Abstract
This study examined whether differential effects of two agents commonly used for hemostatic purposes during cardiac surgery, aprotinin or epsilon-aminocaproic acid (EACA), exist with respect to elevations in proinflammatory interleukins (ILs) and matrix metalloproteinases (MMPs) in patients undergoing coronary artery bypass surgery. Sixty patients were prospectively randomized to receive either aprotinin (1×106 KIU;n=30) or EACA (5gIV;n=30) and blood samples were obtained for IL and MMP levels just before induction of anesthesia (Baseline), 10 minutes after separation from cardiopulmonary bypass (Post) and 6 Hours after surgery (6 Hours). IL-6 was increased at Post in the EACA group and increased further at 6 hours. In the aprotinin group, IL-6 was significantly increased only at 6 Hours. MMP subtypes associated with inflammation, MMP-8 and -9 were increased in the EACA group at Post and remained elevated at 6 Hours. Thus, differential effects on IL and MMP release occurred between aprotinin and EACA, indicative of different mechanisms of action independent of hemostatic effects.
Keywords: matrix metalloproteinases, interleukins, cardiac surgery, aprotinin, epsilon-aminocaproic acid
Introduction
Matrix metalloproteinases (MMPs) represent a group of zinc-dependent enzymes that contribute to extracellular protein degradation (1-4) and increased levels have been associated with pathologic myocardial remodeling, development of aortic aneurysms, and atherosclerotic plaque evolution and rupture (5-11). Acute elevations of MMPs have also been observed in acute inflammatory states and immediately following myocardial ischemia/infarction (9-11). Several past studies have demonstrated that cardiac surgery employing cardiopulmonary bypass (CPB) can result in acute increases in certain MMP subtypes throughout the perioperative period (12-15). While a number of upstream signaling cascades can cause the induction and release of MMPs, inflammatory cytokines such as the interleukins (ILs) have been shown to contribute to this process (15-17). Aprotinin, a serine protease inhibitor, and epsilon- aminocaproic acid, (EACA), an antifibrinolytic agent, have been widely utilized in cardiac surgery requiring CPB, primarily to reduce blood loss (18-22). However, both aprotinin and EACA through inhibition of kallikrein and plasmin, or by plasminogen, respectively, can modify the activity of multiple biological systems (23-26). Past studies have suggested that a differential effect on cytokine release, may occur between aprotinin and EACA administration (24-27). However, there have been no studies which have examined the comparative effects of aprotinin and EACA in relation to cytokine and MMP release in patients following cardiac surgery. This is a particularly pertinent issue since a recent clinical study suggested that aprotinin administration in patients following cardiac surgery may have negative effects on short and long term outcomes (27,28). Accordingly, the goal of the present study was to measure the temporal release of specific ILs and MMP in patients randomized to receive either aprotinin or EACA following elective coronary revascularization procedures requiring CPB.
Methods
Patients
Following approval by the Human Subjects Review Committee, 60 patients undergoing elective coronary artery bypass surgery (CABG) provided informed consent to participate in the study. Patients were prospectively randomized according to surgical protocol to receive either aprotinin (Aprotinin Group; 30 patients) or EACA (EACA Group; 30 patients) immediately after induction of anesthesia. The aprotinin dose consisted of 1×106 kallekrein inhibitory units (KIU) intravenously at the beginning of surgery with an additional 1×106 KIU in the cardiopulmonary bypass circuit; an infusion of 250,000 KIU per hour was started and continued until the end of surgery. Patients in the EACA group received 5 grams of EACA intravenously concurrent with systemic heparinization and an additional 5 grams of EACA placed in the CPB circuit. Another 5 grams of EACA was administered intravenously to the patient immediately after discontinuation of CPB. The dosing regimens utilized in this study are clinically standardized protocols and has been described in detail previously (21,22,26-29). For this study, the surgeon was blinded to the randomization protocol, but due to the differences in dosing regimens, the anesthesiologist was not.
Only patients undergoing elective CABG who had not received any thrombolytic intervention for two weeks (including aspirin) were included in the study. Exclusion criteria consisted of an inability to provide informed consent, emergent procedures, age less than 18 years, multiple procedures (CABG with valve replacement), and exposure to thrombolytic agents, or desire to withdraw from the study. All chronic cardiac medications were continued per usual protocols up until and including the morning of surgery.
Surgery
All patients received intravenous midazolam for sedation in the holding area before surgery. Standard induction and maintenance of anesthesia was accomplished with a combination of sufentanil, midazolam and isoflurane. Intravenous nitroglycerin infusion was administered until CPB. Systemic heparinization was accomplished with a heparin dose of 300 U/kg. Additional heparin was administered during CPB to maintain an activated coagulation time of > 400 seconds. CPB and cardioplegic arrest was performed as previously described (20). Extubation criteria consisted of the patient awake and following commands, inspired oxygen content of less than 40%, spontaneous ventilation with a respiratory rate of less than 25 breaths per minute, and after a 15-minute trial on T-piece an oxygen saturation of > 95% and a PCO2 of <50mmHg. Discharge criteria from the intensive care unit (ICU) included a complete wean from all vasoactive and inotropic infusions, extubation without pulmonary support, and no evidence of major organ failure. Discharge criteria from the hospital included no supplemental oxygen requirement, ambulation and tolerance of oral intake. Packed red blood cells were administered for a hematocrit of < 23%. In the setting of a diffuse coagulopathy after CPB, fresh frozen plasma was administered for an INR of > 1.4, cryoprecipitate was administered for a fibrinogin of < 100 and platelets were provided for a platelet count of < 80×103. Chest tube drainage 24 hours after surgery was documented for each patient. Clinicians responsible for making decisions regarding extubation, ICU discharge, hospital discharge and transfusion were not aware of study group assignments.
Protocol
Blood samples were obtained from the radial artery for interleukin 6 (IL-6), interleukin 10 (Il-10), matrix metalloproteinase 2 (MMP-2), 8 (MMP-8) and 9 (MMP-9) at the following times: just before induction of anesthesia (Baseline), 10 minutes after separation from CPB (Post) and 6 hours after completion of surgery (6 Hour). All samples were placed in EDTA tubes, centrifuged, and plasma was stored at −70°C until assay. At the time of assay, plasma samples were allowed to thaw on ice. Quantification of respective MMP was performed with an enzyme linked immunosorbant assay (ELISA) kit (Amersham Pharmacia Biotech, Buckinghamshire, England). The antisera used for MMP-2 reacts against the proform of MMP-2 (proMMP-2) and does not react against the active form. For MMP-8, the antisera detects both the pro- and active forms of MMP-8. For MMP-9, the antisera detects the proform of the enzyme (proMMP-9). The ELISA procedure was similar for each MMP, using a two-site assay which has been described in detail previously (10-12). Briefly, plasma was diluted 1:10 and added to precoated wells containing the antibody of interest and incubated at 20°C for one hour. The ELISA plate was washed three times and incubated in the primary antisera conjugated to horseradish peroxidase (25°C, 1 hour). After three washes, tetramethylbenzadine (TMB)/hydrogen peroxidase was added to the mixture and the reaction was allowed to proceed for 30 minutes. The ELISA plate was immediately read at a wavelength of 450nm (Labsystems Multiskan MCC/340, Helsinki, Finland). IL levels were detected using Pelikine® Tool Kit, Pelikine® compact Human IL-6 ELISA Kit, and Pelikine® compact Human IL-10 ELISA Kit (Research Diagnostics, Inc., Flanders, NJ). Assay protocols were similar to those described above. The concentration of plasma MMP/IL species was determined using specific MMP/IL concentrations to generate a standard curve with each set of samples. The inter-assay coefficient of variation was less than 10% and the intra-assay coefficient of variation was less than 12% for all ELISA assays. Routine blood chemistry and hematological profiles were also obtained at baseline and at 6 hours post-operatively.
Data Analysis
All variables were tested and confirmed for normality and then reported as the mean ± the standard error of the mean (SEM). The sample sizes for this study were based upon the assumption that aprotinin would reduce relative IL-6 levels by approximately 50% lower than that for EACA. This estimate was predicated upon a past reports that this relative magnitude of reduction in ILs could be achieved by aprotinin (23,24,30). Comparisons between the two groups for one point measurements were made using a two-sample t-test. Comparisons over time were made using an analysis of variance (ANOVA) with pairwise t-tests with Bonferroni correction adjustment for multiple comparisons. All data analysis was performed using STATA 8.0 (College Station, TX).
Results
Demographic data for the 60 patients enrolled in this study are presented in Table 1. The number of distal anastamoses, and duration of cross-camp, CPB and surgery were similar between patients in the aprotinin and EACA groups. Baseline and post-operative values for blood chemistry and hematology are shown in Table 2. There were no differences between groups at baseline for any of these parameters. At 6 hours post-operatively, sodium and chloride levels were increased from baseline, and this increase was similar between the aprotinin and EACA groups. There were no significant differences in plasma creatinine values. Hemoglobin, hematocrit and platelets were significantly reduced compared to baseline, but similar between the aprotinin and EACA groups. Blood loss and blood product usage were documented at 24 hours after surgery (Table 3). The average number of packed red blood cells administered and the quantity of chest tube drainage at 24 hours after surgery was significantly higher in the EACA group. The time to extubation and length of stay in the ICU and hospital were recorded and did not differ between patients in the two study groups.
Table 1.
Patient Demographics Following Randomization to Aprotinin or EACA
| Variable | Aprotinin (n=30) |
EACA (n=30) |
|---|---|---|
| Age (years) | 62±2 | 60±2 |
| Gender (m/f) | 25/5 | 20/10 |
| BSA (m2) | 2.03±.02 | 2.02±.02 |
| LVEF (%) | 49 | 51 |
| Hypertension (%) | 47 | 67 |
EACA: epsilon-aminocaproic acid
BSA: Body surface area
LVEF: Left ventricular ejection fraction
All values reported as MEAN±SEM
Table 2.
Perioperative Chemistry and Hematology Values in Patients Receiving Aprotinin or EACA During Cardiac Surgery
| Variable | Aprotinin (n=30) |
EACA (n=30) |
|
|---|---|---|---|
| Sodium (mmol/L) | Baseline | 143.1±1.5 | 139.2±0.9 |
| Post | 16.27±3.3* | 161.5±4.7* | |
| Potassium (mmol/L) | Baseline | 5.63±0.17 | 5.25±0.13 |
| Post | 5.79+0.15 | 5.53±0.26 | |
| Chloride (mmol/L) | Baseline | 110.4±1.2 | 107.4±0.9 |
| Post | 127.4±2.6* | 126.5±3.7* | |
| Blood Urea Nitrogen (mg/dL) |
Baseline | 13.6±1.0 | 16.6±2.7 |
| Post | 16.3±1.1 | 18.2±2.2 | |
| Creatinine (mg/dL) | Baseline | 0.9±0.0 | 1.2±0.2 |
| Post | 1.1±0.0 | 1.5±0.2 | |
| Hemaglobin (g/dL) | Baseline | 12.8±0.3 | 12.0±0.3 |
| Post | 11.2±0.3* | 10.5±0.2* | |
| Hematocrit (%) | Baseline | 37.0±0.9 | 35.0±0.9 |
| Post | 32.4±0.7* | 30.8±0.6* | |
| Platelet Density (×10−3) | Baseline | 196±10 | 202±11 |
| Post | 148±7* | 141±7* |
EACA: epsilon-aminocaproic acid
Post: 6 hours post operatively
All values reported as MEAN±SEM
p<0.05 vs. Baseline
Table 3.
Cumulative 24 Hour Blood Loss and Blood Product Usage in Patients Receiving Aprotinin or EACA
| Variable | Aprotinin (n=30) |
EACA (n=30) |
|---|---|---|
| 24 Hour PRBC* | 0.56±0.2 | 1.46±0.4 § |
| 24 Hour FFP* | 0.13±0.13 | 0.16±0.10 |
| 24 Hour Plt.* | 0.20±0.20 | 0.60±0.33 |
| 24 Hour Chest Tube Output | 423±249 | 596±370 § |
| % of Patients Transfused | 30 | 50 |
EACA: epsilon-aminocaproic acid
PRBC: Packed Red Blood Cells
FFP: Fresh Frozen Plasma
Plt: Platelets
All values reported as MEAN±SEM
Cryoprecipitate was not given to any of the patients
Average number of units administered per patient
p< 0.05 vs. Aprotinin
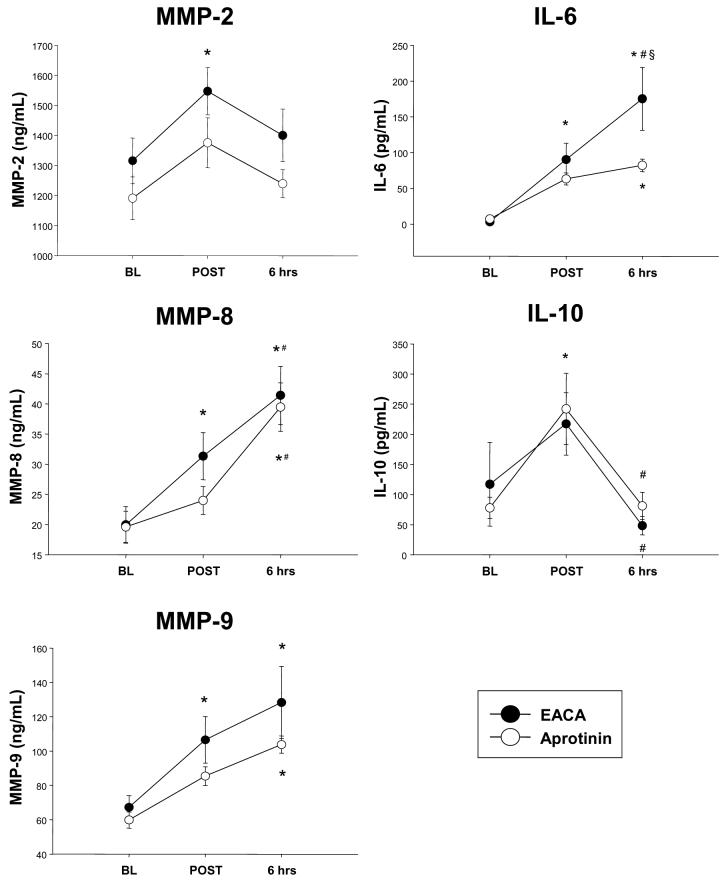
MMP-2, 8 and 9 were measured at baseline, immediately post-operatively, and 6 hours post-operatively (Fig 1). For patients in the EACA group, these MMP types were elevated immediately post-operatively. MMP-9 levels remained elevated at 6 hours post-operatively in the EACA group, while MMP-8 levels increased further at this post-operative time point. In contrast, for patients in the aprotinin group, there was no significant increase in MMP levels in the early post-operative period.
Figure 1.
MMP and IL profiles at baseline (BL), immediately following separation from cardiopulmonary bypass (POST) and at 6 hours post-operatively (6 hrs) in patients receiving either aprotinin or EACA: epsilon-aminocaproic acid. Plasma MMP-2, MMP-8, and MMP-9 levels increased in the EACA group immediately at separation from cardiopulmonary bypass, but not in the aprotinin group. IL-6 levels increased in the EACA group, but were lower in the aprotinin group. All values reported as MEAN±SEM (*p<0.05 vs. Baseline; #p<0.05 vs. Post;§ p<0.05 vs. Aprotinin)
Plasma levels of IL-6 significantly increased during the perioperative period in the EACA group (Fig 1). In the aprotinin group, IL-6 was significantly elevated only at the 6 hour post-operative time point. IL-10 levels were decreased at 6 hours post-operatively in the EACA group (Fig 1). In contrast, IL-10 levels were increased in the aprotinin group immediately post-operatively and then decreased at the 6 hour post-operative time point.
Discussion
Elevations in pro-inflammatory cytokines and matrix metalloproteinases (MMPs) during cardiac surgery may alter vascular permeability and degrade proteins essential for normal tissue homeostasis. Thus, control of the heightened inflammatory state associated with cardiac surgery and cardiopulmonary bypass (CPB) may improve recovery following cardiac surgery. Renewed interest in this subject has resurfaced in light of the fact that agents primarily utilized for hemostatic purposes in cardiac surgery, specifically aprotinin and EACA, may also modify the inflammatory response (22-26). This is particularly relevant in light of the fact that recent controversy surrounds the mechanisms and effects of aprotinin in the post-cardiac surgical setting (27). The unique results of the present study demonstrated that aprotinin administration in patients undergoing cardiac surgery requiring CPB modified the release of certain inflammatory cytokines, specifically interleukin-6 and -10 (IL-6, IL-10) which in turn was associated with an attenuated emergence of certain MMP types such as MMP-8 and MMP-9 within the plasma of patients following CPB. Moreover, the present study demonstrated a unique and differential effect of aprotinin and EACA with respect to the emergence and release of specific ILs and MMPs in the early post-cardiac surgical setting.
In a prior reports by this laboratory and others, increased induction and release of certain MMP subtypes, have been reported in the early post-cardiac surgery period (12-14). Notably, robust plasma levels of MMP-8 and MMP-9 have been reported to occur in patients following separation from CPB (12-14). The increase in MMP subspecies after CPB is related to a number of factors, including increased catecholamines, cytokine activation and degranulation of neutrophils. In the present study, EACA did not prevent progressive and significant elevations of MMP-2, MMP-8 and MMP-9 after CPB. Indeed, the relative increase in these MMP types is concordant with a past study from this laboratory (12). In contrast, aprotinin attenuated the release of these MMP subspecies in the early post-operative period. In the present study, aprotinin prevented increases in the pro-inflammatory cytokine IL-6 during CPB, and resulted in a significant elevation in the anti-inflammatory IL-10 (also termed cytokine secretion inhibitory factor) during CPB, confirming the results from a prior study (30). In contrast, EACA administration was associated with increased IL-6 after CPB, and failed to display an increase in the anti-inflammatory cytokine IL-10. Thus, the increase in IL-6 and the relative reduction in IL-10 would favor a relatively higher proinflammatory state in the EACA patients, which could in turn contribute to a greater relative induction and release of MMPs. It is also likely that aprotinin affected several post-transcriptional processes relevant to MMP release and activation. One of the important MMP steps for full MMP activation is through proteolytic cleavage of the propeptide domain by serine proteases (31). Since aprotinin can inhibit a wide spectrum of serine proteases, then it is plausible that aprotinin may interfere in MMP proteolytic activation. However, it must be recognized that the immunoassay approach utilized in the present study could not adequately differentiate and quantify the proform and active forms of MMPs which emerged in the plasma. Thus, whether and to what degree aprotinin alters MMP activational states, and whether this is different than that of EACA remains speculative. Finally, aprotinin has been shown to reduce the activation of neutrophils within the circulation, and prevent the accumulation of neutrophils within the lung following CPB (23,24,26). This reduced neutrophil activation is important in the control of MMP levels since these inflammatory cells have been demonstrated to release several species of MMPs, including MMP-8 (also known as neutrophil collagenase) and MMP-9 (31-34). Thus, aprotinin likely interfered with post-translational events necessary for MMP activation and release, which in turn reduced the plasma levels of certain MMP types following cardiac surgery.
In the present study, the relative effects of aprotinin and EACA with respect to MMP and IL levels were only measured in the early post-operative period. This was an initial study to examine whether differential effects between these hemostatic agents could be detected when plasma levels of these compounds would be maximal. With respect to the differential effects, aprotinin reduced relative MMP and IL-6 levels in the immediate post-operative period, but not at 6 hours after surgery. This is not surprising considering the pharmacokinetics of aprotinin and the dose of aprotinin utilized in the present study. Specifically, using this aprotinin dosing regimen, it has been shown that plasma aprotinin levels fall below targeted plasmin inhibitory effects at approximately 4 hours post-infusion (35). Moreover, past studies have suggested that the effects of aprotinin with respect to the inflammatory response, are potentially dose dependent (23,24). Based upon the findings of the present study, future studies utilizing higher dose aprotinin regimens along with longer followup periods with respect to plasma MMP and cytokine levels would be appropriate.
Based upon past clinical and basic studies, it is now well recognized that MMPs play a contributory role in a number of cardiovascular remodeling processes which include the coronary vasculature, the aorta, and the myocardium (1-9). Moreover, a distinct change in the plasma levels of MMPs in patients has been demonstrated to occur following myocardial injury and with remodeling (10,11). Thus, in general terms, it has been considered that the induction and release of MMPs is a pathological phenomenon, and contributes to disease progression. However, MMPs are also critical components of the normal wound healing response following injury (36,37). Specifically, MMP activation facilitates tissue extracellular matrix remodeling allowing for the natural ingress of inflammatory cells and eventually tissue healing and scar formation. The elaboration of MMPs following cardiac surgery, as noted in the present study as well as in past reports (12-15), is likely a reflection of the initial biological response to tissue injury and repair. Therefore, it remains unclear whether and to what degree the suppression of MMP release in the early post-cardiac surgery will yield a favorable or deleterious long term outcome.
This study is unique in that this is the first to perform comparative temporal profiles of ILs and MMPs following aprotinin or EACA administration, as well as place these profiles in context with early perioperative outcomes. This study is pertinent in light of the fact that while aprotinin has been widely used for hemostatic control and reducing blood product requirements following cardiac surgery, this pharmacological approach has been called into question. Specifically, meta-analysis has suggested that aprotinin may adversely affect renal function and can potentially contribute to morbidity and mortality (27,28). However, more recent studies have not identified an association between renal dysfunction and aprotinin when other co-variates were considered (38). The present study did not identify any significant differences in plasma creatinine levels in the early post-operative period. However, whether and to what degree aprotinin may have affected renal function over a longer post-operative period could not be addressed by the current study design. Thus, future studies which integrate the cytokine and MMP measurements to renal function with longer post-operative follow-up periods would be appropriate. Nevertheless, the present study demonstrated a temporal disparity in IL and MMP profiles between aprotinin and EACE, underscoring that these hemostatic agents differentially affect other biological pathways independent of the coagulation cascade. These findings hold particular relevance in light of the fact that a comparative study using anti-fibrinolytics in cardiac surgery was halted due to potentially adverse effects of aprotinin (39). Thus, in light of these observations and the findings from the present study, future investigations which identify the biological pathways and mechanisms by which aprotinin as well as other blood conservations strategies alter biological pathways independent of the coagulation cascade are warranted.
ACKNOWLEDGEMENTS
This work was supported by National Heart, Lung, and Blood Institute Grants PO1-HL-48788, RO1-HL-59165, RO1-HL56603 and a Merit Review Award from the Department of Veterans Affairs. The authors wish to acknowledge the assistance of Ms. Laura E. Pope in the preparation of this manuscript.
References
- 1.Spinale FG. Matrix metalloproteinases. Regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 2.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 3.Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006;69:614–624. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Schwimmbeck PL, Tschope C, Leschka S, Husmann L, Rutschow S, Reichenbach F, Noutsias M, Kobalz U, Poller W, Spillmann F, Zeichhardt H, Schultheiss HP, Pauschinger M. Collagen degradation in a murine myocarditis model: relevance of matrix metalloproteinase in association with inflammatory induction. Cardiovasc Res. 2002;56:235–247. doi: 10.1016/s0008-6363(02)00546-1. [DOI] [PubMed] [Google Scholar]
- 5.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, 3rd, Edmunds LH, Jr., Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107:2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 6.Sluijter JP, Pulskens WP, Schoneveld AH, Velema E, Strijder CF, Moll F, de Vries JP, Verheijen J, Hanemaaijer R, de Kleijn DP, Pasterkamp G. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions: a study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke. 2006;37:235–239. doi: 10.1161/01.STR.0000196986.50059.e0. [DOI] [PubMed] [Google Scholar]
- 7.Herman MP, Sukhova GK, Libby P, Gerdes N, Tang N, Horton DB, Kilbride M, Breitbart RE, Chun M, Schonbeck U. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104:1899–1904. doi: 10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- 8.Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007 May 15;139(2):292–307. doi: 10.1016/j.jss.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation. 2003 Feb 4;107(4):618–25. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- 10.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients following myocardial infarction: relation to left ventricular remodeling. Circulation. 2006 Sept 5;114(10):1020–7. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases / tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional & clinical manifestations of hypertensive heart disease. Circulation. 2006 May 2;113(17):2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 12.Joffs C, Gunasinghe HR, Multani MM, Dorman BH, Kratz JM, Crumbley AJ, 3rd, Crawford FA, Jr, Spinale FG. Cardiopulmonary bypass induces the synthesis and release of matrix metalloproteinases. Ann Thorac Surg. 2001 May;71(5):1518–23. doi: 10.1016/s0003-4975(01)02442-0. [DOI] [PubMed] [Google Scholar]
- 13.Mayers I, Hurst T, Puttagunta L, Radomski A, Mycyk T, Sawicki G, Johnson D, Radomski MW. Cardiac surgery increases the activity of matrix metalloproteinases and nitric oxide synthase in human hearts. J Thorac Cardiovasc Surg. 2001 Oct;122(4):746–52. doi: 10.1067/mtc.2001.116207. [DOI] [PubMed] [Google Scholar]
- 14.Lin TC, Li CY, Tsai CS, Ku CH, Wu CT, Wong CS, Ho ST. Neutrophil-mediated secretion and activation of matrix metalloproteinase-9 during cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2005 Jun;100(6):1554–60. doi: 10.1213/01.ANE.0000154307.92060.84. [DOI] [PubMed] [Google Scholar]
- 15.Galley HF, Macaulay GD, Webster NR. Matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 and tumour necrosis factor alpha release during cardiopulmonary bypass. Anaesthesia. 2002 Jul;57(7):659–62. doi: 10.1046/j.1365-2044.2002.02625.x. [DOI] [PubMed] [Google Scholar]
- 16.Deschamps AM, Spinale FG. Pathways of matrix metalloproteinase induction in heart failure: bioactive molecules and transcriptional regulation. Cardiovasc Res. 2006 Feb 15;69(3):666–76. doi: 10.1016/j.cardiores.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Rutschow S, Li J, Schultheiss HP, Pauschinger M. Myocardial proteases and matrix remodeling in inflammatory heart disease. Cardiovasc Res. 2006 Feb 15;69(3):646–56. doi: 10.1016/j.cardiores.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Munoz JJ, Birkmeyer NJ, Birkmeyer JD, et al. Is E-aminocaproic acid as effective as aprotinin in reducing bleeding with cardiac surgery? Circulation. 1999;99:81–89. doi: 10.1161/01.cir.99.1.81. [DOI] [PubMed] [Google Scholar]
- 19.van Oeveren W, Jansen NJ, Bidstrup BP, Royston D, Westaby S, Neuhof H, Wildevuur CR. Effects of aprotinin on hemostatic mechanisms during cardiopulmonary bypass. Ann Thorac Surg. 1987;44:640–5. doi: 10.1016/s0003-4975(10)62153-4. [DOI] [PubMed] [Google Scholar]
- 20.Royston D, Bidstrup BP, Taylor KM, Sapsford RN. Effect of aprotinin on need for blood transfusion after repeat open-heart surgery. Lancet. 1987;2:1289–91. doi: 10.1016/s0140-6736(87)91190-1. [DOI] [PubMed] [Google Scholar]
- 21.Sedrakyan A, Treasure T, Elefteriades JA. Effect of aprotinin on clinical outcomes in a coronary arterty bypass graft surgery: A systematic review and meta-analysis of randomized clinical trials. J Thorac Cardiovasc Surg. 2004;128:442–448. doi: 10.1016/j.jtcvs.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 22.Slaughter TF, Faghih F, Greenberg CS, et al. The effects of epsilon-aminocaproic acid on fibrinolysis and thrombin generation during cardiac surgery. Anesth Analg. 1997;85:1221–1226. doi: 10.1097/00000539-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Mojcik CF, Levy JH. Aprotinin and the systematic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg. 2001;71:745–754. doi: 10.1016/s0003-4975(00)02218-9. [DOI] [PubMed] [Google Scholar]
- 24.Landis RC, Haskard DO, Taylor KM. New anti-inflammatory and platelet-preserving effects of aprotinin. Ann Thorac Surg. 2001;72:S 1808–1813. doi: 10.1016/s0003-4975(01)03193-9. [DOI] [PubMed] [Google Scholar]
- 25.Bull D, Connors R, Albanil A, Reid B, et al. Cardiopulmonary Support and Physiology. Aprotinin preserves myocardial biochemical function during cold storage through suppression of tumor necrosis factor. J Thorac Cardiovasc Surg. 2000;119:242–250. doi: 10.1016/S0022-5223(00)70179-6. [DOI] [PubMed] [Google Scholar]
- 26.Greilich PE, Brouse CF, Rinder CS, et al. Effects of E-aminocaproic acid and aprotinin on leukocyte-platelet adhesion in patients undergoing cardiac surgery. Anesthesiology. 2004;100:1–17. doi: 10.1097/00000542-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Brown JR, Birkmeyer NJ, O'Connor GT. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation. 2007 Jun 5;115(22):2801–13. doi: 10.1161/CIRCULATIONAHA.106.671222. [DOI] [PubMed] [Google Scholar]
- 28.Mangano DT, Tudor IC, Dietzel C. The Risk Associated with Aprotinin in Cardiac Surgery. NEJM. 2006;354:353–65. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 29.Levi M, Cromheecke ME, de Jonge E, Prins MH, de Mol BJ, Briet E, Buller HR. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet. 1999;354:1940–7. doi: 10.1016/S0140-6736(99)01264-7. [DOI] [PubMed] [Google Scholar]
- 30.Greilich PE, Okada K, Latham P, Kumar RR, Jessen ME. Aprotinin but not epsilon-aminocaproic acid decreases interleukin-10 after cardiac surgery with extracorporeal circulation: randomized, double-blind, placebo-controlled study in patients receiving aprotinin and epsilon-aminocaproic acid. Circulation. 2001;104:I265–9. doi: 10.1161/hc37t1.094781. [DOI] [PubMed] [Google Scholar]
- 31.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 32.Pugin J, Widmer MC, Kossodo S, Liang CM, Preas HL, 2nd, Suffredini AF. Human neutrophils secrete gelatinase B in vitro and in vivo in response to endotoxin and proinflammatory mediators. Am J Respir Cell Mol Biol. 1999 Mar;20(3):458–64. doi: 10.1165/ajrcmb.20.3.3311. [DOI] [PubMed] [Google Scholar]
- 33.Gioia M, Fasciglione GF, Marini S, D'Alessio S, De Sanctis G, Diekmann O, Pieper M, Politi V, Tschesche H, Coletta M. Modulation of the catalytic activity of neutrophil collagenase MMP-8 on bovine collagen I. Role of the activation cleavage and of the hemopexin-like domain. J Biol Chem. 2002 Jun 28;277(26):23123–30. doi: 10.1074/jbc.M110873200. [DOI] [PubMed] [Google Scholar]
- 34.Frake PC, Smith HE, Chen LF, Biffl WL. Prestorage leukoreduction prevents accumulation of matrix metalloproteinase 9 in stored blood. Arch Surg. 2006 Apr;141(4):396–400. doi: 10.1001/archsurg.141.4.396. discussion 400. [DOI] [PubMed] [Google Scholar]
- 35.Beath SM, Nuttall GA, Fass DN, Oliver WC, Ereth MH, Oyen LJ. Plasma aprotinin concentrations during cardiac surgery: full- versus half-dose regimens. Anesth Analg. 2000 Aug;91(2):257–64. doi: 10.1097/00000539-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Toy LW. Matrix metalloproteinases: their function in tissue repair. J Wound Care. 2005;14:20–22. doi: 10.12968/jowc.2005.14.1.26720. [DOI] [PubMed] [Google Scholar]
- 37.Steffensen B, Hakkinen L, Larjava H. Proteolytic events of wound-healing--coordinated interactions among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev Oral Biol Med. 2001;12:373–398. doi: 10.1177/10454411010120050201. [DOI] [PubMed] [Google Scholar]
- 38.Furnary AP, Wu Y, Hiratzka LF, Grunkemeier GL, Page US., 3rd Aprotinin does not increase the risk of renal failure in cardiac surgery patients. Circulation. 2007 Sep 11;116(11 Suppl):I127–33. doi: 10.1161/CIRCULATIONAHA.106.681395. [DOI] [PubMed] [Google Scholar]
- 39.American Heart Association Advisory: Bayer suspends marketing of aprotinin (Trasylol) at FDA request. November 2007 Web site: http://www.informz.net/heart/archives/archive_518051.html.