Abstract
The capacity of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) to trigger apoptosis preferentially in cancer cells, although sparing normal cells, has motivated clinical development of TRAIL receptor agonists as anti-cancer therapeutics. The molecular mechanisms responsible for the differential TRAIL sensitivity of normal and cancer cells are, however, poorly understood. Here, we show a novel signalling pathway that activates cytoprotective autophagy in untransformed human epithelial cells treated with TRAIL. TRAIL-induced autophagy is mediated by the AMP-activated protein kinase (AMPK) that inhibits mammalian target of rapamycin complex 1, a potent inhibitor of autophagy. Interestingly, the TRAIL-induced AMPK activation is refractory to the depletion of the two known AMPK-activating kinases, LKB1 and Ca(2+)/calmodulin-dependent kinase kinase-β, but depends on transforming growth factor-β-activating kinase 1 (TAK1) and TAK1-binding subunit 2. As TAK1 and AMPK are ubiquitously expressed kinases activated by numerous cytokines and developmental cues, these data are most likely to have broad implications for our understanding of cellular control of energy homoeostasis as well as the resistance of untransformed cells against TRAIL-induced apoptosis.
Keywords: autophagy, cancer, cell death, signalling, TRAIL
Introduction
Macroautophagy (hereafter referred to as autophagy) is a lysosomal pathway involved in the turnover of long-lived proteins, cytoplasm and whole organelles (Codogno and Meijer, 2005; Kroemer and Jäättelä, 2005; Lum et al, 2005; Xie and Klionsky, 2007). In unstressed cells, autophagy is inhibited by the mammalian target of rapamycin complex 1 (mTORC1). The inhibition of mTORC1 activity (e.g., by starvation or rapamycin) leads to the activation of a set of evolutionarily conserved autophagy-regulating proteins (Atg proteins) and formation of autophagosomes, which then fuse with lysosomes to form autolysosomes, in which the cargo is digested to metabolites and released back to the cytosol for recycling. Accumulating evidence indicates that autophagy can function as an adaptive cell response allowing the cell to survive otherwise lethal challenges by removing damaged organelles and misfolded proteins. Paradoxically, autophagy can also mediate a non-apoptotic type II cell death, called autophagic degeneration.
Tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a multifunctional cytokine originally identified as an apoptosis-inducing member of the TNF superfamily (Falschlehner et al, 2007; Ashkenazi and Herbst, 2008). Prompted by the recent data showing that TRAIL is upregulated during breast morphogenesis and induces accumulation of autophagic vacuoles during lumen formation in an in vitro morphogenesis model (Mills et al, 2004), we hypothesized that autophagy may either contribute to the TRAIL-induced lumen formation by facilitating cell death or it may explain the TRAIL resistance of normal cells. Using immortalized breast and pigment epithelial cells, we show here that TRAIL induces cytoprotective autophagy in untransformed cells through a novel signalling pathway involving TGF-β-activating kinase 1 (TAK1), AMP-activated protein kinase (AMPK) and mTORC1.
Results and discussion
TRAIL triggers functional and cytoprotective autophagy in breast epithelial cells
To study the TRAIL-induced accumulation of autophagosomes in greater detail, we created MCF10A cells (immortalized human breast epithelial cells) stably expressing the autophagosome-associated protein LC3 (also known as Atg8) fused to enhanced green fluorescent protein (eGFP–LC3). In the majority of untreated MCF10A cells, the eGFP–LC3 was diffusely distributed in the cytosol and nucleus, and only approximately 5% of the cells showed a dotted staining pattern of eGFP–LC3 (⩾5 dots per cross-section), indicative of the accumulation of autophagosomes (Figure 1A). TRAIL treatment increased the number of cells with autophagosome accumulation to the level comparable with that seen in rapamycin-treated control cells, whereas the highly related cytokine TNF had no detectable effect on the eGFP–LC3 distribution (Figure 1A). Flow cytometry-based analysis revealed TRAIL receptor 2 (TRAIL-R2) on the surface of MCF10A cells, whereas the expression levels of TRAIL-R1, -R3 and -R4 expression were under the detection limit of the assay (Supplementary Figure S1A). Accordingly, an antagonistic TRAIL-R2 antibody effectively inhibited TRAIL-induced autophagosomes accumulation in MCF10A cells (Figure 1A). This antibody also inhibited TRAIL-induced cell death in MCF10A cells sensitized to TRAIL-induced cytotoxicity by a protein synthesis inhibitor (Supplementary Figure S1B). Thus, TRAIL-R2 is able to activate signalling pathways leading to both autophagosome accumulation and cell death.
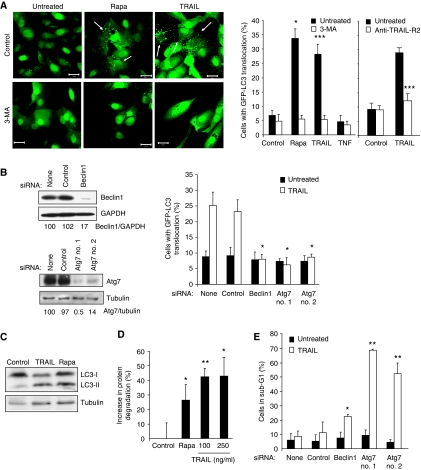
Figure 1.
TRAIL induces cytoprotective autophagy in breast epithelial cells. (A) MCF10A–eGFP–LC3 cells were left untreated (Control) or treated with 5 μM rapamycin (Rapa), 500 ng/ml TRAIL or 10 ng/ml TNF for 24 h. When indicated, 10 mM 3-MA or 5 μg/ml TRAIL-R2 antagonist antibody was added 1 h before the drugs. Representative confocal images (20 μm scale bars) and the percentages of cells with LC3 translocation are shown. (B) MCF10A–eGFP–LC3 cells were transfected with the indicated siRNAs for 48 h and analysed by immunoblotting (left), or stimulated with 500 ng/ml TRAIL for 24 h and analysed for LC3 translocation (right). (C) MCF10A cells left untreated (Control) or treated with 500 ng/ml TRAIL or 5 μM rapamycin (Rapa) for 24 h were analysed for LC3-I, LC3-II and tubulin by immunoblotting. Pepstatin A and E64d were added to the cells 4 h before harvesting. Similar results were obtained in two independent experiments. (D) MCF10A cells were treated with 5 μM rapamycin (Rapa) or indicated concentrations of TRAIL for 24 h, and the increase in the degradation of long-lived proteins as compared with the untreated cultures (Control) was measured. (E) MCF10A–eGFP–LC3 cells transfected with the indicated siRNAs were left untreated 48 h later (Control) or treated with 500 ng/ml TRAIL for 24 h and analysed for the DNA content by flow cytometry. The percentage of apoptotic cells with sub-G1 DNA content is shown. The values represent mean±s.d. for three (A, D) or four (B, E) independent experiments. *P-value <0.05, **P-value <0.01 and ***P-value <0.001 as compared with control sample (A—left panel and D), with TRAIL-treated cells without anti-TRAIL-R2 (A—right panel) or with TRAIL-treated cells without siRNA (B, E).
To test whether the TRAIL-induced autophagosome accumulation was mediated by the known core autophagy machinery, we inhibited the autophagy pathway by pharmacological inhibitors and RNA interference. Co-treatment with 3-methyladenine (3-MA), an inhibitor of class III phosphatidylinositol 3-kinase, which is required for the autophagosome membrane assembly as well as the siRNA-based depletion of two essential autophagy proteins beclin 1 or Atg7, effectively blocked TRAIL-induced eGFP–LC3 translocation (Figure 1A and B). Importantly, TRAIL treatment also induced the appearance of LC3-II, a structural component of autophagosomes (Figure 1C), and the degradation of long-lived proteins (Figure 1D). These data indicate that the LC3 translocation observed in TRAIL-treated MCF10A cells was dependent on the classic autophagy pathway and reflected an increase in functional autophagy.
Tumour necrosis factor-related apoptosis-inducing ligand-induced autophagosome formation was significant as early as 4 h after the addition of 500 ng/ml TRAIL and increased continuously during a 72 h treatment period (Supplementary Figure S2A). Furthermore, the response was dose dependent at TRAIL concentrations ranging from 10 to 500 ng/ml (Supplementary Figure S2A). Notably, TRAIL failed to induce significant cell death in MCF10A cells within these time and concentration ranges, but the inhibition of autophagy by depletion of beclin 1 or Atg7 sensitized the cells to TRAIL-induced cell death as analysed by flow cytometry-based quantification of cells with reduced DNA content and detection of floating dead cells by microscopy (Figure 1E and data not shown). The cytoprotective nature of TRAIL-induced autophagy suggests that it does not contribute to the lumen formation during breast morphogenesis but rather opposes it by inhibiting cell death. Accordingly, autophagy inhibition by depletion of Atg5 or Atg7 has recently been shown to enhance luminal apoptosis in an MCF10A breast morphogenesis model (Fung et al, 2008).
TRAIL-induced autophagy depends on AMPK-mediated inhibition of mTORC1
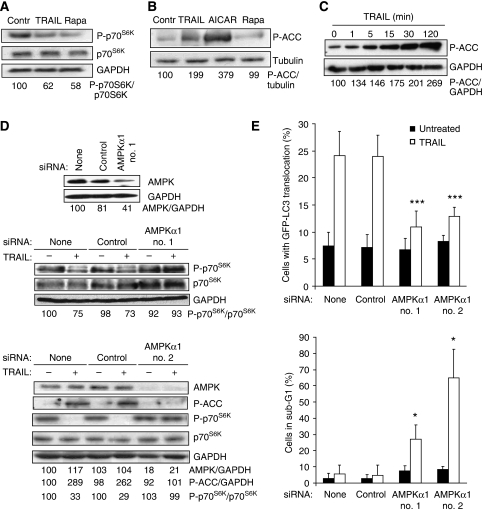
The molecular mechanisms governing TRAIL-induced autophagy have remained unexplored. To test whether TRAIL-induced autophagy was associated with the inhibition of mTORC1 kinase complex, a key negative regulator of autophagy in unstressed cells, we measured the phosphorylation level of an mTORC1 substrate, ribosomal protein S6 kinase 1 (p70S6K). Remarkably, TRAIL inhibited the phosphorylation of p70S6K as effectively as rapamycin, a direct inhibitor of mTORC1 (Figure 2A). AMPK, an evolutionarily highly conserved regulator of cellular energy homoeostasis has recently emerged as a putative activator of autophagy (Kamada et al, 2004; Meley et al, 2006; Høyer-Hansen et al, 2007). The AMPK-mediated autophagy activation may occur through two distinct signalling pathways that lead to the inhibition of mTORC1 (Inoki et al, 2003; Shaw et al, 2004a; Gwinn et al, 2008). AMPK-mediated phosphorylation of tuberous sclerosis complex 2 leads to the inhibition of an mTORC1 activator ras-family GTP-binding protein RHEB, whereas that of an mTOR-binding partner raptor triggers the binding of raptor to 14-3-3 proteins and subsequent inhibition of the ability of mTORC1 to recruit downstream substrates. Thus, we next tested whether AMPK was activated in TRAIL-treated MCF10A cells. Interestingly, TRAIL induced a rapid (detectable at 1 min), sustained (until 24 h) and dose-dependent (10–500 ng/ml) activation of AMPK as shown by an increase in the phosphorylation of an AMPK substrate acetyl-CoA-carboxylase (ACC) (Figure 2B, C and Supplementary Figure S2B). Furthermore, the depletion of the catalytic α1-subunit of AMPK by RNA interference in TRAIL-treated MCF10A cells restored the mTORC1 activity, attenuated autophagosome formation and sensitized the cells to TRAIL-induced cell death, indicating that AMPKα1 mediates the TRAIL-induced cytoprotective autophagy (Figure 2D and E). Even though TRAIL has been reported to activate several kinases (Falschlehner et al, 2007), to our knowledge, these data are the first to report the ability of TRAIL to activate AMPK. Furthermore, the data presented above provide the first genetic evidence that endogenous AMPK, and more precisely AMPKα1, is an essential mediator of autophagy in mammalian cells.
Figure 2.
TRAIL activates AMPK and AMPK-dependent cytoprotective autophagy. (A) Protein lysates from MCF10A cells left untreated or treated with 500 ng/ml TRAIL or 5 μM rapamycin (Rapa, positive control) for 24 h were analysed by immunoblotting for phosphorylated p70S6K (P-p70S6K), total p70S6K and GAPDH (loading control). (B) Immunoblot analysis of the levels of P-ACC and tubulin (loading control) in lysates from MCF10A cells left untreated or treated with 500 ng/ml TRAIL for 24 h, 1 mM AICAR for 4 h (positive control) or 5 μM rapamycin for 24 h (negative control). (C) Immunoblot analysis of the level of P-ACC and GAPDH (loading control) in lysates from MCF10A cells treated with 500 ng/ml TRAIL for the indicated times. (D) MCF10A cells were transfected with indicated siRNAs for 48 h and analysed by immunoblotting for the indicated proteins immediately (upper panel) or after additional 24 h (middle panel) or 2 h (lower panel) incubation with (+) or without (−) 500 ng/ml TRAIL. (E) MCF10A–eGFP–LC3 cells transfected with indicated siRNAs for 48 h and incubated for an additional 24 h with or without 500 ng/ml TRAIL were analysed for LC3 translocation (top) and the DNA content (bottom). The values represent mean±s.d. for four independent experiments. *P-value <0.05 and ***P-value <0.001 as compared with TRAIL-treated cells without siRNA. Similar results were obtained in two (A, B and D) or three (C, D) independent experiments.
TAK1 mediates TRAIL-induced activation of AMPK and autophagy
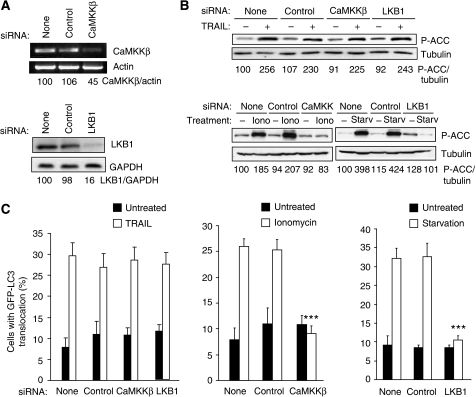
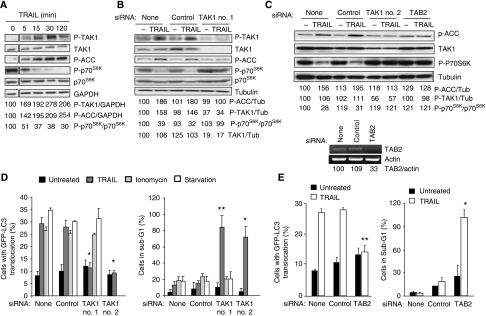
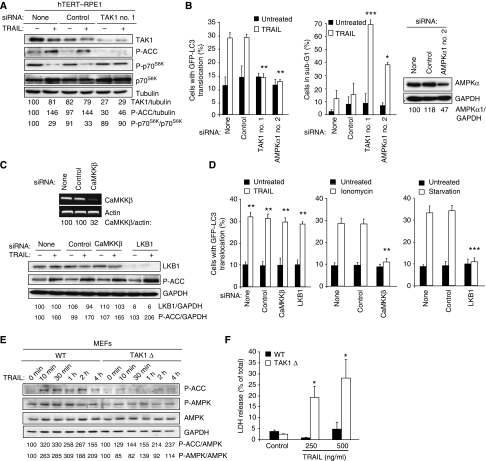
The AMPK can be phosphorylated and activated by tumour suppressor kinase LKB1 when the ratio of AMP/ATP increases and by calcium/calmodulin-dependent protein kinase kinase-β (CaMKKβ) in response to an increase in the cytosolic-free calcium [Ca2+]cyt (Corradetti et al, 2004; Shaw et al, 2004b; Hawley et al, 2005; Woods et al, 2005). Accordingly, LKB1 and CaMKKβ have been reported to mediate autophagy in response to starvation and an increase in the [Ca2+]cyt, respectively (Liang et al, 2007; Høyer-Hansen et al, 2007; Høyer-Hansen and Jäättelä, 2007). Thus, we next studied whether these AMPK kinases were responsible for the TRAIL-induced activation of AMPK and autophagy. Even though the siRNAs used effectively depleted LKB1 and CaMKKβ and completely inhibited the AMPK activation and autophagosome accumulation in starved and ionomycin-treated MCF10A cells, respectively, their depletion influenced neither TRAIL-induced AMPK activation nor autophagy (Figure 3A–C). In spite of some residual LKB1 and CaMKKβ in the siRNA-treated cells, the complete inability of the cells to activate AMPK and autophagosome accumulation upon starvation and ionomycin strongly suggests that TRAIL activates AMPK by a pathway independent of the two known AMPK kinases. Thus, it is interesting to note that TAK1, a cytokine-activated member of the mitogen-activated protein kinase kinase kinase family, has recently been shown to activate the yeast AMPK homologue Snf1 in vivo and mammalian AMPK in vitro, suggesting that it could exert an effect as a third mammalian AMPK kinase (Momcilovic et al, 2006). To investigate the possible function of TAK1 in TRAIL-induced AMPK activation and autophagy, we first examined the effect of TRAIL on TAK1 activation in MCF10A cells by determining TAK1 phosphorylation at Thr184/187, an essential step for the complete kinase activation (Sakurai et al, 2000). Remarkably, TRAIL induced a rapid (detectable at 5 min), sustained (until 24 h) and dose-dependent activation of TAK1 that closely correlated with the activation of the AMPK (Figure 4A–C and Supplementary Figure S2B). Notably, siRNA-based depletion of TAK1 or TAK1-binding subunit 2 (TAB2), an essential co-factor for cytokine-induced activation of TAK1 (Adhikari et al, 2007), effectively abrogated TRAIL-induced AMPK activation, mTORC1 inhibition and autophagosome formation (Figure 4B–E), whereas AMPKα1 depletion did not affect TRAIL-induced phosphorylation of TAK1 (Supplementary Figure S3). Furthermore, TAKI and AMPK activation as well as mTORC1 inhibition were completely inhibited by the antagonistic TRAIL-R2 receptor (Supplementary Figure S1B). Thus, TAK1 is an essential mediator of TRAIL-R2-induced AMPK activation and autophagy in MCF10A cells. Moreover, TAK1- and TAB2-depleted cells were greatly sensitized to TRAIL-induced cell death, further highlighting the importance of the TRAIL-induced autophagy pathway in determining the cellular outcome in response to TRAIL treatment (Figure 4D and E).
Figure 3.
LKB1 and CaMKKβ do not have an important function in TRAIL-induced AMPK activation and autophagy. (A) MCF10A–eGFP–LC3 cells were transfected with indicated siRNAs for 48 h and analysed for the indicated mRNAs (top) and proteins (bottom by RT–PCR and immunoblotting, respectively. Similar results were obtained in three independent experiments. (B) MCF10A–eGFP–LC3 cells transfected with indicated siRNAs for 48 h, and left untreated or treated with 500 ng/ml TRAIL for 2 h, 10 μM ionomycin for 24 h or starved for amino acids and glucose for 24 h (starvation) were analysed for P-ACC and tubulin (loading control) expression by immunoblotting. Similar results were obtained in two independent experiments. (C) Cells transfected as in (B) and left untreated or treated with 500 ng/ml TRAIL, 10 μM ionomycin or starved for amino acids and glucose for 24 h were analysed for LC3 translocation. The values represent mean±s.d. of three independent experiments. ***P-value <0.001 as compared with cells treated in a same way, but transfected without siRNA.
Figure 4.
TAK1 mediates TRAIL-induced AMPK activation and autophagy. (A) The lysates of MCF10A cells treated with 500 ng/ml TRAIL for the indicated times were analysed by immunoblotting. The control lane (0 min TRAIL) originates from the same blot and has been treated and exposed identically. Similar results were obtained in three independent experiments. (B, C) The lysates of MCF10A–eGFP–LC3 cells transfected with indicated siRNAs for 48 h and left untreated (−) or treated with 500 ng/ml TRAIL for 2 h were analysed by immunoblotting. (C, lower panel) MCF10A–eGFP–LC3 cells were transfected with indicated siRNAs for 48 h and analysed for the indicated mRNAs by RT–PCR. Similar results were obtained in two independent experiments. (D, E) MCF10A–eGFP–LC3 cells were transfected with indicated siRNAs for 48 h, treated as in Figure 3C and analysed for LC3 translocation (left) and sub-G1 DNA content (right). The values represent mean±s.d. of a minimum of three independent experiments except for the right panel in E (two experiments). *P-value <0.05 and **P-value <0.01 as compared with cells treated in a same way, but transfected without siRNA.
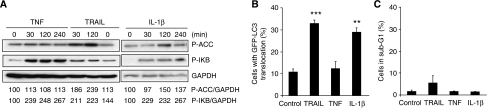
Transforming growth factor-β-activating kinase 1 is an essential mediator of the activation of nuclear factor kappa-B in response to several cytokines (Shim et al, 2005; Adhikari et al, 2007). To investigate whether TAK1 activation is sufficient for the activation of AMPK–autophagy pathway, we tested the ability of TNF and interleukin-1β (IL-1β) to activate this pathway in MCF10A cells. As shown in Figure 5A, both cytokines activated TAK1 as judged by the rapid phosphorylation of the inhibitor of kappa-B (IκB). In the case of TNF, which failed to induce the accumulation of autophagosomes (Figure 1A), we also failed to detect the activation of AMPK, whereas IL-1β activated both AMPK and autophagosome accumulation without detectable cell death (Figure 5A–C). Taken together, our data suggest that TAK1 is essential, but not sufficient for the effective activation of AMPK and autophagy.
Figure 5.
IL-1β activates AMPK and autophagy. (A) Protein lysates from MCF10A cells left untreated (0) or treated with 50 ng/ml TNF, 500 ng/ml TRAIL (positive control) or 10 ng/ml IL-1β for indicated times were analysed by immunoblotting for phosphorylated ACC (P-ACC), phosphorylated IκB (P-IκB) and GAPDH (loading control). The values show the P-ACC/GAPDH and P-IkB/GAPDH ratios as percentages of the ratios in untreated cells (left lanes) and are representative of two independent experiments. (B, C) MCF10A–eGFP–LC3 cells left untreated or treated with 500 ng/ml TRAIL, 50 ng/ml TNF or 10 ng/ml IL-1β for 24 h were analysed for LC3 translocation (B) and the DNA content (C). The values represent mean±s.d. for two independent experiments. **P-value <0.01 and ***P-value <0.001 as compared with untreated cells.
The downstream signalling pathways activated by TAK1 vary greatly, depending on the cell type and the stimulus (Delaney and Mlodzik, 2006). To investigate whether the ability of TRAIL to activate the cytoprotective TAK1–AMPK pathway was restricted to breast epithelial cells, we included human hTERT-immortalized retinal pigment epithelial cells (hTERT–RPE-1) to this study. TRAIL activated AMPK and autophagosome accumulation and inhibited mTORC1 activity in a TAK1-dependent manner also in these cells (Figure 6A and B). Importantly, the inhibition of AMPK activation and autophagosome accumulation by TAK1 and AMPKα1 siRNAs greatly sensitized the hTERT–RPE-1 cells to TRAIL-induced cell death (Figure 6B). Akin to MCF10A cells, TRAIL-induced AMPK activation and autophagosome accumulation were independent of LKB1 and CaMKKβ, whereas the depletion of these kinases effectively inhibited both events induced by starvation and ionomycin, respectively (Figure 6C and D). Moreover, knock-in of a dominant-negative TAK1 mutant in murine embryonic fibroblasts (MEFs) reduced the TRAIL-induced activation of AMPK observed in wild-type MEFs and sensitized the cells to TRAIL-induced cell death (Figure 6E and F). These data further emphasize the function of TAK1 as an activator of AMPK and are in concordance with recent data showing that TAK1-deficient MEFs fail to activate AMPK in response to oligomycin, metformin and 5-aminoimidazole-4-carboxamide riboside (Xie et al, 2006). On the basis of data showing that these drugs fail to activate the AMPK kinase LKB1 in TAK1-deficient cells, Xie et al have suggested that TAK1 functions upstream of LKB1 rather than as a direct AMPK kinase. It should be noted that this study did not test whether TAK1 could activate AMPK1 and LKB1 in parallel. Furthermore, another pharmacological AMPK activator (A-769662) activates AMPK in LKB1-deficient cervix carcinoma cells, but fails to activate it in LKB1-deficient skeletal muscle cells, suggesting that the requirements for the AMPK activation may be cell type specific (Goransson et al, 2007). Our data showing that TRAIL-induced AMPK activation was not affected by an effective depletion of LKB1 indicate that in TRAIL-treated MCF10A and hTERT-RPE-1 cells, TAK1 activates AMPK independent of LKB1. It remains, however, to be studied whether TAK1 is a direct activator of AMPK in this model system. However, its ability to mediate AMPK–mTORC1 autophagy pathway independent of the two known AMPK kinases together and its previously shown ability to directly activate AMPK in vitro support the idea that it may function as an AMPK kinase in TRAIL-treated cells (Momcilovic et al, 2006). It should, however, be noted that TAK1 might not be sufficient to activate AMPK. This is supported by our data showing activation of TAK1 (phosphorylation of IκB) in the absence of AMPK activation (phosphorylation of ACC) in TNF-treated MCF10A cells.
Figure 6.
TRAIL induces TAK1-dependent AMPK activation and autophagy in retinal pigment epithelial cells. (A) The lysates of hTERT–RPE1 cells transfected with indicated siRNAs for 48 h and left untreated or treated with 500 ng/ml TRAIL for 2 h were analysed by immunoblotting. (B) hTERT–RPE1–eGFP–LC3 cells transfected with indicated siRNAs for 48 h were left untreated or treated with 500 ng/ml TRAIL for 24 h and analysed for LC3 translocation (left) and sub-G1 DNA content (middle) and AMPKα expression (right). (C) hTERT–RPE1–eGFP–LC3 cells were transfected with indicated siRNAs for 48 h and analysed for the indicated mRNAs (top). After 48 h, cells were left untreated or treated with 500 ng/ml TRAIL for 2 h, and analysed for P-ACC and GAPDH (loading control) expression by immunoblotting. Similar results were obtained in two independent experiments. (D) Cells transfected as described in (C) and left untreated or treated with 500 ng/ml TRAIL, 10 μM ionomycin or starved for amino acids and glucose for 24 h were analysed for LC3 translocation. (E) Lysates of wild-type (WT) MEFs and MEFs with an inactive TAK1 knock-in (TAK1Δ) treated with 500 ng/ml TRAIL were analysed by immunoblotting. Similar results were obtained in two independent experiments. (F) WT and TAK1Δ MEFs left untreated and treated with 250 or 500 ng/ml TRAIL were analysed for cell death by the LDH release assay. Similar results were obtained in two independent experiments (A, C and E). The values represent mean±s.d. for two (D) or a minimum of three independent experiments (B, F). *P-value <0.05, **P-value <0.01 and ***P-value <0.001 as compared with cells treated in the same way, but transfected without siRNA (B, D) or the wild-type cells (F).
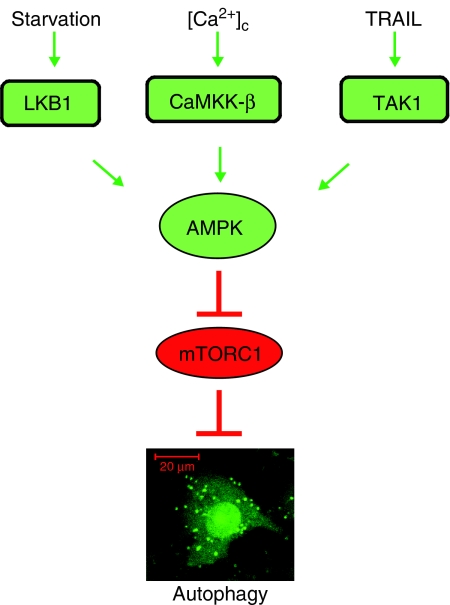
The AMPK sits at a unique position as a proposed energy and stress sensor that can interact with diverse signalling molecules and control processes ranging from macromolecule synthesis to cell polarity and autophagy (Brenman, 2007; Hardie, 2007; Høyer-Hansen and Jäättelä, 2007). Hithertho, LKB1 and CaMKKβ have been identified as AMPK kinases in response to low AMP levels and an increase in the [Ca2+]cyt, respectively. Our data add TRAIL-induced TAK1 activation as a third independent signalling pathway able to activate AMPK. Interestingly, all three signalling pathways leading to AMPK activation can induce autophagy, suggesting that AMPK functions as a universal autophagy activator that can integrate information from various environmental and developmental cues through at least three different signalling pathways (Figure 7).
Figure 7.
A schematic presentation of various signalling pathways leading to AMPK activation and AMPK-dependent autophagy.
Despite the potential of TRAIL receptors as targets for cancer therapy, little is known about the mechanisms regulating the differential sensitivity of normal and tumour cells to TRAIL-induced cytotoxicity (Ashkenazi and Herbst, 2008). The data presented above suggest that the TRAIL-induced TAK1–AMPK signalling pathway leading to an increase in autophagic activity contributes to the protection of normal epithelial cells against TRAIL-induced cell death. This is strongly supported by data showing that transformation of MCF10A cells by activated oncogenes is sufficient to inhibit TRAIL-induced autophagy and to sensitize them to TRAIL-induced apoptosis (our unpublished data). Recent data showing that TRAIL can induce cytoprotective autophagy also in apoptosis-defective cancer cells suggest that TRAIL-induced apoptosis overrides the ability of TRAIL to trigger autophagy in cancer cells (Han et al, 2008). Alternatively, the TRAIL-resistant cancer cells able to cause autophagy induction might have retained the autophagy-competent phenotype normally killed during the transformation process (Høyer-Hansen and Jäättelä, 2008). As the TRAIL-induced cytoprotective autophagy in apoptosis-resistant cancer cells may inspire combination therapies with TRAIL and autophagy inhibitors, it should be emphasized that the data presented here suggest that the clinical safety of TRAIL receptor agonists may depend on their ability to activate cytoprotective autophagy in normal tissues and thereby strongly argue against such combination therapies.
Materials and methods
Cell culture and treatments
The MCF10A breast epithelial cells expressing an ecotropic receptor and eGFP–LC3 fusion protein were described previously (Høyer-Hansen et al, 2007). The human hTERT-immortalized retinal pigment epithelial cell line hTERT–RPE-1 was kindly provided by Dr RM Rios (CABIMER, Sevilla, Spain) and transfected with pEGFP-LC3 plasmid (Høyer-Hansen et al, 2007) to generate a stable pool of hTERT–RPE1–GFP–LC3 cells. The immortalized MEFs from knock-in mice expressing an inactive TAK1 mutant (TAK1Δ) together with the appropriate wild-type MEFs were kindly provided by Dr S Akira (Osaka University, Osaka, Japan) (Sato et al, 2005).
Recombinant human TRAIL was produced as described previously (Harper et al, 2001). TNF and IL-1β were purchased from Peprotech, rapamycin, ionomycin, pepstatin-A, E64d and 3-MA from Sigma-Aldrich (St Louis, MO, USA), 5-aminoimidazole-4-carboxamide riboside (AICAR) from Toronto Research Chemicals (Ontario, Canada), Hank's balanced salt solution starvation medium from GIBCO (CA, USA) and monoclonal antagonist antibody to TRAIL-R2 (HS201, preservative free) from Alexis Biochemicals Corp. (San Diego, CA, USA).
RNA interference
Cells were transfected with indicated siRNAs at 50 nM (except for TAK1 siRNA that was used at 10 nM) using DharmaFECT transfection agent (Dharmacon Research, CO, USA) according to he manufacturer's guidelines. siRNAs corresponding to the human cDNA sequences were as follows: Beclin-1 (5′-CAGTTTGGCACAATCAATAtt-3′), LKB1 (5′-CUGGUGGAUGUGUUAUACAtt-3′) and a control siRNA (5′-CGACCGAGACAAGCGCAAGtt-3′) from Dharmacon Research; CaMKKβ (5′-GGAUCUGAUCAAAGGCAUCtt-3′) and TAK1 no. 1 (5′-GGAGAUCGAGGUGGAAGAGtt-3′) from Ambion (CA, USA); TAK1 no. 2 (5′-UGGCUUAUCUUACACUGGAtt-3′), AMPKα1 no. 1 (5′-UGCCUACCAUCUCAUAAUAtt-3′), AMPKα1 no. 2 (5′-CCUCAAGCUUUUCAGGCAUtt-3′) and control siRNA (5′-CUUUGGGUGAUCUACGUUAtt-3′) from Sigma Proligo (St Louis, MO, USA); Atg7 no. 1 (5′-CAGUGGAUCUAAAUCUCAAACUGAUtt-3′) and Atg7 no. 2 (5′-AAGGAGUCACAGCUCUUCCUUtt-3′) from Invitrogen and TAB2 from Santa Cruz Biotechnology (CA, USA; sc-41049).
Autophagy and cell death detection
The percentage of cells with eGFP–LC3 translocation into dots (a minimum of 100 cells/sample) was counted in eGFP-LC3 expressing cells fixed in 3.7% formaldehyde and 0.19% picric acid (vol/vol) applying Zeiss Axiovert 100 M Confocal Laser Scanning Microscope. Cross-sections with over five dots were considered positive. Long-lived protein degradation assay was performed as described previously (Høyer-Hansen et al, 2005).
Hypodiploid apoptotic cells (sub-G1) were detected by flow cytometry as described previously (Ruiz-Ruiz and Lopez-Rivas, 2002), and cell death was detected by lactate dehydrogenase (LDH) release assay (Roche) essentially as described previously (Foghsgaard et al, 2001).
Immunoblotting
Immunoblot analyses were performed according to standard protocols. For the immunoblot detection of LC3-I and LC3-II, the samples were treated with 10 μg/ml pepstatin A and 10 μg/ml E64d for the last 4 h before the lysis. Quantification of western blot signals was performed by densitometry with ImageQuant TL software after scanning the films on ImageScanner II (GE Healthcare). The relative expression of proteins was normalized to that of loading controls. The values are expressed as percentages of the expression levels in control cells that were arbitrarily set to 100%. The primary antibodies used for immunoblot analysis included murine monoclonal antibodies against beclin-1 (clone 20; Becton Dickinson) from Transduction Laboratories (NJ, USA), GAPDH from Biogenesis (Poole, UK), P-p70S6K (no. 9206) and P-IκB from Cell Signaling Technology Inc. (Danvers, MA, USA), LKB1 from Santa Cruz Biotechnology, LC3 from NanoTools (Germany) and tubulin from Sigma as well as rabbit polyclonals against p70S6K (no. 9202), AMPK (no. 2603), p-AMPK (no. 2535), p-ACC (no. 3661), p-TAK1 (no. 4508), and TAK1 (no. 4505) from Cell Signaling Technology Inc. and Atg7 from ProSci Incorporated (Poway, CA, USA). Appropriate peroxidase-conjugated secondary antibodies were obtained from DAKO A/S (Glostrup, Denmark) and ECL immunoblotting reagents were obtained from Millipore Corporation (Billerica, MA, USA).
RT–PCR
RT–PCR analyses were performed according to standard protocols. The following human primers were applied for the RT–PCR: CaMKKβ: 5′-AGACCAGGCCCGTTTCTACT-3′ and 5′-GAAGATCTTGCGGGTCTCAG-3′; actin: 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ and 5′-CATGAAGCATTTGCGGTGGACGATGGAGGG-3′: TAB2: 5′-CTGACTTGAGCAGTGGGTGA-3′ and 5′-AACGTAAGGGCTTTGGGTCT-3′.
Statistical analysis
Independent experiments were pooled when the coefficient of variance could be assumed identical. Statistical significance was evaluated by using one or two sample t-test (n=number of independent experiments). P-values below 0.05 were considered significant.
Supplementary Material
Supplementary Figures S1–S3
Acknowledgments
We thank K Grøn Henriksen, J Henrichsen, A López and D Sánchez for excellent technical assistance and A Cerami and RM Ríos for invaluable research tools. We are grateful to R Yerbes, G Ortiz-Ferrón, M Pozuelo, A Vela, C Palacios and M Rohde for stimulating discussions. The work was supported by grants from the Danish Cancer Society and Novo Nordisk Foundation (MJ and MHH); Danish National Research Foundation, Danish Medical Research Council, European Commission FP7 (APO-SYS) and Meyer Foundation (MJ); and Ministerio de Educación y Ciencia (MEC) grant SAF2006-00633, Junta de Andalucía grant CTS-211 and Red Temática de Investigación Cooperativa en Cáncer grant RD06/0020/0068 (AL-R). GH-R and CG-G were supported by fellowships from MEC and Consejo Superior de Investigaciones Científicas, respectively. We have no conflicting financial interests.
References
- Adhikari A, Xu M, Chen ZJ (2007) Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 26: 3214–3226 [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Herbst RS (2008) To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest 118: 1979–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE (2007) AMPK/LKB1 signaling in epithelial cell polarity and cell division. Cell Cycle 6: 2755–2759 [DOI] [PubMed] [Google Scholar]
- Codogno P, Meijer AJ (2005) Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 12 (Suppl 2): 1509–1518 [DOI] [PubMed] [Google Scholar]
- Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL (2004) Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz–Jeghers syndrome. Genes Dev 18: 1533–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney JR, Mlodzik M (2006) TGF-beta activated kinase-1: new insights into the diverse roles of TAK1 in development and immunity. Cell Cycle 5: 2852–2855 [DOI] [PubMed] [Google Scholar]
- Falschlehner C, Emmerich CH, Gerlach B, Walczak H (2007) TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol 39: 1462–1475 [DOI] [PubMed] [Google Scholar]
- Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, Elling F, Leist M, Jäättelä M (2001) Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J Cell Biol 153: 999–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung C, Lock R, Gao S, Salas E, Debnath J (2008) Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell 19: 797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K (2007) Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem 282: 32549–32560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Hou W, Goldstein LA, Lu C, Stolz DB, Yin XM, Rabinowich H (2008) Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J Biol Chem 283: 19665–19677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785 [DOI] [PubMed] [Google Scholar]
- Harper N, Farrow SN, Kaptein A, Cohen GM, MacFarlane M (2001) Modulation of tumor necrosis factor apoptosis-inducing ligand-induced NF-kappa B activation by inhibition of apical caspases. J Biol Chem 276: 34743–34752 [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG (2005) Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2: 9–19 [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jäättelä M (2005) Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ 12: 1297–1309 [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jäättelä M (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-β and Bcl-2. Mol Cell 25: 193–205 [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen M, Jäättelä M (2007) AMP-activated protein kinase—a universal regulator of autophagy? Autophagy 3: 381–383 [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen M, Jäättelä M (2008) Autophagy: an emerging target for cancer therapy. Autophagy 4: 574–580 [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590 [DOI] [PubMed] [Google Scholar]
- Kamada Y, Sekito T, Ohsumi Y (2004) Autophagy in yeast: a TOR-mediated response to nutrient starvation. Curr Top Microbiol Immunol 279: 73–84 [DOI] [PubMed] [Google Scholar]
- Kroemer G, Jäättelä M (2005) Lysosomes and autophagy in cell death control. Nat Rev Cancer 5: 886–897 [DOI] [PubMed] [Google Scholar]
- Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB (2007) The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 9: 218–224 [DOI] [PubMed] [Google Scholar]
- Lum JJ, DeBerardinis RJ, Thompson CB (2005) Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol 6: 439–448 [DOI] [PubMed] [Google Scholar]
- Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, Meijer AJ (2006) AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem 281: 34870–34879 [DOI] [PubMed] [Google Scholar]
- Mills KR, Reginato M, Debnath J, Queenan B, Brugge JS (2004) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc Natl Acad Sci USA 101: 3438–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momcilovic M, Hong SP, Carlson M (2006) Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem 281: 25336–25343 [DOI] [PubMed] [Google Scholar]
- Ruiz-Ruiz C, Lopez-Rivas A (2002) Mitochondria-dependent and independent mechanisms in tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis are both regulated by interferon-gamma in human breast tumour cells. Biochem J 365: 825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Miyoshi H, Mizukami J, Sugita T (2000) Phosphorylation-dependent activation of TAK1 mitogen-activated protein kinase kinase kinase by TAB1. FEBS Lett 474: 141–145 [DOI] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S (2005) Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 6: 1087–1095 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC (2004a) The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6: 91–99 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC (2004b) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA 101: 3329–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S (2005) TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev 19: 2668–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D (2005) Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2: 21–33 [DOI] [PubMed] [Google Scholar]
- Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD (2006) A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA 103: 17378–17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ (2007) Autophagosome formation: core machinery and adaptations. Nat Cell Biol 9: 1102–1109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1–S3