Abstract
Analysis of protein and peptide mixtures via capillary electrophoresis is hindered by non-specific adsorption of analytes to the capillary walls, resulting in poor separations and quantitative reproducibility. Phospholipid bilayer (PLB) coatings are very promising for improving protein and peptide separations due to the native resistance to non-specific protein adsorption offered by PLBs; however, these coatings display limited chemical and temporal stability. Here, we show the preparation and characterization of a highly cross-linked, polymerized phospholipid capillary coating prepared using bis-SorbPC. Poly(bis-SorbPC) PLB coatings are prepared in situ within fully enclosed fused silica capillaries via self assembly and radical polymerization. Polymerization of the PLB coating stabilizes the membrane against desorption from the surface and migration in an electric field, improves the temporal and chemical stability, and allows for the separation of both cationic and anionic proteins, while preserving the native resistance to non-specific protein adsorption of natural PLBs.
Introduction
Capillary zone electrophoresis (CZE) is well suited for rapid analysis of small volume samples, although non-specific adsorption of protein and peptide analytes, via non-covalent molecular interactions,1–5 often limits the quantitative and qualitative utility of CZE for protein separations. Additionaly, adsorption of proteins changes the distribution of the charge on the capillary wall, leading to non-uniform zeta potential and variations in electroosmotic flow (EOF), thereby decreasing separation performance,5–8 and introducing significant quantification errors. A variety of strategies have been applied to minimize non-specific adsorption of proteins in CZE, most commonly through alteration of the chemical composition of the buffer1;7;9 or the capillary surface.1–5;9–15 Capillary coatings for protein separations are most commonly grouped into two major categories: (i) permanent coatings, where the coating is covalently linked to the capillary surface, yielding an irreversible coating1;2;5;9–11 and (ii) dynamic or semi-permanent coatings, in which the coating is non-covalently adsorbed to the surface and/or present in the run buffer.2–5;12–15
Non-covalent coatings are most commonly used for CE separations as they offer a number of advantages, including: (i) ease and speed of coating, (ii) high stability and/or easy regeneration, (iii) decreased dependence on capillary surface chemistry and (iv) low cost. The chemical nature of non-covalent capillary coatings vary widely, though most require the addition of the coating material to the run buffer to maintain the surface coating during the time course of the separation.2;3;5;12;13 Alternatively, a number of semi-permanent, physisorbed coatings that withstand multiple runs without addition to the run buffer have been described.4;12;14–18 Among these are double chained surfactants, e.g. didodecyldimethylammonium bromide (DDAB), which are particularly attractive for protein separations, as they are inexpensive and provide sufficient shielding of the capillary surface.14
Biologically-derived alternatives to surfactant coatings have been reported, wherein self-assembled zwitterionic phospholipid bilayers (PLBs) are deposited directly onto the fused silica capillary wall.15–17;19 Phosphorylcholine (PC)-based planar supported PLBs have been studied extensively and possess high resistance to non-specific protein adsorption,20;21 properties that are maintained when planar PC-PLBs are formed on fused silica capillary walls.15;16;19 PC-PLB coatings are effective at masking the charge of the capillary surface, allowing for high efficiency protein separations, reproducible migration times and enhanced protein recoveries.15 Additionally, the zwitterionic headgroup of PC lipids allows for the separation of protein mixtures containing either cationic or anionic proteins.15 Further, PC-PLB coatings present the potential capability to tune the surface chemistry of the PLB structures to create surfaces with engineered surface charge or binding capabilities by doping the PC-PLB with varying headgroup chemistries.22
The primary drawback to PC-PLB coatings is the need to frequently regenerate the coating, typically every one to three separations, due to a lack of PLB stability.15 Furthermore, fluid PLBs are fragile and are readily damaged by brief exposures to common chemical and physical insults that may be encountered in CZE protein separations, including air bubbles, exposure to organic solvents and surfactants and/or elevated temperatures. Moreover, a key impediment to realizing tailored PLB surface chemistries in CZE has been the mobility of charged lipids in electrophoretic fields,23–25 leading to migration of charged lipids throughout the capillary, and alteration of the capillary surface chemistry during the time course of the separation. Thus, the ability to chemically and physically stabilize the PLB would be expected to significantly enhance the analytical capabilities and widespread utilization of this biocompatible coating technology.
A number of strategies have been employed to increase the stability of PLBs, in both vesicle and planar geometries.16;17;19;26–31 Most often stabilization is achieved via addition of biological components to the PLB, e.g. cholesterol.26 Enhanced stability can also be attained via polymerization of reactive monomers, e.g. n-butyl methacrylate (BMA) and ethylene glycol dimethacrylate (EGDMA), incorporated in the hydrophobic lamella of the PLB, though this approach is laborious and has not yet been demonstrated on planar silica substrates.27–30;32
Alternatively, direct polymerization of the phospholipid monomers used to construct the PLB increases PLB stability for native and synthetic PC-PLBs.17;33–38 Highly cross-linked vesicle and planar PLBs have been prepared using a range of PC-based polymerizable phospholipids.22;30;33;34;36;39;40 Diacetylenic phosphorylcholine phospholipids (DAPC) stabilize PLB vesicles37;38 and planar PLBs against a variety of destructive insults (e.g. drying, and surfactant dissolution) via formation of extensive cross-linked polymer networks,35;36;39 though assembly of DAPC requires the use of deposition techniques that are incompatible with fully enclosed, curved surfaces, e.g. fused silica capillaries. Recently, sorbyl-based polymerizable phospholipids,41–43 most notably bis-SorbPC (1,2’-bis[10-(2’,4’-hexadienoyloxy) decanoyl]-sn-glycero-3-phosphocholine), were used to prepare planar, self-assembled PC-PLBs that resist non-specific adsorption of proteins and are stabilized against a variety of chemical and physical insults,22;33;34 suggesting the utility of such a PLB as a stabilized capillary coating.
Here, we show the preparation and characterization of polymerized bis-SorbPC PLB coatings within fully enclosed fused silica capillaries via self assembly and radical polymerization. The resultant poly(bis-SorbPC) PLBs show increased stability compared to non-polymerized PLBs while also allowing for the separation of both cationic and anionic proteins. The polymerization of these PLBs stabilizes the membrane against desorption from the surface and migration in an electric field, while still preserving the native non-specific protein adsorption resistance of unpolymerized PLBs.
Experimental
Materials
Fused silica capillary was obtained from Polymicro Technologies (Phoenix, AZ). DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), DLPC (1,2-dilauroyl-sn-glycero-3-phosphocholine), EggPC (L-α-phosphatidylcholine) and rhodamine-DPPE (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(Lissamine Rhodamine B Sulfonyl)) were obtained from Avanti Polar Lipids (Alabaster, AL). Bis-SorbPC (1,2-bis[10-(2’,4’-hexadieoyloxy)decanoyl]-sn-glycero-2-phosphocholine) was synthesized as previously described.42 FM 1-43 was obtained from Invitrogen Molecular Probes (Eugene, OR). Insulin chain A (ICA), bovine serum albumin (BSA), α-chymotrypsinogen A (α-chymo) and fluorescein isothiocyanate, isomer I (FITC), MOPS buffer, sodium hydrogensulfite and potassium persulfate were obtained from Sigma-Aldrich (St. Louis, MO). Trypsin inhibitor (TI) was obtained from Gibco (Carlsbad, CA). Six-histidine tagged enhanced green fluorescent (6xHis-EGFP) protein was purified in-house using Ni-NTA metal affinity chromatography from transfected E. coli. All H2O was obtained from Barnstead EasyPure UV/UF water purification system at a purity of 18.0 MΩ or better.
Capillary Preparation
Phospholipids were dried of organic solvent using a stream of Ar then dried under vacuum for four hours. The dried phospholipid films were hydrated to a concentration of 1 mg/mL in water and sonicated to clarity to prepare small unilamellar vesicles (SUVs) ca. 50–70 nm in diameter. Prior to coating, the capillaries were cleaned with piranha solution for one hour (70% H2SO4, 30% H2O2), then rinsed with water. Vesicle solutions were introduced into the capillary via gravity-induced flow and allowed to fuse with the capillary surface for 30 minutes. Radical initiator solutions were prepared by mixing 22 mM NaHSO3 and 65 mM K2S2O8 in degassed H2O and introduced to the capillary via gravity-induced flow for a minimum of 3 hours to generate cross-linked poly(bis-SorbPC) films.
Patterned capillaries were prepared via UV-irradiation of self assembled bis-SorbPC films (prepared as described above, minus radical polymerization) using a low-pressure Hg pen lamp (UVP, Inc., Upland, CA) through a stainless steel mask placed directly onto the bis-SorbPC coated capillary.22 The UV source was placed 10 cm from the capillary, which was irradiated for 10 minutes. Unpolymerized bis-SorbPC monomer, from the regions not irradiated through the photomask, was removed using 1% (v/v) Triton X-100 surfactant and water after UV-polymerization.22
Capillary Imaging
Fluorescent images were collected on a Nikon Eclipse TE300 inverted epifluorescence microscope with a 4x / 0.13 N.A. objective. Images were collected using a Cascade 650 front illuminated CCD camera or MicroMAX 512BFT back illuminated CCD camera (Roper Scientific, Tucson, AZ). MetaVue software (Universal Imaging, Downingtown, PA) was used to capture and analyze all images.
Protein Adsorption Studies
Bare silica capillaries were prepared by cleaning the capillary with piranha solution for one hour, then rinsing with water for 1 hour. DOPC capillaries were coated by introducing a 1 mg/mL solution of unilamellar DOPC vesicles to the capillary and allowing the vesicles to fuse for 30 minutes.15 Excess DOPC was rinsed from the capillary with water, and DOPC coated capillaries were incubated with 6xHis-EGFP. Poly(bis-SorbPC) capillaries were prepared as described and rehydrated from dry storage conditions with water for 30 minutes prior to incubation with 6xHis-EGFP. All capillaries were incubated with a 100 µM solution of 6xHis-EGFP for 30 minutes, then rinsed thoroughly with 10 mM phosphate buffer, pH 7.4 before analysis.
Gradient Formation
Patterned poly(bis-SorbPC) capillaries were rehydrated from storage with water. Vesicle solution containing 1% w/w rhodamine-DPPE in 1 mg/mL DOPC was introduced into a capillary containing a poly(bis-SorbPC) pattern for 30 minutes to allow fusion into the bare regions of the pattern. Excess lipid was rinsed from the capillary using electrophoresis buffer. An electric field of 10 V/cm was applied across the capillary. Fluorescence images of the capillary were obtained to visualize the gradient formation.
Atomic Force Microscopy
Topographical studies of polymerized lipid films and bare capillaries were conducted with a Digital Instruments Nanoscope III Atomic Force Microscope (AFM). Tapping mode imaging was performed on samples that were obtained by fracturing bare or poly(bis-SorbPC)-coated 100 µm i.d. fused silica capillaries. With the use of an optical microscope, capillary fragments were positioned on double stick tape with the concave surface of the capillary interior facing outward and imaged.
Protein Separations
Insulin chain A (ICA), trypsin inhibitor (TI), bovine serum albumin (BSA), and α-chymotrypsinogen A (α-chymo) were hydrated in 10 mM NaHCO3, pH 9.2 buffer. Solutions were labeled with FITC at a 1:1 mole ratio of FITC:protein at room temperature for 3 hours. Solutions were diluted in 10 mM MOPS, pH 7.4 prior to separation to a final concentration of 49 nM ICA, 125 nM TI, 31 nM BSA and 45 nM α-chymo. The 488 nm line of a 100 mW argon ion laser (177-G12, Spectra-Physics) was focused into the interior of a 50 µm i.d capillary with a biconvex lens (f = 2 cm, Thor Labs, Newton, NJ). The fluorescence signal was collected with a 20x, 0.4 NA microscope objective (Melles Griot, Irvine, CA), passed through a 495 nm long pass filter (Edmund Optics, Barrington, NJ) and 488 nm holographic notch filter (HNF-488.0–1.0, Kaiser Optical Systems, Inc. Ann Arbor, MI). The signal was detected with a PMT (H8249, Hamamatsu Photonics, Bridgewater, NJ) and collected with an A/D converter (PCI-MIO-16E-4, National Instruments, Austin, TX) using Labview software written in-house (National Instruments). Samples were injected hydrodynamically for 5 sec at a height of 10 cm. Separations were performed at +24 kV using a Spellman CZE1000R power supply (Hauppauge, NJ).
Results and Discussion
PLB coatings are very promising for improving protein and peptide separations due to the high quantitative recovery and reproducibility, coupled with the native resistance to non-specific protein adsorption of these materials.15–16 However, the limited long-term stability of PLB coatings precludes widespread use and likely prevents coupling with MS detectors as desorption of lipids occurs when the network of relatively weak non-covalent interactions between the surface and the lipid and those between individual lipid monomers breaks down. The instability of PLBs in the presence of the organic additives needed to perform ESI-MS and/or potential residual surfactants from protein solubilization may further limit the utility of these promising materials. Recently, oligomerized DOPC PLB coatings were prepared that demonstrated substantial stabilization compared to unpolymerized PLB coatings, with ex situ polymerization increasing the stability of the coating significantly compared in situ polymerization,17 likely due to increased oligomerization efficiency.17
With the goal increasing the stability of PC-PLB coatings, we have explored the use of phospholipids that form highly cross-linked, polymeric PLBs. Polymeric PLBs were fabricated on the walls of fused silica separation capillaries in situ, as shown in Figure 1. SUVs prepared using bis-SorbPC, a reactive phospholipid that forms protein resistant bilayers on silica substrates,22;33;34 were allowed to fuse onto cleaned silica capillary surfaces to form PLBs. Once formed, bis-SorbPC PLBs were polymerized via radical initiated polymerization followed by removal of radical initiator solutions with H2O. The resulting poly(bis-SorbPC) coated capillaries were flushed with air and stored dry until use.
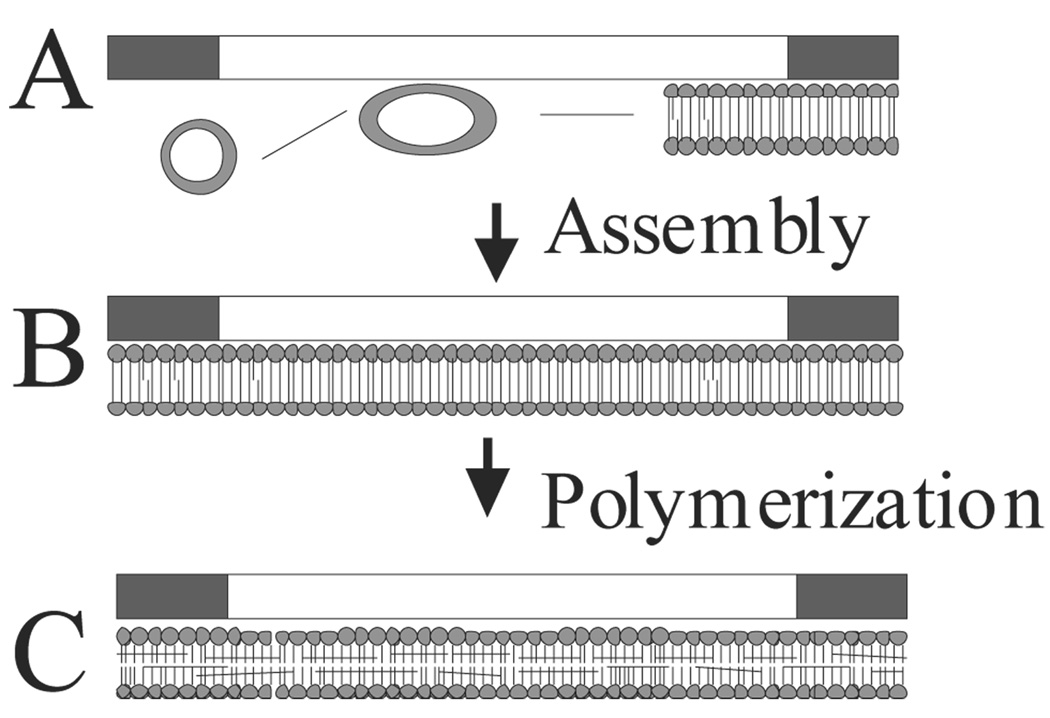
Figure 1.
Schematic of polymeric PLB capillary coating procedure. (A) SUVs are used to form PLBs on the capillary walls via vesicle fusion, resulting in a bilayer on the capillary surface (B). Radical initiators are introduced to the capillary to initiate polymerization of the PLB, resulting in a cross-linked PLB on the capillary wall (C).
Protein Absorption
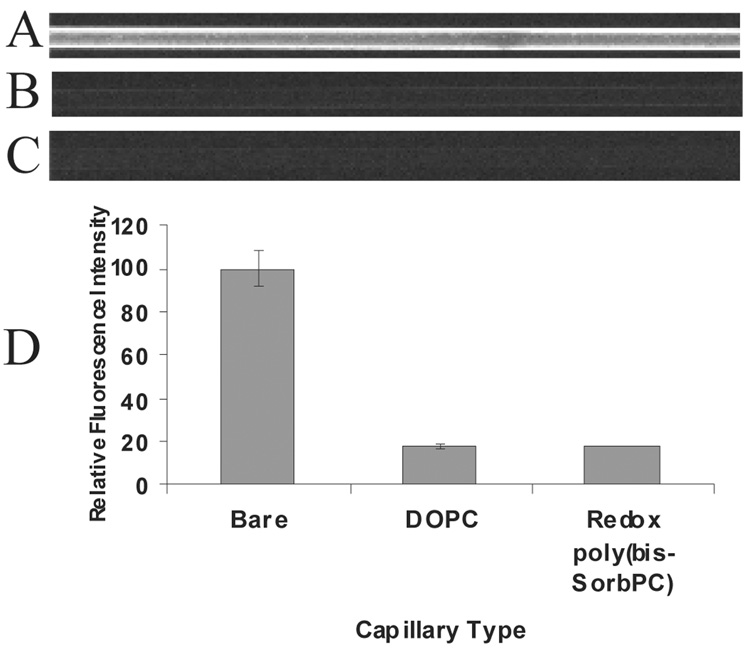
One particularly attractive attribute of zwitterionic PLBs is the native resistance to non-specific protein adsorption exhibited by the bilayer on a surface34 To investigate the reduced non-specific protein adsorption by poly-PLB coatings, capillaries with fluid and polymerized PLB coatings were filled with 100 µM enhanced green fluorescent protein (EGFP) and evaluated via fluorescence microscopy (Figure 2). When bare capillaries were exposed to EGFP, a uniform bright fluorescence was observed throughout the capillary indicating strong adsorption of EGFP (Figure 2A). In contrast, DOPC (Figure 2B) and poly(bis-SorbPC) (Figure 2C) coated capillaries show a marked reduction in fluorescence (18% each) relative to that of a bare capillary (Figure 2D) and are indistinguishable from one another, suggesting substantial reduction in non-specific adsorption of protein. The relative similarity in non-specific protein adsorption levels between DOPC and poly(bis-SorbPC) coated capillaries is in agreement with previous reports showing similar non-specific protein adsorption on poly(bis-SorbPC) and DOPC coated planar surfaces. 34 Unpolymerized bis-SorbPC has also been demonstrated to reduce non-specific adsorption of proteins,34 though the reduced stability of unpolymerized bis-SorbPC compared to native phospholipids likely precludes it from use as a capillary coating material.
Figure 2.
Resistance to non-specific protein absorption on lipid films measured using EGFP in (A) a bare capillary, (B) DOPC coated capillary, (C) redox poly(bis-SorbPC) coated capillary. (D) Mean fluorescence intensity data for bare and coated capillaries following introduction of EGFP (n = 4 for each bar).
Protein Separations
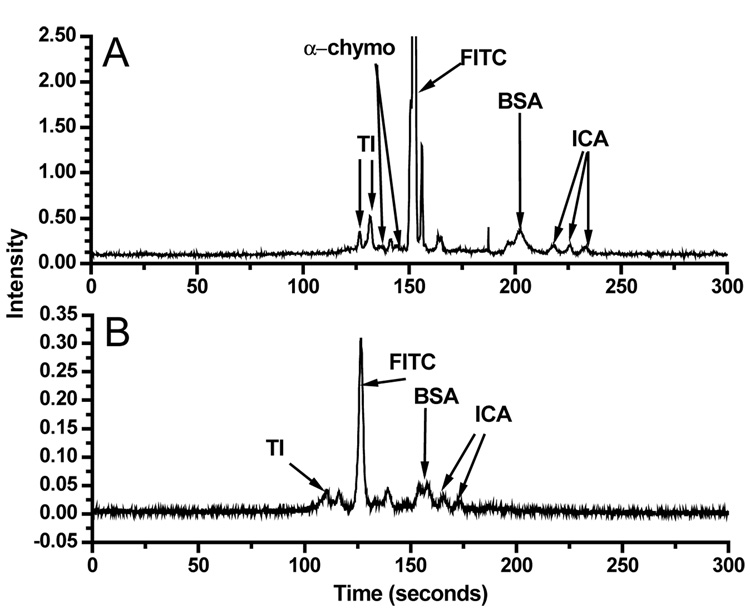
To evaluate the utility of stabilized poly(bis-SorbPC) coatings for protein separations, we performed separations on FITC-labeled cationic and anionic protein mixtures. Figure 3 shows a series of electropherograms obtained for FITC-labeled protein mixtures in poly(bis-SorbPC)-coated capillaries (Figure 3A) and bare capillaries (Figure 3B). As seen in Figure 3, separation of proteins in a poly(bis-SorbPC)-coated capillary provides increased resolution and allows more protein peaks to be observed and/or identified compared to separation in a bare capillary. These advantages are most notable for α-chymotrypsinogen, which was not detected in a bare capillary. Additionally, the doublet resulting from trypsin inhibitor was not resolved, and the third peak associated with insulin chain A was not observed in a bare capillary. Though, the enhanced efficiency, resolution and improved peak recovery are lower than those reported in other PLB coated capillaries,15;17 the enhanced stability of the film may provide advantages that temper the compromises in separation parameters. Importantly, under these separation conditions, bis-SorbPC capillaries could be used to separate proteins for up to 10 hours per day with similar results, as well as the ability to use the same coated capillary for 10 days with no degradation in separation performance (See supporting information). Further, the poly(bis-SorbPC) coating was stable to drying and rehydration, conditions that would destroy a fluid lipid coated capillary, providing for long term storage and utilization of the capillaries.
Figure 3.
Protein separations in coated and uncoated capillaries. (A) Poly(bis-SorbPC) coated capillary separation of four FITC-labeled proteins. (B) Bare capillary separation of same mixture used in A.
EOF Measurements
In previous reports of PLB-coated capillaries, the presence of lipid bilayers on the surface was established through shifts in the electroosmostic flow (EOF). EOF values obtained in (i) bare, (ii) non-polymerizable lipid coated, and (iii) poly(bis-SorbPC) coated capillaries are shown in Table 1. All of the lipid coatings investigated show a reduced EOF compared to a bare capillary. For non-polymerizable PLB coatings (e.g. DLPC, EggPC), EOF values below 1.5 × 10−4 cm2/Vs are routinely obtained, agreeing well with previous reports.15 Poly(bis-SorbPC)-coated capillaries, however, result in EOF values 2–3 times larger that those obtained for non-polymerizable lipids. The difference in EOF values obtained between a DOPC and poly(bis-SorbPC) coatings may result from non-uniform coatings, small film defects (holes) that can occur during the polymerization process or the shielding capacity of the lipid film itself. Importantly, the enhanced EOF and similar reduction in non-specific adsorption compared to PC-PLB coated capillaries allows separation of both cationic and anionic proteins in the same run unlike PC-PLBs where the polarity must be switched and separations performed differentially.
Table 1.
EOF values for PLB-coated capillaries.
| Capillary Coating | EOF (cm2/Vs) |
%RSD | N |
|---|---|---|---|
| Bare | 5.0 × 10−4 | 12 | 8 |
| DLPC | 0.6 × 10−4 | 10 | 3 |
| EggPC | 1.3 × 10−4 | 6 | 5 |
| poly(bis-SorbPC) | 3.4 × 10−4 | 16 | 10 |
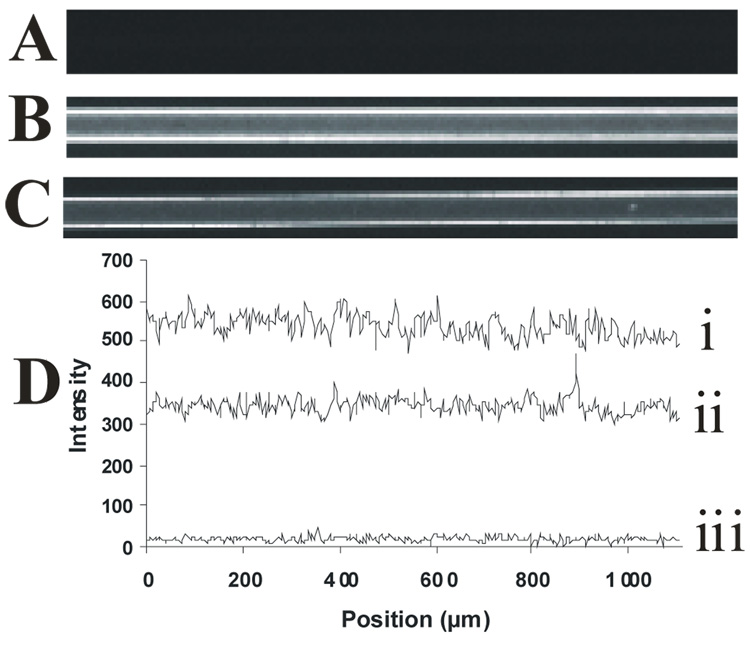
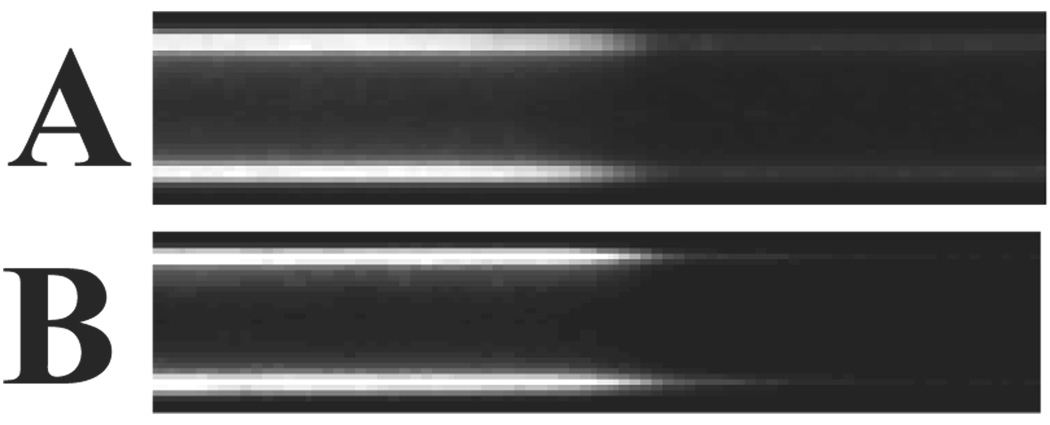
To characterize the continuity of the lipid film within the capillary, a fluorogenic membrane probe (FM 1–43) was used to stain the PLB coatings assembled onto the capillary walls.22 Figure 4 shows fluorescence images of (A) bare, (B) DOPC-coated, and (C) poly(bis-SorbPC)-coated capillaries. The interior walls of the bare fused silica capillary are not fluorescent (Figure 4A), an observation that is consistent with a clean, membrane-free silica surface. In capillaries where PLBs were formed on the capillary walls (Figure 4B, 4C), fluorescence resulting from FM 1–43 is observed via uniform staining throughout the capillary, indicating that the vesicle fusion procedure produces a PLB along the length of the capillary, with no obvious large scale defects. Figure 4D shows a series of line scans corresponding to the fluorescence intensity along a 1 mm length of capillary. Though only 1 mm sections are shown in the images (Figures 4A– 4C), uniform fluorescence was observed along the entire length of the capillary (data not shown). DOPC coated capillaries and poly(bis-SorbPC) coated capillaries show similar fluorescence levels, with the difference in fluorescence intensities between DOPC-coated capillaries and poly(bis-SorbPC)-coated capillaries likely due to differences in FM 1–43 intercalation into the polymerized PLB compared to the unpolymerized PLB. Further, the staining with FM 1–43 confirms that the poly(bis-SorbPC)-PLB is stabilized against rinsing and drying conditions and polymerization conditions, as the poly(bis-SorbPC) coated capillaries were prepared using these conditions.
Figure 4.
Uniformity of PLB coatings formed in situ. Fluorescence images were collected following introduction of the fluorogenic membrane stain, FM 1–43, into (A) bare capillary, (B) DOPC-coated capillary, and (C) poly(bis-SorbPC)-coated capillary. (D) Line scans obtained from regions along the capillary wall, showing the relative fluorescence intensities as a function of position along the capillary i) DOPC capillary, ii) bis-SorbPC capillary, iii) bare capillary. All capillaries are 50 µm i.d.
In the absence of obvious large scale defects in the fluorescence images, the presence of pin-hole or small scale defects leading to increased EOF was investigated. Planar, poly(bis-SorbPC) PLBs form uniform coatings on silica substrates that are defect free on the spatial scale probed by AFM.33;34 Addtionally, we have observed that poly(bis-SorbPC) vesicles contain a number of small defects in the membrane,44 likely due to cleft formation upon polymerization.41;42
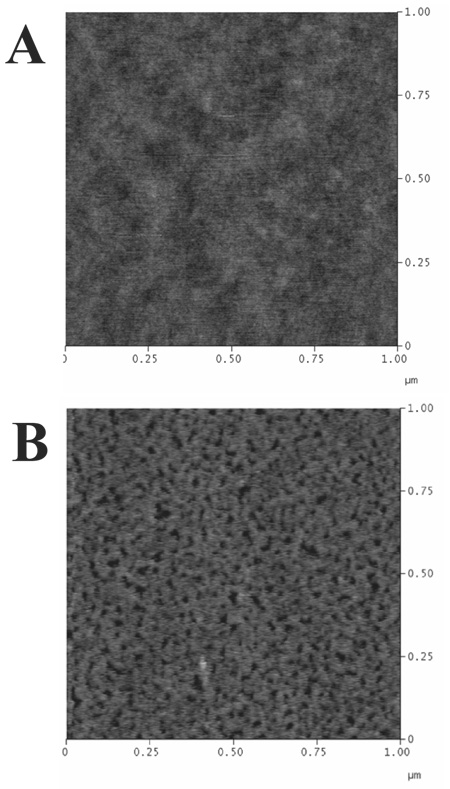
To determine if such PLB coatings are attainable on capillary walls, AFM was used to investigate the topography of the polymer lipid film following assembly on the capillary wall. Sections of capillary were shattered and samples with suitable surface area of exposed inner capillary were carefully selected under an optical microscope for investigation. Figure 5 shows representative AFM images collected for bare (5A) and poly(bis-SorbPC) (5B) coated fused silica capillaries. The AFM image of the bare capillary yielded an rms surface roughness of 0.25 nm, comparable in roughness to a piranha washed standard glass microscope slide or commercially available fused silica coverslip (rms surface roughness of 0.13 nm).34 By comparison, the poly(bis-SorbPC) film exhibited an increased rms surface roughness (0.60 nm), with a number of submicron pits and/or defects apparent, compared to planar silica substrates (rms surface roughness of 0.13 nm).34 It is unlikely that the geometry of the capillary affects the polymerization of bis-SorbPC which is routinely polymerized in small unilamellar vesicles (ca. 100 nm diameter) with markedly higher radius of curvature, though it is possible that polymerization conditions may play a role in the different topography observed. Planar polymerizations involve exposure of the bis-SorbPC film to a quiescent solution of initiators, whereas a steady flow is utilized in capillaries to ensure sufficient initiator is present for polymerization. It is possible that continuous flow may partially destabilize an unpolymerized bis-SorbPC film, which is less stable in the unpolymerized state relative to non-reactive lipids due to the presence of polar groups in the bilayer center.41;42 The presence of these sub-micron defects likely results in the increased EOF observed due to the increased contact of ions with the charged capillary wall and the decreased insulating properties of the bilayer, warranting further investigation of alternative polymerization methods, and/or lipid structures in future studies. Combined, these fluorescence and AFM imaging data strongly support the presence of a stabilized, uniform polymer film, with no observed multilayer structures, large gaps in coverage, or partially ruptured vesicles.
Figure 5.
Tapping mode AFM images of A) bare and B) poly(bis-SorbPC)-coated fused silica capillaries.
Coating Stability
The ability to store coated capillaries greatly reduces the timely regeneration protocol needed for other lipid coatings,15 and allows for direct shelf-to-bench laboratory use. Storage stability can also lead to increased reproducibility since the polymer lipid coating does not require daily regeneration. To determine the suitability of poly(bis-SorbPC) coatings for extended storage, capillaries were prepared that contained alternating regions of bare and poly(bis-SorbPC)-coated capillaries (Figure 6). We have previously shown that UV-polymerized (poly)bis-SorbPC chemical patterns are stable when exposed to solutions of 1% Triton, extensive water/buffer rinses, pH extremes, and drying/rehydration, which allow for long-term storage of the coated capillaries and increased stability needed for separations.22 Further, Saavedra and coworkers have shown the stability of poly(bis-SorbPC) films to a number of organic solvents.33;34 Figure 6 shows representative FM 1–43 stained poly(bis-SorbPC) coated capillaries imaged immediately after fabrication and after 45 days of dry storage. The capillary imaged on day 1 (Figure 6A) was rinsed with pH 7.4 buffer post-polymerization and then immediately stained with FM 1–43. Following imaging, the capillary was rinsed for 5 min with 1% Triton X-100 to remove FM 1–43, rinsed with H2O, purged with air and stored dry until day 45, when it was stained with FM 1–43 and imaged (Figure 6B). Capillaries containing poly(bis-SorbPC) bilayers are stable in excess of one year of dry storage (n = 4). Such storage conditions are not possible with unpolymerized or oligomerized PLB coated capillaries, due to the fragility of fluid PLBs.
Figure 6.
Time stability of poly(bis-SorbPC) coated capillaries. Fluorescent images of the same capillary were collected on day 1 (A) and after 45 days dry storage (B) using FM 1–43.
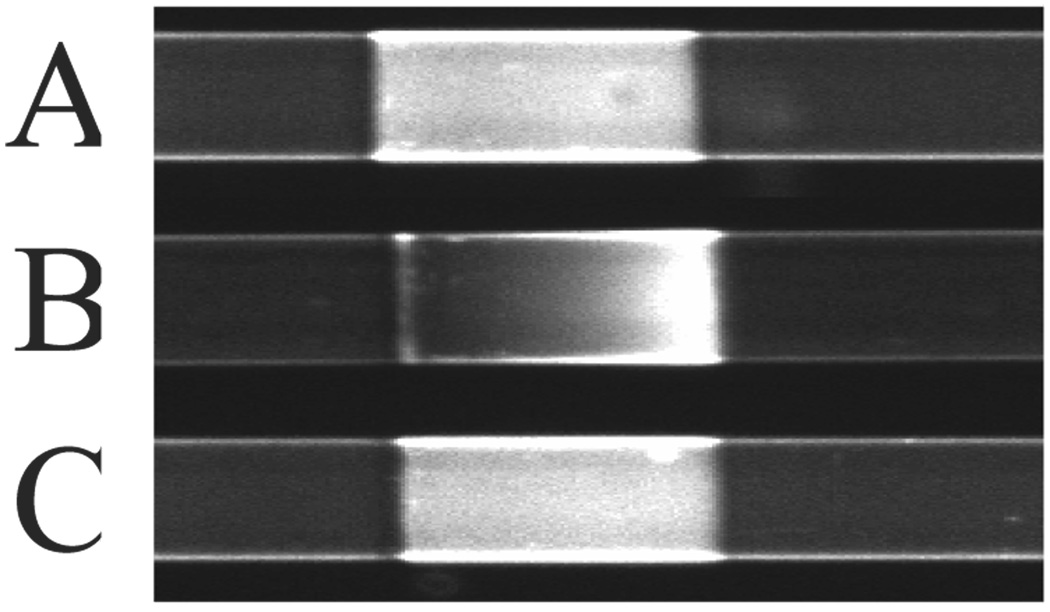
In addition to storage, stability of the poly(bis-SorbPC)-coating under CZE conditions must be considered. A number of investigations have shown that charged lipids in PLBs migrate in the presence of an electric field.23–25 Though zwitterionic PC lipids should exhibit limited lateral mobility in an electric field, a primary advantage of PLB coatings is the ability to add chemical functionality and/or manipulate the surface charge via incorporation of chemically-functionalized lipids. To evaluate the lateral mobility of polymerized lipid films in response to an electric field, a fluid lipid mixture containing 1% rhodamine-DPPE in 99% DOPC was fused into the bare regions of a poly(bis-SorbPC) patterned capillary. Figure 7A shows a fluorescence microscopy image of an alternating pattern of poly(bis-SorbPC) regions (dark) and rhodamine-DPPE doped DOPC (light). Prior to the application of an electric field (Figure 7A), rhodamine-DPPE uniformly fills the area between poly(bis-SorbPC) regions. When an electric field of only 10 V/cm is applied across the capillary (Figure 7B), rhodamine-DPPE moves through the unpolymerized region via electromigration. Upon reaching the poly(bis-SorbPC) boundary, the lipid is corralled resulting in the formation of a gradient of rhodamine-DPPE. The polymerization of bis-SorbPC stabilizes the lipid, allowing it to remain in place and preventing penetration of the rhodamine-DPPE, which clearly migrates through the unpolymerized DOPC. Upon removal of the electric field, the rhodamine-DPPE laterally diffuses to restore the original uniform fluorescence (Figure 7C). Though it is possible to add functionality to fluid lipid PLBs via doping of varying headgroup chemistries, these data demonstrate the need to stabilize the PLB to prevent electromigration and non-uniform surface coverage of the doped lipids, an important first step in the preparation of chemically functionalized PLBs for electrophoretic separations.
Figure 7.
Electric field stability of poly(bis-SorbPC) regions. (a) 1% rhodamine lipid doped DOPC prior to application of electric field (b) after application of 10 V/cm electric field for 10 minutes.
Conclusions
Utilization of highly cross-linked, poly(bis-SorbPC) provides the first example of highly stabilized PLB capillary coatings for protein separations. The resulting coatings display a high degree of resistance to non-specific protein adsorption, coupled with markedly increased capillary stability to harsh chemical conditions, as well as long-term storage. minimal reduction in EOF in the coated capillary provides the basis for simultaneous separation of anionic and cationic proteins. The stability of poly(bis-SorbPC) capillaries is clearly an advantage for protein separations and potentially for multidimensional, post-separation analysis, e.g. CE-MS. Additionally, the ability to incorporate functionalized lipids that may be used to modify the charge or binding capabilities of the surface, without compromising the lipid film, provides a platform upon which a number of novel chemical separations can be designed. Though separation efficiencies are lower than previously reported PC-PLB coatings, the results presented here are promising and warrant further investigation to identify and characterize highly stable PLB coatings that are highly uniform, possess minimal defect density and to increase the quality of the separation.
Supplementary Material
Migration time reproducibility for bare and bis-SorbPC coated capillaries is available as supporting information.
Acknowledgments
This work was supported in part by the National Institutes of Health, GM74522. We thank Dr. Ronald Wysocki in the UA Chemical Synthesis Facility for synthesis of bis-SorbPC used in this work
References
- 1.Towns JK, Regnier FE. Anal. Chem. 1991;63:1126–1132. doi: 10.1021/ac00011a013. [DOI] [PubMed] [Google Scholar]
- 2.Doherty EAS, Meagher RJ, Albarghouthi MN, Barron AE. Electrophoresis. 2003;24:34–54. doi: 10.1002/elps.200390029. [DOI] [PubMed] [Google Scholar]
- 3.Lucy CA, Yeung KKC. Anal. Chem. 1997;69:3435–3441. doi: 10.1021/ac961231k. [DOI] [PubMed] [Google Scholar]
- 4.Iki N, Yeung ES. J Chromatogr. A. 1996;731:273–282. [Google Scholar]
- 5.Gilges M, Kleemiss MH, Schomburg G. Anal. Chem. 1994;66:2038–2046. [Google Scholar]
- 6.Hjertén S, Kubo K. Electrophoresis. 1993;14:390–395. doi: 10.1002/elps.1150140164. [DOI] [PubMed] [Google Scholar]
- 7.McCormick RM. Anal. Chem. 1988;60:2322–2328. doi: 10.1021/ac00172a003. [DOI] [PubMed] [Google Scholar]
- 8.Towns JK, Regnier FE. Anal. Chem. 1992;64:2473–2478. [Google Scholar]
- 9.Bruin GJM, Change JP, Kuhlman RH, Zegers K, Kraak JC, Poppe HJ. Chromatogr. 1989;471:429–436. [Google Scholar]
- 10.Horvath J, Dolnik V. Electrophoresis. 2001;22:644–655. doi: 10.1002/1522-2683(200102)22:4<644::AID-ELPS644>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Belder D, Deege A, Husmann H, Kohler F, Ludwig M. Electrophoresis. 2001;22:3813–3818. doi: 10.1002/1522-2683(200109)22:17<3813::AID-ELPS3813>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Righetti PG, Gelfi C, Verzola B, Castelletti L. Electrophoresis. 2001;22:603–611. doi: 10.1002/1522-2683(200102)22:4<603::AID-ELPS603>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Melanson JE, Baryla NE, Lucy CA. TrAC-Trends in Anal. Chem. 2001;20:365–374. [Google Scholar]
- 14.Yassine MM, Lucy CA. Anal. Chem. 2004;76:2983–2990. doi: 10.1021/ac035372f. [DOI] [PubMed] [Google Scholar]
- 15.Cunliffe JM, Baryla NE, Lucy CA. Anal. Chem. 2002;74:776–783. doi: 10.1021/ac015627u. [DOI] [PubMed] [Google Scholar]
- 16.Varjo SJO, Hautala JT, Wiedmer SK, Riekkola M-L. J. Chromatogr. A. 2005;1081:92–98. doi: 10.1016/j.chroma.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Lucy CA. Anal. Chem. 2005;77:2015–2021. doi: 10.1021/ac0489622. [DOI] [PubMed] [Google Scholar]
- 18.Preisler J, Yeung ES. Anal. Chem. 1996;68:2885–2889. doi: 10.1021/ac960260s. [DOI] [PubMed] [Google Scholar]
- 19.Lindén MV, Wiedmer SK, Hakala RMS, Riekkola M-L. J. Chromatogr. A. 2004;1051:61–68. [PubMed] [Google Scholar]
- 20.Glasmästar K, Larsson C, Höök F, Kasemo B. J. Colloid and Interface Sci. 2002;246:40–47. doi: 10.1006/jcis.2001.8060. [DOI] [PubMed] [Google Scholar]
- 21.Sackmann E. Science. 1996;271:43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- 22.Ross EE, Mansfield E, Aspinwall CA. J. Am. Chem. Soc. 2005;127:16756–16757. doi: 10.1021/ja0539995. [DOI] [PubMed] [Google Scholar]
- 23.Cremer PS, Groves JT, Kung LA, Boxer SG. Langmuir. 1999;15:3893–3896. [Google Scholar]
- 24.Hovis JS, Boxer SG. Langmuir. 2000;16:894–897. [Google Scholar]
- 25.Jung S-Y, Holden MA, Cremer PS, Collier CP. ChemPhysChem. 2005;6:423–426. doi: 10.1002/cphc.200400540. [DOI] [PubMed] [Google Scholar]
- 26.Raffy S, Teissie J. Biophys. Journal. 1999;76:2072–2080. doi: 10.1016/S0006-3495(99)77363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graff A, Winterhalter M, Meier W. Langmuir. 2001;17:919–923. [Google Scholar]
- 28.Hotz J, Meier W. Langmuir. 1998;14:1031–1036. [Google Scholar]
- 29.Nikolelis DP, Drivelos DA, Simantiraki MG, Koinis S. Anal. Chem. 2004;76:2174–2180. doi: 10.1021/ac0499470. [DOI] [PubMed] [Google Scholar]
- 30.Nikolelis DP, Mitrokotsa M. Biosensors and Bioelectronics. 2002;17:565–572. doi: 10.1016/s0956-5663(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 31.Holden MA, Jung S-Y, Yang T, Castellana ET, Cremer PS. J. Am. Chem. Soc. 2004;126:6512–6513. doi: 10.1021/ja048504a. [DOI] [PubMed] [Google Scholar]
- 32.Gomes JFPdS, Sonnen AFP, Kronenberger A, Fritz J, Coelho MÁN, Fournier D, Fournier-Nöel C, Mauzac M, Winterhalter M. Langmuir. 2006;22:7759. doi: 10.1021/la0613575. [DOI] [PubMed] [Google Scholar]
- 33.Ross EE, Rozanski LJ, Spratt T, Liu S, O'Brien DF, Saavedra SS. Langmuir. 2003;19:1752–1765. [Google Scholar]
- 34.Ross EE, Spratt T, Liu S, Rozanski LJ, O'Brien DF, Saavedra SS. Langmuir. 2003;19:1766–1774. [Google Scholar]
- 35.Singh A, Schnur JM. Polymers for Advanced Technologies. 1993;5:358–373. [Google Scholar]
- 36.Morigaki K, Baumgart T, Offenhäusser A, Knoll W. Angew.Chem.Int.Ed. 2001;40:172–174. doi: 10.1002/1521-3773(20010105)40:1<172::AID-ANIE172>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 37.Stanish I, Santos JP, Singh A. J. Am. Chem. Soc. 2001;123:1008–1009. doi: 10.1021/ja0056623. [DOI] [PubMed] [Google Scholar]
- 38.Yan-Lei Su, Jin-Ru Li, Long Jiang. Colloids and Surfaces A-Physicochemical and Engineering Aspects. 2005;257–258:25–30. [Google Scholar]
- 39.Morigaki K, Schonherr H, Frank CW, Knoll W. Langmuir. 2003;19:6994–7002. [Google Scholar]
- 40.Shenoy DK, Barger WR, Singh A, Panchal RG, Misakian M, Stanford VM, Kasianowicz JJ. NanoLetters. 2005;5:1181–1185. doi: 10.1021/nl050481q. [DOI] [PubMed] [Google Scholar]
- 41.Lamparski H, O'Brien DF. Macromolecules. 1995;28:1786–1794. [Google Scholar]
- 42.Lamparski H, Liman U, Barry JA, Frankel DA, Ramaswami V, Brown MF, O'Brien DF. Biochemistry. 1992;31:685–694. doi: 10.1021/bi00118a008. [DOI] [PubMed] [Google Scholar]
- 43.Ross EE, Bondurant B, Spratt T, Conboy JC, O'Brien DF, Saavedra SS. Langmuir. 2001;17:2305–2307. [Google Scholar]
- 44.Cheng Z, D’Ambruoso GD, Aspinwall CA. Langmuir. 2006;22:9507–9511. doi: 10.1021/la061542i. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Migration time reproducibility for bare and bis-SorbPC coated capillaries is available as supporting information.