EMBO J 28, 697–710 (2009); published online 18 March 2009
Neuronal activity regulates dendritic and synaptic development through both a global response in the form of transcription and a local control in the form of synaptic translation. Understanding how transcription and translation might be linked would provide new insight into signals connecting the nucleus to the synapse. Reported in this issue of The EMBO Journal, Fiore et al elucidate a novel mechanism controlling dendritic growth that involves the transcription factor, myocyte-enhancing factor (Mef2), which turns on the expression of a microRNA gene cluster after neuronal depolarization. One of these is miR-134, which turns off the translation of Pumilio2, a known translation repressor. MicroRNAs may thus fine-tune translational and transcriptional output needed for dendritogenesis.
MicroRNAs have emerged as a powerful class of conserved noncoding RNAs that regulate gene expression post-transcriptionally and have important functions in numerous aspects of development. Numerous miRNAs are expressed at high levels in the nervous system; a few of these have been localized to dendrites (Kye et al, 2007), where they are believed to regulate neural development and plasticity. Impaired expression of miRNAs is implicated in several neurological diseases. MicroRNAs are encoded by genes in the form of large transcripts that undergo processing by Drosha and Dicer, two specific RNases in the nucleus and cytoplasm, respectively. The final product is a small RNA molecule (18–22 nt), which forms an imperfect duplexe on their target mRNAs. More commonly this leads to translational inhibition of target mRNAs through the RNA-induced silencing complex (Bartel, 2004). These features enable specific miRNAs to act on the subset of mRNAs and inhibit their translation in a sequence-dependent manner. These responses may be reversible and regulated by physiological signals (Kosik, 2006).
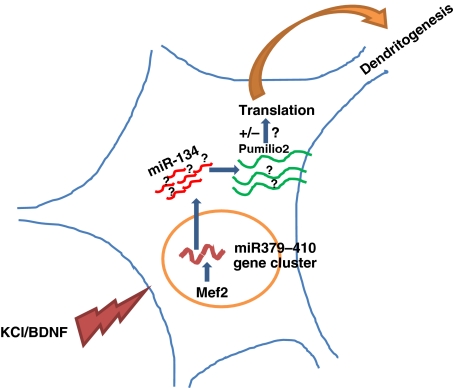
Many microRNA genes are arranged in clusters. In this issue, Fiore et al (2009) show that the gene cluster miR379–410, which includes up to 50 microRNA genes, is induced by neuronal depolarization and has an important function in dendritic development in cultured neurons (Figure 1). miR-134, a member of this cluster, was earlier shown to be localized to dendrites of cultured hippocampal neurons, where it regulates dendritic spine morphology by its reversible inhibitory effect on LimK1 mRNA in response to BDNF (Schratt et al, 2006). Here, Fiore et al provide evidence that these miRNAs are likely expressed from a single transcript, which is induced by neuronal activity (treatment with KCl or BDNF). The transcription factor, Mef2, was shown to bind an upstream element on this miRNA gene cluster and activate transcription upon stimulation. Mef2 was previously shown to be key negative regulator of activity-dependent synapse development (Flavell et al, 2006), whose target genes likely play diverse functions at synapses (Flavell et al, 2008). Chromatin immunoprecipitation assays identified Mef2 as the transcription factor for the miR379–410 cluster. In a series of elegant and well-integrated experiments to inhibit miRNA function, mutate miRNA-binding sites in target reporters, and use of siRNA to knockdown Mef2 expression, the authors provide compelling evidence that Mef2 was necessary for the activation of this microRNA gene cluster. Neuronal depolarization increased a subset of microRNA expression, which appears to have a significant effect on early dendritogenesis in cultured neurons. Increased miRNAs were linked to the increased complexity of dendritic arborization of stimulated neurons. Interestingly, only a few members of this miRNA cluster had a positive impact on the dendritic arborization, while others had no effect. This opens up the possibility that other microRNAs, which are also significantly induced by stimulation, might regulate other aspects of neuronal development and synapse formation. Knockdown of the transcription factor, Mef2, prevented the stimulus-induced increase in microRNAs (from the miR379–410 cluster) and also diminished the dendritic complexity. This finding is significant in its demonstration that a global response (transcriptional activation mediated by Mef2) is necessary for a local effect (dendritic arborization).
Figure 1.
MicroRNAs from the miR379–410 cluster regulate dendritogenesis. Neuronal activity induces several microRNAs from the miR379–410 cluster in an Mef2-mediated pathway. One of these, miR-134, inhibits Pumilio2 protein synthesis essential for dendritogenesis.
Identifying valid mRNA targets for the members of this miR379–410 cluster and elucidating their relevance to dendritogenisis or other aspects of neuronal development will be a challenging task. Potentially, many microRNAs from this cluster might act on various mRNA targets in a coordinated manner to modulate dendritogenesis and spine morphology. Further studies in this direction are needed to clarify how these microRNAs function in neuronal development. In an exciting addition to this story, Fiore et al (2009) have identified Pumilio2 as a target of miR-134 whose inhibition is essential for activity-dependent dendritogenesis. Pumilio2 is localized to dendrites and proposed to act as translation repressor (Vessey et al, 2006). The present study shows that miR-134 inhibits pumilio2 expression in an activity-dependent manner, which supports dendritogenesis. Using a luciferase reporter assay, they show that Mef2 mediates miR-134 induced inhibition of Pumilio2. It is unclear whether the modulation of Pumilio2 mRNA translation by miR-134 is localized to dendrites or restricted to the cell body. The possibility that this mechanism may occur locally is an interesting topic for further exploration, as shown for miR-134 regulation of LimK1 mRNA in dendrites of more mature cultures (Schratt et al, 2006). An intriguing aspect here is the apparent opposite actions of miR-134 on mRNA translation needed for regulation of dendrite and spine morphology at different stages of development. During early stages of culture (DIV5-7), KCl or BDNF stimulation was shown in this study to promote miR-134 mediated inhibition of translation (Pumilio2), which facilitates dendritogenesis; however, at later stages (DIV14), similar stimulation paradigms actually relieve miR-134 mediated translational inhibition (LimK1) leading to increased size of spines (Schratt et al, 2006). The authors provide an interesting speculation that miR-134 inhibition or activation of translation may be under developmental control and/or part of a homeostatic mechanism; either of these possibilities will require further clarification. More work is also needed to understand the mechanistic details of the possible dual nature of miR-134 function (either inducing or relieving the translation inhibition upon stimulation), and whether these activities may be bidirectionally regulated in dendrites in response to physiological patterns of neuronal activity.
The physiological significance of the microRNA pathway and its regulation in neuronal development is evident by its involvement in several neurological disorders, including fragile X syndrome, which is caused by the loss of FMRP. FMRP is an mRNA-binding protein that regulates synaptic protein synthesis and also associated with microRNA pathway (Jin et al, 2004; Xu et al, 2008; Cheever and Ceman, 2009). The significant defects in spine morphology and dendritogenesis seen in disorders such as fragile X syndrome have not been effectively reconciled at a mechanistic level. Further work is needed to understand how mRNA-binding proteins, like FMRP, may interact with miRNAs, or components of the RNAi pathway, to regulate local translation needed for neuronal development and plasticity. The present study motivates further work to elucidate the role of individual and/or clustered microRNAs in the regulation of neuronal function and dysfunction in neurological diseases. It is envisioned that manipulation of miRNA expression and function may be a promising direction for therapeutic intervention in human diseases of abnormal development and differentiation.
References
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297 [DOI] [PubMed] [Google Scholar]
- Cheever A, Ceman S (2009) Phosphorylation of FMRP inhibits association with Dicer. RNA (e-pub ahead of print, 20 January 2009; doi: 10.1261/rna.1500809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore R, Khudayberdiev S, Christensen M, Siegel G, Flavell S, Kim TK, Greenberg ME, Schratt G (2009) Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J 28: 697–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME (2006) Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 311: 1008–1012 [DOI] [PubMed] [Google Scholar]
- Flavell SW, Kim TK, Gray JM, Harmin DA, Hemberg M, Hong EJ, Markenscoff-Papadimitriou E, Bear DM, Greenberg ME (2008) Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron 60: 1022–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Alisch RS, Warren ST (2004) RNA and microRNAs in fragile X mental retardation. Nat Cell Biol 6: 1048–1053 [DOI] [PubMed] [Google Scholar]
- Kosik KS (2006) The neuronal microRNA system. Nat Rev Neurosci 7: 911–920 [DOI] [PubMed] [Google Scholar]
- Kye MJ, Liu T, Levy SF, Xu NL, Groves BB, Bonneau R, Lao K, Kosik KS (2007) Somatodendritic microRNAs identified by laser capture and multiplex RT-PCR. RNA 13: 1224–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME (2006) A brain-specific microRNA regulates dendritic spine development. Nature 439: 283–289 [DOI] [PubMed] [Google Scholar]
- Vessey JP, Vaccani A, Xie Y, Dahm R, Karra D, Kiebler MA, Macchi P (2006) Dendritic localization of the translational repressor Pumilio 2 and its contribution to dendritic stress granules. J Neurosci 26: 6496–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XL, Li Y, Wang F, Gao FB (2008) The steady-state level of the nervous-system-specific microRNA-124a is regulated by dFMR1 in Drosophila. J Neurosci 28: 11883–11889 [DOI] [PMC free article] [PubMed] [Google Scholar]