Abstract
Substantial data have established a sedentary lifestyle as a major modifiable risk factor for coronary heart disease (CHD). Increased levels of physical activity, exercise training, and overall cardiorespiratory fitness have provided protection in the primary and secondary prevention of CHD. This review surveys data from observational studies supporting the benefits of physical activity, exercise training, and overall cardiorespiratory fitness in primary prevention. Clearly, cardiac rehabilitation/secondary prevention (CRSP) programs have been greatly underused by patients with CHD. We review the benefits of CRSP programs on CHD risk factors, psychological factors, and overall CHD morbidity and mortality. These data support the routine referral of patients with CHD to CRSP programs. Patients should be vigorously encouraged to attend these programs.
AHA = American Heart Association; CHD = coronary heart disease; CRF = cardiorespiratory fitness; CRSP = cardiac rehabilitation/secondary prevention; CV = cardiovascular; ET = exercise training; HF = heart failure; hs-CRP = high-sensitivity C-reactive protein; MET = metabolic equivalent; MetS = metabolic syndrome; MI = myocardial infarction; PA = physical activity; SCD = sudden cardiac death
And a healthy physical condition is spoiled by inactivity and inertia, but on the whole, is preserved by exercise.
Plato1
The American Heart Association (AHA) has established a sedentary lifestyle as a major modifiable risk factor for coronary heart disease (CHD).2 Unfortunately, despite overwhelming evidence promoting an active lifestyle, approximately 70% of US adults are sedentary or relatively inactive, and nearly half of young people are not regularly physically active.3
We review the benefits of physical activity (PA), exercise training (ET), and cardiorespiratory fitness (CRF) in primary and secondary CHD prevention, as well as the role of formal cardiac rehabilitation/secondary prevention (CRSP) services. The 1996 National Institutes of Health Consensus Conference Statement on Physical Activity and Cardiovascular Health defined PA as “bodily movement produced by skeletal muscle that requires energy expenditure and promotes health benefits.”4 Exercise or ET was defined as “planned, structured, and repetitive bodily movement done to improve or maintain one or more components of physical fitness.” Peak oxygen uptake or CRF, which can be estimated or directly measured,5 is defined as the capacity to take in and process oxygen for the production of energy via aerobic metabolism for PA or ET. Cardiorespiratory fitness is probably more highly associated with overall risk of CHD than are PA and ET. However, CRF is directly influenced by the performance of regular PA and ET and is also considerably affected by age, sex, and underlying chronic diseases, as well as by genetic factors.6
OBSERVATIONAL STUDIES AND PRIMARY CHD PREVENTION
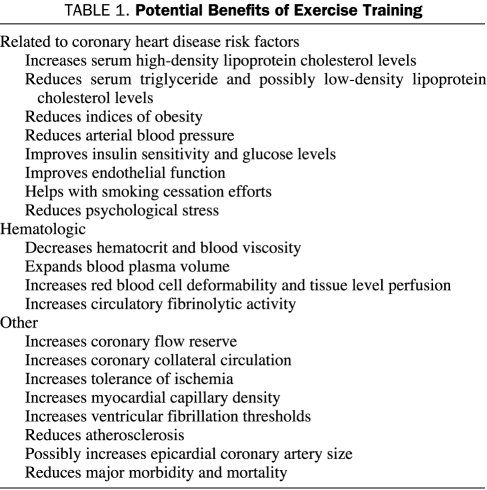
No randomized trials of the effects of PA and ET on the primary prevention of CHD or other cardiovascular (CV) outcomes have been conducted because of various methodological and ethical issues.7 However, substantial evidence from several lines of investigation supports the role of occupational and leisure-time PA and ET as well as CRF in reducing risk of CHD mortality. Observational studies and short-term ET studies suggest a causative role for physical inactivity and low CRF in the development of CV disease. The potential benefits of regular ET are listed in Table 1.
TABLE 1.
Potential Benefits of Exercise Training
Many studies have documented an inverse association between job-related PA and overall CHD risk. Among these studies, Taylor et al8 evaluated PA and CHD risk in over 190,000 US railroad workers and showed that death caused by atherosclerosis was more frequent in the relatively sedentary clerks (relative risk, 2.0) and moderately active switchmen (relative risk, 1.5) than in the section men who had high PA. Morris et al9 studied PA in 667 male London bus drivers and conductors and determined that CHD was much lower for the more active conductors than for the drivers (age-adjusted relative risk, 1.8). Paffenbarger and Hale10 followed up 6351 San Francisco longshoremen for 22 years and determined that death attributable to CHD was inversely associated with job caloric expenditure (relative risk, 1.8 for light PA and 1.7 for moderate PA vs heavy PA). However, these studies are now principally of historical interest because few jobs today require the level of PA described in these studies.
Many studies have examined the effect on CHD end points of varying levels of leisure-time PA. In an analysis of the Harvard Alumni Study, Paffenbarger and Hyde11 used an index of energy expenditure based on self-reported PA and ET in 16,936 men followed up from 1962 to 1978. After adjusting for age, smoking, and hypertension, they showed an inverse association between habitual PA and first myocardial infarction (MI), and they also noted a cardioprotective advantage for more vigorous ET over less strenuous PA. In a subgroup analysis of this cohort, participants who expended more than 1000 kcal/wk had a 20% lower CHD event rate than those who had less PA.12 It was later reported that, after adjusting for total energy expenditure, the duration of individual episodes of PA had no independent effect on CHD risk.13
Other groups have also noted the inverse association between PA and CHD risk. In an analysis of PA quantified by 48-hour recall of 17,944 British male civil servants followed up for an average of 8.5 years, Morris et al14 showed that the age-adjusted relative risk of CHD events for men reporting nonvigorous PA vs those reporting vigorous exercise was 2.2. In an analysis of 72,488 women followed up for 8 years in the Nurses' Health Study, a graded, inverse association existed between the amount of PA and CHD events.15 The multivariate adjusted relative risk for the women with the highest PA vs those with the lowest PA was 0.66. In the Women's Health Study, 39,372 health care professionals were followed up for 5 years; the multivariate adjusted relative risk for the most active women (>1500 kcal/wk) vs the least active women (<200 kcal/wk) was 0.75.16 Importantly, PA was protective for women with CHD risk factors, such as obesity, dyslipidemia, and cigarette smoking. Tanasescu et al17 studied 44,452 US men enrolled in the Health Professionals Follow-Up Study (HPFS) and confirmed a significant inverse dose-response association between total PA and risk of CHD. Moreover, these investigators found that running, rowing, and weight training were associated with reduced CHD risk, and higher intensity of PA was directly related to risk reduction. Therefore, they concluded that increasing the volume of PA, increasing the intensity of PA from low to moderate and from moderate to high, as well as adding weight training to other aerobic PA, were important to reduce CHD risk in men.
Several studies have assessed the effect of PA in older cohorts. Hakim et al18 studied 2678 men aged 71 to 93 years in the Honolulu Heart Program. During a 2- to 4-year follow-up, men who walked less than 0.25 miles per day, 0.25 to 1.5 miles per day, and more than 1.5 miles per day had mortality rates of 5.1%, 4.5%, and 2.5%, respectively. In a study of 73,743 postmenopausal women aged 50 to 79 years in the Women's Health Initiative Observational Study,19 both walking and more vigorous PA or ET were associated with statistically significant and similar reductions in CHD and CV events. Likewise, Kushi et al20 studied 40,417 postmenopausal Iowa women aged 55 to 69 years and showed that PA assessed by a mailed questionnaire was associated with a graded inverse association with all-cause mortality.
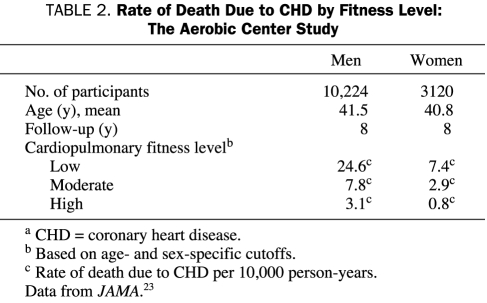
Perhaps the most impressive data on the association between PA and ET and CV risk comes from studies that estimate CRF by maximal graded treadmill stress testing. Although many investigators have used self-reported questionnaires to show the importance of PA on CV and CHD morbidity and mortality, Blair et al,21-23 who studied more than 13,000 men and women at the Cooper Clinic with approximately 8 years of follow-up, have shown that CRF as determined by maximal treadmill testing is a better predictor of prognosis than self-reported PA scores. Blair et al23 categorized patients by CRF as low, moderate, or high on the basis of treadmill exercise performance. Although relatively few CHD deaths occurred in this population of relatively young people, the CHD death rate decreased dramatically with increased levels of CRF in both sexes (Table 2). Although persons who are overweight or obese have lower levels of CRF than those who are lean, Wei et al24 showed that obese persons with at least moderate CRF have lower rates of CV disease and about half the all-cause mortality compared with unfit peers of normal weight. These results are remarkably similar regardless of which obesity indices are used (eg, body mass index, percent body fat, total fat mass, or waist circumference) and are consistent in both sexes and for men with type 2 diabetes mellitus.21,25-27 Moreover, Blair et al28 also showed that, relative to persons who remained unfit over time, persons who improved their CRF category over time experienced an age-adjusted relative risk of 0.48.
TABLE 2.
Rate of Death Due to CHD by Fitness Level: The Aerobic Center Study
Other groups have confirmed the association between CRF and CHD risk. In a cohort of 1960 Norwegian men who were followed up for an average of 16 years, a strong inverse association between CRF and CHD risk was found, with relative risks for quartiles 2, 3, and 4 of 0.59, 0.45, and 0.41, respectively, compared with men in the lowest CRF quartile.29 Roger et al30 followed up 1452 men and 741 women from Olmsted County, Minnesota, for approximately 6 years; after multivariate adjustment, only peak treadmill workload or estimated metabolic equivalents (METs) were associated with their outcome measures of cardiac death, nonfatal MI, and heart failure (HF). For every 1-MET increase in CRF, CV events were reduced by 25% in both sexes. Goraya et al31 followed up 3107 Olmsted County, Minnesota, residents for a median of 6 years and found that each 1-MET increase in CRF reduced CV events by 14% and 18% for younger and older (>65 years) persons, respectively. In a study of 2534 men without a history of CV disease and with a “normal” exercise treadmill result, Myers et al32 reported that each 1-MET increase in exercise capacity conferred a 16% improvement in survival. Gulati et al33 investigated a cohort of 5721 asymptomatic women who underwent a baseline treadmill test to assess CRF in 1992 and who were followed up until the end of 2000; they found that the Framingham risk score-adjusted mortality risk decreased by 17% for every 1-MET increase in CRF. From the same cohort, these authors established the prognostic value of a nomogram for exercise capacity in women; the risk of death among asymptomatic women whose exercise capacity was less than 85% of that predicted for their age was twice that among women whose exercise capacity was at least 85% of the age-predicted value (P<.0001).34 They also confirmed this effect in a referral population of 4471 additional women who underwent symptom-limited stress testing for CV symptoms. Meta-analyses of 24 studies have shown a powerful association between PA and overall CRF and major health outcomes.21,35
A detailed discussion of all factors to consider in clinical treadmill testing is beyond the scope of this review. Although clinicians often focus on whether findings on a stress test indicate ischemia or its absence on the basis of ST-segment responses or imaging modalities, many other factors, including heart rate recovery, heart rate reserve, and ventricular ectopy, strongly affect the prognostic value of this testing.36-38 Perhaps the most powerful predictor of prognosis from treadmill testing comes from the estimation of exercise capacity or CRF, a factor often neglected when evaluating the results of this testing.
EXERCISE TRAINING IN SECONDARY CHD PREVENTION
Those who think they have no time for bodily exercise will sooner or later have to find time for illness.
Earl of Derby39
Several observational studies as well as randomized trials have assessed the benefits of PA and ET in cohorts with established CHD.40 Perhaps the best example of ET and secondary CHD prevention comes from the formal CRSP programs. We and others have published findings supporting the beneficial effects of ET for CRSP in patients with CHD as well as peripheral arterial disease. Table 3 summarizes the benefits of such ET programs, and Table 4 identifies the types of patients who are candidates for them; this topic has been reviewed in detail elsewhere.40-47
TABLE 3.
Benefits of Cardiac Rehabilitation and Exercise Training Programsa
TABLE 4.
Indications for Early Outpatient Cardiac Rehabilitation and Exercise Training Programs
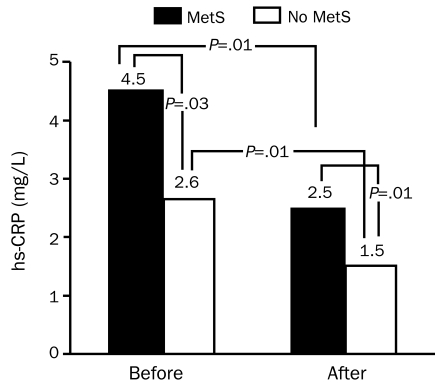
One of the potential benefits of CRSP services is the promotion of weight reduction. The “obesity paradox” has created controversy regarding the role of weight reduction for patients with established CV diseases. Several studies have suggested that obese patients with CHD, HF, and hypertension have a better prognosis than lean patients with CV disease.48-52 Other studies have shown the safety and efficacy of weight loss for patients with CHD.53,54 Participation in a CRSP program has been shown to reduce the prevalence of the metabolic syndrome (MetS) by 37%.55 A substantial body of data points to the importance of elevated high-sensitivity C-reactive protein (hs-CRP) as a CHD risk factor and predictor of CHD events.56 Patients with MetS have considerably higher levels of hsCRP than do other patients (Figure 1),55 and both groups show significant reductions in hs-CRP after CRSP programs. Although improvements in hs-CRP appear to be independent of weight loss and statin therapy,56 we recently noted marked reductions in hs-CRP levels in obese patients with CHD after a CRSP program, whereas lean patients had only slight and nonsignificant declines in hs-CRP after the program.57 In a small group of 45 obese patients with 5% or more weight loss (average 10%) after CRSP, we noted significantly more improvement in exercise capacity and plasma lipid levels than in the 81 obese patients who did not lose weight.58 In a preliminary analysis of a much larger sample, we noted that overweight or obese patients with CHD who lost weight showed significantly greater improvements in CHD risk factors, including levels of hs-CRP, blood lipids, and glucose, and this group had slightly lower mortality, but the difference was not statistically significant.59 Most importantly, a recent Mayo Clinic study of 377 consecutive patients enrolled in CRSP services showed the benefit of weight loss on composite end points (mortality plus major CV events), including benefits even among those with a body mass index less than 25 kg/m2, as well as in patients with CHD who were overweight or obese.60 Despite the obesity paradox, data from CRSP programs confirm the safety and efficacy of purposeful weight loss in secondary CHD prevention.
FIGURE 1.
Median levels of high-sensitivity C-reactive protein (hs-CRP) before and after cardiac rehabilitation and exercise training in patients with and without the metabolic syndrome (MetS). From Am J Cardiol,55 with permission.
Through CRSP programs, CRF can be improved. Major discrepancies may exist between estimated exercise capacity (estimated METs) and exercise capacity directly measured by gas exchange (peak oxygen consumption or measured METs) in patients with CHD. In general, studies have shown marked overestimation of CRF, particularly after a training program.61,62 However, both studies using estimates and direct measurements of CRF have shown that CRF strongly predicts subsequent prognosis.5,7,40 In studies of 12,169 men63 and 2380 women64 referred for CRSP services, a 1-mL/kg/min increase in peak oxygen consumption directly measured by gas exchange was associated with an approximately 10% decrease in CV mortality. For every 1% increase in peak oxygen consumption after ET, CV mortality is reduced by 2%.65 Although patients with lower baseline exercise capacity may derive greater improvements in CRF after CRSP, patients with higher baseline exercise capacity also derive substantial benefits.66
Essentially all patients with CHD take statins and have low-density lipoprotein cholesterol levels at or near goal. However, many patients have abnormal high-density lipoprotein cholesterol and/or triglyceride levels, which significantly improve (+6% and -15%, respectively, with much greater improvements noted in subgroups with greater baseline abnormalities) after CRSP.67-69 Likewise, we and others have shown significant beneficial effects of CRSP on markers of inflammation,55,56 autonomic function,70-72 blood rheology,73 homocysteine levels,74 and endothelial function.75
Perhaps the most important effect of CRSP is on major CV morbidity and mortality. Unfortunately, most of the many randomized controlled trials of CRSP that included ET have not been large enough to adequately assess major CV events.7 However, several meta-analyses have addressed this issue. In 1989, O'Connor et al76 performed a meta-analysis of 22 randomized studies of ET and CHD risk factor intervention in 4554 post-MI patients followed up for an average of 3 years. Compared with control patients, those randomized to ET had reductions in total mortality, CV mortality, and fatal MI of 20%, 22%, and 25%, respectively. There was also a significant 37% reduction in sudden cardiac death (SCD) at 1 year with strong trends in favor of CRSP at 2 and 3 years that were not statistically significant. No significant effect on the risk of nonfatal MI was found. A more recent meta-analysis by Jolliffe et al,77 including 8440 participants from 32 trials, was published in the Cochrane database. It found a 31% reduction in CV mortality associated with CRSP. A recent study from Olmsted County, Minnesota, reviewed 1821 patients with MI, 55% of whom participated in CRSP (a many-fold higher participation rate than in most of the United States).78 Although women and elderly people had lower participation rates (odds ratio, 0.45 and 0.23, respectively), 3-year survival adjusted for baseline factors was considerably better in participants (95% vs 64%; P<.001); most importantly, the benefits of CRSP were even more marked in 1998 than in 1982. Suaya79 assessed CRSP among 600,000 Medicare patients, finding that CRSP participants had a 34% reduction in mortality during 1- to 5-year follow-up; the benefit was 19% greater in those who attended at least two-thirds of the CRSP sessions. Moreover, those with HF had a greater benefit than those without HF. Finally, in a preliminary report from Japan, supervised ET has been reported to improve survival in patients with peripheral arterial disease. After a mean follow-up of 5.4 years, event-free survival was higher in those who completed supervised ET compared with those who did not (81% vs 57%).80
PSYCHOLOGICAL RISK FACTORS AND CRSP SERVICES
It is exercise alone that supports the spirit, and keeps the mind in vigor.
Cicero81
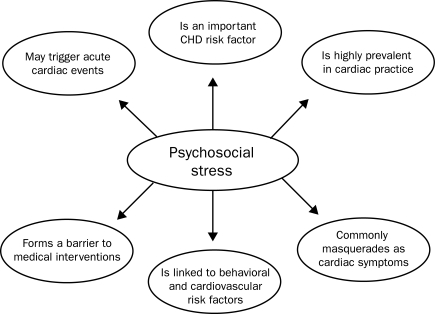
Considerable evidence indicates that psychological factors are strong risk factors for CHD and adversely affect recovery after major CHD events (Figure 2).82,83 In fact, the AHA has recently targeted the evaluation and treatment of depression in patients with established CHD,84 listing CRSP services as a major therapeutic modality to decrease depression and its associated risks.85 Although most of the attention has been directed at depression,84,86 other adverse psychological characteristics, including anxiety,87,88 hostility,88-90 and total psychosocial stress,82,91,92 may also be significant CHD risk factors. In this regard, we have demonstrated reductions of between 40% and 70% in the prevalence of depression, anxiety, and hostility after formal, early outpatient CRSP services.85-91
FIGURE 2.
Several reasons for interest by medical practices in the evaluation and treatment of psychosocial stress. CHD = coronary heart disease. Adapted from J Am Coll Cardiol,82 with permission.
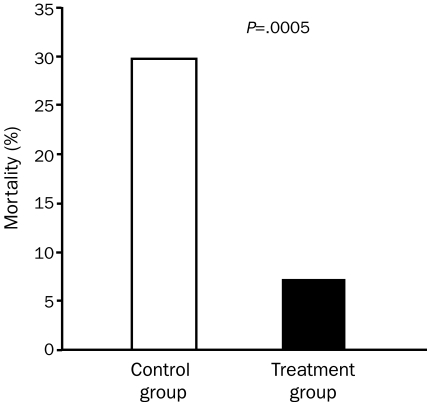
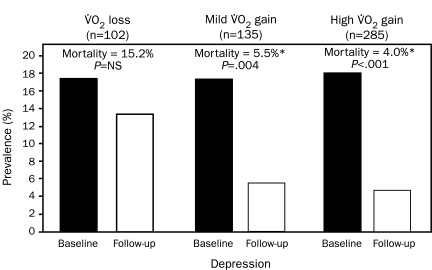
Depressed patients with CHD have 3-fold higher mortality than do nondepressed patients.85,86 Moreover, depressed patients with CHD who attend formal CRSP services have nearly a 70% reduction in mortality risk (Figure 3).85 We also showed that patients whose functional capacity does not significantly improve continue to have a high prevalence of depression and a high mortality risk. In contrast, patients who have either a mild improvement in functional capacity (eg, 0%-10% improvement in peak oxygen consumption) or who have a more marked improvement in functional capacity (>10% improvement in peak oxygen consumption) have equal and marked improvements in the prevalence of depression and mortality risk (Figure 4). These results suggest that only small improvements in exercise capacity may produce profound improvements in depression and depression-related mortality. Although these data were specifically collected in patients with established CHD, we think that these data may also be applicable in primary CHD prevention.
FIGURE 3.
Effect of cardiac rehabilitation and exercise training on mortality rates in 139 patients with baseline depression. From Am J Med,85 with permission.
FIGURE 4.
Prevalence of depression and subsequent mortality based on changes in peak oxygen consumption (VO2) during cardiac rehabilitation and exercise training. *P<.001 compared with VO2 loss. From Am J Med,85 with permission.
UNDERUSE OF CRSP PROGRAMS
There are risks and costs to a program of action, but they are far less than the long-range risks and costs of comfortable inaction.
John F. Kennedy93
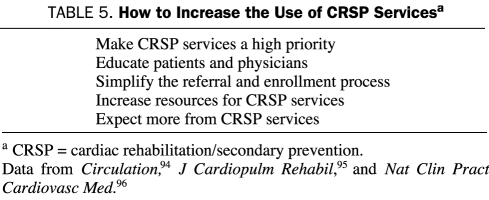
Despite the well-proven benefits of CRSP programs, substantial data suggest that many eligible patients are not referred to these programs and others are not vigorously encouraged to attend; as a result, an extremely large number of patients who survive a major CHD event do not receive CRSP services.45,94-99 In fact, Suaya et al100 reported that only 50,000 (18.7%) of 267,427 Medicare-eligible patients older than 65 years with a major CHD event (MI or coronary artery bypass graft surgery) in 1997 actually participated in a CRSP program, a number that probably has not changed substantially over the past 15 to 20 years. Although program participation was very low in nearly all subgroups assessed, participation was particularly low in elderly persons, women, nonwhites, those of lower socioeconomic status, those with substantial comorbidities, and those with a long travel distance from the patient's home to the CRSP center, with particularly low rates of attendance in the southern compared with the midwestern United States. Although Suaya's study100 documented that more than 80% of eligible patients older than 65 years did not attend CRSP programs, other studies have suggested the limited use of these services in younger patients.79,94,97 Improvement in the use and effect of CRSP services will require intensive efforts and likely a multifactorial approach (Table 5).
TABLE 5.
How to Increase the Use of CRSP Servicesa
EXERCISE TRAINING IN CHRONIC HF AND VALVULAR HEART DISEASE
For patients with stable, compensated chronic HF, ET has provided substantial benefits, reducing symptoms, improving CRF, decreasing neurohormonal activation, improving quality of life, and possibly reducing CV morbidity and mortality.21,101 More than 30 randomized controlled trials of ET in patients with HF have been published; however, the number of participants in the individual trials was quite small.21,102 A meta-analysis reported that average improvement in peak oxygen consumption was 17%. ExTraMATCH (Exercise Training Meta-analysis of Trials with Chronic Heart Failure) was a collaborative meta-analysis of nonparallel controlled trials of ET in 801 patients with HF.103 During a mean follow-up of just under 2 years, those randomized to ET had a 35% reduction in all-cause mortality. Taken together, these data have allowed ET to have a class I indication in HF.
A major randomized trial of ET and HF (HF-ACTION [A randomized Controlled Trial Investigating Outcomes of Exercise Training]) has just been completed.104 This land-mark trial randomized 2331 patients with HF to supervised ET for 3 months followed by nonsupervised ET vs usual care. Most of these patients had class II HF and an average ejection fraction of only 25%; in 52%, the HF had an ischemic etiology. Patients were treated with contemporary maximal HF medications. Importantly, ET was shown to be safe in these patients with advanced HF. Those randomized to ET had significant improvements in time on the cardiopulmonary stress test as well as peak oxygen consumption (P<.0001). However, the primary end point of all-cause mortality or hospitalizations was reduced by only 7% in the ET group (P=.13). After preplanned adjustment for confounding factors (baseline exercise capacity, ejection fraction, Beck Depression Inventory, and history of atrial fibrillation or flutter), there was an 11% reduction in the primary end point among patients randomized to ET (P=.03). The unadjusted secondary end point of CV mortality and HF hospitalization was also not significantly reduced (P=.06) but was reduced by 15% after adjustment (P<.02) in the ET group. Unfortunately, peak oxygen consumption increased only 5% in the ET group, much less than in the smaller randomized trials. One of the biggest problems with this trial was the low “dose” of exercise maintained by the intervention group, especially during the nonsupervised ET program, and the possibly higher than expected level of ET in the usual care arm. Nevertheless, these data provide further support for the safety of ET and its at least modest efficacy in preventing major CV events in HF, in addition to its more substantial effects on functional capacity and health-related quality of life.
Detailed discussions of the role of CRSP services in management of valvular heart disease have already been published,105-107 and such a discussion is beyond the scope of this review. Patients with valvular heart disease also often have concomitant CHD, and CRSP services are now reimbursed by Medicare and other payors.
RISKS DURING INTENSE ET
As already discussed, regular ET and PA provide protection against major CV events. However, during an intense bout of ET, the risk of SCD or MI is transiently increased.108 Unfortunately, in the lay press, this information often receives much more publicity than do the marked benefits of ET and PA.109 In an analysis of 1228 survivors of acute MI, 5% of cases seemed to be triggered by heavy exertion.110 However, the annual incidence of exercise-related SCD in previously healthy persons is only 5.4 per 100,000.108 This incidence is 56 times higher than at rest for sedentary men, whereas the risk is only 5 times higher for physically active men.111 The risk of MI during ET is increased 2 to 6 times higher than at rest, more so in relatively sedentary persons than in those who engage in regular ET.108 Women, however, experience a much lower risk of SCD during heavy ET than do men.112
Survey data from the 1980s and 1990s enable analysis of the incidence of major CV events in patients with CHD enrolled in CRSP programs.113 Although these events are very serious when they occur, the rate per 1,000,000 patient-hours of ET for cardiac arrest, acute MI, and cardiac death were only 8.6, 4.5, and 1.3, respectively. In all likelihood, better medical management with revascularization, statin therapy, and more potent antiplatelet agents has probably further reduced these rates during the past 2 decades.
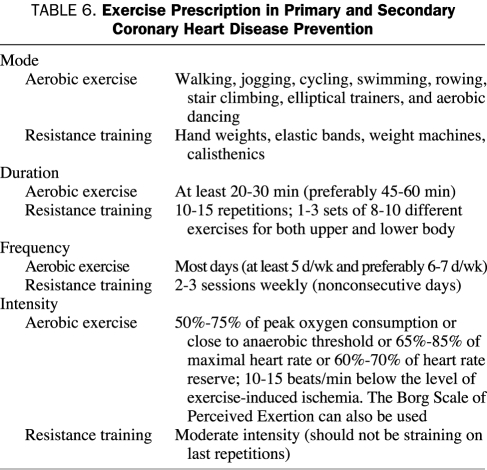
EXERCISE PRESCRIPTION
Prescription of aerobic exercise involves 4 factors: modes of activity, frequency, duration, and intensity (Table 6).114 Similar recommendations are appropriate for both primary and secondary prevention of CHD in middle-aged persons. Contraindications to ET are similar to contraindications for exercise stress testing and include unstable coronary or cerebral ischemia, decompensated HF, recent aortic dissection, uncontrolled and malignant hypertension, unstable medical conditions (eg, active gastrointestinal bleeding or severe anemia), and the inability to exercise due to musculoskeletal and neuromuscular disorders.
TABLE 6.
Exercise Prescription in Primary and Secondary Coronary Heart Disease Prevention
The most common modes of aerobic exercise are walking, jogging, cycling, swimming, rowing, stair climbing, using elliptical trainers, and aerobic dancing. Recumbent cycles, treadmills, and recumbent combination arm/leg machines are commonly available in CRSP programs. Resistance (strength) training 2 to 3 times weekly on nonconsecutive days, consisting of 8 to 15 slow repetitions to moderate fatigue using 8 to 10 different exercises for both upper and lower body, has been shown to be safe and to improve strength and quality of life for our patients.115 A detailed update of the health benefits and safety of resistance training, its effect on CV structure and function, its role in modifying risk factors, its benefits in special populations, and prescriptive methods has recently been published by Williams et al116 of the AHA. Resistance training may also reduce fractures and falls in elderly patients and improve insulin sensitivity and glucose metabolism.
Patients should be advised to exercise for at least 30 minutes (preferably 45-60 minutes to provide more caloric burning) on most days (at least 5 and preferably 6 or 7 days per week). In CRSP programs, we usually provide intensity recommendations on the basis of cardiopulmonary stress test results, ie, the target heart rate is set close to the anaerobic or ventilatory threshold (60%-70% of peak oxygen consumption).117,118 In patients with an exercise stress test performed without gas exchange, the target heart rate is set at 65% to 85% of the maximal heart rate or 60% to 70% of the heart rate reserve and at least 10 beats/min below the level of any exercise-induced symptomatic or silent ischemia.114 In patients who are able to prove that their heart rate corresponds to a certain level of perceived exertion, the Borg Scale of Perceived Exertion can be used to more easily monitor exercise intensity.42,114 Because stress test results may be unavailable in primary prevention, ET intensity can be recommended at a moderate level (the patient should be able to speak during ET but should be exercising with enough intensity to prefer not to speak for most of the ET session). If a 10-point scale is used to describe ET intensity, with 0 describing rest and 10 describing the highest intensity possible, most exercise should be performed in the 5 to 7 intensity range.
CONCLUSION
Regular ET and PA and maintenance of high levels of CRF have a role in reducing CHD risk in both primary and secondary prevention. Although an intense bout of heavy exercise transiently increases risk of a CV event, the absolute risk is very small, and the long-term benefits of regular exercise clearly outweigh these risks.
In patients with established CHD, CRSP services are grossly underused. Patients who are most likely to benefit include those with recent acute MI, those who have undergone myocardial revascularization, those with stable angina pectoris, and probably those with compensated HF. Evidence suggests that CRSP programs are associated with increased exercise and functional capacity as well as improvements in obesity indices, plasma lipid levels, MetS, glucose metabolism, inflammation, autonomic function, blood rheology, and psychological risk factors. In addition, CRSP services have also been proven to reduce major CV morbidity and mortality by approximately 20% to 25%. Through automatic referral and incentive-based systems, greater emphasis must be placed on the referral of all appropriate patients to CRSP programs after major CHD events.
Acknowledgments
The authors acknowledge the excellent work of the CRSP staff at Ochsner Clinic and Mayo Clinic, including physicians, exercise physiologists, physical therapists, nurses, dietitians, and secretaries, during patient care and data collection. Also, a special tribute is given to Gerald T. Gau, MD, a master CV disease specialist and formerly the Medical Director of the Cardiovascular Health Clinic at Mayo Clinic, who also provided clinical and academic mentorship to several of the authors.
REFERENCES
- 1.Plato Theaetetus Waterfield R, trans-ed. New York, NY: Penguin Classics; 1987:33 [Google Scholar]
- 2.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003;107(24):3109-3116 [DOI] [PubMed] [Google Scholar]
- 3.US Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion http://www.cdc.gov/NCCDPHP/sgr/pdf/sgraag.pdf. Physical activity and health: a report of the Surgeon General: 1996. Accessed January 29, 2009.
- 4.Leon AS, ed. Physical Activity and Cardiovascular Health: A National Consensus Champaign, IL: Human Kinetics; 1997:3-4 [Google Scholar]
- 5.Lavie CJ, Milani RV. Metabolic equivalent (MET) inflation—not the MET we used to know [editorial]. J Cardiopulm Rehabil Prev. 2007;27(3):149-150 [DOI] [PubMed] [Google Scholar]
- 6.Bouchard C, Dione FT, Simoneau JA, Boulay MR. Genetics of aerobic and anaerobic performances. In: Holloszy JO, ed. Exercise and Sport Sciences Reviews Baltimore, MD: Williams & Wilkins; 1992:27-58 [PubMed] [Google Scholar]
- 7.Squires RW, Hamm LF. Exercise and the coronary heart disease connection. In: Hamm LF, Berra K, Kavanagh T, eds. AACVPR Cardiac Rehabilitation Resource Manual Champaign, IL: Human Kinetics; 2006. [Google Scholar]
- 8.Taylor HL, Klepetar E, Keys A, Parlin W, Blackburn H, Puchner T. Death rates among physically active and sedentary employees of the railroad industry. Am J Public Health Nations Health 1962;52(Oct):1697-1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris JN, Kagan A, Pattison DC, Gardner MJ. Incidence and prediction of ischemic heart disease in London busmen. Lancet 1966;2(7463):553-559 [DOI] [PubMed] [Google Scholar]
- 10.Paffenbarger RS, Hale WE. Work activity and coronary heart mortality. N Engl J Med. 1975;292(11):545-550 [DOI] [PubMed] [Google Scholar]
- 11.Paffenbarger RS, Jr, Hyde RT. Exercise in the prevention of coronary heart disease. Prev Med. 1984;13(1):3-22 [DOI] [PubMed] [Google Scholar]
- 12.Sesso HD, Paffenbarger RS, Jr, Lee IM. Physical activity and coronary heart disease in men: the Harvard Alumni Health Study. Circulation 2000;102(9):975-980 [DOI] [PubMed] [Google Scholar]
- 13.Lee IM, Sesso HD, Paffenbarger RS., Jr Physical activity and coronary heart disease risk in men: does the duration of exercise episodes predict risk? Circulation 2000;102(9):981-986 [DOI] [PubMed] [Google Scholar]
- 14.Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure time: protection against coronary heart disease. Lancet 1980;2(8206):1207-1210 [DOI] [PubMed] [Google Scholar]
- 15.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341(9):650-658 [DOI] [PubMed] [Google Scholar]
- 16.Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE. Physical activity and coronary heart disease in women: is “no pain, no gain” passé? JAMA 2001;285(11):1447-1454 [DOI] [PubMed] [Google Scholar]
- 17.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA 2002;288(16):1994-2000 [DOI] [PubMed] [Google Scholar]
- 18.Hakim AA, Curb JD, Petrovitch H, et al. Effects of walking on coronary heart disease in elderly men: the Honolulu Heart Program. Circulation 1999;100(1):9-13 [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347(10):716-725 [DOI] [PubMed] [Google Scholar]
- 20.Kushi LH, Fee RM, Folsom AR, Mink PJ, Anderson KE, Sellers TA. Physical activity and mortality in postmenopausal women. JAMA 1997;277(16):1287-1292 [PubMed] [Google Scholar]
- 21.Blair SN, Church TS. The fitness, obesity, and health equation: is physical activity the common denominator [editorial]? JAMA 2004;292(10):1232-1234 [DOI] [PubMed] [Google Scholar]
- 22.Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33(6)(suppl):S379-S399 [DOI] [PubMed] [Google Scholar]
- 23.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA 1989;262(17):2395-2401 [DOI] [PubMed] [Google Scholar]
- 24.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132(8);605-611 [DOI] [PubMed] [Google Scholar]
- 25.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69(3):373-380 [DOI] [PubMed] [Google Scholar]
- 26.Farrell SW, Braun L, Barlow CE, Cheng YJ, Blair SN. The relation of body mass index, cardiorespiratory fitness, and all-cause mortality in women. Obes Res. 2002;10(6):417-423 [DOI] [PubMed] [Google Scholar]
- 27.Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004;27(1):83-88 [DOI] [PubMed] [Google Scholar]
- 28.Blair SN, Kohl HW, III, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality: a prospective study of healthy and unhealthy men. JAMA 1995;273(14):1093-1098 [PubMed] [Google Scholar]
- 29.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med. 1993;328(8):533-537 [DOI] [PubMed] [Google Scholar]
- 30.Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation 1998;98(25):2836-2841 [DOI] [PubMed] [Google Scholar]
- 31.Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med. 2000;132(11):862-870 [DOI] [PubMed] [Google Scholar]
- 32.Myers J, Prakash M, Froelicher V, Do D, Partington B, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793-801 [DOI] [PubMed] [Google Scholar]
- 33.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation 2003September30;108(13):1554-1559 Epub 2003 Sep 15 [DOI] [PubMed] [Google Scholar]
- 34.Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353(5):468-475 [DOI] [PubMed] [Google Scholar]
- 35.Blair SN, Brodney S. Effects of physical inactivity and obesity on morbidity and mortality: current evidence and research issues. Med Sci Sports Exerc. 1999:31(11)(suppl):S646-S662 [DOI] [PubMed] [Google Scholar]
- 36.Aktas MK, Ozduran V, Pothier CE, Lang R, Lauer MS. Global risk scores and exercise testing for predicting all-cause mortality in a preventive medicine program. JAMA 2004;292(12):1462-1468 [DOI] [PubMed] [Google Scholar]
- 37.Aijaz B, Babuin L, Squires RW, et al. Long-term mortality with multiple treadmill exercise test abnormalities: comparison between patients with and without cardiovascular disease. Am Heart J 2008October;156(4):783-789 Epub 2008 Jul 11 [DOI] [PubMed] [Google Scholar]
- 38.Lyerly GW, Sui X, Church TS, Lavie CJ, Hand GA, Blair SN. Maximal exercise electrocardiography responses and coronary heart disease mortality among men with diabetes mellitus. Circulation 2008May27;117(21):2734-2742 Epub 2008 May 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edward Stanley, Earl of Derby. The Conduct of Life Address at Liverpool College, December20, 1873. [Google Scholar]
- 40.Squires RW, Hamm LF. Exercise and the coronary heart disease connection. In: AACVPR Cardiac Rehabilitation Resource Manual Champaign, IL: Human Kinetics; 2006:53-62 [Google Scholar]
- 41.Artham SM, Lavie CJ, Milani RV, Chi YW, Goldman CK. Benefits of exercise training in secondary prevention of coronary and peripheral arterial disease. Vasc Dis Prev. 2008;5(3):156-168 [Google Scholar]
- 42.Squires RW, Gau GT, Miller T, Allison T, Lavie CJ. Cardiovascular rehabilitation: status, 1990. Mayo Clin Proc. 1990;65(5):731-755 [DOI] [PubMed] [Google Scholar]
- 43.Williams MA, Ades PA, Hamm LF, et al. Clinical evidence for a health benefit from cardiac rehabilitation: an update. Am Heart J. 2006;152(5):835-841 [DOI] [PubMed] [Google Scholar]
- 44.Ades PA. Cardiac rehabilitation and secondary prevention of coronary heart disease. N Engl J Med. 2001;345(12):892-902 [DOI] [PubMed] [Google Scholar]
- 45.Thomas RJ, King M, Lui K, et al. AACVPR/ACC/AHA 2007 performance measures on cardiac rehabilitation for referral to and delivery of cardiac rehabilitation/secondary prevention services endorsed by the American College of Chest Physicians, American College of Sports Medicine, American Physical Therapy Association, Canadian Association of Cardiac Rehabilitation, European Association for Cardiovascular Prevention and Rehabilitation, Inter-American Heart Foundation, National Association of Clinical Nurse Specialists, Preventive Cardiovascular Nurses Association, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2007;50(14):1400-1433 [DOI] [PubMed] [Google Scholar]
- 46.Lavie CJ, Milani RV. Cardiac rehabilitation update 2008—biological, psychological, and clinical benefits. US Cardiol. 2008;5(1):72-75 [Google Scholar]
- 47.Wenger NK. Current status of cardiac rehabilitation. J Am Coll Cardiol. 2008;51(17):1619-1631 [DOI] [PubMed] [Google Scholar]
- 48.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease—risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. In press [DOI] [PubMed]
- 49.Artham SM, Lavie CJ, Milani RV, Ventura HO. The obesity paradox: impact of obesity on the prevalence and prognosis of cardiovascular diseases. Postgrad Med. 2008;120(2):34-41 [DOI] [PubMed] [Google Scholar]
- 50.Todd Miller M, Lavie CJ, White CJ. Impact of obesity on the pathogenesis and prognosis of coronary heart disease. J Cardiometab Syndr. 2008;3(3):162-167 [DOI] [PubMed] [Google Scholar]
- 51.Artham SM, Lavie CJ, Patel HM, Ventura HO. Impact of obesity on the risk of heart failure and its prognosis. J Cardiometab Syndr. 2008;3(3):155-161 [DOI] [PubMed] [Google Scholar]
- 52.Lavie CJ, Milani RV, Ventura HO. Obesity, heart disease, and favorable prognosis—truth or paradox [editorial]? Am J Med. 2007;120(10):825-826 [DOI] [PubMed] [Google Scholar]
- 53.Lavie CJ, Milani RV. Cardiac rehabilitation and exercise training programs in metabolic syndrome and diabetes. J Cardiopulm Rehabil. 2005;25(2):59-66 [DOI] [PubMed] [Google Scholar]
- 54.Blaha MJ, Bansal S, Rouf R, Golden SH, Blumenthal RS, DeFilippis AP. A practical “ABCDE” approach to the metabolic syndrome. Mayo Clin Proc. 2008;83(8):932-941 [DOI] [PubMed] [Google Scholar]
- 55.Milani RV, Lavie CJ. Prevalence and profile of metabolic syndrome in patients following acute coronary events and effects of therapeutic lifestyle change with cardiac rehabilitation. Am J Cardiol. 2003;92(1):50-54 [DOI] [PubMed] [Google Scholar]
- 56.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43(6):1056-1061 [DOI] [PubMed] [Google Scholar]
- 57.Lavie CJ, Morshedi-Meibodi A, Milani RV. Impact of cardiac rehabilitation on coronary risk factors, inflammation, and the metabolic syndrome in obese coronary patients. J Cardiometab Syndr. 2008;3(3):136-140 [DOI] [PubMed] [Google Scholar]
- 58.Lavie CJ, Milani RV. Effects of cardiac rehabilitation, exercise training, and weight reduction on exercise capacity, coronary risk factors, behavioral characteristics, and quality of life in obese coronary patients. Am J Cardiol. 1997;79(4):397-401 [DOI] [PubMed] [Google Scholar]
- 59.Lavie CJ, Jr, Milani RV, Artham SM. Combating the obesity paradox: benefits and safety of purposeful weight loss in overweight and obese coronary patients [abstract 1035-218]. J Am Coll Cardiol. 2008;51(10)(suppl 1):A367 [Google Scholar]
- 60.Sierra-Johnson J, Romero-Corral A, Somers VK, et al. Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil. 2008;15(3):336-340 [DOI] [PubMed] [Google Scholar]
- 61.Milani RV, Lavie CJ, Spiva H. Limitations of estimating metabolic equivalents in exercise assessment in patients with coronary artery disease. Am J Cardiol. 1995;75(14):940-942 [DOI] [PubMed] [Google Scholar]
- 62.Lavie CJ, Milani RV. Disparate effects of improving aerobic exercise capacity and quality of life after cardiac rehabilitation in young and elderly coronary patients. J Cardiopulm Rehabil. 2000;20(4):235-240 [DOI] [PubMed] [Google Scholar]
- 63.Kavanagh T, Mertens DJ, Hamm LF, et al. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation 2002;106(6):666-671 [DOI] [PubMed] [Google Scholar]
- 64.Kavanagh T, Mertens DJ, Hamm LF, et al. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am Coll Cardiol. 2003;42(12):2139-2143 [DOI] [PubMed] [Google Scholar]
- 65.Vanhees L, Fagard R, Thijs L, Amery A. Prognostic value of training-induced change in peak exercise capacity in patients with myocardial infarcts and patients with coronary bypass surgery. Am J Cardiol. 1995;76(14):1014-1019 [DOI] [PubMed] [Google Scholar]
- 66.Lavie CJ, Milani RV. Patients with high baseline exercise capacity benefit from cardiac rehabilitation and exercise training programs. Am Heart J 1994;128(6, pt 1):1105-1109 [DOI] [PubMed] [Google Scholar]
- 67.Lavie CJ, Milani RV. Effects of nonpharmacologic therapy with cardiac rehabilitation and exercise training in patients with low levels of high-density lipoprotein cholesterol. Am J Cardiol. 1996;78(11):1286-1289 [DOI] [PubMed] [Google Scholar]
- 68.Milani RV, Lavie CJ. Prevalence and effects of nonpharmacologic treatment of “isolated” low-HDL cholesterol in patients with coronary artery disease. J Cardiolpulm Rehab 1995;15(6):439-444 [DOI] [PubMed] [Google Scholar]
- 69.Lavie CJ, Milani RV. Effects of cardiac rehabilitation and exercise training on low density lipoprotein cholesterol in patients with hypertriglyceridemia and coronary artery disease. Am J Cardiol. 1994;74(12):1192-1195 [DOI] [PubMed] [Google Scholar]
- 70.Lucini D, Milani RV, Costantino G, Lavie CJ, Porta A, Pagani M. Effects of cardiac rehabilitation and exercise training on autonomic regulation in patients with coronary artery disease. Am Heart J 2002;143(6):977-983 [DOI] [PubMed] [Google Scholar]
- 71.Tygesen H, Wettervik C, Wennerblom B. Intensive home-based exercise training in cardiac rehabilitation increases exercise capacity and heart rate variability. Int J Card 2001;79(2-3):175-182 [DOI] [PubMed] [Google Scholar]
- 72.Earnest CP, Lavie CJ, Blair SN, Church TS. Heart rate variability characteristics in sedentary postmenopausal women following six months of exercise training: the DREW study. PLoS ONE 2008;3(6):e2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Church TS, Lavie CJ, Milani RV, Kirby GS. Improvements in blood rheology after cardiac rehabilitation and exercise training in patients with coronary heart disease. Am Heart J 2002;143(2):349-355 [DOI] [PubMed] [Google Scholar]
- 74.Ali A, Mehra MR, Lavie CJ, et al. Modulatory impact of cardiac rehabilitation on hyperhomocysteinemia in patients with coronary artery disease and “normal” lipid levels. Am J Cardiol. 1998;82(12):1543-1545, A8 [DOI] [PubMed] [Google Scholar]
- 75.Hambrecht R, Wolf A, Gielen S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342(7):454-460 [DOI] [PubMed] [Google Scholar]
- 76.O'Connor GT, Buring JE, Yusuf S, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation 1989;80(2):234-244 [DOI] [PubMed] [Google Scholar]
- 77.Jolliffe JA, Rees K, Taylor RS, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2001;1:CD001800 [DOI] [PubMed] [Google Scholar]
- 78.Witt BJ, Jacobsen SJ, Weston SA, et al. Cardiac rehabilitation after myocardial infarction in the community. J Am Coll Cardiol. 2004;44(5):988-996 [DOI] [PubMed] [Google Scholar]
- 79.Suaya J. Survival benefits and dose-response effect of cardiac rehabilitation in Medicare beneficiaries after a cardiac event or revascularization [abstract 405-8]. J Am Coll Cardiol. 2008;51(10)(suppl 1);A373 [Google Scholar]
- 80.Sakamoto S, Yokoyama N, Kasai S, et al. Long-term clinical outcome of supervised exercise rehabilitation among patients with peripheral arterial disease [abstract 804-6]. J Am Coll Cardiol. 2007;49(9)(suppl 1):351A [Google Scholar]
- 81.Cicero On Old Age Vol 9, Part 2 The Harvard Classics Shuckburgh ES, trans-ed. New York, NY: PF Collier and Son; 1909-14:10 [Google Scholar]
- 82.Rozanski A, Blumental JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45(5):637-651 [DOI] [PubMed] [Google Scholar]
- 83.Lavie CJ, Milani RV, Lavie TJ. Impact of cardiac rehabilitation, exercise training, and fitness on psychological distress. In: Sher L, ed. Psychological Factors and Cardiovascular Disorders: The Role of Psychiatric Pathology & Maladaptive Personality Features Hauppauge, NY: Nova Science Publishers; 2009:312-329 [Google Scholar]
- 84.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation 2008October21;118(17):1768-1775 Epub 2008 Sep 29 [DOI] [PubMed] [Google Scholar]
- 85.Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med. 2007;120(9):799-806 [DOI] [PubMed] [Google Scholar]
- 86.Milani RV, Lavie CJ, Cassidy MM. Effects of cardiac rehabilitation and exercise training program on depression in patients after major coronary events. Am Heart J 1996;132(4):726-732 [DOI] [PubMed] [Google Scholar]
- 87.Lavie CJ, Milani RV. Prevalence of anxiety in coronary patients with improvement following cardiac rehabilitation and exercise training. Am J Cardiol. 2004;93(3):336-339 [DOI] [PubMed] [Google Scholar]
- 88.Lavie CJ, Milani RV. Adverse psychological and coronary risk profiles in young patients with coronary artery disease and benefits of formal cardiac rehabilitation. Arch Intern Med. 2006;166(17):1878-1883 [DOI] [PubMed] [Google Scholar]
- 89.Lavie CJ, Milani RV. Effects of cardiac rehabilitation and exercise training programs on coronary patients with high levels of hostility. Mayo Clin Proc. 1999;74(10):959-966 [DOI] [PubMed] [Google Scholar]
- 90.Lavie CJ, Milani RV. Prevalence of hostility in young coronary artery disease patients and effects of cardiac rehabilitation and exercise training. Mayo Clin Proc. 2005;80(3):335-342 [DOI] [PubMed] [Google Scholar]
- 91.Artham SM, Lavie CJ, Milani RV. Cardiac rehabilitation programs markedly improve high-risk profiles in coronary patients with high psychological distress. South Med J 2008;101(3):262-267 [DOI] [PubMed] [Google Scholar]
- 92.Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51(13):1237-1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kennedy JF. http://quoteland.com/search.asp. Accessed August 3, 2007.
- 94.Thomas RJ. Cardiac rehabilitation/secondary prevention programs a raft for the rapids: why have we missed the boat [editorial]? Circulation 2007;116(15):1644-1646 [DOI] [PubMed] [Google Scholar]
- 95.Thomas RJ, Witt BJ, Lopez-Jimenez F, King ML, Squires RW. Quality indicators in cardiovascular care: the case for cardiac rehabilitation. J Cardiopulm Rehabil. 2005;25(5):249-256 [DOI] [PubMed] [Google Scholar]
- 96.Squires RW. Are cardiac rehabilitation programs underutilized by patients with coronary heart disease? Nat Clin Pract Cardiovasc Med. 2008April5(4):192-193 Epub 2008 Feb 12 [DOI] [PubMed] [Google Scholar]
- 97.Thomas RJ, Miller NH, Lamendola C, et al. National Survey on Gender Differences in Cardiac Rehabilitation Programs: patient characteristics and enrollment patterns. J Cardiopulm Rehabil. 1996;16(6):402-412 [DOI] [PubMed] [Google Scholar]
- 98.Ades PA, Waldmann ML, McCann WJ, Weaver SO. Predictors of cardiac rehabilitation participation in older coronary patients. Arch Intern Med. 1992;152(5):1033-1035 [PubMed] [Google Scholar]
- 99.Cortés O, Arthur HM. Determinants of referral to cardiac rehabilitation program in patients with coronary artery disease: a systematic review. Am Heart J 2006;151(2):249-256 [DOI] [PubMed] [Google Scholar]
- 100.Suaya JA, Shepard DS, Normand SL, Ades P, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007October;116(15):1653-1662 Epub 2007 Sep 24 [DOI] [PubMed] [Google Scholar]
- 101.McKelvie RS. Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail Rev. 2008;13(1):3-11 [DOI] [PubMed] [Google Scholar]
- 102.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116(10):693-706 [DOI] [PubMed] [Google Scholar]
- 103.Piepoli MF, Davos C, Francis DP, Coats AJ, ExTraMATCH Collaborative Exercise training meta-analysis of trials with chronic heart failure (ExTraMATCH). BMJ 2004January24;328(7433):189 Epub 2004 Jan 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whellan DJ, O'Connor CM.HF-ACTION. Efficacy and safety of exercise training as a treatment modality in patients with chronic heart failure: results of A randomized Controlled Trial Investigating Outcomes of exercise traiNing (HF-ACTION) Paper presented at: American Heart Association Scientific Sessions, New Orleans, LA; November8-12, 2008 [Google Scholar]
- 105.Jairath N, Salerno T, Chapman J, Dornan J, Weisel R. The effect of moderate exercise training on oxygen uptake post-aortic/mitral valve surgery. J Cardiopulm Rehabil. 1995;15(6):424-430 [DOI] [PubMed] [Google Scholar]
- 106.Gohlke-Bärwolf C, Gohlke H, Samek L, et al. Exercise tolerance and working capacity after valve replacement. J Heart Valve Dis. 1992;1(2):189-195 [PubMed] [Google Scholar]
- 107.Sire S. Physical training and occupational rehabilitation after aortic valve replacement. Eur Heart J 1987;8(11):1215-1220 [DOI] [PubMed] [Google Scholar]
- 108.Thompson PD, Moore GE. The cardiac risks of vigorous physical activity. In: Leon AS, ed. Physical Activity and Cardiovascular Health: A National Consensus Champaign, IL: Human Kinetics; 1997:137-142 [Google Scholar]
- 109.Lavie CJ. Making exercise and fitness a high priority. Ochsner J 2007;7(4):154-157 [PMC free article] [PubMed] [Google Scholar]
- 110.Mittleman MA, Maclure M, Tolfer GH, Sherwood JB, Goldberg RJ, Muller JE, Determinants of Myocardial Infarction Onset Study Investigators Triggering of acute myocardial infarction by heavy physical exertion: protection against triggering by regular exertion. N Engl J Med. 1993;329(23):1677-1683 [DOI] [PubMed] [Google Scholar]
- 111.Siscovick DS, Weiss NS, Fletcher RH, Lasky T. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311(14):874-877 [DOI] [PubMed] [Google Scholar]
- 112.Whang W, Manson JE, Hu FB, et al. Physical exertion, exercise, and sudden cardiac death in women. JAMA 2006;295(12):1399-1403 [DOI] [PubMed] [Google Scholar]
- 113.Thompson PD, Franklin BA, Balady GJ, et al. American College of Sports Medicine Exercise and acute cardiovascular events: placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 2007;115(17):2358-2368 [DOI] [PubMed] [Google Scholar]
- 114.Lavie CJ, Milani RV, Marks P, deGruiter H. Exercise and the heart: risks, benefits, and recommendations for providing exercise prescriptions. The Ochsner Journal 2001;3(4):207-213 [PMC free article] [PubMed] [Google Scholar]
- 115.Lavie CJ, Milani RV. Aerobic and resistance exercise training in the elderly. Am J Geriatr Cardiol. 2007;16(1):36-37 [DOI] [PubMed] [Google Scholar]
- 116.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007July;116(5):572-584 Epub 2007 Jul 16 [DOI] [PubMed] [Google Scholar]
- 117.Milani RV, Lavie CJ, Mehra MR. Cardiopulmonary exercise testing: how do we differentiate the cause of dyspnea? Circulation 2004;110(4):e27-e31 [DOI] [PubMed] [Google Scholar]
- 118.Milani RV, Lavie CJ, Mehra MR, Ventura HO. Understanding the basics of cardiopulmonary exercise testing. Mayo Clin Proc. 2006;81(12):1603-1611 [DOI] [PubMed] [Google Scholar]