a clinical role depends on extrapolation of studies that have assessed the impact of ezetimibe on important intermediate steps in the process of atherosclerosis.
Although ezetimibe lowers LDL-C levels, whether it affects any other traditional CVD risk factors is unclear. Limited reports suggest that ezetimibe may reduce the inflammatory process of atherosclerosis when used in combination with statin therapy, but ezetimibe appears to have no beneficial effects on thrombotic factors. Results of published studies on the effect of ezetimibe on markers of subclinical atherosclerosis are somewhat mixed. Studies assessing ezetimibe's impact on endothelial function and arterial stiffness have been generally positive. Data assessing the impact of ezetimibe on CIMT are limited.
On the basis of the limited published data, it appears appropriate to use ezetimibe as a second- or third-line agent while trying to achieve treatment targets of LDL-C. However, ezetimibe should be used with the understanding that evidence regarding its impact on the intermediate steps in the atherosclerotic pathway is somewhat mixed and that evidence regarding its impact on clinical cardiovascular events is lacking. It is hoped that longer-term studies, when they become available, will help clarify the impact of ezetimibe on cardiovascular morbidity and mortality. Until that time, the jury is still out regarding its true impact on CVD pathways and outcomes.
Footnotes
Dr Kopecky is a consultant for Bayer, Fibrex, ParinGenix, Pinnacle Care, Prime Therapeutics, and sanofi-aventis and is a member of the medical advisory board of Biophysical Corp. He has received research grant support from AtheroGenics, Integrium, and Reliant. Dr Thomas has received research grant support from Omron, Blue Cross and Blue Shield of Minnesota, and the Marriott Family Foundation.
REFERENCES
- 1.Corti R, Fayad ZA, Fuster V, et al. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation 2001;104(3):249-252 [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe JH, Bybee KA, Lavie CJ. Intensive lipid intervention in the post-ENHANCE era [editorial]. Mayo Clin Proc. 2008;83(8):867-869 [DOI] [PubMed] [Google Scholar]
- 3.Albert MA, Danielson E, Rifai N, Ridker PM, PRINCE Investigators Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001;286(1):64-70 [DOI] [PubMed] [Google Scholar]
- 4.Egashira K, Hirooka Y, Kai H, et al. Reduction in serum cholesterol with pravastatin improves endothelium-dependent coronary vasomotion in patients with hypercholesterolemia. Circulation 1994;89(6):2519-2524 [DOI] [PubMed] [Google Scholar]
- 5.Goldberg AC, Sapre A, Liu J, Capece R, Mitchel YB, Ezetimibe Study Group Efficacy and safety of ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2004;79(5):620-629 [DOI] [PubMed] [Google Scholar]
- 6.Ballantyne CM. Low-density lipoproteins and risk for coronary artery disease. Am J Cardiol. 1998;82(9A):3Q-12Q [DOI] [PubMed] [Google Scholar]
- 7.Lavie CJ, Milani RV, O'Keefe JH. Statin wars: emphasis on potency vs event reduction and safety [editorial]? Mayo Clin Proc. 2007;82(5):539-542 [DOI] [PubMed] [Google Scholar]
- 8.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17(1):1-12 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Calvo M, Lisnock J, Bull HG, et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci U S A 2005June7;102(23):8132-8137 Epub 2005 May 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudhop T, Lütjohann D, Kodal A, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 2002;106(15):1943-1948 [DOI] [PubMed] [Google Scholar]
- 11.Bays HE, Neff D, Tomassini JE, Tershakovec AM. Ezetimibe: cholesterol lowering and beyond. Expert Rev Cardiovasc Ther. 2008;6(4):447-470 [DOI] [PubMed] [Google Scholar]
- 12.Bays HE, Moore PB, Drehobl MA, et al. Ezetimibe Study Group Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies [published correction appears in Clin Ther. 2001;23(9):1601] Clin Ther. 2001;23(8):1209-1230 [DOI] [PubMed] [Google Scholar]
- 13.Knopp RH, Dujovne CA, Le Beaut A, Lipka LJ, Suresh R, Veltri EP, Ezetimbe Study Group Evaluation of the efficacy, safety, and tolerability of ezetimibe in primary hypercholesterolaemia: a pooled analysis from two controlled phase III clinical studies. Int J Clin Pract. 2003;57(5):363-368 [PubMed] [Google Scholar]
- 14.Gagné C, Gaudet D, Bruckert E, Ezetimbe Study Group Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation 2002;105(21):2469-2475 [DOI] [PubMed] [Google Scholar]
- 15.Melani L, Mills R, Hassman D, et al. Ezetimbe Study Group Efficacy and safety of ezetimibe coadministered with pravastatin in patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Eur Heart J 2003;24(8):717-728 [DOI] [PubMed] [Google Scholar]
- 16.Deushi M, Nomura M, Kawakami A, et al. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett. 2007December11;581(29):5664-5670 Epub 2007 Nov 20 [DOI] [PubMed] [Google Scholar]
- 17.Dagli N, Yavuzkir M, Karaca I. The effects of high dose pravastatin and low dose pravastatin and ezetimibe combination therapy on lipid, glucose metabolism and inflammation. Inflammation 2007December;30(6):230-235 Epub 2007 Aug 9 [DOI] [PubMed] [Google Scholar]
- 18.González-Ortiz M, Martinez-Abundis E, Kam-Ramos AM, Hernández-Salazar E, Ramos-Zavala MG. Effect of ezetimibe on insulin sensitivity and lipid profile in obese and dyslipidaemic patients. Cardiovasc Drugs Ther. 2006;20(2):143-146 [DOI] [PubMed] [Google Scholar]
- 19.Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001;103(3):357-362 [DOI] [PubMed] [Google Scholar]
- 20.Yamakawa T, Takano T, Tanaka S, Kadonosono K, Terauchi Y. Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb 2008;15(5):269-275 [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa M, Namiki A, Kubota T, et al. Effect of pravastatin and atorvastatin on glucose metabolism in nondiabetic patients with hypercholesterolemia. Intern Med. 2006;45(2):51-55 Epub 2006 Feb 15 [DOI] [PubMed] [Google Scholar]
- 22.Libby P. Inflammation in atherosclerosis. Nature 2002;420(6917):868-874 [DOI] [PubMed] [Google Scholar]
- 23.Paoletti R, Gotto AM, Jr, Hajjar DP. Inflammation in atherosclerosis and implications for therapy. Circulation 2004;109(23)(suppl 1):III20-III26 [DOI] [PubMed] [Google Scholar]
- 24.Koenig W, Sund M, Fröhlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation 1999;99(2):237-242 [DOI] [PubMed] [Google Scholar]
- 25.Zebrack JS, Anderson JL, Maycock CA, Horne BD, Bair TL, Muhlestein JB, Intermountain Heart Collaborative (IHC) Study Group Usefulness of high-sensitivity C-reactive protein in predicting long-term risk of death or acute myocardial infarction in patients with unstable or stable angina pectoris or acute myocardial infarction. Am J Cardiol. 2002;89(2):145-149 [DOI] [PubMed] [Google Scholar]
- 26.Musunuru K, Kral BG, Blumenthal RS, et al. The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat Clin Pract Cardiovasc Med. 2008October;5(10):621-635 Epub 2008 Aug 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008November20;359(21):2195-2207 Epub 2008 Nov 9 [DOI] [PubMed] [Google Scholar]
- 28.Donner-Banzhoff N, Sönnichsen A. Statins and primary prevention of cardiovascular events [editorial]. BMJ 2008;337:a2576 [DOI] [PubMed] [Google Scholar]
- 29.Sager PT, Melani L, Lipka L, et al. Ezetimbe Study Group Effect of coadministration of ezetimibe and simvastatin on high-sensitivity C-reactive protein. Am J Cardiol. 2003;92(12):1414-1418 [DOI] [PubMed] [Google Scholar]
- 30.Pearson T, Ballantyne C, Sisk C, Shah A, Veltri E, Maccubbin D. Comparison of effects of ezetimibe/simvastatin versus simvastatin versus atorvastatin in reducing C-reactive protein and low-density lipoprotein cholesterol levels. Am J Cardiol. 2007June15;99(12):1706-1713 Epub 2007 May 2 [DOI] [PubMed] [Google Scholar]
- 31.Bays HE, Ose L, Fraser N, et al. Ezetimbe Study Group A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther. 2004;26(11):1758-1773 [DOI] [PubMed] [Google Scholar]
- 32.Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40(12):2125-2134 [DOI] [PubMed] [Google Scholar]
- 33.Efrati S, Averbukh M, Dishy V, Faygenzo M, Friedensohn L, Golik A. The effect of simvastatin, ezetimibe and their combination on the lipid profile, arterial stiffness and inflammatory markers. Eur J Clin Pharmacol. 2007February;63(2):113-121 Epub 2007 Jan 3 [DOI] [PubMed] [Google Scholar]
- 34.Ballantyne CM, Houri J, Notarbartolo A, et al. Ezetimbe Study Group Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003May;107(19):2409-2415 Epub 2003 Apr 28 [DOI] [PubMed] [Google Scholar]
- 35.Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study [published correction appears in Am Heart J. 2005;149(5):882] Am Heart J 2005;149(3):464-473 [DOI] [PubMed] [Google Scholar]
- 36.Galin ID, Smith DA. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) Study [letter]. Am Heart J 2006;151(5):e1 [DOI] [PubMed] [Google Scholar]
- 37.Catapano AL, Davidson MH, Ballantyne CM, et al. Lipid-altering efficacy of the ezetimibe/simvastatin single tablet versus rosuvastatin in hypercholesterolemic patients. Curr Med Res Opin. 2006;22(10):2041-2053 [DOI] [PubMed] [Google Scholar]
- 38.Ballantyne CM, Weiss R, Moccetti T, et al. EXPLORER Study Investigators Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). Am J Cardiol. 2007March1;99(5):673-680 Epub 2007 Jan 4 [DOI] [PubMed] [Google Scholar]
- 39.Kinlay S. Low-density lipoprotein-dependent and -independent effects of cholesterol-lowering therapies on C-reactive protein: a meta-analysis. J Am Coll Cardiol. 2007May22;49(20):2003-2009 Epub 2007 May 4 [DOI] [PubMed] [Google Scholar]
- 40.Carvalho AC, Colman RW, Lees RS. Platelet function in hyper-lipoproteinemia. N Engl J Med. 1974;290(8):434-438 [DOI] [PubMed] [Google Scholar]
- 41.Haramaki N, Ikeda H, Takenaka K, et al. Fluvastatin alters platelet aggregability in patients with hypercholesterolemia: possible improvement of intraplatelet redox imbalance via HMG-CoA reductase. Arterioscler Thromb Vasc Biol. 2007June;27(6):1471-1477 Epub 2007 Mar 22 [DOI] [PubMed] [Google Scholar]
- 42.Piorkowski M, Fischer S, Stellbaum C, et al. Treatment with ezetimibe plus low-dose atorvastatin compared with higher-dose atorvastatin alone: is sufficient cholesterol-lowering enough to inhibit platelets? J Am Coll Cardiol. 2007March13;49(10):1035-1042 Epub 2007 Feb 23 [DOI] [PubMed] [Google Scholar]
- 43.Hussein O, Minasian L, Itzkovich Y, Shestatski K, Solomon L, Zidan J. Ezetimibe's effect on platelet aggregation and LDL tendency to peroxidation in hypercholesterolaemia as monotherapy or in addition to simvastatin. Br J Clin Pharmacol. 2008May;65(5):637-645 Epub 2008 Jan 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young IS, McEneny J. Lipoprotein oxidation and atherosclerosis. Biochem Soc Trans. 2001;29(pt 2):358-362 [DOI] [PubMed] [Google Scholar]
- 45.Oka H, Ikeda S, Koga S, Miyahara Y, Kohno S. Atorvastatin induces associated reductions in platelet P-selectin, oxidized low-density lipoprotein, and interleukin-6 in patients with coronary artery diseases. Heart Vessels 2008July;23(4):249-256 Epub 2008 Jul 23 [DOI] [PubMed] [Google Scholar]
- 46.Staprans I, Pan XM, Rapp JH, Moser AH, Feingold KR. Ezetimibe inhibits the incorporation of dietary oxidized cholesterol into lipoproteins. J Lipid Res. 2006November;47(11):2575-2580 Epub 2006 Aug 7 [DOI] [PubMed] [Google Scholar]
- 47.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146(6):483-494 [DOI] [PubMed] [Google Scholar]
- 48.Prati P, Tosetto A, Vanuzzo D, et al. Carotid intima media thickness and plaques can predict the occurrence of ischemic cerebrovascular events. Stroke 2008September;39(9):2470-2476 Epub 2008 Jul 10 [DOI] [PubMed] [Google Scholar]
- 49.Bots ML. Carotid intima-media thickness as a surrogate marker for cardiovascular disease in intervention studies. Curr Med Res Opin. 2006;22(11):2181-2190 [DOI] [PubMed] [Google Scholar]
- 50.Blankenhorn DH, Selzer RH, Crawford DW, et al. Beneficial effects of colestipol-niacin therapy on the common carotid artery: two- and four-year reduction of intima-media thickness measured by ultrasound. Circulation 1993;88(1):20-28 [DOI] [PubMed] [Google Scholar]
- 51.Nolting PR, de Groot E, Zwinderman AH, Buirma RJ, Trip MD, Kastelein JJ. Regression of carotid and femoral artery intima-media thickness in familial hypercholesterolemia: treatment with simvastatin. Arch Intern Med. 2003;163(15):1837-1841 [DOI] [PubMed] [Google Scholar]
- 52.Langenfeld MR, Forst T, Hohberg C, et al. Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus: results from a controlled randomized study. Circulation 2005May;111(19):2525-2531 Epub 2005 May 9 [DOI] [PubMed] [Google Scholar]
- 53.Lonn E, Yusuf S, Dzavik V, et al. SECURE Investigators Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation 2001;103(7):919-925 [DOI] [PubMed] [Google Scholar]
- 54.Okada K, Maeda N, Tatsukawa M, Shimizu C, Sawayama Y, Hayashi J. The influence of lifestyle modification on carotid artery intima-media thickness in a suburban Japanese population. Atherosclerosis 2004;173(2):329-337 [DOI] [PubMed] [Google Scholar]
- 55.Terpstra WF, May JF, Smit AJ, de Graeff PA, Crijns HJ. Effects of nifedipine on carotid and femoral arterial wall thickness in previously untreated hypertensive patients. Blood Press Suppl. 2003;1:22-29 [DOI] [PubMed] [Google Scholar]
- 56.Furberg CD, Adams HP, Jr, Applegate WB, et al. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Circulation 1994;90(4):1679-1687 [DOI] [PubMed] [Google Scholar]
- 57.Crouse JR, III, Raichlen JS, Riley WA, et al. METEOR Study Group Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA 2007;297(12):1344-1353 [DOI] [PubMed] [Google Scholar]
- 58.National Institutes of Health ClinicalTrials.gov Web site. Carotid Atorvastatin Study in Hyperlipidemic Post-menopausal Women http://clinicaltrials.gov/ct2/show/NCT00163163 Accessed March 2, 2009
- 59.Kastelein JJ, Akdim F, Stroes ES, et al. ENHANCE Investigators Simvastatin with or without ezetimibe in familial hypercholesterolemia [published correction appears in N Engl J Med. 2008;358(18):1977] N Engl J Med. 2008April3;358(14):1431-1443 Epub 2008 Mar 30 [DOI] [PubMed] [Google Scholar]
- 60.Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001;357(9256):577-581 [DOI] [PubMed] [Google Scholar]
- 61.Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation 2002;106(16):2055-2060 [DOI] [PubMed] [Google Scholar]
- 62.O'Keefe JH, Bybee KA, Lavie CJ. Is carotid intima-media thickness a reliable clinical predictor [reply]? Mayo Clin Proc. 2008;83(11):1300-1301 [DOI] [PubMed] [Google Scholar]
- 63.Howard BV, Roman MJ, Devereux RB, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA 2008;299(14):1678-1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fleg JL, Mete M, Howard BV, et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J Am Coll Cardiol. 2008;52(25):2198-2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J 1999;138(5, pt 2):S419-S420 [DOI] [PubMed] [Google Scholar]
- 66.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109(23, suppl 1):III27-III32 [DOI] [PubMed] [Google Scholar]
- 67.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation 2005;111(3):363-368 [DOI] [PubMed] [Google Scholar]
- 68.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 2006;113(5):657-663 [DOI] [PubMed] [Google Scholar]
- 69.Dupuis J, Tardif JC, Cernacek P, Théroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes: the RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation 1999;99(25):3227-3233 [DOI] [PubMed] [Google Scholar]
- 70.Raison J, Rudnichi A, Safar ME. Effects of atorvastatin on aortic pulse wave velocity in patients with hypertension and hypercholesterolaemia: a preliminary study. J Hum Hypertens 2002;16(10):705-710 [DOI] [PubMed] [Google Scholar]
- 71.Shinohara K, Shoji T, Kimoto E, et al. Effect of atorvastatin on regional arterial stiffness in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2005;12(4):205-210 [DOI] [PubMed] [Google Scholar]
- 72.Settergren M, Böhm F, Rydén L, Pernow J. Cholesterol lowering is more important than pleiotropic effects of statins for endothelial function in patients with dysglycaemia and coronary artery disease. Eur Heart J 2008July;29(14):1753-1760 Epub 2008 Apr 25 [DOI] [PubMed] [Google Scholar]
- 73.Fichtlscherer S, Schmidt-Lucke C, Bojunga S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for ‚pleiotropic‘ functions of statin therapy. Eur Heart J 2006May;27(10):1182-1190 Epub 2006 Apr 18 [DOI] [PubMed] [Google Scholar]
- 74.Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 2005May10;111(18):2356-2363 Epub 2005 May 2 [DOI] [PubMed] [Google Scholar]
- 75.Mäki-Petäjä KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007August28;50(9):852-858 Epub 2007 Aug 13 [DOI] [PubMed] [Google Scholar]
- 76.Bulut D, Hanefeld C, Bulut-Streich N, Graf C, Mügge A, Spiecker M. Endothelial function in the forearm circulation of patients with the metabolic syndrome—effect of different lipid-lowering regimens. Cardiology 2005;104(4):176-180 Epub 2005 Sep 7 [DOI] [PubMed] [Google Scholar]
- 77.Dhawan SS, Quyyumi AA. Rheumatoid arthritis and cardiovascular disease. Curr Atheroscler Rep. 2008;10(2):128-133 [DOI] [PubMed] [Google Scholar]
- 78.Rossebø AB, Pedersen TR, Boman K, et al. SEAS Investigators Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008September25;359(13):1343-1356 Epub 2008 Sep 2 [DOI] [PubMed] [Google Scholar]
- 79.Kashani A, Sallam T, Bheemreddy S, Mann DL, Wang Y, Foody JM. Review of side-effect profile of combination ezetimibe and statin therapy in randomized clinical trials. Am J Cardiol. 2008June1;101(11):1606-1613 Epub 2008 Apr 9 [DOI] [PubMed] [Google Scholar]
- 80.Fux R, Mörike K, Gundel UF, Hartmann R, Gleiter CH. Ezetimibe and statin-associated myopathy [letter]. Ann Intern Med. 2004;140(8):671-672 [DOI] [PubMed] [Google Scholar]
- 81.Castellote J, Ariza J, Rota R, Girbau A, Xiol X. Serious drug-induced liver disease secondary to ezetimibe. World J Gastroenterol. 2008;14(32):5098-5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slim H, Thompson PD. Ezetimibe-related myopathy: a systematic review. J Clin Lipidol. 2008;2(5):328-334 [DOI] [PubMed] [Google Scholar]
- 83.Peto R, Emberson J, Landray M, et al. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008September25;359(13):1357-1366 Epub 2008 Sep 2 [DOI] [PubMed] [Google Scholar]