Abstract
Ovariectomized rats were hormonally primed with 10 μg estradiol benzoate or with estradiol benzoate plus 500 μg progesterone. Rats received a bilateral infusion with 200 ng of the 5-HT1B/1D receptor antagonist, N-[4-methoxy-3-(4-methyl-1-piperazinyl)phenyl]-2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)-1-1′-biphenyl-4-carboxamide hydrochloride (GR 127935), into the ventromedial nucleus of the hypothalamus (VMN), followed by a 5 min restraint or home cage experience. In estrogen-primed females that had experienced minimal handling between ovariectomy and use in the experiment, infusion with the water vehicle transiently inhibited lordosis behavior, and the 5-HT1B/1D receptor antagonist amplified this inhibition. There were no effects in rats hormonally primed with estrogen and progesterone. Handling for two days before the experiment reduced the effects of the infusions in estrogen-primed rats. However, when a 5 min restraint experience followed infusion with GR 127935, there was a significant decline in lordosis behavior that persisted for 10 to 15 min after the experience. Regardless of the prior experience or type of infusion, the addition of progesterone to the hormonal priming completely prevented the lordosis inhibition. These findings are consistent with prior evidence that progesterone protects against the inhibitory effects of a 5 min restraint experience on lordosis behavior. Moreover, these are the first experiments to demonstrate an inhibitory effect of a selective 5-HT1B/1D receptor antagonist in the VMN on lordosis behavior of estrogen primed, but not estrogen and progesterone primed, ovariectomized rats.
Keywords: stress, restraint, sexual behavior, reproduction, hypothalamus, ventromedial nucleus
Introduction
The lordosis reflex is a supraspinal reflex, made by sexually receptive female rodents in response to somatosensory stimuli by the male (Pfaff and Modianos, 1985). The reflex is orchestrated by estrogen and progesterone priming, but only estrogen is required (Pfaff and Modianos, 1985; Sodersten, 1981). Progesterone increases lordosis frequency when combined with relatively low doses of estrogen (Sodersten, 1981), but a variety of neurotransmitters, including serotonin (5-HT), influence whether or not lordosis behavior will occur (Bethea, et al., 2002; Etgen, et al., 2001; Fink, et al., 1996; Frye, 2007; Kow and Pfaff, 1985; McQueen, et al., 1997). Depending on the receptor subtype activated, 5-HT receptor agonists can either inhibit or facilitate lordosis behavior (Mendelson, 1992; Uphouse, 2000). Activation of 5-HT1A receptors in the ventromedial nucleus of the hypothalamus (VMN) are primarily responsible for the lordosis-inhibiting effects of 5-HT (Uphouse, et al., 1996; Uphouse and Caldarola-Pastuszka, 1993). In contrast, activation of 5-HT2A/2C receptors facilitates the behavior (Gonzalez, et al., 1997; Wolf, et al., 1998) and attenuates lordosis inhibition mediated by 5-HT1A receptors in the VMN (Uphouse, et al., 1994). Hormonal priming that facilitates lordosis behavior reduces functioning of 5-HT1A receptors (Mize and Alper, 2000; Mize, et al., 2003) and enhances that of 5-HT2A/2C receptors (Ansonoff and Etgen, 1998; Cyr, et al., 1998; Gorosito and Cambiasso, 2008; Karp, et al., 2007; Marino, et al., 2006; Sumner and Fink, 1995; Sumner and Fink, 1997).
Nevertheless, in a naturally cycling rat, 5-HT1A receptor agonists continue to be effective in reducing lordosis behavior of sexually receptive, proestrous female rats (Uphouse, 2000). Therefore, additional mechanisms must be involved to reduce 5-HT1A receptor-mediated inhibition so that sexual behavior emerges. One such mechanism may reside in the modulation of extracellular 5-HT in the mediobasal hypothalamus (MBH). As the naturally cycling female moves toward the sexually receptive state, there is a decline in extracellular 5-HT in the mediobasal hypothalamus (MBH) that appears to result from the effects of progesterone (Farmer, et al., 1996; Gundlah, et al., 1998; Maswood, et al., 1999). Consequently, while estrogen may reduce 5-HT1A receptor efficiency within the MBH, progesterone decreases the amount of 5-HT available to activate the inhibitory 5-HT1A receptors.
Since 5-HT1B receptors, located on terminals of 5-HT neurons, function as autoreceptors to control 5-HT release (Daws, et al., 2000; Engel, et al., 1986; Maura, et al., 1986; Riad, et al., 2000), these receptors might be expected to play a positive role in control of lordosis behavior. Furthermore, progesterone has been suggested to increase 5-HT1B receptors in the MBH (Frankfurt, et al., 1994). A facilitatory role for 5-HT1B receptors in the 5-HT nerve terminal region of the VMN has been suggested (Frankfurt et al., 1994; Mendelson, 1992; Mendelson and Gorzalka, 1986), and a variety of nonselective 5-HT1B receptor agonists, but not all (Hunter, et al., 1985), can increase lordosis behavior (Aiello-Zaldivar, et al., 1992; Mendelson, 1992; Mendelson and Gorzalka, 1990). However, until recently, selective 5-HT1B agents were not available for evaluation of this suggestion (Sari, 2004). In the following experiments, the 5-HT1B/1D receptor antagonist, N-[4-methoxy-3-(4-methyl-1-piperazinyl)phenyl]-2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)-1-1′-biphenyl-4-carboxamide hydrochloride (GR 127935), (Liao, et al., 2000) was employed. If rats, hormonally primed with estrogen and progesterone (EP rats), have enhanced functioning and/or density of 5-HT1B receptors in the VMN, EP rats should be less sensitive to a 5-HT1B receptor antagonist agonist than rats hormonally primed only with estrogen (EO rats). As reported below, this appears to be the case.
Additionally, experiments are described that were designed to test the hypothesis that progesterone’s modulation of 5-HT1B receptors contributes to the hormone’s protection against a 5 min restraint stress. In previous studies, we reported that 5 min restraint transiently reduced lordosis behavior of EO rats but had no effect in EP rats (Truitt, et al., 2003; White and Uphouse, 2004). Since stress increases release of 5-HT in a variety of brain areas (Beekman, et al., 2005; Fujino, et al., 2002; Kirby, et al., 1997), including the MBH (Shimizu, et al., 1992), a progesterone-mediated enhancement of terminal 5-HT1B receptor functioning could be involved in the EP rats’ resistance to stress. It was, therefore, hypothesized that antagonism of 5-HT1B receptor function would enhance the vulnerability of EP rats to this restraint. However, while GR 127935 did amplify the effect of restraint in EO rats, there was no such amplification in EP rats.
Methods
Materials
Estradiol benzoate, progesterone, sesame seed oil, and GR 127935 (5-HT1B/1D receptor antagonist; N-[4-methoxy-3-(4-methyl-1-piperazinyl)phenyl]-2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)-1-1′-biphenyl-4-carboxamide hydrochloride) were purchased from Sigma-Aldrich Co. (St. Louis, MO). Isoflurane (AErrane®) and suture material (vicryl) were purchased from Henry Schein (Melville, NY). Intracranial (i.c.) cannulae were obtained from Plastics One (Roanoke, VA) and dental acrylic was purchased from Reliance Dental (Worth, IL). Disposable Decapicone® restrainers were purchased from Braintree Scientific Inc. (Braintree, MA). All other supplies came from Fisher Scientific (Houston, TX).
Animals and housing
Female, adult Fischer (F-344) rats were purchased from Charles River Laboratories (Wilmington, MA). Rats were housed 2 or 3 per cage in polycarbonate shoebox cages in a colony room maintained at 25 °C and 55% humidity, with lights on from 12 midnight to 12 noon. Food and water were available ad lib. All procedures were conducted according to PHS policy and were approved by the IACUC at Texas Woman’s University.
Surgical procedures and hormone treatments
Females, weighing between 140 and 170 g (approximately 60 to 90 days of age), were anesthetized with AErrane® and stereotactically implanted bilaterally with 22-gauge stainless steel cannulae directed toward the VMN [atlas coordinates AP 4.38; DV - 7.8; ML ± 0.4 (König and Klippel, 1963)] as previously described (Uphouse, et al., 1992). Two weeks later, rats were ovariectomized under AErrane® anesthesia (White and Uphouse, 2004) and injected two weeks later with 10 μg of estradiol benzoate followed 48 hr later with sesame seed oil or 500 μg progesterone. Hormones were dissolved in sesame seed oil and were injected subcutaneously (s.c.) in a volume of 0.1 ml/rat. Behavioral testing occurred 4 to 6 hr after progesterone or oil treatment.
Testing for sexual receptivity
On the morning of testing (prior to lights out), rats were moved to the testing room where the males were housed. Testing for sexual behavior, as previously described (Uphouse et al., 1992), was initiated within 1 to 3 hr after colony lights off and 4 to 6 hr after the progesterone or oil injection. Visibility was aided by red lighting. In a pretest for sexual receptivity, females were placed in the home cages of sexually experienced Sprague-Dawley male rats and behavior was monitored until the male had accomplished 10 mounts; rats with a pretest lordosis to mount (L/M) ratio of 0.7 or higher were included in the remaining experiments. Lordosis quality and proceptivity, as previously described (Hardy and DeBold, 1971; White and Uphouse, 2004), were also recorded. Following the pretest, rats were infused with GR 127935 or water and treated as described in the specific experiments. GR 127935 was dissolved in distilled/deionized water immediately before use. Infusions were delivered at a rate of 0.24–0.26 μl/min to a final infusion volume of 0.5 μl per cannula. Intracranial drug concentrations are indicated as the amount of drug infused per bilateral cannula or one-half the concentration per animal. Sexual behavior testing continued for 30 consecutive min as previously described (Uphouse et al., 2003).
Specific experimental procedures
A total of 111 rats were used in three separate experiments. The number of rats used in the first, second and third experiments, respectively, were 26, 32 and 53. In the first experiment, rats were not specifically handled between ovariectomy and use in the experiment. EO or EP rats were infused intracranially with water or 200 ng GR 127935 and tested immediately after infusion. In the second and third experiments, rats were specifically handled, as described below, prior to the day of infusion. In the second experiment, rats were infused and tested as in the first experiment. In the third experiment, rats were either restrained or remained in the home cage for 5 min following infusion and were tested immediately after the 5 min experience.
Histological procedures
After behavioral testing, rats were anesthetized with AErrane® and perfused with 0.9% saline followed by 10% buffered formalin. Brain tissue was placed into 10% buffered formalin for at least 24 h before sectioning. Coronal sections (100 μm) were stained with cresyl violet and examined for cannulae placement (König and Klippel, 1963). Only data from rats with cannulae in the MBH in the vicinity of the VMN were included in the study.
Handling procedures
On the day of the estrogen injection, each rat was picked up, held for 15 sec and turned as in preparation for an intraperitoneal injection. This procedure was then repeated and constituted a handling session. After all rats in the cage had received a handling session, the procedure was repeated until each rat had received 5 handling sessions. The entire process was repeated the following day.
Restraint procedures
Restraint procedures were as previously described (Uphouse, et al., 2007). After infusion, the female was placed head first into the Decapicone® so that her nose was flush with the small opening at the tip of the cone. A longitudinal slit was made along the cone to allow room for the top of the guide cannulae. The slit was then secured with lab tape after the female’s nose was flush with the air hole. The base of the cone was gathered around the female’s tail and secured tightly with tape. The process of wrapping the female required between 30 and 60 sec. The wrapped female was set aside for 5 min. Nonrestrained rats were returned to their home cages for 5 min.
Statistical procedures
For statistical purposes, L/M ratios, lordosis quality scores and number of mounts by the male were grouped into the pretest interval and five-min intervals after infusion or restraint. Data were evaluated by repeated measures ANOVA with hormone, type of infusion and/or type of experience as the independent factors. Time after infusion (or experience in experiment 3) was the repeated factor. Differences between treatment groups, within time intervals, were compared with Tukey’s test. Comparisons within groups, across time, were made with Dunnett’s test to the pretest interval. An alpha level of 0.05 was required for rejection of the null hypothesis (Zar, 1999).
Results
Experiment 1
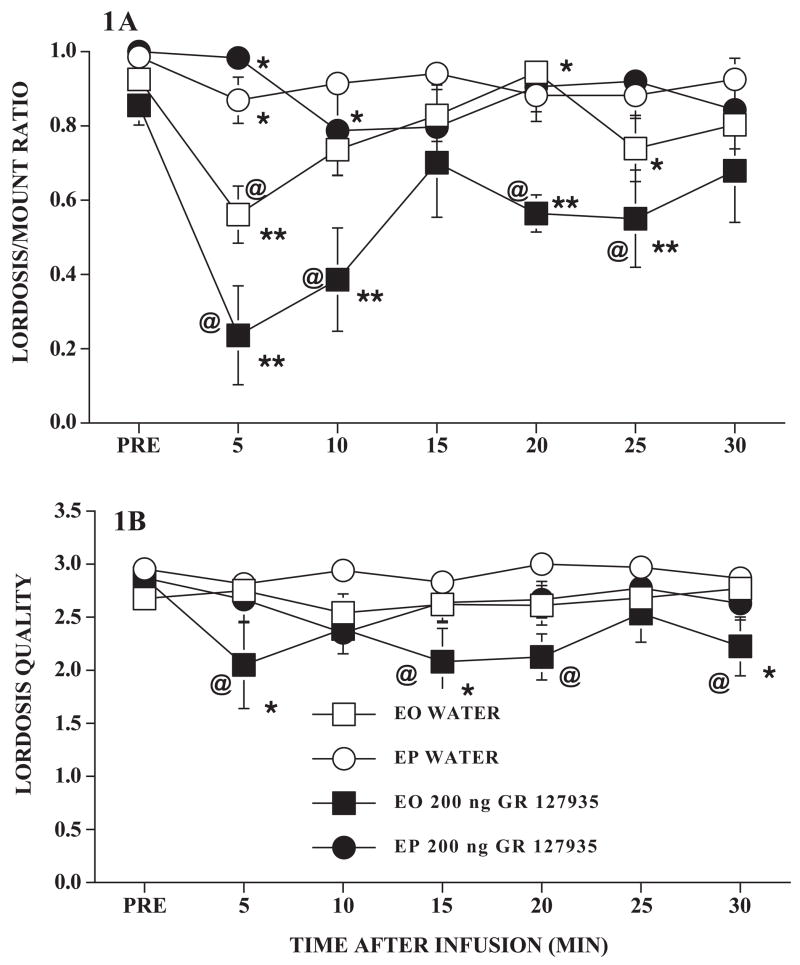
As expected, GR 127935 reduced lordosis behavior of EO, but not EP, rats (Figure 1A). However, infusion with the water vehicle also reduced sexual behavior of EO rats. There were significant effects of hormone treatment (F1,22 = 25.6, P ≤ 0.0003), time after infusion (F6,132 = 6.47, P ≤ 0.0001), and their interaction (F6,132 = 4.88, P ≤ 0.0002). Infusion with GR 127935 led to greater decrements in the L/M ratio than did the water infusion (F1,22 = 7.83, P ≤ 0.02) that was primarily evident in the EO rats (interaction between type of infusion and hormone, F1,22 = 5.12, P ≤ 0.04). The water infusion reduced L/M ratios of EO rats at the 5 min test time (Dunnett’s q120,7 = 3.75, P ≤ 0.05) while infusion with GR 127935 reduced the L/M ratios of EO rats at 5, 10, 20 and 25 min after infusion (Dunnett’s q120,7 ≥ 2.60, all P ≤ 0.05). In contrast, neither the water nor the GR 127935 infusion reduced L/M ratios of EP rats. After water infusion, EO and EP rats differed significantly only at the 5 min test interval (Tukey’s q120,4 = 4.52, P ≤ 0.05), but after GR 127935 infusion, a hormonal effect was present at 5, 10, 20 and 25 min after the infusion (Tukey’s q120,4 ≥ 3.685, P ≤ 0.05).
Figure 1. Infusion and GR 127935 effects in nonhandled, hormonally primed, ovariectomized rats.
Ovariectomized rats with bilateral cannulae near the VMN were hormonally primed with 10 μg estradiol benzoate and oil (EO) or with estradiol benzoate and 500 μg progesterone (EP). After a pretest (PRE) for sexual receptivity, rats were infused with 200 ng GR 127935 or the water vehicle. Lordosis behavior was monitored for 30 consecutive min thereafter. Data are the mean ± S.E. L/M ratios (1A) or lordosis quality (1B) for the pretest and 5 min intervals after infusion. N’s for EO rats infused with water or GR 127935, respectively, were 8 and 5; for EP rats, N’s were 7 and 6. @ indicates difference from the pretest interval; * indicates effect of hormone within drug and time; ** indicates effect of GR 127935 within hormone and time.
One EO rat infused with GR 127935 had an L/M ratio of zero during the testing and was, therefore, excluded from analysis of lordosis quality. For the remaining rats, lordosis quality was lower in EO rats than in EP rats (F1,21 = 12.99, P ≤ 0.002; Figure 1B). However, this difference was primarily due to the effect of GR 127935 in EO rats leading to a significant effect of the type of infusion (F1,21 = 13.70, P ≤ 0.002). None of the interactions terms were significant (all P > 0.05).
There was no significant effect of any of the treatments or their interactions on mounting behavior (all P > 0.05). For GR 127935 and water, respectively, in EO rats, the mean ± S.E. number of mounts per interval were 6.46 ± 0.75 and 5.28 ± 0.79. For EP rats, the average mounts were 5.83 ± 0.43 and 6.25 ± 0.34.
Experiment 2
The transient decrease in L/M ratios in EO rats infused with water was reminiscent of prior evidence that an acute 5 min stressor produced a transient decline in L/M ratios in EO, but not EP, rats (Truitt et al., 2003; White and Uphouse, 2004). Since rats had not been specifically handled between ovariectomy and testing, we considered the possibility that the infusion procedure, itself, was acting as an acute stressor in the implant EO rats and that GR 127935 had amplified this effect.
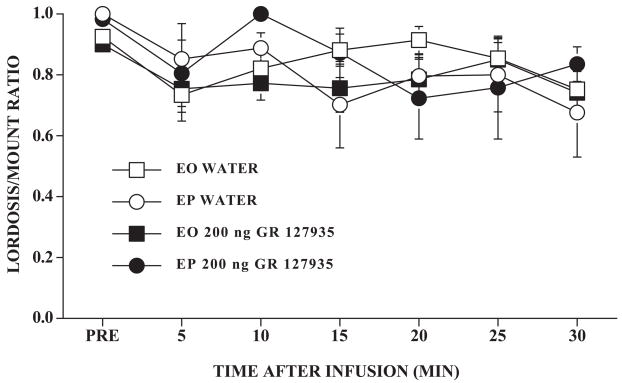
We, therefore, examined the effect of a water or GR 127935 infusion in ovariectomized rats that had been repeatedly handled (see Methods) prior to the experiment. There was a small, but significant, time-dependent decline in the L/M ratio (F6,174 = 3.59, P ≤ 0.05) after infusion, but no main effects and none of the interaction terms were significant (P > 0.05). However, as can be seen in Figure 2, even in handled rats, EO rats continued to show a small response to the infusion. Similarly, none of the main effects were significant for lordosis quality (data not shown). However, there was a slight time-dependent decline in the number of mounts received (F6,174 = 16.04, P ≤ 0.05) which was accentuated in EO rats (time × hormone interaction, F6,174 = 2.91, P ≤ 0.05). The mean ± S.E. number of mounts per interval were 5.8 ± 0.34 and 5.33 ± 0.38 for EO GR 127935 and water groups, respectively. For EP rats, GR 127935 and water groups had 6.0 ± 0.49 and 6.51 ± 0.60 mounts per 5 min interval.
Figure 2. Infusion and GR 127935 effects in handled, hormonally primed, ovariectomized rats.
Ovariectomized rats with bilateral cannulae near the VMN were hormonally primed with 10 μg estradiol benzoate and oil (EO) or with estradiol benzoate and 500 μg progesterone (EP). Beginning with the day of estrogen priming, rats were handled for two consecutive days as described in the Methods. Behavioral testing proceeded as described in Figure 1. Data are the mean ± S.E. L/M ratios for the pretest (PRE) and 5 min intervals after infusion. N’s for EO rats infused with water or GR 127935, respectively, were 12 and 5; for EP rats, N’s were 10 and 6.
In a posthoc omnibus ANOVA, the L/M ratios from the first and second experiments were combined for analysis to assess the effect of handling. Although the main effect of handling was not significant, there was a significant interaction between hormone and handling (F1,51 = 9.02, P ≤ 0.005) and a significant interaction between time and handling (F6,306 = 2.72, P ≤ 0.02). This interaction reflected primarily higher L/M ratios of handled relative to nonhandled EO rats.
Experiment 3
The third experiment was designed to test the hypothesis that effects of restraint on L/M ratios would be accentuated by treatment with GR 127935. All rats were handled prior to the experiment. GR 127935 amplified the effects of restraint in EO but not in EP rats (Figure 3). There was a significant effect of hormone (F1,45 = 33.78, P ≤ 0.0001), time (F6,270 = 10.29, P ≤ 0.0001), time × type of infusion (F6,270 = 3.84, P ≤ 0.002) and time × type of 5 min experience (F6,270 = 2.32, P ≤ 0.04). Supporting the idea that the infusion procedure was mildly stressful even in handled EO rats, both water and GR 127935 EO rats showed a decrease in their L/M ratio during the first 5 min after infusion (Dunnett’s all q ≥ 2.57, P ≤ 0.05, Figure 3A). Restraint plus GR 127935 accentuated and prolonged this decrease, but EO water restraint and GR 127935 restraint rats differed significantly only at the 5 min interval (q270,8 = 4.69, P ≤ 0.05). L/M ratios of EP rats were never significantly different from their respective pretest values (Figure 3B, all P > 0.05).
Figure 3. Effects of water or GR 127935 infusion in restrained rats.
Ovariectomized rats with bilateral cannulae near the VMN were hormonally primed with 10 μg estradiol benzoate and oil (EO) or with estradiol benzoate and 500 μg progesterone (EP). Beginning with the day of estrogen priming, rats were handled for two consecutive days as described in the Methods. After the pretest (PRE) for sexual receptivity, rats were infused with 200 ng GR 127935 or the water vehicle. Rats were then restrained for 5 min or returned to their home cage for 5 min. Lordosis behavior was monitored for 30 consecutive min thereafter. Data for EO rats are shown in 3A; data for EP rats are shown in 3B. Data are the mean ± S.E. L/M ratios for the pretest and 5 min intervals after the restraint or home cage experience. For EO rats, N’s for water home cage, water restraint, GR 127935 home cage, and GR 127935 restraint, respectively, were 7, 9, 5, and 7. For EP rats, N’s were 7, 7, 6, and 5, respectively. @ indicates difference from the pretest interval; * indicates difference from EO water and GR 127935 restraint.
Coincident with the decline in L/M ratios, females became resistive to the male’s attempts to mount and engaged in fighting, boxing and escape behaviors. Proceptivity was low throughout the experiment and did not differ across infusion or experience.
For 10 EO rats (5 EO drug and restraint, 3 EO drug no restraint, 2 EO water restraint, and 1 EO water no restraint), the L/M ratio fell to zero during the test so that lordosis quality could not be examined. For the remaining rats, lordosis quality was slightly lower in EO relative to EP rats (F1,35 = 5.06, P ≤ 0.04) and lordosis quality declined during the test period (F6,210 = 3.10, P ≤ 0.007) (data not shown). EO rats received slightly fewer mounts than EP rats (F1,45 = 5.25, P ≤ 0.03; respectively mean ± S.E. for EO and EP rats = 6.08 ± 0.23 and 7.59 ± 0.34) and GR 127935 slightly reduced the number of mounts (F1,45 = 4.10, P ≤ 0.05; respectively for GR 127935 and water, mean ± S.E. = 6.08 ± 0.26 and 7.34 ± 0.29).
Cannulae locations for rats infused with water or GR 127935 and then restrained for 5 min are shown in Figure 4. Nonrestrained rats had cannulae located in comparable positions.
Figure 4. Cannulae locations for restrained rats.
In the figure are shown cannulae locations for restrained rats used in the third experiment. Coronal sections are taken from König and Klippel (1963). Cannula location was assigned to the brain section containing the most ventral location of the cannula track. Filled circles on each section indicate bilateral locations from rats that were infused with 200 ng GR 127935. Open circles indicate cannulae locations for rats infused with the water vehicle. Sites located in EO rats are shown on the left and those for EP rats are shown on the right.
Three additional EP rats were infused with 1000 ng GR 127935 and restrained for 5 min. Even with this high dose of the drug, the L/M ratio did not decline (P > 0.05).
Discussion
There were two major objectives of the current studies: (1) to determine if EO rats were more vulnerable to the lordosis-inhibiting effect of a 5-HT1B receptor antagonist than EP rats and (2) to determine if the 5-HT1B receptor antagonist would eliminate the resistance of EP rats to the lordosis-inhibiting effect of 5 min restraint. Although GR 127935 can act as a partial agonist under some conditions, it is primarily recognized as a selective 5-HT1B/1D receptor antagonist (Beer, et al., 1998; de Groote, et al., 2003). Nevertheless, further study will be required to confirm that the effects of GR 127935, seen in the current experiments, result from an antagonist action of the drug.
Previously, we have shown that 5 min restraint transiently inhibits female rat lordosis behavior of EO, but not EP, rats (Truitt et al., 2003; White and Uphouse, 2004). Since stress increases extracellular 5-HT (Beekman et al., 2005; Fujino et al., 2002; Kirby et al., 1997; Lanfumey, et al., 2008; Shimizu et al., 1992) and 5-HT infusion into the VMN inhibits lordosis behavior (Foreman and Moss, 1978; Uphouse, et al., 1993), we hypothesized that the inhibition present in EO rats resulted from a stress-induced increase in release of 5-HT leading to activation of inhibitory 5-HT1A receptors in the VMN. We reasoned that terminal 5-HT1B receptors, if enhanced by progesterone, should reduce the stress-induced elevation of 5-HT and thereby attenuate the lordosis inhibition. If so, then EP rats should be less sensitive to the effects of a 5-HT1B receptor antagonist. This appeared to be the case. A greater sensitivity of EO than EP rats to stress has been previously documented (Figueiredo, et al., 2002; Truitt et al., 2003) and 5-HT1B receptors have been implicated in modulating the response to stress (Clark and Neumaier, 2001; Clark, et al., 2004). GR 127935’s amplification of effects of stress in EO rats is consistent with the greater stress responsiveness of EO rats and with prior findings that corticosterone treatment increased a GR 127935-induced elevation in extracellular 5-HT in the rat hypothalamus (Gur, et al., 2001). However, the effects of restraint were only amplified in EO rats, and not EP rats, infused with the 5-HT1B receptor antagonist. Moreover, an unexpected finding in the current studies was the effect of the water vehicle infusion in EO rats. This apparent infusion stress was also amplified by GR 127935 in nonhandled EO rats, but not in handled, EO rats. The similarity between the effects of 5 min restraint and effects of the infusion allow the suggestion that the infusion was also a stressor and that in EO rats, a 5-HT1B receptor antagonist enhanced the response to stress.
While these findings are consistent with prior suggestions that 5-HT1B receptors may contribute to the facilitation of lordosis behavior of EO rats (Mendelson, 1992), 5-HT1B receptors appear to be less important in EP rats. If an amplification of 5-HT1B receptors were responsible for progesterone’s attenuation of the effects of stress, EP rats infused with the 5-HT1B receptor antagonist should behave like EO rats after the 5 min restraint. This did not appear to be the case. Therefore, progesterone’s protection against restraint stress must include mechanisms in addition to an increase in 5-HT1B receptors. A concentration of GR 127935 that was 5-fold higher than required to inhibit lordosis behavior of EO rats was ineffective in EP rats. Either 5-HT1B receptors of EP rats remained unsaturated even with the highest concentration of GR 127935 or 5-HT1B receptors, alone, are not responsible for the different vulnerabilities of EO and EP rats to the 5 min restraint.
Progesterone’s ability to facilitate lordosis behavior includes effects mediated through intracellular progesterone receptors and through progesterone metabolites, such as 3 alpha-hydroxy-5alpha-pregnan-20-one (allopregnanolone) (Frye, 2001; Frye and Vongher, 2001). Progesterone metabolites have anxiolytic actions and have been reported to reduce the response to stress (Frye, 2007; Pinna, et al., 2008). Since brain concentrations of allopregnanolone are higher in EP than in EO animals (Frye, et al., 1998), it is possible that elevations in progesterone metabolites in EP rats compensated for GR 127935 blockade of 5-HT1B receptors. In addition to their role as terminal autoreceptors, 5-HT1B receptors also function as heteroceptors on terminals of nonserotonergic neurons (Barnes and Sharp, 1999; Bramley, et al., 2005; Fink and Gothert, 2007; Sari, 2004). 5-HT1B heteroceptors and progesterone metabolites could provide redundant control of neurotransmitters, in addition to 5-HT, that reduce lordosis behavior. If so, antagonism of 5-HT1B receptors would have minimal effectiveness in the presence of such progesterone metabolites. It would be interesting to test this hypothesis in EP rats in the presence of an inhibitor of 5-alpha reductase to reduce accumulation of the progesterone metabolites.
Therefore, while the rationale for these studies was based on the recognized role that terminal 5-HT1B receptors play in controlling 5-HT release (Daws et al., 2000; Engel et al., 1986; Maura et al., 1986; Riad et al., 2000), the findings lead us to believe that receptors other than (or in addition to) terminal 5-HT1B autoreceptors contribute to the observed differences between EO and EP rats. In addition, we cannot rule out a possible role of 5-HT1D receptors in the response to GR 127935.
Finally, although GR 127935 is recognized as a relatively selective 5-HT1B/1D receptor antagonist, there is evidence that it can act as a partial agonist under some conditions (Beer, et al., 1998; de Groote, et al., 2003). Therefore, additional experiments will be required to define the precise role of 5-HT1B receptors in modulation of lordosis behavior of EO rats. Nevertheless, these experiments are important in expanding the understanding of serotonin’s role in modulation of female rat lordosis behavior and may implicate VMN 5-HT1B/1D receptors as one of the components involved in the effects of progesterone.
In summary, 5-HT1B/1D receptors may be important in preventing lordosis inhibition but are not required for maintenance of the behavior in a rat with optimal hormone (estrogen and progesterone) priming. This dichotomy between effects of a 5-HT receptor antagonist in suboptimally hormone primed and maximally primed rats has also been reported for 5-HT2 receptors (Uphouse, 2000) and underscores the different vulnerability of EO and EP rats to lordosis disruption.
Acknowledgments
Special appreciation is given to Mr. Dan Wall and Ms. Karolina Blaha-Black for animal care and to Mr. Brandon Hurlburt for technical assistance. The research was supported by NIH R01 HD28419 and by GM 55380 and by the Department of Biology at TWU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello-Zaldivar M, Luine V, Frankfurt M. 5,7-DHT facilitated lordosis: effects of 5-HT agonists. Neuroreport. 1992;3(6):542–4. doi: 10.1097/00001756-199206000-00024. [DOI] [PubMed] [Google Scholar]
- Ansonoff MA, Etgen AM. Estradiol elevates protein kinase C catalytic activity in the preoptic area of female rats. Endocrinology. 1998;139(7):3050–6. doi: 10.1210/endo.139.7.6088. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Beekman M, Flachskamm C, Linthorst AC. Effects of exposure to a predator on behaviour and serotonergic neurotransmission in different brain regions of C57bl/6N mice. Eur J Neurosci. 2005;21(10):2825–36. doi: 10.1111/j.1460-9568.2005.04107.x. [DOI] [PubMed] [Google Scholar]
- Beer MS, Heald MA, McAllister G, Stanton JA. Pharmacological characterisation of a cloned dog 5-HT1B receptor cell line. Eur J Pharmacol. 1998;360(1):117–21. doi: 10.1016/s0014-2999(98)00698-0. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23(1):41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bramley JR, Sollars PJ, Pickard GE, Dudek FE. 5-HT1B receptor-mediated presynaptic inhibition of GABA release in the suprachiasmatic nucleus. J Neurophysiol. 2005;93(6):3157–64. doi: 10.1152/jn.00770.2004. [DOI] [PubMed] [Google Scholar]
- Clark MS, Neumaier JF. The 5-HT1B receptor: behavioral implications. Psychopharmacol Bull. 2001;35(4):170–85. [PubMed] [Google Scholar]
- Clark MS, Vincow ES, Sexton TJ, Neumaier JF. Increased expression of 5-HT1B receptor in dorsal raphe nucleus decreases fear-potentiated startle in a stress dependent manner. Brain Res. 2004;1007(1–2):86–97. doi: 10.1016/j.brainres.2004.01.070. [DOI] [PubMed] [Google Scholar]
- Cyr M, Bosse R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829–36. doi: 10.1016/s0306-4522(97)00445-4. [DOI] [PubMed] [Google Scholar]
- Daws LC, Gould GG, Teicher SD, Gerhardt GA, Frazer A. 5-HT(1B) receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo. J Neurochem. 2000;75(5):2113–22. doi: 10.1046/j.1471-4159.2000.0752113.x. [DOI] [PubMed] [Google Scholar]
- de Groote L, Klompmakers AA, Olivier B, Westenberg HG. An evaluation of the effect of NAS-181, a new selective 5-HT(1B) receptor antagonist, on extracellular 5-HT levels in rat frontal cortex. Naunyn Schmiedebergs Arch Pharmacol. 2003;367(2):89–94. doi: 10.1007/s00210-002-0685-0. [DOI] [PubMed] [Google Scholar]
- Engel G, Gothert M, Hoyer D, Schlicker E, Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol. 1986;332(1):1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav. 2001;40(2):169–77. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- Farmer CJ, Isakson TR, Coy DJ, Renner KJ. In vivo evidence for progesterone dependent decreases in serotonin release in the hypothalamus and midbrain central grey: relation to the induction of lordosis. Brain Res. 1996;711(1–2):84–92. doi: 10.1016/0006-8993(95)01403-9. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology. 2002;143(7):2534–40. doi: 10.1210/endo.143.7.8888. [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner BE, Rosie R, Grace O, Quinn JP. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325–44. doi: 10.1007/BF02088099. [DOI] [PubMed] [Google Scholar]
- Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59(4):360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- Foreman MM, Moss RL. Role of hypothalamic serotonergic receptors in the control of lordosis behavior in the female rat. Horm Behav. 1978;10(1):97–106. doi: 10.1016/0018-506x(78)90028-4. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, McKittrick CR, Mendelson SD, McEwen BS. Effect of 5,7-dihydroxytryptamine, ovariectomy and gonadal steroids on serotonin receptor binding in rat brain. Neuroendocrinology. 1994;59(3):245–50. doi: 10.1159/000126665. [DOI] [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37(1–3):201–22. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007;86(2):209–19. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3alpha, 5alpha-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808(1):72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. Progesterone and 3alpha, 5alpha-THP enhance sexual receptivity in mice. Behav Neurosci. 2001;115(5):1118–28. [PubMed] [Google Scholar]
- Fujino K, Yoshitake T, Inoue O, Ibii N, Kehr J, Ishida J, Nohta H, Yamaguchi M. Increased serotonin release in mice frontal cortex and hippocampus induced by acute physiological stressors. Neurosci Lett. 2002;320(1–2):91–5. doi: 10.1016/s0304-3940(02)00029-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Greengrass P, Russell M, Wilson CA. Comparison of serotonin receptor numbers and activity in specific hypothalamic areas of sexually active and inactive female rats. Neuroendocrinology. 1997;66(6):384–92. doi: 10.1159/000127277. [DOI] [PubMed] [Google Scholar]
- Gorosito SV, Cambiasso MJ. Axogenic effect of estrogen in male rat hypothalamic neurons involves Ca(2+), protein kinase C, and extracellular signal-regulated kinase signaling. J Neurosci Res. 2008;86(1):145–57. doi: 10.1002/jnr.21466. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Simon LD, Auerbach SB. Differences in hypothalamic serotonin between estrous phases and gender: an in vivo microdialysis study. Brain Res. 1998;785(1):91–6. doi: 10.1016/s0006-8993(97)01391-7. [DOI] [PubMed] [Google Scholar]
- Gur E, Dremencov E, Lerer B, Newman ME. Functional effects of corticosterone on 5-HT(1A) and 5-HT(1B) receptor activity in rat brain: in vivo microdialysis studies. Eur J Pharmacol. 2001;411(1–2):115–122. doi: 10.1016/s0014-2999(00)00911-0. [DOI] [PubMed] [Google Scholar]
- Hardy DF, DeBold JF. The relationship between levels of exogenous hormones and the display of lordosis by the female rat. Horm Behav. 1971;2:287–297. [Google Scholar]
- Hunter AJ, Hole DR, Wilson CA. Studies into the dual effects of serotonergic pharmacological agents on female sexual behaviour in the rat: preliminary evidence that endogenous 5HT is stimulatory. Pharmacol Biochem Behav. 1985;22(1):5–13. doi: 10.1016/0091-3057(85)90477-0. [DOI] [PubMed] [Google Scholar]
- Karp G, Maissel A, Livneh E. Hormonal regulation of PKC: estrogen up-regulates PKCeta expression in estrogen-responsive breast cancer cells. Cancer Lett. 2007;246(1–2):173–81. doi: 10.1016/j.canlet.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760(1–2):218–30. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- König J, Klippel R. A Stereotaxic Atlas of the Forebrain and Lower Parts of the Brain Stem. Williams and Wilkins; Baltimore: 1963. The Rat Brain. [Google Scholar]
- Kow LM, Pfaff DW. Estrogen effects on neuronal responsiveness to electrical and neurotransmitter stimulation: an in vitro study on the ventromedial nucleus of the hypothalamus. Brain Res. 1985;347(1):1–10. doi: 10.1016/0006-8993(85)90883-2. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32(6):1174–84. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Liao Y, Bottcher H, Harting J, Greiner H, van Amsterdam C, Cremers T, Sundell S, Marz J, Rautenberg W, Wikstrom H. New selective and potent 5-HT(1B/1D) antagonists: chemistry and pharmacological evaluation of N-piperazinylphenyl biphenylcarboxamides and biphenylsulfonamides. J Med Chem. 2000;43(3):517–25. doi: 10.1021/jm990397l. [DOI] [PubMed] [Google Scholar]
- Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7(8):497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maswood S, Truitt W, Hotema M, Caldarola-Pastuszka M, Uphouse L. Estrous cycle modulation of extracellular serotonin in mediobasal hypothalamus: role of the serotonin transporter and terminal autoreceptors. Brain Res. 1999;831(1–2):146–54. doi: 10.1016/s0006-8993(99)01439-0. [DOI] [PubMed] [Google Scholar]
- Maura G, Roccatagliata E, Raiteri M. Serotonin autoreceptor in rat hippocampus: pharmacological characterization as a subtype of the 5-HT1 receptor. Naunyn Schmiedebergs Arch Pharmacol. 1986;334(4):323–6. doi: 10.1007/BF00569364. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Fink G. Estradiol-17 beta increases serotonin transporter (SERT) mRNA levels and the density of SERT-binding sites in female rat brain. Brain Res Mol Brain Res. 1997;45(1):13–23. doi: 10.1016/s0169-328x(96)00233-1. [DOI] [PubMed] [Google Scholar]
- Mendelson SD. A review and reevaluation of the role of serotonin in the modulation of lordosis behavior in the female rat. Neurosci Biobehav Rev. 1992;16(3):309–50. doi: 10.1016/s0149-7634(05)80204-0. [DOI] [PubMed] [Google Scholar]
- Mendelson SD, Gorzalka BB. 5-HT1A receptors: differential involvement in female and male sexual behavior in the rat. Physiol Behav. 1986;37(2):345–51. doi: 10.1016/0031-9384(86)90244-1. [DOI] [PubMed] [Google Scholar]
- Mendelson SD, Gorzalka BB. Sex differences in the effects of 1-(m-trifluoromethylphenyl) piperazine and 1-(m-chlorophenyl) piperazine on copulatory behavior in the rat. Neuropharmacology. 1990;29(8):783–6. doi: 10.1016/0028-3908(90)90133-c. [DOI] [PubMed] [Google Scholar]
- Mize AL, Alper RH. Acute and long-term effects of 17beta-estradiol on G(i/o) coupled neurotransmitter receptor function in the female rat brain as assessed by agonist-stimulated [35S]GTPgammaS binding. Brain Res. 2000;859(2):326–33. doi: 10.1016/s0006-8993(00)01998-3. [DOI] [PubMed] [Google Scholar]
- Mize AL, Young LJ, Alper RH. Uncoupling of 5-HT1A receptors in the brain by estrogens: regional variations in antagonism by ICI 182, 780. Neuropharmacology. 2003;44(5):584–91. doi: 10.1016/s0028-3908(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Modianos D. Neural mechanisms of female reproductive behavior. In: Adler D, Pfaff D, Goy RW, editors. Handbook of behavioral neurobiology. Plenum Press; New York: 1985. pp. 423–493. [Google Scholar]
- Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid Biosynthesis Regulates Sexually Dimorphic Fear and Aggressive Behavior in Mice. Neurochem Res. 2008 doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417(2):181–94. [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28(6):565–82. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Take S, Hori T, Oomura Y. In vivo measurement of hypothalamic serotonin release by intracerebral microdialysis: significant enhancement by immobilization stress in rats. Brain Res Bull. 1992;28(5):727–34. doi: 10.1016/0361-9230(92)90252-s. [DOI] [PubMed] [Google Scholar]
- Sodersten P. Estradiol-progesterone interactions in the reproductive behavior of female rats. In: Ganten D, Pfaff D, editors. Current Topics in Neuroendocrinology: Actions of Progesterone on the Brain. Springer-Verlag; New York: 1981. pp. 141–174. [Google Scholar]
- Sumner BE, Fink G. Estrogen increases the density of 5-hydroxytryptamine 2A receptors in cerebral cortex and nucleus accumbens in the female rat. J Steroid Biochem Molec Biol. 1995;54:15–20. doi: 10.1016/0960-0760(95)00075-b. [DOI] [PubMed] [Google Scholar]
- Sumner BE, Fink G. The density of 5-hydoxytryptamine2A receptors in forebrain is increased at pro-oestrus in intact female rats. Neurosci Lett. 1997;234(1):7–10. doi: 10.1016/s0304-3940(97)00651-4. [DOI] [PubMed] [Google Scholar]
- Truitt W, Harrison L, Guptarak J, White S, Hiegel C, Uphouse L. Progesterone attenuates the effect of the 5-HT1A receptor agonist, 8-OH-DPAT, and of mild restraint on lordosis behavior. Brain Res. 2003;974(1–2):202–11. doi: 10.1016/s0006-8993(03)02581-2. [DOI] [PubMed] [Google Scholar]
- Uphouse L. Female gonadal hormones, serotonin, and sexual receptivity. Brain Res Rev. 2000;33(2–3):242–57. doi: 10.1016/s0165-0173(00)00032-1. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Andrade M, Caldarola-Pastuszka M, Jackson A. 5-HT1A receptor antagonists and lordosis behavior. Neuropharmacology. 1996;35(4):489–95. doi: 10.1016/0028-3908(95)00196-4. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Andrade M, Caldarola-Pastuszka M, Maswood S. Hypothalamic infusion of the 5-HT2/1C agonist, DOI, prevents the inhibitory actions of the 5-HT1A agonist, 8-OH-DPAT, on lordosis behavior. Pharmacol Biochem Behav. 1994;47(3):467–70. doi: 10.1016/0091-3057(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Caldarola-Pastuszka M. Female sexual behavior following intracerebral infusion of the 5-HT1A agonist, 8-OH-DPAT, into the medial preoptic area. Brain Res. 1993;601(1–2):203–8. doi: 10.1016/0006-8993(93)91711-z. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Caldarola-Pastuszka M, Montanez S. Intracerebral actions of the 5-HT1A agonists, 8-OH-DPAT and buspirone and of the 5-HT1A partial agonist/antagonist, NAN-190, on female sexual behavior. Neuropharmacology. 1992;31(10):969–81. doi: 10.1016/0028-3908(92)90097-9. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Caldarola-Pastuszka M, Moore N. Inhibitory effects of the 5-HT1A agonists, 5-hydroxy- and 5-methoxy-(3-di-n-propylamino)chroman, on female lordosis behavior. Neuropharmacology. 1993;32(7):641–51. doi: 10.1016/0028-3908(93)90077-g. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Hiegel C, Perez E, Guptarak J. Serotonin receptor involvement in effects of restraint on female rat lordosis behavior. Pharmacol Biochem Behav. 2007;86(4):631–6. doi: 10.1016/j.pbb.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S, Uphouse L. Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Horm Behav. 2004;45(3):201–8. doi: 10.1016/j.yhbeh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Wolf A, Caldarola-Pastuszka M, Uphouse L. Facilitation of female rat lordosis behavior by hypothalamic infusion of 5-HT(2A/2C) receptor agonists. Brain Res. 1998;779(1–2):84–95. doi: 10.1016/s0006-8993(97)01082-2. [DOI] [PubMed] [Google Scholar]
- Zar J. Biostatistical Analysis. 4. Prentice-Hall; Englewood Cliffs, NJ: 1999. [Google Scholar]