Abstract
Background
We examined the potential clinical utility of using a cytomegalovirus (CMV)–specific T cell immunoassay to determine the risk of developing new-onset CMV retinitis (CMVR) in patients with acquired immunodeficiency syndrome (AIDS).
Methods
CMV-specific T cell assays were performed by multiparameter flow cytometry using stored peripheral blood mononuclear cells that had been obtained in an observational study 2–6 months before new-onset CMVR was diagnosed in case patients (at a study visit during which a dilated ophthalmologic examination revealed no evidence of CMVR) and at the same study visit in control subjects (matched by absolute CD4+ T cell count at entry) who did not subsequently develop retinitis during 1–6 years of study follow-up.
Results
There were no significant differences in CMV-specific CD4+ or CD8+ T cell interferon-γ or interleukin-2 expression in peripheral blood mononuclear cells from case patients and control subjects. Although there were trends toward lower percentages and absolute numbers of CMV-specific, cytokine-expressing CD8+ T cells with a “late memory” phenotype (CD27−CD28−) as well as with an “early memory” phenotype (CD27+CD28+CD45RA+) in case patients than in control subjects, these differences were not statistically significant.
Conclusions
Many studies have reported that CMV-specific CD4+ and CD8+ T cell responses distinguish patients with active CMVR (i.e., who lack CMV-protective immunity) from those with inactive CMVR after immune restoration by antiretroviral treatment (i.e., who have CMV-protective immunity). However, the multiple CMV-specific immune responses we measured do not appear to have clinical utility for predicting the risk for patients with AIDS of developing new-onset CMVR with sufficient accuracy to be used in guiding therapeutic management.
Before the availability of HAART regimens, cytomegalovirus (CMV) end-organ disease, primarily retinitis (CMVR), was a common complication of AIDS, occurring in 40% of patients with absolute CD4+ T cell counts <50 cells/μL [1-3]. Before HAART, retinitis progressed despite systemic anti-CMV drug therapy [4]. The incidence of CMVR has decreased substantially in Western countries, where HAART became widely available [5, 6], although it subsequently has stabilized at ∼25% of its pre-HAART incidence [5-7]. Nevertheless, CMVR remains an important complication of AIDS internationally, reported in up to 8.5% of patients with AIDS in parts of Africa [8], 15% of patients with AIDS in Thailand [9], and 17% of patients with AIDS in India [10]. Thus, interventions that could prevent or more cost-effectively treat CMVR could have worldwide impact. In particular, the ability to identify patients at very high risk of developing CMVR in the near future could allow either anti-CMV or more intensive antiretroviral therapeutic interventions to be made before irreversible retinal necrosis occurs.
Because CMV-specific T cells may be important in controlling replication of CMV, numerous studies have investigated their role in controlling disease, both in patients with AIDS and in those undergoing transplantation [11-20]. We recently reported results of measuring both CMV-specific CD4+ and CD8+ T cell IFN-γ and IL-2 responses and the maturational status of CMV-specific IFN-γ–expressing CD8+ T cells in 2 groups of patients with CMVR—one with clear evidence of absent CMV-protective immunity (i.e., patients with active CMVR) and one with clear evidence of restored CMV-protective immunity (i.e., patients with CMVR clinically immunorestored after receipt of antiretroviral therapy who were able to discontinue anti-CMV therapy without further reactivation or progression of retinitis) [21]. In comparison with those with active retinitis, immunorestored patients had higher levels of circulating CD4+ and CD8+ T cells expressing IL-2 and IFN-γ in response to combined CMV pp65 and immediate-early-1 (IE-1) peptide pool stimulation. Immunorestored patients, compared with those with active retinitis, had increased numbers of circulating CMV-specific CD8+ T cells with “early” (CD27+CD28+CD45RA+, CD27+CD28+CD45RA−) and “intermediate” (CD27−CD28+CD45RA−) phenotypes. In particular, 3 CMV-specific T cell subsets—CD8+IFN-γ+CD27+CD28+, CD4+IFN-γ+IL-2+, and CD4+IFN-γ+IL-2− T cells—were very depleted or absent in 100% of patients with active CMVR, whereas 85% of patients with immunorestored CMVR had a positive value for at least one of these subsets. We now report the results of testing the potential clinical utility of these CMV-specific T cell subsets in identifying patients with advanced AIDS who would develop new-onset CMVR within the subsequent 6 months.
PATIENTS, MATERIALS, AND METHODS
Study participants
Frozen PBMC specimens, cryopreserved from acid citrate dextrose–treated whole blood as described elsewhere [12], were obtained from the Longitudinal Studies of the Ocular Complications of AIDS (LSOCA), a prospective, multicenter, observational study of patients with AIDS [22]. Case patients had no evidence of CMVR at the entry ophthalmologic examination, developed incident CMVR during follow-up, and had a PBMC specimen obtained at a visit within 6 months before CMVR diagnosis that was available in the LSOCA repository. During the visit at which the PBMC specimen was obtained from case patients, a dilated ophthalmologic examination was done and revealed no evidence of CMVR. Two control subjects were selected to match each case patient for absolute CD4+ T cell count at the baseline visit and for the time interval between first AIDS diagnosis and baseline visit. Matched control subjects had no evidence of CMVR at the entry ophthalmologic examination and did not develop incident CMVR during follow-up (which included dilated ophthalmoscopic examinations every 6 months) and were required to have a duration of follow-up longer than the matching case patient's, as well as a stored PBMC specimen available in the LSOCA repository from the same study visit as the case patient's PBMC specimen. No control subjects or case patients were receiving anti-CMV therapy at the visit when the PBMC specimen was obtained. Informed consent was obtained from all patients participating in the study. The human experimentation guidelines of the US Department of Health and Human Services and of participating institutions were followed in conducting this research.
CMV-specific multiparameter cytokine flow cytometry assay
For each subject, one aliquot (10×106 cryopreserved PBMCs) was processed and immunoassayed as described elsewhere [21], except that pp65 and IE-1 peptide pools were mixed together for PBMC stimulation at final concentrations of 1.7 and 2.5 μg/mL per peptide, respectively. Samples with <50% viability or <10% recovery by Viacount assay (Guava PCA) before peptide stimulation were discarded. After peptide stimulation and antibody staining, ethidium monoazide bromide (Molecular Probes)–positive events were gated out to remove dead cells from the analysis. Samples with >50% dead cells were not analyzed.
Positive cutoff values for the percentage of CD4+ and CD8+ T cells expressing IFN-γ or both IFN-γ and IL-2 after CMV pp65/IE-1 stimulation were determined by assaying cryopreserved PBMCs from 10 healthy CMV-seronegative volunteers. The background-corrected percentages of CMV-specific T cells in all 10 samples were as follows: CD4+ IFN-γ+, <0.03%; CD4+IFN-γ+IL-2+, <0.03%; CD8+IFN-γ+, <0.02%; and CD8+IFN-γ+IL-2+, <0.01%.
Data analysis
Statistical significance of between-group comparisons was determined by Fisher's exact test for categorical results and by the t test for quantitative results. The risks of CMVR occurrence associated with key baseline characteristics and measurements made at the visit before CMVR diagnosis were analyzed by conditional logistic regression, using the ranks for continuous variables.
RESULTS
Viability and recovery of cells from archived PBMC samples
Of 13 PBMC specimens from case patients, 11 had adequate viability and recovery after thawing when assessed by the Guava PCA Viacount assay, as well as (after resting and stimulation) by ethidium monoazide bromide staining, and were considered to be evaluable. For each case patient, 2 matched control samples were thawed. Overall, 11 of 13 PBMC specimens from case patients versus 17 of 26 control samples were evaluable (P=.16, by 2-tailed Fisher's exact test). For 1 evaluable case patient, both control specimens were evaluable. For the other 10 evaluable case patients, only 1 of the 2 control PBMC specimens were evaluable.
Characteristics of evaluable subjects
Characteristics of evaluable case patients and control subjects are summarized in table 1. The case patients developed new-onset CMVR a median of 448 days (range, 55–1526 days) after enrollment in LSOCA; the median duration of follow-up on study for the control subjects who never developed CMVR was 1163 days (range, 348–2401 days) after enrollment in LSOCA. The visit from which the PBMC specimens were obtained for immunoassay occurred 2–6 months before CMVR was diagnosed in the case patients; a PBMC specimen from the same LSOCA visit was used for the matched control subjects. All PBMC specimens were obtained during LSOCA visits that occurred between 1999 and 2003 for case patients and between 1999 and 2004 for control subjects. All subjects had a dilated ophthalmologic examination at this visit, which showed no evidence of CMVR. Ten of 11 case patients and 9 of 12 control subjects were receiving HAART at this visit.
Table 1.
Characteristics of evaluable case patients with cytomegalovirus (CMV) retinitis (CMVR) diagnosed during follow-up and control subjects who did not develop CMVR during follow-up.
| Factor | Case patients (n = 11) |
Control subjects (n = 12) |
|
|---|---|---|---|
| Age, median years (range) | 42 (35–52) | 42 (33–59) | |
| Percentage of men | 91 | 67 | |
| Race, percentage of subjects | |||
| White | 73 | 67 | |
| Black | 27 | 8 | |
| Hispanic | 0 | 25 | |
| Time from AIDS diagnosis to LSOCA entry, median years (range) | 5.6 (1.6–12) | 4.9 (0.2–11.7) | |
| Plasma HIV RNA, median log copies/mL (range)a | 5.534 (1.699–6.000) | 4.891 (1.491–5.875) | |
| No. of subjects with detectable plasma CMV DNA level/total no. of subjectsa | 3/11 | 1/8 | |
| Hemoglobin level, median g/dLa | 12.4 | 12.9 | |
| Absolute T cell count, median cells/mL (range) | |||
| CD4+ T cell count at entry | 13 (0–351) | 12 (2–349) | |
| CD4+ T cell count on date that the PBMC specimen was obtained | 16 (2–336) | 44 (2–830) | |
| CD8+ T cell count on date that the PBMC specimen was obtained | 366 (62–890) | 491 (163–1584) | |
NOTE. LSOCA, Longitudinal Studies of the Ocular Complications of AIDS.
On date that the PBMC specimen was obtained (2–6 months before CMVR diagnosis in case patients).
CD4+ and CD8+ T cell IFN-γ and IL-2 expression in response to stimulation by pp65 and IE-1
In our previous study, we constructed receiver operator curves to determine optimal cutoff values for discriminating patients with active CMVR from patients with CMVR clinically immunorestored by antiretroviral therapy [21]. These values for combined CMV pp65- and IE-1–specific, cytokine-expressing T cells were >50 CD4+IFN-γ+IL-2+ T cells/mL, >70 CD4+IFN-γ+IL-2− T cells/mL, >100 CD8+IFN-γ+IL-2+ T cells/mL, and >4000 CD8+IFN-γ+IL-2− T cells/mL [21]. Among patients with active CMVR, 0%–33% had a result above the cutoff value, compared with 76%–85% of patients with clinically immunorestored CMVR. However, in the current study, when these same values were used to define a protective CMV-specific cytokine response, there were no differences between case patients and control subjects for any of these 4 CMV-specific T cell responses (table 2, comparison A). CD4+ T cell responses were low or undetectable in PBMCs obtained from case patients and control subjects. CD8+ T cell responses were maintained in the majority of PBMCs obtained from both case patients and control subjects. We also compared the percentage of pp65/IE-1–specific CD4+ and CD8+ T cells using the cutoff value that defined a negative result in healthy CMV-seronegative volunteers. This analysis also showed no differences between results for PBMCs obtained from case patients and control subjects (table 2, comparison B). Because CMV-specific CD4+ and CD8+ T cell IFN-γ−IL-2+ responses were extremely low in all subjects, these data were not included in any analysis.
Table 2.
Proportions of case patients and control subjects with positive cytomegalovirus (CMV) pp65 and immediate-early–1 (IE-1)–specific T cell cytokine responses in PBMCs obtained 2–6 months before diagnosis of CMV retinitis (CMVR) in case patients and at the same visit in control subjects who did not subsequently develop CMVR.
| Proportion of subjects |
|||
|---|---|---|---|
| Comparison, T cell response | Case patients |
Control subjects |
|
| A | |||
| CD4+IFN-γ+IL-2+ >50 cells/mL | 3/11 | 2/12 | |
| CD4+IFN-γ+IL-2− >70 cells/mL | 3/11 | 4/12 | |
| CD8+IFN-γ+IL-2+ >100 cells/mL | 8/11 | 8/12 | |
| CD8+IFN-γ+IL-2− >4000 cells/mL | 8/11 | 7/12 | |
| B | |||
| CD4+IFN-γ+IL-2+ >0.03% | 4/11 | 4/12 | |
| CD4+IFN-γ+ >0.03% | 6/11 | 5/12 | |
| CD8+IFN-γ+IL-2+ >0.01% | 11/11 | 8/12 | |
| CD8+IFN-γ+ >0.02% | 11/11 | 8/12 | |
| C | |||
| CD8+IFN-γ+CD27+CD28+CD45RA+ >12 cells/mL | 3/11 | 6/8 | |
| CD8+IFN-γ+CD27+CD28+CD45RA+ >0.002% | 5/11 | 7/8 | |
| CD8+IFN-γ+IL-2+CD27+CD28+CD45RA+ >0 cells/mL | 3/11 | 6/8 | |
| CD8+IFN-γ+IL-2+CD27+CD28+CD45RA+ >0% | 3/11 | 6/8 | |
| D | |||
| CD8+IFN-γ+CD27−CD28− >15,300 cells/mL | 3/11 | 5/8 | |
| CD8+IFN-γ+CD27−CD28− >3% | 4/11 | 6/8 | |
| CD8+IFN-γ+IL-2+CD27−CD28− >2000 cells/mL | 1/11 | 5/8 | |
| CD8+IFN-γ+IL-2+CD27−CD28− >0.44% | 2/11 | 5/8 | |
NOTE. Percentage results refer to the percentage of all CD4+ or CD8+ T cells that expressed the specific cytokines and maturational markers after pp65/IE-1 stimulation. All results were corrected by subtracting background results in a simultaneous, nonstimulated, control well. Comparisons A and B, optimal cutoff values from previous study [21]; comparisons C and D, optimal cutoff values of other CMV-specific CD8+ T cell maturational subsets.
Maturational status of IFN-γ+IL-2+ and IFN-γ+IL-2− CMV-specific CD8+ T cells.
In our previous study, we analyzed CMV-specific CD8+IFN-γ+ T cell maturational subsets and found that the subset expressing CD28 and CD27 (independent of CD45RA and IL-2 expression) had a maturational profile that discriminated patients with active CMVR from patients with CMVR after antiretroviral immunorestoration. The optimal cutoff value for a protective value for CMV-specific CD8+IFN-γ+CD27+CD28+ T cells was >1100 cells/mL. However, in the current study, 2 of 11 case patients versus 0 of 8 control subjects had this positive result in PBMCs obtained 2–6 months before the diagnosis of CMVR.
We also explored whether other CMV-specific CD8+ T cell maturational subsets might better predict subsequent incident CMVR. By inspection, it appeared that there were trends toward lower percentages and absolute numbers of CMV-specific, cytokine-expressing CD8+ T cells with a “late memory” phenotype (CD27−CD28−) as well as with an “early memory” phenotype (CD27+CD28+CD45RA+) in case patients than in control subjects. Thus, we constructed receiver operator curves to determine optimal cutoff values for discriminating case patients from control subjects, which confirmed these trends (table 2, comparisons C and D). However, the differences in proportions of case patients and control subjects with responses above cutoff values were not statistically significant after adjustments were made for the multiple comparisons. In addition, these analyses were potentially biased by the lack of control data for 4 of the 11 case patients.
Effect of other covariables on risk of developing CMVR
We used conditional logistic regression analysis to examine the risk of CMVR incidence associated with key baseline characteristics (demographics, baseline absolute CD4+ cell count, and time since AIDS diagnosis) as well as CMV-specific CD4+ and CD8+ T cell cytokine responses and other potential covariables (absolute CD4+ and CD8+ T cell count, the presence of CMV DNA, plasma HIV RNA load, hemoglobin level, and use of antiretroviral therapy) determined at the visit before CMVR diagnosis in case patients and the same study visit in control subjects. There were insufficient data about the maturational status of IFN-γ+IL-2+ and IFN-γ+IL-2− CMV-specific CD8+ T cells to include these parameters in this analysis. Although no predictive parameters were statistically significant, the 3 variables most strongly associated with subsequent CMVR incidence were the absolute CD4+ T cell count (P = .16), plasma HIV RNA load (P p .17), and percentage of CMV-specific CD8+ T cells that expressed IFN-γ but not IL-2 (P = .22) at the visit before CMVR diagnosis.
Changes in CMV-specific T cell responses at the time of CMVR diagnosis.
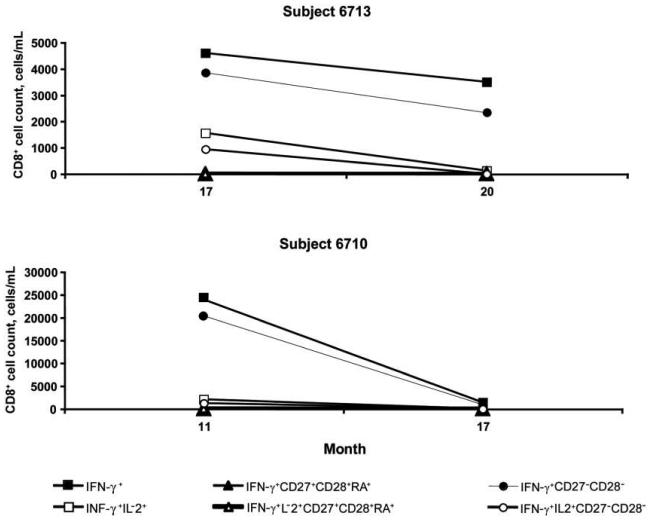
Because CMV-specific T cell responses in PBMCs obtained 2–6 months before CMVR diagnosis were not predictive of CMVR, we wished to examine whether there was a change in responses obtained at this visit versus the subsequent visit when CMVR was diagnosed. There were 2 case patients who, as part of our previous study, did have CMV-specific T cell responses measured in PBMCs obtained at the time of CMVR diagnosis [21]. Therefore, we compared the complete panel of CMV-specific T cell responses and the maturational status of CMV-specific CD8+ T cells at the visit before CMVR diagnosis and at the subsequent visit when CMVR was diagnosed in these 2 case patients (figure 1). Both subjects lacked CMV-specific CD4+ T cell IFN-γ and IL-2 responses at both visits. However, both case patients had detectable CMV-specific CD8+ T cell IFN-γ and IL-2 responses and quantifiable CD8+ maturational subsets that decreased, with the same pattern for both subjects, between the 2 visits. This decline was particularly large for IFN-γ–expressing CMV-specific CD8+ T cells that had a late differentiation phenotype (CD27−CD28−).
Figure 1.
Changes in the absolute number of cytomegalovirus-specific CD8+ T cell subsets from the last visit before diagnosis of cytomegalovirus retinitis to the time of diagnosis of cytomegalovirus retinitis in 2 subjects.
DISCUSSION
We previously observed that patients with AIDS who had CMVR and clinical evidence of restored CMV-protective immunity had higher levels of circulating CMV-specific CD4+ and CD8+ T cells expressing IL-2 and IFN-γ and higher levels of circulating CMV-specific CD8+ T cells with “early” or “inter-mediate” phenotypes than did patients with active CMVR [21]. These results, as well as those of other studies reporting that CMV-specific CD4+ and CD8+ T cell responses distinguish patients with active CMVR from those who have been immunorestored by antiretroviral therapy and have no retinitis activity when not taking anti-CMV therapy, suggest that monitoring such CMV-specific immune responses might be useful in predicting risk of developing CMVR in patients with AIDS [12-17]. However, when we measured CMV-specific CD4+ and CD8+ T cell IFN-γ and IL-2 expression, and the maturational status of C MV-specific cytokine-expressing CD8+ T cells, in PBMCs obtained from 11 patients with AIDS 2–6 months before CMVR was diagnosed and compared these responses to those of well-matched control subjects, we did not observe differences that would likely lead to clinical utility.
Although the sample size in this study was small, the power to distinguish significantly (2-sided type I error = 0.05) between 2 independent groups in which 80% of case patients did not have a particular CMV-specific T cell subset versus 10% of control subjects was 88%. As clinicians, we consider that candidate CMV-specific immune markers would require this degree of accurate discrimination between at-risk patients who would versus would not develop CMVR within the next 6 months to have clinical utility in determining whether new treatment interventions (e.g., valganciclovir) should be initiated.
The correlation between factors such as low absolute CD4+ cell count, high plasma HIV RNA load, and the presence of CMV DNA in plasma, which have been clearly shown in observational studies with larger sample sizes to constitute risk factors for CMV disease [23-25], was not significant in our study because of the small sample size. However, the strength of the association of these factors with CMV disease has recently been demonstrated, in a randomized clinical trial [26], to be insufficient for clinical utility in determining whether valganciclovir treatment should be initiated.
For 2 case patients, we also had results of CMV-specific T cell responses measured in PBMCs subsequently obtained at the time of CMVR diagnosis. For these patients, CMV-specific CD8+ T cell IFN-γ and IL-2 responses—and, in particular, the subset of CD8+ T cells with a late memory phenotype—decreased between visits. This suggests that the poor predictive value of these assays may be due to a rapid decrease in CMV-specific CD8+ T cell subsets occurring in the weeks immediately before the onset of CMVR (perhaps due to exhaustion of CMV-specific CD8+ T cells, migration of CMV-specific effectors to sites of active CMV replication, or an immunosuppressive effect of increased CMV replication). If this explanation is true, immunologic monitoring of these CMV-specific T cell responses would have to be done more frequently than every 2–3 months to accurately detect the risk of developing CMV disease—a strategy that would not likely be cost-effective or clinically feasible.
Our findings do raise the possibility that measurement of other antigen-specific CD8+ T cell functions, such as the ability to proliferate or degranulate, the expression of other cytokines (e.g., TNF-α) or markers of exhaustion (e.g., PD-1), or the antigenic diversity of the CMV-specific CD8+ T cell repertoire might reveal deficits that precede the late drop in CMV-specific CD8+ T cell numbers we observed just before CMVR diagnosis and thus might be more temporally suitable for monitoring in terms of potential clinical utility [13, 27-31]. It is also possible that a different assay of CMV-specific CD8+ T cell cytokine response, such as the ELISPOT, might be superior to our cytokine flow cytometry assay for this indication [17]. In addition, analysis of CD8+ T cell responses to individual antigens may have better clinical utility for predicting outcome in the 3–6-month window before CMV end-organ disease occurs in patients with AIDS [32]. In the current study, we combined pp65 and IE-1 stimulation to maximize the detection of CMV-specific CD8+ T cells; however, in a previous study comparing patients with AIDS with active CMVR and those with CMVR who had been immunorestored by antiretroviral therapy, we found that the immunorestored group had higher numbers of CD8+ T cells in response to IE-1, whereas there was no difference between the 2 groups in their response to pp65 [21]. Finally, the use of fresh PBMCs, instead of frozen samples, or the use of a different source of reagents could potentially improve the accuracy of the assay in identifying patients who would develop CMVR.
The presence of CMV-specific CD4+ T cells is necessary to sustain CMV-specific cytotoxic CD8+ T cell activity in bone marrow transplant recipients [20]. Bronke et al. [16] reported disappearance of CMV-specific CD4+ T cells 1 year before the onset of CMV end-organ disease in patients with AIDS. However, when we tested the potential clinical utility of longitudinally monitoring CMV-specific CD4+ T cell proliferation and IFN-γ expression in patients with active and immunorestored CMVR, these assays did not appear to have clinical utility [11]. In the present study, in which subjects were matched for absolute CD4+ T cell count, both case patients and control subjects had low CD4+ T cell responses to CMV, suggesting that lack of these responses may facilitate progression to retinitis but that this change alone is insufficient to predict short-term likelihood of CMVR occurring.
In conclusion, although we previously identified CMV-specific T cell parameters that discriminated well between patients with active versus immunorestored CMVR, these parameters were not predictive of CMVR when measured 2–6 months before disease diagnosis. CD4+ T cell IFN-γ and IL-2 responses to CMV were often lost before the development of CMVR; however, loss of these responses in subjects who did not develop CMV disease also frequently occurred. CMV-specific CD8+ T cell responses, although absent at the time of CMVR diagnosis, were present in the months preceding incident disease. Although the sample size in our study had power to detect only large differences in CMV-specific T cell responses, the multiple CMV-specific immune responses we measured do not appear to have clinical utility in predicting the risk of developing CMVR in patients with AIDS with the sufficient discrimination and accuracy requisite for use in guiding therapeutic management. Observational studies of other CMV-specific CD8+ T cell responses that may be more temporally suited for clinical diagnosis (e.g., proliferation, degranulation, and expression of markers of cellular exhaustion) should be examined for predictive value and potential clinical utility in defining CMV-protective immunity and guiding clinical management for HIV-infected patients with a history of CMVR or at risk for developing CMVR.
LSOCA KEY PERSONNEL
Participating clinical centers
Baylor College of Medicine, Cullen Eye Institute, Houston, Texas: Richard Alan Lewis, director; Pamela Frady, Ronald Gross, Silvia Orengo-Nania, Tobias C. Samo, Allison Schmidt, Laura Shawver, Benita Slight, Rachel Sotuyo, and Steven Spencer, members; Richard C. Allen, James W. Shigley, and Stephen Travers, former members. Emory University Eye Center, Atlanta, Georgia: Daniel F. Martin, director; David Furukuwa, Allison Gibbs, Deborah Gibbs, and Bob Myles, members; Antonio Capone, Jr., and Baker Hubbard, former members. Indiana University, Indianapolis: Mitchell Goldman, director; Jean Craft, Paul Fry, Hua Gao, Samir Gupta, Janet Hernandez, Linda Pratt, and Beth Zwickl, members; Janice Brown, Thomas Ciulla, Ronald Danis, Debra Poe, James D. Richardson (former director), Tim Steffens, and L. Joseph Wheat (former director), former members. Johns Hopkins University School of Medicine, Baltimore, Maryland: J. P. Dunn, director; Patricia Barditch-Crovo, Joseph B. Brodine, Diane M. Brown, Lisa M. Brune, Dennis Cain, David Emmert, Anat Galor, Douglas A. Jabs, Sanjay R. Kedhar, Henry A. Leder, Kisten D. Nolan, George B. Peters, III, Richard D. Semba, and Jennifer E. Thorne, members; Rebecca Becker, Marie-Lyne Bélair, Stephen G. Bolton, Jared Christopher, Terry George, John H. Kempen, Stephen J. Kim, Susan M. LaSalvia, Paul Latkany, Laura G. Neisser, Quan D. Nguyen, Armando L. Oliver, Faqir A. Qazi, Aruna Subramanian, and Susan Wittenberg, former members. Louisiana State University Medical Center, New Orleans: Bruce Barron, director; Robin Bye, Rebecca Clark, Robin Cooper, Larry Dillon, Christine Jarrott, and Lynn Otillio, members; Mandi Conway and Gholman Peyman, former members. New Jersey Medical School, Newark: Ronald Rescigno, director; Neelakshi Bhagat, Rosa Paez-Boham, and Marta Paez-Quinde, members. New York Hospital-Cornell Medical Center, New York: Murk-Hein Heinemann, director; Cynthia Chiu, Susana Coleman, Jasmine Elison, Roberta Janis, Andrzej Kozbial, Joseph Murphy, Christina Peroni, Diane Iglesias Rivera, and Kent Sepkowitz, members; Kenneth Boyd, Charles Doering, Sangwoo Lee, Joseph Murphy, Firas M. Rahhal, and Ashok Reddy, former members. New York University Medical Center, New York: Dorothy N. Friedberg, director; Adrienne Addessi, Douglas Dieterich, Richard Hutt, Monica Lorenzo-Latkany, and Maria Pei, members; Alex McMeeking, former member. Northwestern University, Chicago, Illinois: Alice T. Lyon, director; Lori Ackarz, Manjot Gill, Lori Kaminski, Robert Murphy, Frank Palella, and Jonathan Shankle, members; Alexander Habib, Jill Koecher, Annmarie Muñana, Jeevan Mathura, Michele Till, David V. Weinberg, and James Yuhr, former members. Rush University, Chicago, Illinois: Mathew W. MacCumber, director; Bruce Gaynes, Pamela Hulvey, Pauline Merrill, Allen Tenorio, and Denise Voskuil-Marre, members; Harold Kessler, Andrea Kopp, Frank Morini, and Nada Smith, former members. University of California, Irvine: Baruch D. Kuppermann, director; Bogdan Alexandiescu, Babak Fardin, Donald N. Forthal, Jeff Grijalva, Rosie Magallon, and Bret Trump, members; Faisal Jehan, Karen Lopez, Nader Moinfar, Melody Vega, and Randy Williams, former members. University of California, Los Angeles: Gary N. Holland, director; Robert D. Almanzor, Margrit E. Carlson, Ann K. Johiro, David LeBeck, Kristen Lipka, Germán Pinñón, Susan S. Ransome, Kayur H. Shah, and Jean D. Vaudaux, members; Suzette A. Chafey, Alexander C. Charonis, Angela Sanderson, Ardis A. Moe, Robert Stalling, and Dennis Thayer, former members. University of California, San Diego: William R. Freeman, director; Tom Clark, Randall L. Gannon, Victoria Morrison, Nicole Reagan, members; Sunan Chaidhawanqual, Lingyun Cheng, Mark Cleveland, Claudio Garcia, Daniel Goldberg, Marietta Karavellas, Brian Kosobucki, Mi-Kyoung Song, Francesca Torriani, Dorothy Wong, and Tekeena Young, former members. University of California, San Francisco: Jacque Duncan, director; Fermin Ballesteros, Robert Bhisitkul, David Clay, Michael Deiner, Donald Eubank, Alexander Irvine, Mark Jacobson, Mary Lew, Todd Margolis, and Michael Narahara, members; Judith Aberg, Jacqueline Hoffman, James Larson, Jody Lawrence, and James O. O'Donnell (former director), former members. University of North Carolina, Chapel Hill: Travis A. Meredith, director; Kelly DeBoer, Sandy Janowski, Angela Jeffries, Maurice B. Landers, Kean T. Oh, and David Wohl, members; Stephanie Betran, David Eifrig, John Foley, Jan Kylstra, Barbara Longmire, Sharon Myers, Jeremy Pantell, Susan Pedersen, Cadmus Rich, Cecilia A. Sotelo, Charles van der Horst, and Samir Wadhvania, former members. University of Pennsylvania Medical Center, Philadelphia: Charles W. Nichols, director; Mark Bardsley, Cheryl C. Devine, Jay Kostman, Albert Maguire, William Nyberg, and Leslie Smith, members; RobRoy MacGregor, Chris Helker, Karen McGibney, and Keith Mickelberg, former members. University of Southern California, Los Angeles: Jennifer I. Lim, director; Rizwan Bhatti, Lawrence Chong, Amani Fawzi, Jesus Garcia, Todd Klesert, Margaret Podilla, Len Richine, Danny Romo, Srinivas Sadda, Richard Scartozzi, A. Frances Walonker, and Alexander Walsh, members; John Canzano, Thomas S. Chang, Alexander Charonis, Robert Equi, Christina Flaxel, Francoise Kramer, Lori Levin, Tracy Nichols, Christopher Pelzek, Robert See, Kevin Shiramizu, Mark Thomas, and Ziquiang Wu, former members. University of South Florida, Tampa: Peter Reed Pavan, director; Burton Goldstein, Sandra Gompf, Amy Kramer, Scott E. Paulter, Wyatt Saxon, and Nancy Walker, members; Bonnie Hernandez, JoAnn Leto, Sharon Millard, and Jeffrey Nadler, former members. University of Texas Medical Branch, Galveston: Helen K. Li, director; John Horna, Zbigniew Krason, Beverly B. Mizell, Lan-Chi Nguyen, David Paar, and Anne Stewart, members; Wael M. Abdelghani, Robert Blem, Celia Hutchinson, Vivian Keys, Michelle Onarato, and Sami Uwaydat, former members. Johns Hopkins University School of Medicine, Baltimore, Maryland (Chairman's Office): Douglas A. Jabs, study chairman; Judy C. Southall, and Maria Stevens, members; Wanda M. Chaney, Nancy Davidson, Jacqueline Harden, Joan L. Klemstine, and Lana D. Kramer, former members. Johns Hopkins University Bloomberg School of Hygiene and Public Health, Baltimore, Maryland (Coordinating Center): Curtis L. Meinert, director; Debra A. Amend-Libercci, Karen L. Collins, Betty J. Collison, Joan Dodge, John Dodge, Michele Donithan, Kevin Frick, Judith Harle, Janet T. Holbrook, Milana R. Isaacson, Rosetta M. Jackson, John H. Kempen, Hope Livingston, Barbara K. Martin, Jennifer Meyers, Wai Ping Ng, Michael Smith, Paul Smith, Jennifer E. Thorne, James A. Tonascia, Mark L. Van Natta, and Albert Wu, members; Carley Benham, Gregory Foster, Adele M. Kaplan Gilpin, Nancy Min, Laurel Murrow, Maria J. Oziemkowska, Pamela E. Scott, Erica Smothers, Emily West, Claudine Woo, and Alice Zong, former members. Fundus Photograph Reading Center, University of Wisconsin, Madison: Matthew D. Davis, director; Michael Altaweel, Jane Armstrong, Sheri Glaeser, Larry Hubbard, Dolores Hurlburt, Jeffrey Joyce, Linda Kastorff, Michael Neider, Nancy Robinson, Therese Traut, Marilyn Vanderhoof-Young, and Hugh Wabers, members. National Eye Institute, Bethesda, Maryland: Natalie Kurinij, member.
Officers of the study
Douglas A. Jabs, chair; Matthew D. Davis, Janet T. Holbrook, Natalie Kurinij, and Curtis L. Meinert; James A. Tonascia, former member.
Steering Committee
Douglas A. Jabs, chair; Lisa Brune, Tom Clark, Matthew D. Davis, James P. Dunn, Janet T. Holbrook, Larry Hubbard, Mark Jacobson, Natalie Kurinij, Daniel F. Martin, Curtis L. Meinert, Travis A. Meredith, Frank Palella, and Steven Spencer, members; Adrienne Addessi, Beverly L. Alston, Rebecca Clark, Janet Davis, William R. Freeman, Dorothy Friedberg, James Gilman, John Horna, Annmarie Muñana, Robert Murphy, P. Reed Pavan, Tim Steffens, Dennis Thayer, Michele Till, James A. Tonascia, Francesca Torriani, Charles van der Horst, and Fran Wallach, former members.
Policy and Data Monitoring Board
John P. Phair, chair; Brian P. Conway, Barry R. Davis, Matthew D. Davis, Argye Hillis, Janet T. Holbrook, Douglas A. Jabs, Natalie Kurinij, Curtis L. Meinert, Robert Nussenblatt, Harmon Smith, and Richard Whitley, members; Beverly L. Alston, B. William Brown, Jr., James Grizzle, and James A. Tonascia, former members.
Visual Function Quality Assurance Committee
Steven Spencer, chair; Robert D. Almanzor, Deborah Gibbs, Milana Isaacson, Richard Alan Lewis, advisor, members; and Rosa Paez-Boham; Ferman Ballesteros, Jeff Grijalva, Karen Lopez, and Laura G. Neisser, former members.
Acknowledgments
Financial support. R01 EY015651, 5R01AI047062, National Center for Research Resources (UL1 RR024131–01), and Center for AIDS Research (5P30AI027763–14, UCSF-GIVI ) at the National Institutes of Health. The Studies of Ocular Complications of AIDS Research Group is supported by cooperative agreements from the National Eye Institute to the Johns Hopkins University School of Medicine (U10 EY 08052), the Johns Hopkins University Bloomberg School of Public Health (U10 EY 08057), and the University of Wisconsin, Madison, School of Medicine (U10 EY 08067), with additional support provided by National Center for Research Resources through General Clinical Research Center (5M01 RR 00350 to Baylor College of Medicine; 5M01 RR 05096 to LSU/Tulane/Charity Hospital; 5M01 RR00096 to New York University Medical Center, New York; 5M01 RR 00865 to University of California, Los Angeles; 5M01 RR00046 to University of North Carolina; 5M01 RR00043 to University of Southern California; and 5M01 RR00047 to Weill Medical College of Cornell University) and through cooperative agreements (U01 AI 27674 to Louisiana State University/Tulane; U01 AI 27660 to University of California, Los Angeles; U01 AI 27670 to University of California, San Diego; U01 AI 27663 to University of California, San Francisco; U01 AI25868 to University of North Carolina; and U01 AI32783 to University of Pennsylvania).
Footnotes
Potential conflicts of interest. M.I. and H.T.M. are employees of BD Biosciences, a manufacturer of some of the research and diagnostic reagents used in the study described herein. All other authors: no conflicts.
References
- 1.Hoover DR, Saah AJ, Bacellar H, et al. Clinical manifestations of AIDS in the era of pneumocystis prophylaxis. N Engl J Med. 1993;329:1922–6. doi: 10.1056/NEJM199312233292604. [DOI] [PubMed] [Google Scholar]
- 2.Gallant JE, Moore RD, Richman DD, Keruly J, Chaisson RE. Zidovudine Epidemiology Study Group. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. J Infect Dis. 1992;166:1223–7. doi: 10.1093/infdis/166.6.1223. [DOI] [PubMed] [Google Scholar]
- 3.Pertel P, Hirschtick R, Phair J, Chmiel J, Poggensee L, Murphy R. Risk of developing cytomegalovirus retinitis in persons infected with the human immunodeficiency virus. J Acquir Immune Defic Syndr. 1992;5:1069–74. [PubMed] [Google Scholar]
- 4.Studies of Ocular Complications of AIDS Research Group Mortality in patients with the acquired immunodeficiency syndrome treated with either foscarnet or ganciclovir for cytomegalovirus retinitis. N Engl J Med. 1992;326:213–20. doi: 10.1056/NEJM199201233260401. [DOI] [PubMed] [Google Scholar]
- 5.Ledergerber B, Egger M, Erard V, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA. 1999;282:2220–6. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson MA, Stanley H, Holtzer C, Margolis TP, Cunningham ET. Natural history and outcome of new AIDS-related cytomegalovirus retinitis diagnosed in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30:231–3. doi: 10.1086/313612. [DOI] [PubMed] [Google Scholar]
- 7.Palella FJ, Chmiel JS, Moorman AC, Holmberg SD. HIV Outpatient Study Investigators. Durability and predictors of success of highly active antiretroviral therapy for ambulatory HIV-infected patients. AIDS. 2002;16:1617–26. doi: 10.1097/00002030-200208160-00007. [DOI] [PubMed] [Google Scholar]
- 8.Kestelyn P. The epidemiology of CMV retinitis in Africa. Ocul Immunol Inflamm. 1999;7:173–7. doi: 10.1076/ocii.7.3.173.4002. [DOI] [PubMed] [Google Scholar]
- 9.Ausayakhun S, Watananikorn S, Ittipunkul N, Chaidaroon W, Patikulsila P, Patikulsila D. Epidemiology of the ocular complications of HIV infection in Chiang Mai. J Med Assoc Thai. 2003;86:399–406. [PubMed] [Google Scholar]
- 10.Biswas J, Madhavan HN, George AE, Kumarasamy N, Solomon S. Ocular lesions associated with HIV infection in India: a series of 100 consecutive patients evaluated at a referral center. Am J Ophthalmol. 2000;129:9–15. doi: 10.1016/s0002-9394(99)00415-8. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson MA, Schrier R, McCune JM, et al. Cytomegalovirus (CMV)-specific CD4+ T lymphocyte immune function in long-term survivors of AIDS-related CMV end-organ disease receiving potent, antiretroviral therapy. J Infect Dis. 2001;183:1399–404. doi: 10.1086/319854. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson MA, Maecker HT, Orr PL, et al. Results of a cytomegalovirus (CMV)-specific CD8+/interferon-γ+ cytokine flow cytometry assay correlate with clinical evidence of protective immunity in patients with AIDS with CMV retinitis. J Infect Dis. 2004;189:1362–73. doi: 10.1086/382964. [DOI] [PubMed] [Google Scholar]
- 13.Sacre K, Carcelain G, Cassoux N, et al. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J Exp Med. 2005;201:1999–2010. doi: 10.1084/jem.20042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torriani FJ, Freeman WR, Macdonald JC, et al. CMV retinitis recurs after stopping treatment in virological and immunological failures of potent antiretroviral therapy. AIDS. 2000;14:173–80. doi: 10.1097/00002030-200001280-00013. [DOI] [PubMed] [Google Scholar]
- 15.Komanduri KV, Viswanathan MN, Wieder ED, et al. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat Med. 1998;4:953–6. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- 16.Bronke C, Palmer NM, Jansen CA, et al. Dynamics of cytomegalovirus (CMV)–specific T cells in HIV-1–infected individuals progressing to AIDS with CMV end-organ disease. J Infect Dis. 2005;191:873–80. doi: 10.1086/427828. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg A, Tierney C, Kendall MA, et al. Cytomegalovirus-specific immunity and protection against viremia and disease in HIV-infected patients in the era of highly active antiretroviral therapy. J Infect Dis. 2006;193:488–93. doi: 10.1086/499826. [DOI] [PubMed] [Google Scholar]
- 18.Bunde T, Kirchner A, Hoffmeister B, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031–6. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–92. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 20.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair E, Tan QX, Sharp M, et al. Protective immunity to cytomegalovirus (CMV) retinitis in AIDS is associated with CMV-specific T cells that express interferon-γ and interleukin-2 and have a CD8+ cell early maturational phenotype. J Infect Dis. 2006;194:1537–46. doi: 10.1086/508997. [DOI] [PubMed] [Google Scholar]
- 22.Jabs DA, Van Natta ML, Kempen JH, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2002;133:48–61. doi: 10.1016/s0002-9394(01)01322-8. [DOI] [PubMed] [Google Scholar]
- 23.Erice A, Tierney C, Hirsch M, et al. Cytomegalovirus (CMV) and human immunodeficiency virus (HIV) burden, CMV end-organ disease, and survival in subjects with advanced HIV infection (AIDS Clinical Trials Group Protocol 360) Clin Infect Dis. 2003;37:567–78. doi: 10.1086/375843. [DOI] [PubMed] [Google Scholar]
- 24.Wohl DA, Zeng D, Stewart P, et al. Cytomegalovirus viremia, mortality, and end-organ disease among patients with AIDS receiving potent antiretroviral therapies. J Acquir Immune Defic Syndr. 2005;38:538–44. doi: 10.1097/01.qai.0000155204.96973.c3. [DOI] [PubMed] [Google Scholar]
- 25.Salmon-Ceron D, Mazeron MC, Chaput S, et al. Plasma cytomegalovirus DNA, pp65 antigenaemia, and a low CD4 cell count remain risk factors for cytomegalovirus disease in patients receiving highly active antiretroviral therapy. AIDS. 2000;14:1041–9. doi: 10.1097/00002030-200005260-00017. [DOI] [PubMed] [Google Scholar]
- 26.Wohl D, Kendall M, Andersen J, et al. 14th Conference on Retroviruses and Opportunistic Infections: program and abstracts (Denver) Foundation for Retroviruses and Human Health; Alexandria, VA: 2006. Randomized, placebo-controlled trial of valganciclovir to prevent CMV end-organ disease among HIV-infected subjects with detectable plasma CMV DNA PCR: ACTG 5030 [abstract 150] [Google Scholar]
- 27.Lieberman J, Shankar P, Manjunath N, Andersson J. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood. 2001;98:1667–77. doi: 10.1182/blood.v98.6.1667. [DOI] [PubMed] [Google Scholar]
- 28.Gruener NH, Lechner F, Jung MC, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–8. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel TU, Allen TM, Altman JD, Watkins DI. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J Virol. 2001;75:2458–61. doi: 10.1128/JVI.75.5.2458-2461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozdemir E, John LS, Gillespie G, et al. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood. 2002;100:3690–7. doi: 10.1182/blood-2002-05-1387. [DOI] [PubMed] [Google Scholar]
- 32.La Rosa C, Limaye AP, Krishnan A, Longmate J, Diamond DJ. Longitudinal assessment of cytomegalovirus (CMV)–specific immune responses in liver transplant recipients at high risk for late CMV disease. J Infect Dis. 2007;195:633–44. doi: 10.1086/511307. [DOI] [PubMed] [Google Scholar]