Abstract
Regulation of BCR signalling strength is crucial for B-cell development and function. Bright is a B-cell-restricted factor that complexes with Bruton's tyrosine kinase (Btk) and its substrate, transcription initiation factor-I (TFII-I), to activate immunoglobulin heavy chain gene transcription in the nucleus. Here we show that a palmitoylated pool of Bright is diverted to lipid rafts of resting B cells where it associates with signalosome components. After BCR ligation, Bright transiently interacts with sumoylation enzymes, blocks calcium flux and phosphorylation of Btk and TFII-I and is then discharged from lipid rafts as a Sumo-I-modified form. The resulting lipid raft concentration of Bright contributes to the signalling threshold of B cells, as their sensitivity to BCR stimulation decreases as the levels of Bright increase. Bright regulates signalling independent of its role in IgH transcription, as shown by specific dominant-negative titration of rafts-specific forms. This study identifies a BCR tuning mechanism in lipid rafts that is regulated by differential post-translational modification of a transcription factor with implications for B-cell tolerance and autoimmunity.
Keywords: B cell, immunity, signal transduction
Introduction
B-cell development and response to antigen depend on signalling through the B-cell antigen receptor (BCR) complex (Gauld et al, 2002; Meyer-Bahlburg et al, 2008). BCR signalling directs positive and negative selection of immature B cells and their progression through transitional (T) stages into mature B cells. Surface markers allow the resolution of three non-proliferative immature B-cell subpopulations: T1, T2 and T3 (Allman et al, 2001; Sims et al, 2005). The lineage origins and signalling requirements of these intermediate stages of B cells are the subject of considerable interest and debate (Matthias and Rolink, 2005; Teague et al, 2007; Welner et al, 2008). It is generally agreed that sequential progression requires an increasingly higher threshold level of BCR signalling; that is, low or ‘tonic' threshold signals promote T1 to T2, whereas relatively higher levels of signalling are needed for T2 to progress to FO or MZB (Petro et al, 2002; Su and Rawlings, 2002; Hoek et al, 2006). Strong BCR signalling also is required to direct non-transitional, fetal progenitors to B-1 fate (Loder et al, 1999; Cariappa et al, 2001; Casola et al, 2004). The amplitude of BCR signalling is positively and negatively regulated by coreceptors (Carter and Fearon, 1992; Cherukuri et al, 2001; Ravetch and Bolland, 2001) and crosstalk between the antigen receptors and other pathways, particularly BAFF (Guo and Rothstein, 2005; Venkatesh et al, 2006).
A spatially continuous but mobile unit of critical size within the plasma membrane is required for efficient initiation of BCR activation by multivalent antigen (Dintzis et al, 1976). Engagement of the antigen receptor yields ‘microclusters' that can be found in highly ordered domains within the plasma membrane, known as lipid rafts (Dykstra et al, 2003; Saeki et al, 2003; Harwood and Batista, 2008). Size and composition of these platforms of BCR signalling are dynamic and responsive to signalling events mediated by the actin cytoskeleton through plasma membrane linker proteins, such as Ezrin (Stoddart et al, 2002; Gupta et al, 2006; Sohn et al, 2006).
Bright (B-cell regulator of IgH transcription/Dril1/ARID3A) is the founder of the AT-rich interaction domain (ARID) super-family of DNA-binding proteins (Herrscher et al, 1995; Wilsker et al, 2005). Bright shuttles between the cytoplasm and the nucleus in a Crm1- and cell cycle-dependent fashion (Kim and Tucker, 2006). Bright transactivates the IgH intronic enhancer (Eμ) and certain IgH promoters by binding as a tetramer to ATC motifs within nuclear matrix associating regions (Webb et al, 1999; Kim et al, 2007; Lin et al, 2007). DNA binding and IgH transcriptional activities of Bright are stimulated by its interaction with Btk and transcription initiation factor-II (TFII-I), a direct substrate of Btk (Webb et al, 2000; Rajaiya et al, 2005, 2006). TFII-I also undergoes nucleocytoplasmic shuttling (Hakre et al, 2006), and, within the cytoplasm, it associates with PLCγ to inhibit Ca2+ mobilisation (Caraveo et al, 2006).
Bright is lineage and stage-specifically expressed with high basal levels in immature B cells and in mitogen or cytokine-induced mature B cells (Webb et al, 1991a, 1991b, 1998; Nixon et al, 2004a, 2004b). Shankar et al (2007) recently demonstrated the pathological consequences of loss of this tight control. Transgenic (TG) mice that over-express wild-type (WT) Bright specifically within the B lineage display spontaneous autoimmunity. This intrinsic B-cell autoreactivity was not accompanied by global increase in serum Ig. Instead, a markedly expanded population of T1 and MZB cells was observed.
These observations, along with the extranuclear expression of Bright, TFII-I and their functional association with Btk, prompted us to examine whether Bright is used in BCR signal transduction. We show here that a pool of Bright acts within lipid rafts as a ‘brake' to set a signalling threshold on the BCR.
Results
Association of Bright with mIgM on B-cell membranes is reduced after antigen receptor stimulation
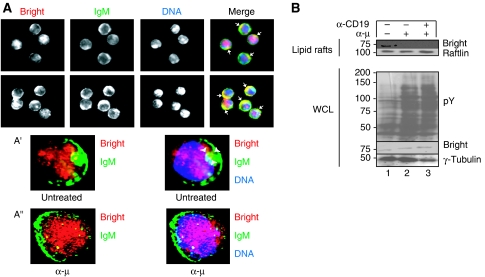
Immunostaining of murine B splenocytes indicated that a fraction of the non-nuclear Bright pool colocalised with mIgM, suggesting cortical and/or membrane-associated localisation (Figure 1A and readdressed below). This observation was confirmed by computerised 3D reconstructions of the immunofluorescence data (Figure 1A′ and Supplementary Video 1).
Figure 1.
Bright accumulates within lipid rafts of resting but not stimulated B cells. (A) Association of Bright with mIgM on B-cell membranes is reduced after antigen receptor stimulation. CD43− B cells from spleens of BALB/c adult mice were fixed and stained for Bright (red), mIgM (green) and DNA (blue). Arrows point to areas (yellow) where Bright colocalises with membrane IgM. (A′, A″) Engagement of the antigen receptor reduces the colocalisation between Bright and mIgM. CD43− B cells (∼1 × 104) from spleens of BALB/c adult mice were left untreated (A′) or stimulated for 5 min (A″) with 10 pg α-μ, followed by immunostaining as described above. Deconvoluted images are shown with arrows pointing to areas (yellow) where Bright colocalises with mIgM. (B) BCR engagement leads to a discharge of Bright from lipid rafts. CD43− B cells (∼2 × 106) were stimulated with either 2 ng α-μ or 2 ng α-μ+2 ng α-CD19 for 5 min. Lipid rafts or whole cell lysates (WCL) were prepared from half of each sample. Proteins from each fraction were analysed by SDS–PAGE/western blot using the antibodies indicated.
To determine whether this colocalisation remains intact after engagement of the BCR, cells were stimulated for 5 min with α-μ. Only modest colocalisation of Bright and IgM was retained, as assessed by computerised 3D reconstructions of the immunofluorescence data (Figure 1A″ and Supplementary Video 2). Inspection of these and additional images (data not shown) indicated that the observed redistribution of mIgM-associated Bright in stimulated B cells was not accompanied by significant alteration in either its nuclear or its cytoplasmic levels (data not shown).
Bright accumulates within lipid rafts of resting but not stimulated B cells
Because lipid rafts serve as platforms for BCR signalling, we assayed purified plasma membranes and lipid rafts (Supplementary Figure 1A) for the presence of Bright. A small pool of Bright resides in lipid rafts purified from unstimulated CD43− B cells (Figure 1B, upper panel). Consistent with the imaging results, Bright was not detected within lipid rafts after BCR engagement that was sufficient to elicit a phosphotyrosine (pY) response (Figure 1B, lower panel). This suggested that the presence or absence of Bright within lipid rafts might influence BCR signalling.
Levels of Bright within lipid rafts determine BCR signalling threshold
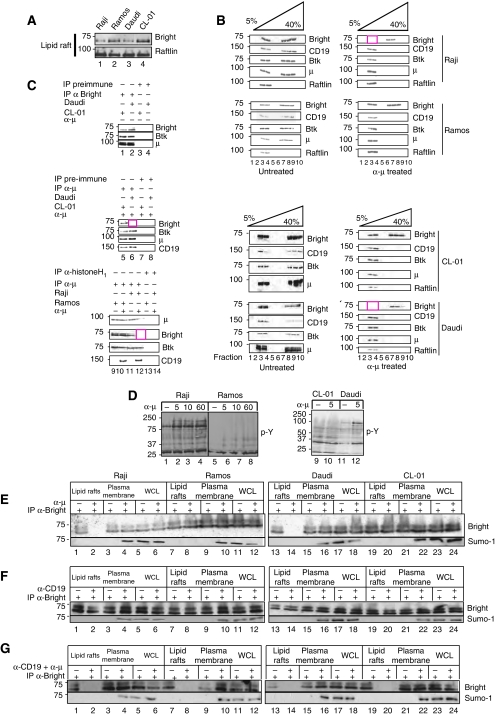
Normal B cells and mature B-cell lines were examined semi-quantitatively for lipid raft content of Bright using the B cell-specific lipid rafts component, Raflin (Saeki et al, 2003), as an internal control (Supplementary Figure 1B and data not shown). We estimated that raft-localised Bright accounted for 1–10% of total cellular Bright, consistent with percentages previously estimated for mIgM concentrations in lipid rafts (Sproul et al, 2000; Putnam et al, 2003). Lipid rafts of Raji and Daudi cells contained ∼10-fold less Bright than those of CL01 or Ramos (Figure 2A). However, no significant differences were observed in other subcellular fractions among these lines (Supplementary Figure 1B).
Figure 2.
Levels of Bright within lipid rafts determine BCR signalling threshold. (A) Bright levels within lipid rafts vary among B-cell lines. Lipid rafts were prepared from the indicated exponentially growing human B-cell lines (107 cells) and probed for Bright and (as loading control) Raftlin. (B, C) Antigen receptor engagement results in a discharge of Bright from lipid raft-localised BCR complexes. (B) The indicated cell lines (∼5 × 108) were stimulated for 5 min with 500 ng of α-μ, followed by preparation of lipid rafts using discontinuous gradient centrifugation. Aliquots of each fraction were analysed directly by western or (C) were extracted with RIPA buffer and subjected to co-IP/western using the antibodies indicated. (D) Raji, Daudi, Ramos and CL01 cells respond differentially to BCR stimulation. The indicated cells were stimulated (100 ng α-μ for 108 cells) for 5, 10 and 60 min (Raji and Ramos; left panel) or for 5 min (CL01 and Daudi; right panel), as indicated, and whole cell extracts were blotted with α-phosphotyrosine (pY). Equal loading was confirmed by staining of the filters with India Ink (data not shown). (E–G) BCR+CD19 stimulation leads to Bright discharge from lipid rafts and accumulation of Sumo-I-Bright in plasma membranes. Raji, Ramos, CL01 and Daudi (∼108 cells) were stimulated for 5 min with (E) 100 ng α-μ, (F) 100 ng α-CD19 or (G) 100 ng α-μ+100 ng α-CD19. Lipid rafts (Raft), plasma membranes (membrane) and whole cell lysates (WCL) were analysed by IP/western using the antibodies indicated.
To achieve maximal BCR responses under minimal antibody concentrations and minimal receptor internalisation, an approach using an anti-IgM mAb in the absence of secondary cross-linking was optimised (Supplementary Figures 2B and 4B; Materials and methods; data not shown). Lipid rafts from resting and stimulated cell lines were purified on sucrose gradients, and fractions were analysed for Bright and other signalosome occupants (Figure 2B). In agreement with published reports (Saeki et al, 2003; Depoil et al, 2008), levels of mIgM, CD19, and the Bright-interacting partner Btk increased in lipid rafts after α-μ stimulation (Figure 2B). As observed for normal B cells, Bright moved in the opposite manner. However, its discharge from lipid rafts was complete only in cell lines in which its starting levels in lipid rafts were low (Daudi and Raji, Figure 2B, fractions 3 and 4; boxed in red).
These differences in trafficking could reflect differences in composition of raft-localised complexes, or artifacts resulting from increased resistance to solubilisation, as BCR ligation is known to induce coalescence of lipid rafts (Gupta et al, 2006). Therefore, we compared profiles obtained from RIPA-solubilised versus non-soluble lipid rafts immunoprecipitated (IP) with α-μ, α-Raftlin and α-Btk (Supplementary Figure 2E). We observed that, as previously published (Saeki et al, 2003), a complex containing Raflin and IgM was seen only in non-solubilised rafts (Supplementary Figure 2E); this indicated that our solubilisation conditions were sufficient. However, unexpectedly, Raflin did IP with Btk under both conditions, suggesting that an IP complex containing Btk and Raflin is not disrupted by RIPA solubilisation of lipid rafts (readdressed below). Importantly, Bright remained in a complex with mIgM and Btk in solubilised lipid rafts of all unstimulated cells (Figure 2C) but was lost only in α-μ stimulated cells (Daudi and Raji) that contained lower starting levels in their lipid rafts (Figure 2C, lanes 6 and 12; boxed in red).
These results suggested that B cells that contain more lipid rafts-associated Bright (Ramos and CL01) would be less sensitive (higher threshold) to BCR ligation. This was confirmed by the pY responses of these cell lines to α-μ stimulation (Figure 2D). Ramos and CL01 also responded less vigorously to pro-apoptotic signals shown previously (Chen et al, 1999) to result from long-term stimulation by α-μ (Supplementary Figure 2C).
Ligation of the BCR coreceptor, CD19, is known to synergistically enhance antigen receptor-mediated signalling (Carter and Fearon, 1992; Cherukuri et al, 2001; Depoil et al, 2008). Accordingly, all cell lines responded to α-μ+α-CD19 costimulation with robust responses (Supplementary Figure 2B). BCR costimulation was required to expel Bright from lipid rafts of the less sensitive (higher threshold) cell lines Ramos and CL01 (Figure 2E–G). That Bright migrates as a doublet is apparent in these experiments (addressed below in the context of the sumoylation observations).
Thus, engagement of the BCR results in a significant and specific reduction of the small pool of lipid rafts-localised Bright. This pool is lost from lipid rafts, as Btk and other signalosome components accumulate there, in proportion to BCR signalling strength.
Entry of Bright into lipid rafts does not require interaction with Btk but does require palmitoylation
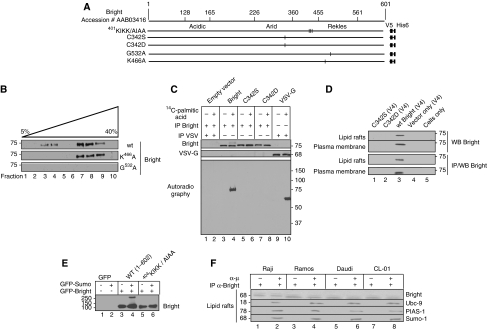
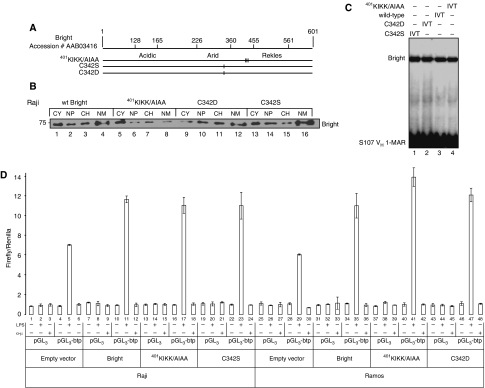
Bright was readily detected in lipid rafts prepared from retrovirally transduced NIH/3T3 fibroblasts and other non-B cells (Figure 3B; data not shown). This indicated that even though Bright associates (at least transiently) with Btk and other signalsome components in α-μ stimulated lipid rafts (Figure 2C and addressed further below), these B-cell-restricted proteins are not required to designate or retain Bright in lipid rafts. Bright point mutants (Figure 3A; Kim and Tucker, 2006) that are retained either within the cytoplasm (K466A) or the nucleus (G532A) did not localise to rafts (Figure 3B). Thus, the rafts-localised pool of Bright is not directly diverted from the cytoplasmic pool, suggesting that nucleocytoplasmic shuttling (Kim and Tucker, 2006) is required.
Figure 3.
Entry and exit of Bright from lipid rafts requires nucleocytoplasmic shuttling, alternative post-translational modifications, but not association with Btk. (A) Schematic of Bright indicating domains and positions of substitution mutations. (B) Cytoplasmic-nuclear shuttling is a requirement for Bright's inclusion into lipid rafts. NIH/3T3 fibroblasts were transfected with constructs encoding wild type and nuclear export signal-defective (NES, G532A) and nuclear localisation signal-defective (NLS, K466A) Bright. Fractions from discontinuous (5–40%) sucrose gradient purification of lipid rafts were analysed by western for Bright. (C) Palmitoylation of Bright requires cysteine 342. Bright constructs (indicated at the top) were transfected into Cos-7 cells, and after 48 h, were incubated as indicated with 14C palmitic acid. Whole cell lysates were subjected to immunoprecipitation using antibodies against Bright and VSV as indicated (left). SDS–PAGE separated proteins were transferred onto nitrocellulose, and the metabolic incorporation of 14C palmitic acid was determined by autoradiography. Solvent control is indicated by −. (D) Specification of Bright to lipid rafts requires cysteine 342. NIH/3T3 fibroblasts were transfected with constructs indicated in the figure. Crude plasma membrane and lipid rafts, prepared as described in previous legends, were analysed by anti-Bright western blotting. (E) Sumo-I modification of Bright is lost after mutation of 401KIKK. Cos-7 cells were transfected with GFP-SUMO-I and GFP-Bright expression constructs, as indicated. Whole cell lysates were prepared using RIPA buffer and analysed by western using α-Bright anti-serum. An arrow points to a GFP-Sumo-I conjugated species of GFP-Bright. (F) Bright forms a transient, stimulation-specific complex with Sumo-I-conjugation enzymes PIAS1 and Ubc9 in lipid rafts. Indicated B cells (∼108) were stimulated mildly (30 s; 100 ng α-μ). Lipid rafts were collected on gradients, subjected to immunoprecipitation with anti-Bright antiserum, and then analysed by western using the antibodies indicated.
Palmitoylation of cysteine residues is a feature shared by a number of lipid raft occupants (Simons and Toomre, 2000; Ashery et al, 2006). Bright contains a single cysteine (C342) in its ARID DNA-binding domain, which is conserved among all identified orthologues and paralogues (Figure 3A; Wilsker et al, 2005). After transfection into fibroblasts, WT Bright, but not point mutants (C342S and C342D), were palmitoylated (Figure 3C).
Sumoylation of Bright regulates its discharge from lipid rafts into membranes after BCR stimulation
Yeast 2-hybrid cDNA library screening and additional analyses (Supplementary Figure 3A; data not shown) detected strong and specific Bright interactions with Sumo-I conjugating enzymes Ubc9 and PIAS1. Further investigations indicated that Bright is conjugated to Sumo-I at a consensus motif (ΨKxE, Sampson et al, 2001; Gocke et al, 2005; Bossis and Melchior, 2006) 401KIKKE (Figure 3A) both in cultured cell lines and in vitro (Figure 3E; Supplementary Figure 3B).
Sumo-I-Bright was readily detected in α-Bright IPs of whole cell lysates prepared from B-cell lines and normal B cells (Figures 2E–G; Supplementary Figures 2D, 3B and C). We found no Sumo-I-Bright in lipid rafts regardless of cell source and stimulation regime; only membranes prepared from stimulated B cells contained Sumo-I-Bright (Figure 2E–G; Supplementary Figure 2D). Yet Sumo-I-deficient (401KIKK/AIAA) Bright was capable of entering lipid rafts and membranes as efficiently as WT in transfected fibroblasts (Supplementary Figure 3D).
These results prompted us to speculate that the Sumo-I-Bright pool within stimulated plasma membranes might derive from a sumoylation reaction initiated in B cell rafts immediately after BCR ligation. If so, a transient sumoylation initiation complex might be trapped in lipid rafts under much weaker BCR stimulation conditions. To test this, we isolated lipid rafts following conditions (30 s; α-μ). Consistent with our hypothesis, Bright was detected in these mildly stimulated rafts in an IP complex with sumolyation E2 and E3 components, Ubc-9 and PIAS-1 (Figure 3F; Schwarz et al, 1998; Kahyo et al, 2001).
Dominant-negative, lipid rafts localisation-defective mutants modulate BCR signalling
Because Bright exists primarily as a homo-tetramer, we established the basis for a dominant-negative approach by pulling down endogenous Sumo-I-Bright using V5 tagged 401KIKK/AIAA-Bright (Supplementary Figure 4A; Herrscher et al, 1995; Kim and Tucker, 2006). Thus, we reasoned that over-expression of palmitoylation-defective C342S or C342D Bright should titrate the small pool of palmitoylated Bright tetramers destined for lipid rafts while sparing tetramers destined for the nucleus. Conversely, the inability to sumoylate Bright should trap it in lipid rafts, leading to suppression of BCR signalling.
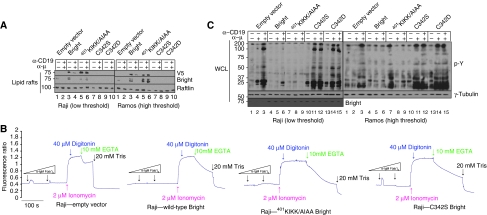
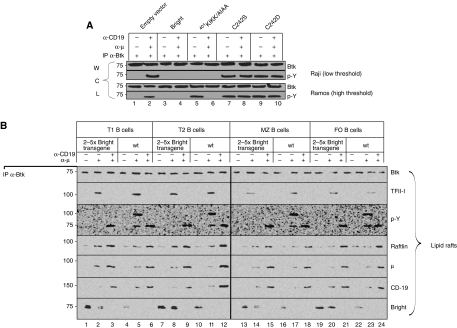
As shown in Figure 4A, rafts prepared from WT transductants contained increased levels (relative to mock controls) of V5-tagged retroviral Bright, whereas those expressing C342 substitutions were virtually depleted. Over-expression of 401KIKK/AIAA-Bright in lipid rafts (Figure 4A) led to retention of both retroviral and endogenous Bright within rafts after BCR stimulation.
Figure 4.
Dominant-negative, lipid rafts localisation-defective mutants modulate BCR signalling. Raji and Ramos cells (5 × 108) were infected with retroviruses encoding wild type and mutant forms of Bright and were then stimulated for 5 min with 500 ng α-μ, 500 ng α-CD19 or 500 ng α-μ+500 ng α-CD19. (A) Levels of Bright in lipid rafts are altered by dominant-negative forms. Lipid rafts levels of total Bright (endogenous+ectopic) were measured by anti-Bright western. Levels of ectopic V5-tagged wild-type Bright, a palmitoylation-defective (C342S/D) form, which is unable to enter lipid rafts, and a Sumo-I-mutant form (401KIKK/AIAA), unable to be discharged from rafts, were detected by anti-V5 western. Raftlin was used as a loading control. (B) Intracellular free [Ca2+] is increased by palmitoylation-deficient and decreased by wild type or Sumo-I-deficient titration of endogenous Bright. Transduced Raji B cells (1 × 106 cells/ml) were loaded with 2 μM Indo-1 and stimulated with 1 ng or 40 μg of α-μ (indicated as low and high concentrations, respectively, by triangles). Fluorescence signal was plotted against time (scale bar=100 s). Internal calibration was performed as detailed in Materials and methods. Downward arrows indicate times of reagent addition. (C) Dominant-negative Bright mutants alter global phosphotyrosine responses. Whole cell lysates prepared from cell lines established and stimulated in (A) were analysed by anti-pY, Bright and tubulin (loading control) western.
We next measured the effect of the dominant-negative titrations on BCR signalling. As shown in Figure 4B and C, signalling was markedly increased in Bright C342S and C342D-infected cells. Notably, the signalling threshold of the Raji BCR was converted from low to high, because weak stimulation (α-μ only) now resulted in significantly reduced signalling (Figure 4; Supplementary Figure 4B). Conversely, over-expression of WT Bright and the 401KIKK/AIAA dominant-negative mutant form virtually eliminated BCR-stimulated Ca2+ flux and pY activity. Yet, unstimulated 401KIKK/AIAA-transduced Ramos cells appeared to be constitutively ‘hyperactivated' with respect to pY signals (Figure 4C, lane 7). This was a consistent result (please see Figure 7A and discussion below) that we suspect derived from Ramos-specific, off-target (i.e., non-BCR mediated) effects of this dominant negative.
Figure 7.
BCR-mediated activation of Btk and TFII-I depends on the levels of Bright in lipid rafts. (A) Dominant-negative Bright mutants alter Btk phosphorylation in B-cell lines. Whole cell lysates prepared from retrovirally transduced cell lines established in Figure 4A were stimulated for 5 min using either 500 ng α-μ or 500 ng α-μ+500 ng α-CD19 for 5 × 108 cells as indicated. After anti-Btk immunoprecipitation, westerns were performed with anti-Btk (loading control) and anti-pY anti-sera. (B) Transgenic Bright over-expression inhibits Btk and TFII-I phosphorylation in B-cell subpopulations. Lipid raft extracts prepared using RIPA buffer (described in Figure 6D; equivalent of ∼106 cells per lane) were subjected to α-Btk IP, followed by western blotting using the antibodies indicated.
We conclude that a palmitoylated pool of Bright is dispatched to lipid rafts to dampen BCR signalling, and sumoylation-triggered discharge of Bright is essential for relieving this inhibition.
Bright regulates signalling and IgH transcription independently
As the small lipid rafts-localised pool of Bright is diverted from its nucleocytoplasmatic shuttling pool (Figure 3B; Kim and Tucker, 2006), we examined the potential nuclear consequences of the dominant-negative-mediated signalling perturbations. Neither nuclear-cytoplasmic ratios nor in vitro DNA binding of Bright to a target VH-associated promoter were significantly altered (Figure 5B and C).
Figure 5.
Wild type and dominant-negative mutant forms of Bright are indistinguishable in DNA-binding, subcellular localisation and transcriptional activity. (A) Schematic illustration of Bright amino acid substitution mutations. (B) Subcellular fractionation of Bright is unaltered by dominant-negative transduction. Raji (107 cells), expressing wild type and the indicated mutant forms of Bright, were subjected to fractionation into cytoplasm (CY), nucleoplasm (NP), chromatin (CH) and nuclear matrix (NM) and analysed by western using α-Bright anti-serum. (C) Substitution mutants bind indistinguishably to IgH promoter sites. The indicated forms of Bright were prepared by in vitro transcription/translation (IVT) and subjected to electrophoretic mobility shift assays using 32P labelled S107 VH1-MAR probe. Specificity of binding was demonstrated by α-Bright super-shift and cold probe competition (not shown) as previously described (Herrscher et al, 1995; Zong et al, 2000; Kaplan et al, 2001). (D) Bright transactivation of inducible IgH promoter activity is not changed by dominant-negative transduction. Exponentially growing Raji and Ramos cells, stably transduced with retroviruses encoding empty vector (control) or wild type and mutant forms of Bright (as indicated), were transfected by electroporation with either a Firefly luciferase reporter (pGL3) or a pGL3-derived Bright-responsive (Kim and Tucker, 2006) reporter (VH1-MAR-pGCL3) driven by the S107 VH1-MAR-containing promoter plus Renilla luciferase. After culture for 2 days, cells were either untreated or stimulated for 5 days with 20 μg/ml LPS or stimulated for 5 min with anti-IgM F(ab′)2 fragment, as described in the Materials and methods. Dual luciferase activity was then measured as described (Rajaiya et al, 2006) and expressed as the Firefly/Renilla ratio.
In vivo, Bright does not modulate basal levels of IgH transcription, but like several other trans-activators requires accessory proteins induced during differentiation (reviewed in Webb et al, 1999). Thus, we assayed Bright-binding-dependent luciferase reporter activity in LPS stimulated Raji and Ramos before and after dominant-negative transduction. As shown in Figure 5D, LPS-induced increase in reporter activity above endogenous levels (lanes 5 and 29) was equally enhanced by over-expression of WT (lanes 11 and 35) or substitution-mutant forms of Bright (lanes 17, 23, 41 and 47). Five minutes of stimulation using α-μ sufficient to influence BCR signalling (Figure 4) failed to elevate reporter activity above background levels (Figure 5D, lanes 6, 12, 18, 24, 30, 36, 42 and 48). We conclude that the dominant-negative effects of C342S/D and 401KIKK/AIAA are limited to the rafts-destined pool, and that Bright functions independently as both an inducible transactivator of IgH and a BCR signalling regulator.
Over-expression of Bright impairs BCR signalling of normal B-cell subpopulations
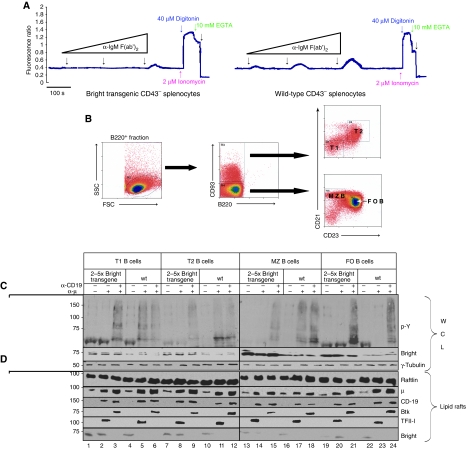
What is the consequence of manipulating Bright levels within lipid rafts of normal B cells? Splenic B cells purified from Bright-TG mice express 3–5-fold higher levels of Bright within lipid rafts and whole cells lysates (Figure 6A). TG B cells were markedly reduced relative to WT in Ca2+ and pY responses over a wild range of α-μ doses with concomitant depletion kinetics of Bright from stimulated rafts (Figure 6A; Supplementary Figure 5A).
Figure 6.
Transgenic over-expression of Bright decreases BCR signalling of normal B cells. (A) Mobilisation of intracellular Ca2+ is reduced by Bright over-expression. Wild type and Bright transgenic mice were sacrificed, splenic CD43− B cells were prepared by negative selection, loaded with Indo-1 and then subjected to measurements of intracellular Ca2+ using 1 ng (low), 500 ng (medium) or 40 μg α-μ (high) to stimulate 106 cells; Ionomycin, Digitonin, EGTA and Tris were used for internal calibration. (B) Purification of T1, T2, FO and MZ B-cell populations. B cells were prepared from single cell suspensions of transgenic (shown here) and wild-type (not shown) splenocytes. B220+ B-cell subpopulations were defined as T1 (CD93+ CD23− CD21−), T2 (CD93+ CD23+ CD21+), FO (CD93− CD23+ CD21+) and MZ (CD93− CD23− CD21+). (C) Over-expression of Bright inhibits global phosphotyrosine responses of isolated B-cell populations. Each indicated subpopulation (∼106 cells) was stimulated for 5 min using 1 ng α-μ, 1 ng α-CD19 or 1 ng α-μ+1 ng α-CD19. Whole cell lysates (WCL) were prepared from each and then were subjected to SDS–PAGE/western blotting using α-pY, α-Bright and α-Tubulin (loading control) anti-sera. (D) Trafficking of BCR signalling components in and out of lipid rafts is not disturbed in transgenic B-cell populations. The indicated subpopulations (∼2 × 106 cells each) were stimulated for 5 min using 2 ng α-μ, 2 ng α-CD19 or 2 ng α-μ+2 ng α-CD19. The entire preparation of each was then used for the isolation of lipid rafts. Fractions from the gradient centrifugation were collected and divided into two equal parts. One part was analysed directly by SDS–PAGE/western (lipid rafts from ∼106 cells per lane) using the antibodies indicated to serve as an input control for the IP performed with the other half of the RIPA extracted samples (shown in Figure 7B). Here, increased levels of coprecipitated μ, Raftlin and CD19 indicated coalescence of lipid rafts upon BCR engagement (Depoil et al, 2008).
MZB and immature B-cell populations are significantly elevated in Bright TG, leading to development of spontaneously autoimmunity during aging (Shankar et al, 2007). TG and WT B splenocytes were sorted under conditions that avoid BCR activation into immature (T1 and T2), MZB, and FO populations (Figure 6B). Elevated levels of Bright (∼2–5-fold) were observed in whole cell lysates and in lipid rafts (Figure 6C and D) prepared from all resting TG populations except FO. We confirmed that our α-μ stimulation conditions induced no changes in proliferation or differentiation of these subpopulations, such as that observed by others under prolonged stimulation (data not shown; Petro et al, 2002; readdressed in Discussion).
The movement of mIgM, Btk and CD19 into lipid rafts was unaffected by the starting levels of Bright, as all TG and WT populations were indistinguishable (Figure 6D). Likewise, all WT and TG populations responded to strong (α-μ+α-CD19) BCR costimulation with a complete discharge of Bright from lipid rafts (Figure 6D) and a loss of membrane colocalisation with mIgM (Supplementary Figure 5B and data not shown). However, the BCR signalling threshold of immature and MZB populations, as judged by their response to weak (α-μ) stimulation, correlated inversely with their lipid raft content of Bright. As shown in Figure 6C, global pY responses of T1, T2 and MZB TG B cells were reduced relative to WT controls, consistent with the fact that weak stimulation was insufficient to discharge Bright from their lipid rafts (Figure 6D). pY responses of FO WT B cells were, as expected (Li et al, 2001), relatively less robust (Figure 6C). Resting FO B cells contained slightly lower levels of total or lipid raft-localised Bright (Figure 6C and D; Shankar et al, 2007) and displayed less colocalisation between Bright and mIgM than the other subpopulations (Supplementary Figure 5B). Nonetheless, weak TG FO signalling was consistently dampened in response to α-μ stimulation, and Bright was not fully discharged from their lipid rafts (Figure 6C and D).
We conclude that lipid rafts-localised Bright increases the signalling threshold of MZB, immature, and, to a lower extent, FO B cells. We further suggest that, as BCR signalling strength contributes to B-cell subset development (Loder et al, 1999; Cariappa et al, 2001; Niiro and Clark, 2002; Petro et al, 2002; Su and Rawlings, 2002; Casola et al, 2004; Su et al, 2004; Hoek et al, 2006), the skewed T1 and MZB populations in Bright-over-expressing B-cells derived, at least in part, from impaired signalling.
Phosphorylation of Btk and TFII-I within lipid rafts is inhibited by Bright
Btk interacts with Bright (Webb et al, 2000; Rajaiya et al, 2005) to modulate its transcriptional activity in the nucleus (Rajaiya et al, 2006). Thus, it seemed particularly informative to determine how Bright levels outside the nucleus affect Btk activation. First, we examined whole cell extracts prepared from the dominant-negative transduced cell lines (Figure 7A). When Bright entry into lipid rafts was blocked by over-expression of the palmitoylation-defective C342S/D mutant, pY-Btk was robustly detected after BCR stimulation of either the less sensitive (Ramos) or the more sensitive (Raji) cell line. Under conditions in which lipid rafts levels of Bright were increased (by either WT over-expression or Bright-401KIKK/AIAA retention), pY of Btk was inhibited (Figure 7A). Note that the constitutive hyperactivation phenotype observed for global pTyr (Figure 4C, lane 7) was confirmed in this independent set of 401-KIKK/AIAA Ramos transductants.
These results suggested that Btk activation by pY in lipid rafts is inhibited by Bright. Consistent with this hypothesis, pY-Btk was detected in α-Btk IPs prepared from lipid rafts of all α-μ stimulated WT B-cell populations but in none of the corresponding Bright-over-expressing TG B cells (Figure 7B). As predicted by the global pY results, all WT and TG B-cells responded with robust pY-Btk after strong (α-μ+α-CD19) BCR ligation (Figure 6C).
Btk pY is required for TFII-I function as a nuclear transcription factor (Rajaiya et al, 2005, 2006) as well as for its cytoplasmic interaction with PLCγ and subsequent inhibition of PLCγ-mediated Ca2+ mobilisation (Guo et al, 2004; Caraveo et al, 2006). As observed for Btk, TFII-I moved into lipid rafts of TG and WT B-cell subsets after α-μ stimulation (Figures 6D and 7B). However, while stronger stimulation (α-μ+α-CD19) further concentrated Btk and other signalosome components there, TFII-I was codischarged with Bright (Figures 6D and 7B). Notably, phosphorylation of TFII-I and Btk was inhibited in α-μ stimulated rafts of Bright-over-expressing TG B cells (Figure 7B).
These results indicate that lipid rafts-localised Bright contributes to dampening of BCR responses by decoupling Btk activation and downstream immediate early events, such as tyrosine phosphorylation of TFII-I.
Discussion
We have shown that 1–10% of Bright, a B-cell-specific transcriptional activator of IgH transcription, associates with the BCR complex in lipid rafts prepared from transformed mature B-cell lines or from primary B-cell populations purified from mouse splenocytes or from human PBL. We demonstrated that Bright is palmitoylated at a single cysteine residue and that this modification is required for its localisation in lipid rafts. Our data indicate that the lipid raft concentration of Bright is used for BCR threshold signalling, as the sensitivity to BCR stimulation, as measured by calcium flux and transmission of global and Btk-mediated pTyr signals, depends on the amount of Bright discharged from the rafts. An inducible, rafts-specific association between Bright and Sumo-I E2/Ubc-9 and E3/PIAS-1 enzymes, along with accumulation of sumoylated Bright in the plasma membrane only after BCR stimulation, led us to test whether this post-translational modification triggered Bright discharge. Signalling alterations observed in TG B cells that over-express Bright, or in B-cells transduced with sumoylation-insensitive and rafts localisation-defective dominant-negative Bright retroviruses, support this notion. The data suggest a model in which Bright acts as a ‘brake' to set a signalling threshold that is regulated by alternative post-translational modification.
The strength of BCR-derived signals determines the sequential development of immature to mature B cells and their subsequent fates in the spleen (Casola et al, 2004; Gazumyan et al, 2006; Patterson et al, 2006; Pao et al, 2007). Shankar et al reported that T1 and MZB cells are statistically increased relative to other B-cell populations in Bright-over-expressing TG mice. Although serum Ig levels were increased only modestly, spontaneous autoimmunity ensued (Shankar et al, 2007). Petro et al (2002) showed that under conditions of prolonged (24 h) BCR engagement with high concentrations of α-μ (∼10 μg/ml/105 cells), normal T1 B cells undergo apoptosis, whereas T2 B cells proliferate. Under the same conditions, T2 B cells display a higher threshold for α-μ-elicited signals than T1 cells (Petro et al, 2002; Hoek et al, 2006; Meyer-Bahlburg et al, 2008). To focus on early signalling events and to circumvent apoptotic/proliferation complications, we used ∼103-fold lower concentrations of F(ab′)2 in most of our stimulation assays. We found that over-expression of Bright led to an elevated level of Bright in lipid raft-associated signalosomes of all B-cell populations except FO and increased signalling thresholds to our low concentrations of α-μ. In contrast to the imaging results of Chung et al (2001), we found that this level of stimulation was adequate to induce translocation of BCR components (mIgM, CD19, and Btk) into rafts from all subpopulations. However, our biochemical approach did not allow us to address their contention that there are fewer lipid rafts in immature B cells. That T1 and MZB were particularly sensitive suggests that, in Bright TG mice, these populations would be compromised in their ability to undergo appropriate apoptotic responses to self-antigens in vivo. This would account for the elevated numbers of TG T1 and MZB—established predecessors for autoreactive B cells (Atencio et al, 2004; Samuels et al, 2005; Yurasov et al, 2005a, 2005b; Quinn et al, 2006)—and provide a unique mechanism by which a B-cell-restricted transcription factor could contribute intrinsically to B-cell tolerance. On the other hand, and not mutually exclusive, the selective production of antibodies associated with the autoimmune syndromes of Bright TGs (Shankar et al, 2007) could be a direct result of as yet unidentified, non-IgH transcriptional targets of Bright.
Bright interacts with a well-defined signalling molecule, Btk (Webb et al, 2000; Rajaiya et al, 2005, 2006). Previously we hypothesised (Webb et al, 1999) that their interaction in the cytoplasm allowed Bright to deliver Btk to nuclear IgH promoters. There, Btk could phosphorylate and activate TFII-I, which at the time, was the only defined substrate for Btk (Novina et al, 1999). Subsequent studies by Webb and colleagues (Webb et al, 2000; Rajaiya et al, 2005, 2006) support the notion that Bright delivers Btk to places of active TFII-I transcription in the nucleus.
Extension of the hypothesis would predict that Btk is required for Bright's inclusion into lipid rafts and, taken to the extreme, into lipid raft-localised BCR signalosomes. However, in Btk-deficient non-B cells, exogenously expressed Bright accumulated within lipid rafts, indicating that its localisation is independent of Btk or other B-cell-specific factors. This led us to hypothesise that Bright might function in lipid raft-localised BCR complexes to limit or increase the concentration of Btk. Bright–Btk association was, indeed, observed in lipid raft-localised BCR complexes. BCR engagement resulted in reversed trafficking patterns in and out of lipid rafts; that is, Btk accumulated in lipid raft-localised BCR complexes as Bright was being depleted. Thus, Bright does not function to limit signalosome-associated Btk.
Alternatively and in contrast to their kinase-dependent collaboration in the nucleus (Rajaiya et al, 2006), we reasoned that Bright–Btk complexes in lipid rafts may be catalytically inactive or compromised, such that Bright has to be removed from Btk in order for the Tec kinase to achieve full activity. Consistent with this notion, activation of Btk by tyrosine phosphorylation is stimulated when Bright's entry to lipid rafts is blocked by palmitoylation-defective mutants and inhibited when Bright is trapped in lipid rafts by a sumoylation-defective mutant. In further support, we found that loss of Btk activation occurs in lipid rafts, and inactive Btk (non-phosphorylated) is associated with Bright there. This suggested that Bright-containing BCR complexes are signalling-impaired because the presence of Bright in lipid rafts raises the threshold of Btk-dependent BCR signalling. Accordingly, Btk phosphorylation of a direct downstream substrate, TFII-I, was inhibited within Bright-rich lipid rafts.
The lineage relationships between FO and MZB and the role that Btk plays in this are controversial (Matthias and Rolink, 2005; Teague et al, 2007; Welner et al, 2008). MZB cells have been ascribed to develop either directly from T1 cells (Debnath et al, 2007) or from a subpopulation of CD21int T2 B cells (Meyer-Bahlburg et al, 2008). Others contend that both MZB and FO derive from a long lived, post-transitional follicular B-cell subset described as Follicular Type II (Cariappa et al, 2007; Allman and Pillai, 2008). Although Xid phenotypic CBA/N mice show significantly greater loss of FO (Hardy et al, 1982), their MZB numbers are also reduced (Liu et al, 1988; Cariappa et al, 2001). The enrichment of certain Ag specificities into MZB requires functional Btk (Martin and Kearney, 2000; Kanayama et al, 2005). Our findings support a common progenitor model and suggest that if MZB require an intact Btk signalling pathway, Bright has a function in this regulation.
Bright is the first transcription factor shown to function in lipid rafts, but its residence there is not unprecedented. Small cytoplasmic fractions of Stat1 and Stat3 constitutively localise to lipid rafts and have been suggested to function there during early stages of cytokine signalling (Sehgal et al, 2002). An isoform of OCA-B, an IgH transcriptional coactivator, localises as a myristoylated form to the cytoplasm and to plasma membranes but not to lipid rafts per se (Yu et al, 2001, 2006). Similarly, TFII-I was previously detected in cytoplasmic complexes with PLCγ (Caraveo et al, 2006). Btk-dependent phosphorylation of TFII-I was required for its interaction with and concomitant inhibition of PLCγ-catalysed Ca2+ mobilisation (Guo et al, 2004). Both TFII-I and Bright are regulated by nucleocytoplasmic shuttling (Novina et al, 1999; Nore et al, 2000; Kim and Tucker, 2006). Lipid rafts-designated Bright derives from a palmitoylated pool that requires continual shuttling, as neither NLS nor NES mutants accumulate in rafts. That nucleocytoplasmic shuttling is also required for Bright transcriptional activity (Kim and Tucker, 2006) raises the possibility that its occupancy in lipid rafts is prerequisite for assembly of and subsequent transfer of Bright–Btk–TFII-I complexes to the nucleus. Consistent with this notion, Bright is codischarged with activated TFII-I from lipid rafts after BCR ligation as a Sumo-I-modified form. Although sumoylation has been ascribed to mediate nuclear import/export and activity of transcription factors (Liu et al, 2006), the membrane-localised metabotropic glutamate receptor is targeted by the sumoylation machinery (Tang et al, 2005). Similarly, the sumo pathway was shown to control the activity of the potassium channel K2P1 (Rajan et al, 2005).
Our observations suggest a new avenue for signal propagation between the membrane and the nucleus. They emphasise the need to describe the properties of the compartmentalised pools, their temporal and spatial regulation and the molecular requirement(s) for the signalling networks involved. Our results extend and enrich the notion that mice with an artificially altered BCR threshold are more likely to display features of autoimmunity (Grimaldi et al, 2005; Goodnow, 2007). We identify the transcription factor Bright as an unsuspected component of such a network and implicate it as regulator of an early event in BCR signalling and immunologic tolerance.
Materials and methods
Cells
Raji (EBV+; McConnell et al, 1992; Miller et al, 1993; Caldwell et al, 1998; Cerimele et al, 2005; Bernasconi et al, 2006), Daudi (EBV+; Wang et al, 1990), Ramos (EBV−; Cerimele et al, 2005) and CL01 (EBV−; Laskov et al, 2006) were obtained from ATCC (Manassas, Virginia) and maintained as described (Fell et al, 1986). CD43-B cells were prepared by negative selection of whole human blood (Gulf Coast Regional Blood Center, Houston, Texas) or from ∼10-wk-old BALB/c murince splenocytes (Webb et al, 1998). Preparative sorts were executed according to Webb et al (1998) and Shankar et al (2007). B cells were stained as described by Kim and Tucker (2006), and deconvolution was performed according to Kuhn and Poenie (2002) and Combs et al (2006).
Molecular and cellular biology
Mutant forms of Bright were generated using the site directed mutagenesis kit (Stratagene, CA) and transferred into the retroviral construct pVxy (Ngo et al, 2006).
In vitro translation, sumoylation assays and transduction of B cells were performed as described (Kienker et al, 1998; Rosas-Acosta et al, 2005a, 2005b; Kim and Tucker, 2006). Specificity of sumoylation reactions was confirmed by cleavage of modified Bright by Ulp-1 (Li and Hochstrasser (2003). Stabilisation of Sumo-1 modified Bright was achieved by alkylation with iodoacetic acid sodium salt (Byrd and Hruby, 2005).
To assay for palmitoylation, WT and mutant forms of Bright as well as VSV-G were transfected into Cos-7 cells and processed as described (Rose et al, 1984; α-VSV was kindly provided by Dr Michael G Roth, U.T. Southwestern Medical Center, Dallas; Yu and Roth, 2002).
Preparation of stable retrovirally transduced dominant-negative B-cell lines
Stable transductants were established by employment of the Phoenix-A retroviral system. We plated 3 × 105 amphitrophic Phoenix-A packaging cells in 4 ml of DMEM supplemented with 10% fetal bovine serum (FBS) in 60-mm plates. After one day of culture, cells were transfected using pBabe constructs using FuGene6, and viral supernatant was harvested 2 days post-transfection, centrifuged, and filtered to remove live cells and debris. Target cells (3 × 105) were plated into 60-mm plates and growth medium was replaced with viral mixture. Stable cell lines were established by selection with 2 μg/ml of puromycin from day 2 post-infection.
Transcriptional analysis
The transcriptional activity of WT Bright and mutant forms were assayed by luciferase assays (Kim and Tucker, 2006; Rajaiya et al, 2006). Raji or Ramos cells (∼5 × 105) stably transduced with empty vector, WT or one of the mutant forms of Bright (401KIKK/AIAA, C342S or C342D) were mixed with 125 ng of pRL (Renilla) and 750 ng of either pGL3 or pGL3btp in 300 μl of RPMI. Cells were incubated for 15 min at room temperature, transferred into electroporation cuvettes and subjected to electroporation at 975 μF and 260 V. Electroporated cells were left in the cuvette at room temperature for 15 min and cultured in RPMI complete growth medium for 5 h. Cells were then treated with LPS (20 μg/ml) for 3 days in complete growth medium to stimulate cofactors required for Bright transactivation (Nixon et al, 2004a). Anti-IgM stimulation was performed for 5 min using 500pg α-μ for 5 × 105 cells. Luciferase activity was then measured and normalised according to the Dual Luciferase Reporter Assay Kit from Promega.
B-cell stimulation
To measure signalling effects at low doses of anti-IgM where receptor internalisation is minimised, we used monoclonal anti-IgM antibodies in the absence of secondary cross-linking (please see Supplementary Figures 2B, 4B and 5 for optimisation and further considerations). To stimulate B cells, 500 ng of F(ab')2 fragments of α-μ (clone JDC-15; Dako [α-human]; OB1022; Southern Biotech [α-mouse]) and α-CD19 (clone HD37; Dako [α-human]; clone SJ25-C1 [α-mouse]) were added to 5 × 108 cells for 5 min at 37°C (or other times as indicated in the figures). We determined by FACS analysis (data not shown) and semi-quantitative western of lipid raft-associated mIgM (Supplementary Figure 5A) that under these conditions ∼1–5% of mIgM in rafts and membranes are engaged.
Measurement of free intracellular calcium
Approximately 1 × 106 cells/ml were loaded with 2 μM Indo-1 (Molecular Probes) in HEPES buffered RPMI/1% FBS (pH 7.1) for 30 min at 37°C and stimulated successively with 1 ng or 40 μg (indicated in Figures 4B and 6A as low or high α-μ, respectively. Fluorescence was excited at 340 and 380 nm, and the resulting signal at each excitation wavelength was plotted against time. Internal calibration was performed using final concentrations of 2 μM Ionomycin, 40 μM Digitonin, 10 mM EGTA/pH 7 and 20 mM Tris exactly as described (Vorndran et al, 1995). At the end of each calcium trace, dye calibration to insure comparable loading and dye responses were performed by treatment with 2 μM ionomycin followed by 40 μM digitonin to release Indo-1 into the medium that contains 1 mM calcium. These levels of calcium saturate the Indo-1 to give the apparent Rmax. Subsequently, excess EGTA/pH7 (10 mM) is added, followed by 20 mM Tris base to convert EGTA from the -2 or -1 form at pH 7 (and below) to the -4 form, greatly increasing its chelating ability. That this procedure was sufficient to achieve Rmin was confirmed by the fact that, in the presence of a large excess of EGTA (10 mM), further additions of Tris base (i.e., beyond two equivalents) did not lower Indo-1 ratios.
Preparation of lipid rafts
Approximately 500 mg of wet cell pellet were washed twice in ice-cold phosphate buffered solution (PBS) and homogenised in 5 ml of 10 mM Tris/Cl (pH 7.4), 1 mM EDTA, 250 mM sucrose, 1 mM phenylmethylsulfonyl fluoride and 1 μg/ml leupeptin (all from Sigma, St Louis, Montana) in a tightly fitted Dounce homogeniser using five strokes (Shelton et al, 1982; Short and Barr, 2000). The resulting homogenate was centrifuged at 900 g for 10 min at 4°C, the resulting supernatant was then subjected to centrifugation at 110 000 g for 90 min at 4°C (Nagamatsu et al, 1992). The resulting membrane pellet was resuspended in ice cold 500 μl TNE buffer (10 mM Tris/Cl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 1% Triton X-100 [Sigma], 10 × protease inhibitors [Complete tablets, Roche, Indianapolis, IN]). Sucrose gradients for the preparation of lipid rafts were assembled exactly as described in an earlier publication (Fuentes-Pananá et al, 2005). Lipid rafts were isolated by flotation on discontinuous sucrose gradients. Membrane pellets were extracted for 30 min on ice in TNE buffer. For the discontinuous sucrose gradient, 1 ml of cleared supernatant was mixed with 1 ml of 85% sucrose in TNE and transferred to the bottom of an ultracentrifugation tube, followed by overlay with 6 ml of 35% sucrose in TNE and 3.5 ml of 5% sucrose in TNE. Samples were spun at 200 000 g for 30 h at 4°C; fractions were collected from the top of the gradient and analysed using western blotting and/or coimmunoprecipitation, as described in an earlier publication (Kim and Tucker, 2006).
Immunoprecipitation/western analyses
Unabridged BCR complexes have been successfully immunoprecipitated using either stringent RIPA-based buffers (Indraccolo et al, 2002; Gazumyan et al, 2006) or mild conditions, such as Digitonin or NP-40 (Hombach et al, 1988; Batista et al, 1996). We used a stringent RIPA formulation of 500 mM NaCl; 10 mM Tris–Cl pH 8; 0.1% SDS; 5 mM EDTA, pH 8; 10 × protease inhibitor (Complete tablet, Roche) to solubilise lipid rafts for subsequent immunoprecipitation experiments. Briefly, buoyant fractions, taken from the discontinuous gradient centrifugation, were pooled and incubated with the same volume of RIPA buffer on ice for 15 min. Resulting extracts were pre-cleared by rocking with 1 ml of a 5% slurry of RIPA equilibrated Protein A beads CL-4B (Amersham Pharmacia, Uppsala) for 4 h at 4°C and removal of the precipitate. The resulting supernatant was then subjected to IP/western assays as described (Kim and Tucker, 2006). The following antibodies were used: α-CD19 (clone 6D5, Dako), α-Bright, α-IgM (BD Pharmingen), α-V5 (Sigma), α-Raftlin (Dr Akihiko Yoshimura; Fukuoka, Japan; Saeki et al, 2003), α-Sumo-1 (Sigma), α-phosphotyrosine (Sigma), α-caspase-3 (Cell Signalling) and anti-TFII-I (kindly provided by Dr Carol Webb; Rajaiya et al, 2005).
Apoptosis
To assay for DNA fragmentation, 1 × 108 cells were treated with 100 ng α-μ for 72 h and processed as described by Duke et al (1983). In sum, cells were harvested by centrifugation (200 g; 10 min at room temperature), washed twice with PBS and resuspended in 2 ml lysis buffer (100 mM NaCl, 10 mM Tris, 1 mM EDTA, 0.5% NP-40, and 0.5% SDS, pH 7.4) for 10 min at room temperature. DNA was extracted twice with an equal volume of phenol followed by a single extraction with an equal volume of chloroform. DNA fragments were resolved in a 0.75% agarose gel in TBE running buffer (90 mM Tris, 90 mM boric acid, 1.5 mM EDTA pH 8.4) and visualised by staining with ethidium bromide and photography under UV illumination.
Note added in proof
Nixon et al (2008) recently reported that dominant inhibition of Bright DNA binding activity in vivo inhibits antibody response to phosphorylcholine of B1 B cells-a subset whose development and function are highly dependent on Btk signaling. These data provide additional support for a mechanistic link between Btk and both nuclear and lipid rafts-localized Bright.
Supplementary Material
Supplementary Video 1
Supplementary Video 2
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figures 1–5
Acknowledgments
The authors are indebted to Paul Das for administrative assistance. We thank Chhaya Das, Maya Ghosh and June V Harriss for expert technical assistance. We thank Martin P Kracklauer and Dr Mark A Brown for critically reading the manuscript and all members of the Tucker lab for comments on the paper. SM is the recipient of an Undergraduate Research Fellowship from UT Austin. Financial aid from the NIH is acknowledged by GCI (CA110624), CFW (AI044215), MP (AA015437) and PWT (Marie Betzner Morrow Centennial Endowment, NIH grants AI64886 and CA031534).
References
- Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR (2001) Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol 167: 6834–6840 [DOI] [PubMed] [Google Scholar]
- Allman D, Pillai S (2008) Peripheral B cell subsets. Curr Opin Immunol 20: 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashery U, Yizhar O, Rotblat B, Kloog Y (2006) Nonconventional trafficking of Ras associated with Ras signal organization. Traffic 7: 119–126 [DOI] [PubMed] [Google Scholar]
- Atencio S, Amano H, Izui S, Kotzin BL (2004) Separation of the New Zealand Black genetic contribution to lupus from New Zealand Black determined expansions of marginal zone B and B1a cells. J Immunol 172: 4159–4166 [DOI] [PubMed] [Google Scholar]
- Batista FD, Anand S, Presani G, Efremov DG, Burrone OR (1996) The two membrane isoforms of human IgE assemble into functionally distinct B cell antigen receptors. J Exp Med 184: 2197–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi M, Berger C, Sigrist JA, Bonanomi A, Sobek J, Niggli FK, Nadal D (2006) Quantitative profiling of housekeeping and Epstein-Barr virus gene transcription in Burkitt lymphoma cell lines using an oligonucleotide microarray. Virol J 3: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossis G, Melchior F (2006) Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell 21: 349–357 [DOI] [PubMed] [Google Scholar]
- Byrd CM, Hruby DE (2005) Development of an in vitro cleavage assay system to examine vaccinia virus I7L cysteine proteinase activity. Virol J 2: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RG, Wilson JB, Anderson SJ, Longnecker R (1998) Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9: 405–411 [DOI] [PubMed] [Google Scholar]
- Caraveo G, van Rossum DB, Patterson RL, Snyder SH, Desiderio S (2006) Action of TFII-I outside the nucleus as an inhibitor of agonist-induced calcium entry. Science 314: 122–125 [DOI] [PubMed] [Google Scholar]
- Cariappa A, Boboila C, Moran ST, Liu H, Shi HN, Pillai S (2007) The recirculating B cell pool contains two functionally distinct, long-lived, posttransitional, follicular B cell populations. J Immunol 179: 2270–2281 [DOI] [PubMed] [Google Scholar]
- Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S (2001) The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity 14: 603–615 [DOI] [PubMed] [Google Scholar]
- Carter RH, Fearon DT (1992) CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science 256: 105–107 [DOI] [PubMed] [Google Scholar]
- Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K (2004) B cell receptor signal strength determines B cell fate. Nat Immunol 5: 317–327 [DOI] [PubMed] [Google Scholar]
- Cerimele F, Battle T, Lynch R, Frank DA, Murad E, Cohen C, Macaron N, Sixbey J, Smith K, Watnick RS, Eliopoulos A, Shehata B, Arbiser JL (2005) Reactive oxygen signaling and MAPK activation distinguish Epstein-Barr Virus (EBV)-positive versus EBV-negative Burkitt's lymphoma. Proc Natl Acad Sci USA 102: 175–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Wang HG, Srinivasula SM, Alnemri ES, Cooper NR (1999) B cell apoptosis triggered by antigen receptor ligation proceeds via a novel caspase-dependent pathway. J Immunol 163: 2483–2491 [PubMed] [Google Scholar]
- Cherukuri A, Cheng PC, Sohn HW, Pierce SK (2001) The CD19/CD21 complex functions to prolong B cell antigen receptor signaling from lipid rafts. Immunity 14: 169–179 [DOI] [PubMed] [Google Scholar]
- Chung JB, Baumeister MA, Monroe JG (2001) Cutting edge: differential sequestration of plasma membrane-associated B cell antigen receptor in mature and immature B cells into glycosphingolipid-enriched domains. J Immunol 166: 736–740 [DOI] [PubMed] [Google Scholar]
- Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, Poenie M (2006) Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci USA 103: 14883–14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath I, Roundy KM, Weis JJ, Weis JH (2007) Defining in vivo transcription factor complexes of the murine CD21 and CD23 genes. J Immunol 178: 7139–7150 [DOI] [PubMed] [Google Scholar]
- Depoil D, Fleire S, Treanor BL, Weber M, Harwood NE, Marchbank KL, Tybulewicz VL, Batista FD (2008) CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol 9: 63–72 [DOI] [PubMed] [Google Scholar]
- Dintzis HM, Dintzis RZ, Vogelstein B (1976) Molecular determinants of immunogenicity: the immunon model of immune response. Proc Natl Acad Sci USA 73: 3671–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke RC, Chervenak R, Cohen JJ (1983) Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acac Sci USA 80: 6361–6365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK (2003) Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol 21: 457–481 [DOI] [PubMed] [Google Scholar]
- Fell HP, Smith RG, Tucker PW (1986) Molecular analysis of the t(2;14) translocation of childhood chronic lymphocytic leukemia. Science 232: 491–494 [DOI] [PubMed] [Google Scholar]
- Fuentes-Pananá EM, Bannish G, van der Voort D, King LB, Monroe JG (2005) Igα/Igβ complexes generate signals for B cell development independent of selective plasma membrane compartmentalization. J Immunol 174: 1245–1252 [DOI] [PubMed] [Google Scholar]
- Gauld SB, Dal Porto JM, Cambier JC (2002) B cell antigen receptor signaling: roles in cell development and disease. Science 296: 1641–1642 [DOI] [PubMed] [Google Scholar]
- Gazumyan A, Reichlin A, Nussenzweig MC (2006) Igβ tyrosine residues contribute to the control of B cell receptor signaling by regulating receptor internalization. J Exp Med 203: 1785–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocke CB, Yu H, Kang J (2005) Systematic identification and analysis of mammalian small ubiquitin-like modifier substrates. J Biol Chem 280: 500–512 [DOI] [PubMed] [Google Scholar]
- Goodnow CC (2007) Multistep pathogenesis of autoimmune disease. Cell 130: 25–35 [DOI] [PubMed] [Google Scholar]
- Grimaldi CM, Hill L, Xu X, Peeva E, Diamond B (2005) Hormonal modulation of B cell development and repertoire selection. Mol Immunol 42: 811–820 [DOI] [PubMed] [Google Scholar]
- Guo B, Rothstein TL (2005) B cell receptor (BCR) cross-talk: IL-4 creates an alternate pathway for BCR-induced ERK activation that is phosphatidylinositol 3-kinase independent. J Immunol 174: 5375–5381 [DOI] [PubMed] [Google Scholar]
- Guo S, Ferl GZ, Deora R, Riedinger M, Yin S, Kerwin JL, Loo JA, Witte ON (2004) A phosphorylation site in Bruton's tyrosine kinase selectively regulates B cell calcium signaling efficiency by altering phospholipase C-γ activation. Proc Natl Acad Sci USA 101: 14180–14185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Wollscheid B, Watts JD, Scheer B, Aebersold R, DeFranco AL (2006) Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat Immunol 7: 625–633 [DOI] [PubMed] [Google Scholar]
- Hakre S, Tussie-Luna MI, Ashworth T, Novina CD, Settleman J, Sharp PA, Roy AL (2006) Opposing functions of TFII-I spliced isoforms in growth factor-induced gene expression. Mol Cell 24: 301–308 [DOI] [PubMed] [Google Scholar]
- Hardy RR, Hayakawa K, Haaijman J, Herzenberg LA (1982) B-cell subpopulations identified by two-colour fluorescence analysis. Nature 297: 589–591 [DOI] [PubMed] [Google Scholar]
- Harwood NE, Batista FD (2008) New insights into the early molecular events underlying B cell activation. Immunity 28: 609–619 [DOI] [PubMed] [Google Scholar]
- Herrscher RF, Kaplan MH, Lelsz DL, Das C, Scheuermann R, Tucker PW (1995) The immunoglobulin heavy-chain matrix-associating regions are bound by Bright: a B cell-specific trans-activator that describes a new DNA-binding protein family. Genes Dev 9: 3067–3082 [DOI] [PubMed] [Google Scholar]
- Hoek KL, Antony P, Lowe J, Shinners N, Sarmah B, Wente SR, Wang D, Gerstein RM, Khan WN (2006) Transitional B cell fate is associated with developmental stage-specific regulation of diacylglycerol and calcium signaling upon B cell receptor engagement. J Immunol 177: 5405–5413 [DOI] [PubMed] [Google Scholar]
- Hombach J, Leclercq L, Radbruch A, Rajewsky K, Reth M (1988) A novel 34kD protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J 7: 3451–3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indraccolo S, Minuzzo S, Zamarchi R, Calderazzo F, Piovan E, Amadori A (2002) Alternatively spliced forms of Igα and Igβ prevent B cell receptor expression on the cell surface. Eur J Immunol 32: 1530–1540 [DOI] [PubMed] [Google Scholar]
- Kahyo T, Nishida T, Yasuda H (2001) Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Mol Cell 8: 713–718 [DOI] [PubMed] [Google Scholar]
- Kanayama N, Cascalho M, Ohmori H (2005) Analysis of marginal zone B cell development in the mouse with limited B cell diversity: role of the antigen receptor signals in the recruitment of B cells to the marginal zone. J Immunol 174: 1438–1445 [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Zong RT, Herrscher RF, Scheuermann RH, Tucker PW (2001) Transcriptional activation by a matrix associating region-binding protein. contextual requirements for the function of bright. J Biol Chem 276: 21325–21330 [DOI] [PubMed] [Google Scholar]
- Kienker LJ, Ghosh MR, Tucker PW (1998) Regulatory elements in the promoter of a murine TCRD V gene segment. J Immunol 161: 791–804 [PubMed] [Google Scholar]
- Kim D, Probst L, Das C, Tucker PW (2007) REKLES is an ARID3-restricted multifunctional domain. J Biol Chem 282: 15768–15777 [DOI] [PubMed] [Google Scholar]
- Kim D, Tucker PW (2006) A regulated nucleocytoplasmic shuttle contributes to Bright's function as a transcriptional activator of immunoglobulin genes. Mol Cell Biol 26: 2187–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn JR, Poenie M (2002) Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity 16: 111–121 [DOI] [PubMed] [Google Scholar]
- Laskov R, Berger N, Scharff MD, Horwitz MS (2006) Tumor necrosis factor-α and CD40L modulate cell surface morphology and induce aggregation in Ramos Burkitt's lymphoma cells. Leuk Lymphoma 47: 507–519 [DOI] [PubMed] [Google Scholar]
- Li SJ, Hochstrasser M (2003) The Ulp1 SUMO isopeptidase: distinct domains required for viability, nuclear envelope localization, and substrate specificity. J Cell Biol 160: 1069–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Martin F, Oliver AM, Kearney JF, Carter RH (2001) Antigen receptor proximal signaling in splenic B-2 cell subsets. J Immunol 166: 3122–3129 [DOI] [PubMed] [Google Scholar]
- Lin D, Ippolito GC, Zong RT, Bryant J, Koslovsky J, Tucker P (2007) Bright/ARID3A contributes to chromatin accessibility of the immunoglobulin heavy chain enhancer. Mol Cancer 6: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ippolito GC, Wall JK, Niu T, Probst L, Lee BS, Pulford K, Banham AH, Stockwin L, Shaffer AL, Staudt LM, Das C, Dyer MJ, Tucker PW (2006) Functional studies of BCL11A: characterization of the conserved BCL11A-XL splice variant and its interaction with BCL6 in nuclear paraspeckles of germinal center B cells. Mol Cancer 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Lortan JE, Oldfield S, MacLennan IC (1988) CBA/N mice have marginal zone B cells with normal surface immunoglobulin phenotype. Adv Exp Med Biol 237: 105–111 [DOI] [PubMed] [Google Scholar]
- Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R (1999) B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med 190: 75–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Kearney JF (2000) Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity 12: 39–49 [DOI] [PubMed] [Google Scholar]
- Matthias P, Rolink AG (2005) Transcriptional networks in developing and mature B cells. Nat Rev Immunol 5: 497–508 [DOI] [PubMed] [Google Scholar]
- McConnell FM, Shears SB, Lane PJ, Scheibel MS, Clark EA (1992) Relationships between the degree of cross-linking of surface immunoglobulin and the associated inositol 1,4,5-trisphosphate and Ca2+ signals in human B cells. Biochem J 284: 447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ (2008) Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med 205: 155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Longnecker R, Kieff E (1993) Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J Virol 67: 3087–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu S, Kornhauser JM, Burant CF, Seino S, Mayo KE, Bell GI (1992) Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem 26: 467–472 [PubMed] [Google Scholar]
- Ngo VN, Davis RE, Lamy L, Yu X, Zhao H, Lenz G, Lam LT, Dave S, Yang L, Powell J, Staudt LM (2006) A loss-of-function RNA interference screen for molecular targets in cancer. Nature 441: 106–110 [DOI] [PubMed] [Google Scholar]
- Niiro H, Clark EA (2002) Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol 2: 945–956 [DOI] [PubMed] [Google Scholar]
- Nixon JC, Rajaiya J, Webb CF (2004a) Mutations in the DNA-binding domain of the transcription factor Bright act as dominant negative proteins and interfere with immunoglobulin transactivation. J Biol Chem 279: 52465–52472 [DOI] [PubMed] [Google Scholar]
- Nixon JC, Ferrell S, Miner C, Oldham AL, Hochgeschwender U, Webb CF (2008) Transgenic mice expressing dominant-negative bright exhibit defects in B1 B cells. J Immunol 181: 6913–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon JC, Rajaiya JB, Ayers N, Evetts S, Webb CF (2004b) The transcription factor, Bright, is not expressed in all human B lymphocyte subpopulations. Cell Immunol 228: 42–53 [DOI] [PubMed] [Google Scholar]
- Nore BF, Vargas L, Mohamed AJ, Brandén LJ, Bäckesjö CM, Islam TC, Mattsson PT, Hultenby K, Christensson B, Smith CI (2000) Redistribution of Bruton's tyrosine kinase by activation of phosphatidylinositol 3-kinase and Rho-family GTPases. Eur J Immunol 30: 145–154 [DOI] [PubMed] [Google Scholar]
- Novina CD, Kumar S, Bajpai U, Cheriyath V, Zhang K, Pillai S, Wortis HH, Roy AL (1999) Regulation of nuclear localization and transcriptional activity of TFII-I by Bruton's tyrosine kinase. Mol Cell Biol 19: 5014–5024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K (2007) B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity 27: 35–48 [DOI] [PubMed] [Google Scholar]
- Patterson HC, Kraus M, Kim YM, Ploegh H, Rajewsky K (2006) The B cell receptor promotes B cell activation and proliferation through a non-ITAM tyrosine in the Igα cytoplasmic domain. Immunity 25: 55–65 [DOI] [PubMed] [Google Scholar]
- Petro JB, Gerstein RM, Lowe J, Carter RS, Shinners N, Khan WN (2002) Transitional type 1 and 2 B lymphocyte subsets are differentially responsive to antigen receptor signaling. J Biol Chem 277: 48009–48019 [DOI] [PubMed] [Google Scholar]
- Putnam MA, Moquin AE, Merrihew M, Outcalt C, Sorge E, Caballero A, Gondré-Lewis TA, Drake JR (2003) Lipid raft-independent B cell receptor-mediated antigen internalization and intracellular trafficking. J Immunol 170: 905–912 [DOI] [PubMed] [Google Scholar]
- Quinn WJ, Noorchashm N, Crowley JE, Reed AJ, Noorchashm H, Naji A, Cancro MP (2006) Cutting edge: impaired transitional B cell production and selection in the nonobese diabetic mouse. J Immunol 176: 7159–7164 [DOI] [PubMed] [Google Scholar]
- Rajaiya J, Hatfield M, Nixon JC, Rawlings DJ, Webb CF (2005) Bruton's tyrosine kinase regulates immunoglobulin promoter activation in association with the transcription factor Bright. Mol Cell Biol 25: 2073–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaiya J, Nixon JC, Ayers N, Desgranges ZP, Roy AL, Webb CF (2006) Induction of immunoglobulin heavy-chain transcription through the transcription factor Bright requires TFII-I. Mol Cell Biol 26: 4758–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA (2005) Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell 121: 37–47 [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Bolland S (2001) IgG Fc receptors. Annu Rev Immunol 19: 275–290 [DOI] [PubMed] [Google Scholar]
- Rosas-Acosta G, Langereis MA, Deyrieux A, Wilson VG (2005a) Proteins of the PIAS family enhance the sumoylation of the papillomavirus E1 protein. Virology 331: 190–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Acosta G, Russell WK, Deyrieux A, Russell DH, Wilson VG (2005b) A universal strategy for proteomic studies of SUMO and other ubiquitin-like modifiers. Mol Cell Proteomics 4: 56–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Adams GA, Gallione CJ (1984) The presence of cysteine in the cytoplasmic domain of the vesicular stomatitis virus glycoprotein is required for palmitate addition. Proc Natl Acad Sci USA 81: 2050–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki K, Miura Y, Aki D, Kurosaki T, Yoshimura A (2003) The B cell-specific major raft protein, Raftlin, is necessary for the integrity of lipid raft and BCR signal transduction. EMBO J 22: 3015–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson DA, Wang M, Matunis MJ (2001) The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem 276: 21664–21669 [DOI] [PubMed] [Google Scholar]
- Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E (2005) Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med 201: 1659–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz SE, Matuschewski K, Liakopoulos D, Scheffner M, Jentsch S (1998) The ubiquitin-like proteins SMT3 and SUMO-1 are conjugated by the UBC9 E2 enzyme. Proc Natl Acad Sci USA 95: 560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal PB, Guo GG, Shah M, Kumar V, Patel K (2002) Cytokine signaling: STATS in plasma membrane rafts. J Biol Chem 277: 12067–12074 [DOI] [PubMed] [Google Scholar]
- Shankar M, Nixon JC, Maier S, Workman J, Farris AD, Webb CF (2007) Antinuclear antibody production and autoimmunity in transgenic mice that overexpress the transcription factor Bright. J Immunol 178: 2996–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KR, Guthrie VH, Cochran DL (1982) Oligomeric structure of the major nuclear envelope protein lamin B. J Biol Chem 257: 4328–4332 [PubMed] [Google Scholar]
- Short B, Barr FA (2000) The Golgi apparatus. Curr Biol 10: R583–R585 [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39 [DOI] [PubMed] [Google Scholar]
- Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE (2005) Identification and characterization of circulating human transitional B cells. Blood 105: 4390–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn HW, Tolar P, Jin T, Pierce SK (2006) Fluorescence resonance energy transfer in living cells reveals dynamic membrane changes in the initiation of B cell signaling. Proc Natl Acad Sci USA 103: 8143–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproul TW, Malapati S, Kim J, Pierce SK (2000) Cutting edge: B cell antigen receptor signaling occurs outside lipid rafts in immature B cells. J Immunol 165: 6020–6023 [DOI] [PubMed] [Google Scholar]
- Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK, Brodsky FM (2002) Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17: 451–462 [DOI] [PubMed] [Google Scholar]
- Su TT, Guo B, Wei B, Braun J, Rawlings DJ (2004) Signaling in transitional type 2 B cells is critical for peripheral B-cell development. Immunol Rev 197: 161–178 [DOI] [PubMed] [Google Scholar]
- Su TT, Rawlings DJ (2002) Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J Immunol 168: 2101–2110 [DOI] [PubMed] [Google Scholar]
- Tang Z, El Far O, Betz H, Scheschonka A (2005) Pias1 interaction and sumoylation of metabotropic glutamate receptor 8. J Biol Chem 280: 38153–38159 [DOI] [PubMed] [Google Scholar]
- Teague BN, Pan Y, Mudd PA, Nakken B, Zhang Q, Szodoray P, Kim-Howard X, Wilson PC, Farris AD (2007) Cutting edge: Transitional T3 B cells do not give rise to mature B cells, have undergone selection, and are reduced in murine lupus. J Immunol 178: 7511–7515 [DOI] [PubMed] [Google Scholar]
- Venkatesh J, Peeva E, Xu X, Diamond B (2006) Cutting edge: hormonal milieu, not antigenic specificity, determines the mature phenotype of autoreactive B cells. J Immunol 176: 3311–3314 [DOI] [PubMed] [Google Scholar]
- Vorndran C, Minta A, Poenie M (1995) New fluorescent calcium indicators designed for cytosolic retention or measuring calcium near membranes. Biophys J 69: 2112–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gregory C, Sample C, Rowe M, Liebowitz D, Murray R, Rickinson A, Kieff E (1990) Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol 64: 2309–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C, Zong RT, Lin D, Wang Z, Kaplan M, Paulin Y, Smith E, Probst L, Bryant J, Goldstein A, Scheuermann R, Tucker P (1999) Differential regulation of immunoglobulin gene transcription via nuclear matrix-associated regions. Cold Spring Harb Symp Quant Biol 64: 109–118 [DOI] [PubMed] [Google Scholar]
- Webb CF, Das C, Eaton S, Calame K, Tucker PW (1991a) Novel protein-DNA interactions associated with increased immunoglobulin transcription in response to antigen plus interleukin-5. Mol Cell Biol 11: 5197–5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CF, Das C, Eneff KL, Tucker PW (1991b) Identification of a matrix-associated region 5′ of an immunoglobulin heavy chain variable region gene. Mol Cell Biol 11: 5206–5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CF, Smith EA, Medina KL, Buchanan KL, Smithson G, Dou S (1998) Expression of bright at two distinct stages of B lymphocyte development. J Immunol 160: 4747–4754 [PubMed] [Google Scholar]
- Webb CF, Yamashita Y, Ayers N, Evetts S, Paulin Y, Conley ME, Smith EA (2000) The transcription factor Bright associates with Bruton's tyrosine kinase, the defective protein in immunodeficiency disease. J Immunol 165: 6956–6965 [DOI] [PubMed] [Google Scholar]
- Welner RS, Pelayo R, Kincade PW (2008) Evolving views on the genealogy of B cells. Nat Rev Immunol 8: 95–106 [DOI] [PubMed] [Google Scholar]
- Wilsker D, Probst L, Wain HM, Maltais L, Tucker PW, Moran E (2005) Nomenclature of the ARID family of DNA-binding proteins. Genomics 86: 242–251 [DOI] [PubMed] [Google Scholar]
- Yu S, Roth MG (2002) Casein kinase I regulates membrane binding by ARF GAP1. Mol Biol Cell 13: 2559–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang L, Luo Y, Roeder RG (2001) Identification and characterization of a novel OCA-B isoform. Implications for a role in B cell signaling pathways. Immunity 14: 157–167 [PubMed] [Google Scholar]
- Yu X, Siegel R, Roeder RG (2006) Interaction of the B cell-specific transcriptional coactivator OCA-B and Galectin-1 and a possible role in regulating BCR-mediated B cell proliferation. J Biol Chem 281: 15505–15516 [DOI] [PubMed] [Google Scholar]
- Yurasov S, Hammersen J, Tiller T, Tsuiji M, Wardemann H (2005a) B-cell tolerance checkpoints in healthy humans and patients with systemic lupus erythematosus. Ann NY Acad Sci 1062: 165–174 [DOI] [PubMed] [Google Scholar]
- Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC (2005b) Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med 201: 703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong RT, Das C, Tucker PW (2000) Regulation of matrix attachment region-dependent, lymphocyte-restricted transcription through differential localization within promyelocytic leukemia nuclear bodies. EMBO J 19: 4123–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1
Supplementary Video 2
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figures 1–5